Abstract
Background/Objective:
High cervical spinal cord hemisection interrupts descending respiratory drive from the rostral ventral respiratory group in the medulla to the ipsilateral phrenic motoneurons. Hemisection results in the paralysis of the ipsilateral hemidiaphragm. Chronic administration of rolipram, a specific phosphodiesterase-IV inhibitor, promotes synaptic plasticity and restores phrenic nerve function after a high cervical spinal cord lesion. Here, we test the hypothesis that an acute administration of rolipram will increase spinal and medullary levels of 3′,5′-cyclic adenosine monophosphate (cAMP) and induce phrenic nerve recovery after cervical (C2) spinal cord hemisection.
Methods:
Male Sprague-Dawley rats were subjected to left C2 hemisection surgery 1 week before experimentation. Bilateral phrenic nerve activity was recorded in anesthetized, vagotomized, and pancuronium paralyzed rats, and rolipram was intravenously applied (2 mg/kg).
Results:
Intravenous administration of rolipram increased phrenic nerve output in uninjured control and left C2 spinal cord–hemisected rats. In addition, rolipram restored respiratory-related activity to the left phrenic nerve made quiescent by the hemisection. In both uninjured and hemisected rats, rolipram significantly enhanced phrenic inspiratory burst amplitude and burst area compared with predrug values. Also, rolipram concomitantly increased spinal and medullary cAMP.
Conclusions:
These results suggest that a phosphodiesterase inhibitor capable of elevating cAMP levels can enhance phrenic nerve output and restore respiratory-related phrenic nerve function after high cervical spinal cord injury. Thus, targeting the cAMP signaling cascade can be a useful therapeutic approach in promoting synaptic efficacy and respiratory recovery after cervical spinal cord injury.
Keywords: Spinal cord injuries, cervical; Cyclic adenosine monophosphate; Phosphodiesterase, inhibition; Respiration; Phrenic nerve, pathway; Rolipram; Theophylline; Rat
INTRODUCTION
Respiratory function is often compromised subsequent to high cervical spinal cord injury. In mammals, the diaphragm, the major inspiratory muscle, is innervated by phrenic motoneurons through the phrenic nerves. Phrenic motoneurons are located rostro-caudally in the cervical spinal cord in rats (C3–C6). Spinal cord injury above the level of the phrenic nucleus interrupts the major descending respiratory pathways from the rostral ventral respiratory group (rVRG) of neurons in the medulla to the phrenic motoneurons (1), consequently resulting in the paralysis of the diaphragm. In an animal model of spinal cord injury, it has been shown that a left spinal cord hemisection at the second cervical level disrupts the major bulbospinal respiratory pathways from the rVRG to the ipsilateral phrenic motoneurons and paralyzes the left hemidiaphragm (2,3). However, it has been shown that a functionally ineffective respiratory pathway that crosses the spinal midline caudal to the level of the hemisection can be strengthened either physiologically or pharmacologically to restore function to the once paralyzed hemidiaphragm (4–7).
Previously, it has been shown in our laboratory that, after a left C2 spinal cord hemisection, the ineffective respiratory pathway can be activated pharmacologically through the acute and chronic systemic administration of theophylline (6,8,9). Theophylline acts as a nonselective adenosine receptor antagonist and as an inhibitor of phosphodiesterase enzymes (10,11). Although theophylline has been used extensively for the treatment of respiratory problems such as asthma and chronic obstructive pulmonary disease (12,13), theophylline's mode of action in our model is not very apparent. Several studies have indicated that synaptic plasticity in a wide variety of species is dependent on signaling pathways stimulated by an increase in intracellular 3′,5′-cyclic monophosphate (cAMP) concentration (14–16). Also, elevation of cAMP levels through the administration of phosphodiesterase inhibitors has been shown to promote regeneration and functional recovery after spinal cord injury (17–20).
Recently, we have shown that, after a left C2 spinal cord lesion, chronic inhibition of phosphodiesterase activity (over a 3-day period) can induce long-lasting phrenic nerve recovery in the previously quiescent left phrenic nerve (21). Furthermore, other studies have implicated the cAMP signaling cascade as a possible stimulator of respiratory neurons (22,23). However, no one has examined the effect of acute phosphodiesterase inhibition on respiratory plasticity and phrenic nerve recovery after spinal cord injury.
Specifically, in this study, we tested the hypothesis that the acute systemic administration of the specific phosphodiesterase-IV inhibitor, rolipram, in left C2 spinal cord–hemisected rats will elevate cAMP levels in the spinal cord segments containing the phrenic nucleus and in the region of the ventral medulla containing the rVRG. Furthermore, we hypothesized that this upregulation of cAMP will be accompanied by phrenic nerve recovery.
METHODS
All animal husbandry and surgical procedures were approved by the Animal Investigation Committee at Wayne State University. Forty male Sprague-Dawley rats (Harlan, 3–6 months old) were divided equally into 4 groups. Rats in Groups 1 and 2 underwent left C2 spinal cord hemisections, whereas the other 2 groups served as uninjured controls.
Animals were anesthetized with an intraperitoneal injection of ketamine (70 mg/kg) and xylazine (20 mg/kg) and prepared for left C2 spinal cord hemisection surgery. Once the dorsal surface of the neck was shaved and cleansed with povidone-iodine solution and rubbing alcohol, a 3- to 4-cm midline incision was made to expose the paravertebral muscles. These muscles were separated in the midline and retracted laterally. After the retraction of the paravertebral muscles, a laminectomy of the second cervical vertebra and a durotomy of the underlying dura mater were performed to expose the spinal cord. Microscissors were used to make a left C2 spinal cord hemisection caudal to the left C2 dorsal roots. Care was taken to extend the lesion from the midline to the most lateral extend of the spinal cord. The overlaying muscles were sutured with polyglactin suture (3.0 Vicryl), and the skin was stapled closed with wound clips. Immediately after surgery, animals were given a subcutaneous injection of an analgesic (buprenorphine 0.01 mg/kg) dissolved in 10 mL of saline. The saline administration helped prevent dehydration after surgery until the animals were able to consume adequate amounts of water. Animals were placed on a heating blanket for a short period (30–60 minutes) and returned to individual cages and given food and water ad libitum.
One week after the hemisection, the hemisected rats (n = 20) and uninjured controls (n = 20) were anesthetized with urethane (1.6 g/kg intraperitoneally). Approximately 10 minutes before urethane administration, all rats were injected intramuscularly with atropine sulfate (0.01 mg/kg) to reduce secretions in the respiratory passages. The femoral vein and artery were cannulated to administer fluids and monitor blood pressure throughout the experiment, respectively. After tracheotomy, animals were ventilated using a rodent mechanical ventilator (Harvard Apparatus, model 683, Harvard Bioscience, Holliston, MA) supplied with an equal mixture of oxygen and air. The vagus nerves were sectioned bilaterally to eliminate mechanoreceptor feedback, and the animals were paralyzed with pancuronium bromide (0.5 mg/kg, IV). End-tidal CO2 (Model 1265, Capnogard, Novametrix, Wallingford, CT) and blood pressure (pressure monitor BP-1, World Precision Instruments, Sarasota, FL) were monitored throughout the experiment. Body temperature was monitored and maintained at 37 ± 1°C with a rectal thermometer and a heating pad.
Using a ventral approach, both the left and right phrenic nerves were isolated, desheathed, transected, and placed on silver bipolar electrodes. Phrenic nerve activity was amplified (10 K) and band pass filtered (0.1–3 kHz) using a Tektronix 502 AM amplifier (Tektronix, Beaverton, OR). Phrenic nerve signals were recorded and analyzed using a Cambridge Electronic Design data acquisition system and a Spike 2 computer program (v 4.13, Cambridge Electronic Design, Cambridge, UK).
Once the phrenic nerves were placed on the electrodes and before starting the experimental protocol, the rats were allowed to stabilize at an end-tidal CO2 value of 40 ± 3 mmHg for approximately 30 minutes. At this time, the apneic threshold was determined by slowly increasing the frequency and the volume of the ventilator until respiratory bursting ceased to occur. Once apnea was reached, the ventilator rate and volume were gradually decreased until phrenic bursting reappeared. The end-tidal CO2 at which phrenic bursting ceased was considered the apneic threshold, and thereafter the CO2 value was maintained at 4 to 5 mmHg above this value by adjusting the ventilator. Baseline phrenic nerve activity was recorded for approximately 20 minutes before the systemic administration of drugs through the femoral vein.
Once baseline phrenic nerve recording was established, left C2-hemisected rats received an intravenous injection of either rolipram (2 mg/kg, n = 10) or an equal volume of vehicle that was 10% dimethyl sulfoxide (DMSO) dissolved in saline (≤1 mL, n = 10). Similarly, the effect of rolipram (2 mg/kg, n = 10) or 10% DMSO dissolved in saline (≤1 mL, n = 10) on phrenic nerve output was monitored in uninjured control rats. Phrenic nerve activity was monitored for approximately 1 to 2 hours after drug application.
Phrenic nerve activity was rectified and integrated and quantitatively analyzed using spike 2 data analysis software. Integrated phrenic nerve waveforms were assessed for inspiratory burst frequency, burst amplitude, and burst area for a period of 30 seconds. To determine the effect of rolipram on phrenic nerve activity in uninjured control rats, comparisons of percent changes in inspiratory burst frequency, burst amplitude, and burst area were made before drug administration and then again after drug administration (when peak drug effects were stabilized approximately 30 minutes after drug application). The effects of rolipram administration on left phrenic nerve recovery were characterized as a percentage of right phrenic nerve output of the same animal before drug administration.
At the conclusion of electrophysiologic measurement of respiratory output, approximately 2 hours after rolipram administration, the ventral segments of the cervical spinal cord (C3–C6) containing the phrenic nucleus and the ventral medulla containing the neurons of the rVRG of uninjured (N = 6) and left C2-hemisected rats that were treated with either rolipram (N = 6) or vehicle control solution (N = 6) were rapidly dissected and frozen in liquid nitrogen for analysis of cAMP levels. Frozen tissue was ground in liquid nitrogen, weighed, and homogenized in a solution of 0.1 M HCl. The samples were centrifuged at 600g at room temperature and diluted in 0.1 M HCl. cAMP content was determined with a commercially available kit (CA201, cAMP Enzyme Immunoassay Kit, Sigma-Aldrich Co, St Louis, MO) based on an enzyme-linked immunoassay using a nonacetylation procedure.
All data are expressed as the mean ± SE, and statistics were performed using commercially available software (10.2, Systat, Richmond, CA). Paired Student's t test was used to determine the effect of drug on phrenic motor output in uninjured control rats. In left C2-hemisected rats, rolipram-induced effects were compared using a 1-way analysis of variance (ANOVA). Levels of cAMP in ventral cervical spinal segments (C3–C5) and medullae of uninjured control, hemisected, and rolipram-treated rats were also compared using a 1-way ANOVA. Statistical significance was set at P ≤ 0.05.
RESULTS
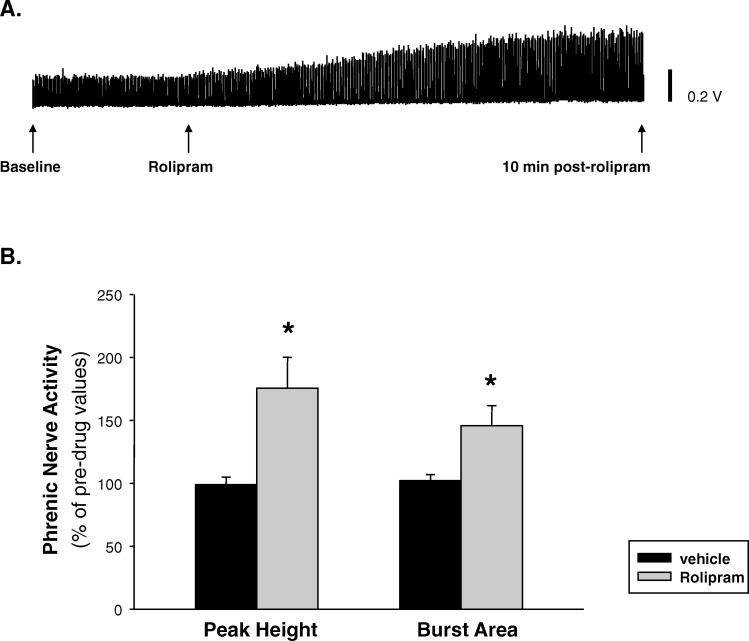
One week after the left C2-hemisection surgery, the apneic threshold of hemisected rats (33 ± 1 mmHg) did not significantly differ from the apneic threshold of uninjured control rats (34 ± 1 mmHg; P = 0.442). Mean arterial pressure at 1 and 30 minutes after saline treatment was 90 ± 8 and 87 ± 10 mmHg, respectively, and this was not significantly different from the baseline mean arterial pressure of 90 ± 8 mmHg. At 1 and 30 minutes after rolipram administration, mean arterial pressures were 91 ± 8 and 91 ± 9 mmHg, respectively. Rolipram also failed to alter mean arterial blood pressure compared with baseline (87 ± 10 mmHg). As mentioned in “Methods,” temperature was monitored and maintained at about 37 ± 1°C using a heating pad. There was no significant change in temperature in response to the drug treatment. In uninjured control rats, systemic administration of rolipram (2 mg/kg, IV) enhanced phrenic nerve activity in 9 of 10 animals. This enhancement in phrenic nerve output was evident as early as 10 minutes after rolipram administration (Figure 1). The rolipram-induced increase in phrenic nerve output of uninjured control rats was expressed as a percentage of the predrug baseline values. In uninjured control rats, rolipram significantly increased peak burst amplitude (P = 0.04) and burst area (P = 0.0041) compared with predrug values (Figure 1). Intravenous administration of vehicle control solution (10% DMSO in saline, 0.5–1.0 mL) did not alter phrenic nerve output (data not shown).
Figure 1. Effects of acute administration of rolipram (2 mg/kg) on phrenic nerve activity of uninjured control rats. (A) Example of phrenic nerve activity recorded during baseline, rolipram administration, and 10 minutes after rolipram administration in an uninjured rat. (B) Rolipram significantly enhanced inspiratory burst amplitude and area compared with predrug values. *P < 0.05.
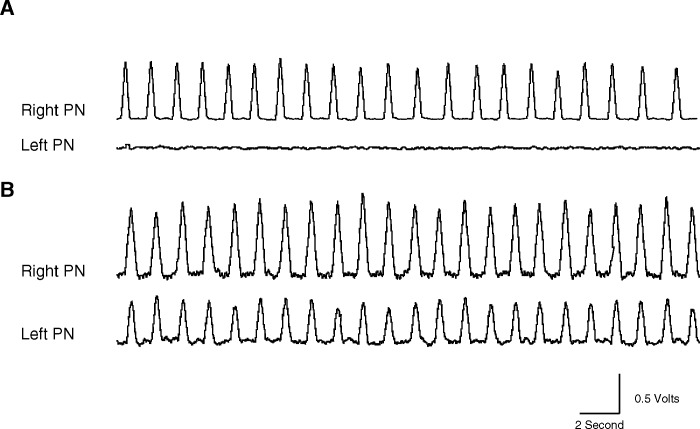
One week after injury and before the administration of rolipram, the right phrenic nerve in all left C2-hemisected rats showed distinct respiratory-related activity, whereas the left phrenic nerve had no discernible respiratory bursts, indicating that the major respiratory pathways were functionally interrupted by the hemisection. Figure 2A is a representative integrated neurogram tracing taken from a rat before drug administration showing a lack of respiratory-related activity in the left phrenic nerve.
Figure 2. Integrated waveform tracings from an animal 1 week after left C2 hemisection. (A) One week after left C2 hemisection and before drug administration, respiratory-related activity is present in the right phrenic nerve, whereas there is a lack of respiratory-related activity in the left phrenic nerve, showing that the hemisection was functionally complete. (B) Thirty minutes after rolipram (2 mg/kg) administration in the same animal, right phrenic nerve activity is enhanced, and respiratory-related activity is restored in the previously quiescent left phrenic nerve. PN, phrenic nerve.
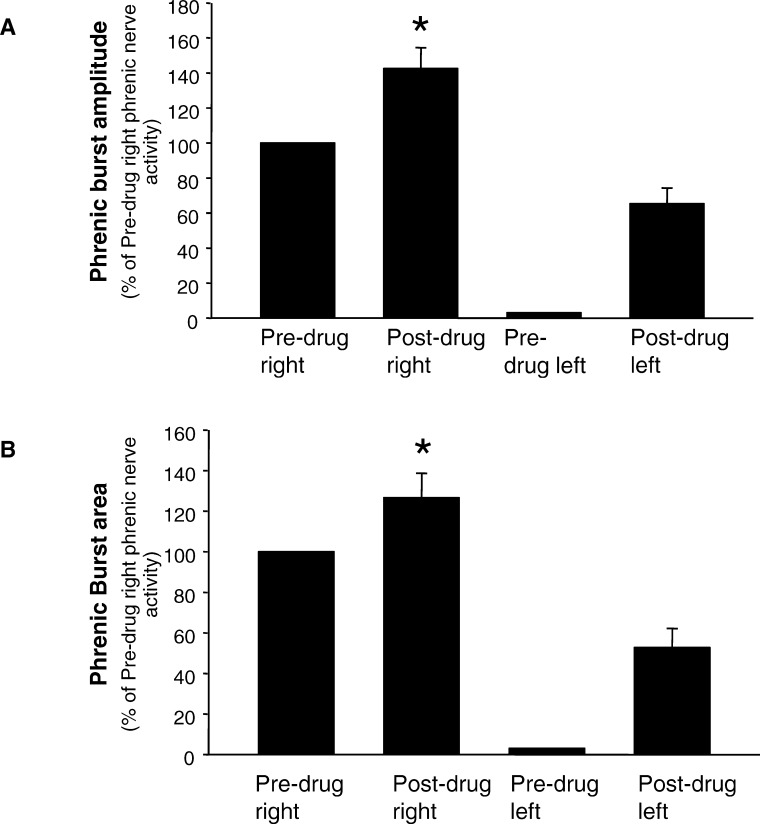
One week after the hemisection surgery, intravenous administration of rolipram resulted in the activation of respiratory-related activity ipsilateral to the hemisection in 8 of the 10 rats (Figure 2B). In 2 of the 10 hemisected rats, rolipram failed to induce activity in the phrenic nerve ipsilateral to the hemisection. However, the above-mentioned rats did exhibit an increase in phrenic nerve output contralateral to the hemisection. When rolipram-induced increases were seen on both right and left phrenic nerves, increases in the contralateral phrenic nerve output occurred earlier than the onset of respiratory-related bursting in the ipsilateral phrenic nerve. Rolipram-induced effects on the ipsilateral and contralateral phrenic nerves were maintained for up to 2 hours. At this time, the experiment was terminated. The restored activity in the left phrenic nerve was quantified and expressed as a percentage of predrug right phrenic nerve activity (Figure 3).
Figure 3. Effect of rolipram on the right and left phrenic nerves in hemisected rats. Both the right and left phrenic nerve activity after rolipram administration is expressed as a percent of predrug right phrenic nerve activity. Rolipram significantly increased peak amplitude and burst area bilaterally in hemisected rats. Bars represent mean ± SE. *P < 0.05.
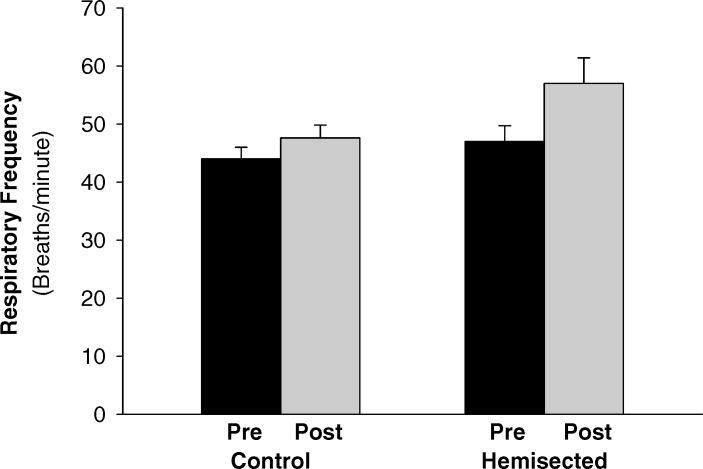
Rolipram administration increased both the inspiratory burst amplitude (P = 0.007) and area (P = 0.017) of the left phrenic nerve. In addition, rolipram-induced changes in right phrenic nerve activity was also quantified and expressed as a percentage of predrug right phrenic nerve activity (Figure 3). In response to intravenous administration of rolipram, contralateral phrenic nerve burst amplitude (P = 0.008) and burst area (P = 0.009) were also significantly enhanced. In uninjured control rats, rolipram administration did not significantly change respiratory frequency (P = 0.256; Figure 4). Similar to the uninjured control rats, rolipram did not significantly alter respiratory frequency in the hemisected animals (P = 0.146; Figure 4).
Figure 4. Changes in breathing frequency in response to acute rolipram administration in uninjured control and left C2-hemisected rats. Respiratory frequency was not significantly different from baseline in both uninjured control and left C2-hemisected rats. Bars represent mean ± SE. Significance was set at P < 0.05.
Finally, using ELISA, cAMP levels were measured in the ventral segments of the cervical spinal cord (C3–C6) containing the phrenic nucleus and in the ventral medulla containing the rVRG neurons. Levels of cAMP in the spinal cord and medulla of hemisected rats treated with saline or rolipram were compared with cAMP levels in uninjured control rats (Table 1; Figure 5). Hemisection surgery alone had no significant effect on cAMP levels in both the spinal cord and the medulla. Rolipram administration significantly increased cAMP levels in the ventral segments of the cervical spinal cord (C3–C6) compared with uninjured control rats (P = 0.0435) and hemisected rats treated with saline (P = 0.0213). Rolipram administration resulted in increasing medullary levels of cAMP (1.51 ± 0.21 pg/mg) compared with uninjured control rats (P = 0.0256). The specific values of cAMP in each group are shown in Table 1.
Table 1.
Effects of Rolipram on cAMP Concentrations (pg/mg) of Rat Cervical Spinal Cord (C3–C6) and Medulla
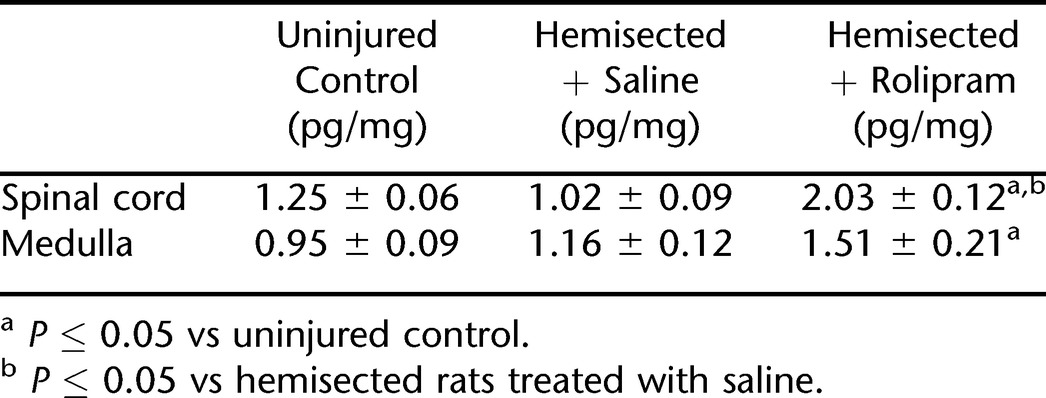
Figure 5. Effects of rolipram on cAMP concentrations of rat cervical spinal cord (C3–C6) and medulla. Bars represent mean ± SE. *P ≤ 0.05 vs uninjured control; #P < 0.05 vs hemisected rats treated with saline.
DISCUSSION
Spinal cord injuries occurring at the level of the cervical spinal cord often result in respiratory complications. Respiratory complications are among the leading causes of morbidity and mortality in individuals with spinal cord injuries (25–28). Most injuries are incomplete, and as such, there are usually axonal connections passing through the lesion that are spared after the injury. Over the years, using our hemisection model of spinal cord injury, we have shown that multiple approaches may be used to activate spared but latent motor pathways to restore respiratory motor function (4,5,7,29–32). The anesthetized, vagotomized, pancuronium paralyzed, and mechanically ventilated rat preparation has been used extensively by us and others to evaluate respiratory neural function after drug administration. Although the preparation may not reflect normal physiologic conditions, the results obtained with this approach have provided valuable information regarding respiratory mechanisms and the effect of various drugs on respiratory neural output. It is also well established that crossed phrenic activity detected in the phrenic nerve translates into both diaphragmatic and actual breathing function (30,33,34).
In a previous study, we showed that chronic administration of rolipram for 3 days leads to long-lasting restoration of phrenic nerve motor function after high cervical spinal cord hemisection. The results of this study show that the acute systemic administration of rolipram, a phosphodiesterase-IV–specific inhibitor, is capable of modulating cAMP levels and concomitantly increases phrenic motor output in uninjured control rats while restoring ipsilateral phrenic nerve activity in left C2-hemisected rats. Left C2 spinal cord hemisection had no significant effect on cAMP levels in both the ventral cervical spinal cord segments (C3–C6) containing the phrenic nucleus or the area of the medulla that contains the respiratory premotor neurons of the rVRG. However, administration of rolipram significantly increased levels of cAMP in both the medulla and the ventral cervical spinal cord segments of rolipram-treated rats.
Initially, we hypothesized that spinal cord injury would decrease cAMP levels in the cervical spinal cord because of the interruption of afferent connections. In another model of spinal cord injury, cAMP levels were found to be reduced for up to 2 weeks after injury in the spinal cord, sensorimotor cortex, and brainstem (18). Therefore, it was surprising that we did not find a significant change in cAMP levels both in the ventral segments of the cervical spinal cord and the medulla 1 week after the left C2-hemisection injury. However, the differences between our findings may be partially explained by the fact that, in our study, we measured cAMP levels in cervical spinal cord segments caudal to the injury site, whereas the above study examined cAMP levels rostral to the injury. Also, in the above study, the authors may have looked at the entire brainstem, whereas we only examined the ventrolateral medulla.
In this study, we were able to enhance phrenic motor output and promote phrenic nerve recovery ipsilateral to the spinal cord lesion through the acute administration of rolipram; however, the exact cellular events associated with the rolipram-induced effects on phrenic nerve output were not delineated. Nonetheless, we did find that an increase in cAMP levels in the spinal cord and medulla containing respiratory neurons resulted in respiratory recovery. This suggests the possibility that increasing cAMP levels leads to an increase in respiratory drive from the inspiratory centers in the medulla and/or strengthening spared ineffective synaptic connections at the level of the phrenic nucleus. Although we did see an increase in medullary cAMP levels associated with rolipram administration, we did not observe an enhancement in respiratory frequency, and as such, this does not directly implicate medullary plasticity, but we certainly cannot rule it out. However, the data do suggest strengthening of ineffective synaptic connections within the phrenic nucleus in the spinal cord.
In support of our observations, cAMP has been shown to play a critical role in a variety of forms of synaptic plasticity in numerous other models (14,35–37). Rolipram in particular has been shown to improve scopolamine- and MK-801–induced memory deficits in rats (38,39). In addition, rolipram has been shown to reduce neuronal cell death in the hippocampus after acute ischemia (40,41). In the respiratory system, activation of the cAMP signaling cascade has been shown to stimulate respiratory neurons and reverse respiratory depression (22).
After spinal cord injury, targeting the cAMP signaling cascade may be a promising strategy for the development of drugs that can strengthen spared or alternate synaptic connections in a variety of motor systems including the respiratory system. For example, after an incomplete cervical spinal cord lesion in rats, rolipram treatment resulted in an improved distal and proximal control of forelimb use (17). In the above study, rolipram treatment was combined with embryonic tissue transplants to promote functional recovery. In another study, using a contusion model of spinal cord injury, Pearse et al (18) showed that combining cAMP elevation and Schwann cell transplantation promotes improved hind limb function. Although the authors in the above studies attribute the cAMP-induced functional improvements mainly to regenerative and neuroprotective strategies, it is possible that rolipram may be acting through multiple mechanisms to enhance neural function. Therefore, we cannot rule out the possibility that, after spinal cord injury, rolipram may be strengthening existing spared axonal connections in addition to promoting regeneration of injured axons. The above studies have used a combinatory approach to improve functional recovery after spinal cord injury. In this study and in a previous study from our laboratory (21), we showed that rolipram administration alone can bring forth recovery of motor function after spinal cord injury.
The results of this study have extended a previous study from our laboratory in which we reported that long-term (3-day) administration of phosphodiesterase inhibitors such as pentoxifylline and rolipram can bring about long-lasting phrenic nerve recovery in animals subjected to a left C2 spinal cord lesion (21). These studies provide support for the exploration of cAMP-elevating agents as an effective approach in alleviating some of the respiratory insufficiency that is associated with high cervical spinal cord injury. Our conclusions are supported by other laboratories that have also shown the effectiveness of activating the cAMP pathway in improving functional recovery after spinal cord injury (17,18).
CONCLUSION
Acute systemic administration of the specific phosphodiesterase inhibitor, rolipram, increased cAMP levels in the spinal cord and medulla and concomitantly enhanced phrenic motor output and activated the latent crossed phrenic pathways. Results from this study suggest that, after spinal cord injury, functional recovery can be promoted pharmacologically by facilitating the intrinsic ability of the spinal cord to strengthen existing but ineffective synaptic connections. The results from this study and our previous study strongly suggest that phosphodiesterase inhibitors may be therapeutically useful in alleviating respiratory dysfunctions that ensue after spinal cord injury.
Footnotes
This study was supported by NIH Grant HD31550.
REFERENCES
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347(1):64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Aserinsky E. Effects of usage of a dormant respiratory nerve pathway upon its subsequent activity. Exp Neurol. 1961;3:467–475. doi: 10.1016/0014-4886(61)90022-x. [DOI] [PubMed] [Google Scholar]
- Guth L. Functional plasticity in the respiratory pathway of the mammalian spinal cord. Exp Neurol. 1976;51(2):414–420. doi: 10.1016/0014-4886(76)90265-x. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Olson EB, Jr, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci. 2003;23(7):2993–3000. doi: 10.1523/JNEUROSCI.23-07-02993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94(2):795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, Goshgarian HG. Theophylline-induced recovery in a hemidiaphragm paralyzed by hemisection in rats: contribution of adenosine receptors. Neuropharmacology. 1998;37(1):113–121. doi: 10.1016/s0028-3908(97)00190-1. [DOI] [PubMed] [Google Scholar]
- Alilain WJ, Goshgarian HG. MK-801 upregulates NR2A protein levels and induces functional recovery of the ipsilateral hemidiaphragm following acute C2 hemisection in adult rats. J Spinal Cord Med. 2007;30(4):346–354. doi: 10.1080/10790268.2007.11753950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantwi KD, Basura GJ, Goshgarian HG. Effects of long-term theophylline exposure on recovery of respiratory function and expression of adenosine A1 mRNA in cervical spinal cord hemisected adult rats. Exp Neurol. 2003;182(1):232–239. doi: 10.1016/s0014-4886(03)00109-2. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy A, Goshgarian HG. Actions of systemic theophylline on hemidiaphragmatic recovery in rats following cervical spinal cord hemisection. Exp Neurol. 1996;140(1):53–59. doi: 10.1006/exnr.1996.0114. [DOI] [PubMed] [Google Scholar]
- Schwabe U, Ukena D, Lohse MJ. Xanthine derivatives as antagonists at A1 and A2 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1985;330(3):212–221. doi: 10.1007/BF00572436. [DOI] [PubMed] [Google Scholar]
- Daly JW, Padgett W, Shamim MT, Butts-Lamb P, Waters J. 1,3-Dialkyl-8-(p-sulfophenyl)xanthines: potent water-soluble antagonists for A1- and A2-adenosine receptors. J Med Chem. 1985;28(4):487–492. doi: 10.1021/jm00382a018. [DOI] [PubMed] [Google Scholar]
- Newman D, Tamir J, Speedy L, Newman JP, Ben-Dov I. Physiological and neuropsychological effects of theophylline in chronic obstructive pulmonary disease. Isr J Med Sci. 1994;30(11):811–816. [PubMed] [Google Scholar]
- Mulloy E, McNicholas WT. Theophylline improves gas exchange during rest, exercise, and sleep in severe chronic obstructive pulmonary disease. Am Rev Respir Dis. 1993;148((4 Pt 1)):1030–1036. doi: 10.1164/ajrccm/148.4_Pt_1.1030. [DOI] [PubMed] [Google Scholar]
- Alberini CM, Ghirardi M, Huang YY, Nguyen PV, Kandel ER. A molecular switch for the consolidation of long-term memory: cAMP-inducible gene expression. Ann N Y Acad Sci. 1995;758:261–286. doi: 10.1111/j.1749-6632.1995.tb24833.x. [DOI] [PubMed] [Google Scholar]
- Inan M, Lu HC, Albright MJ, She WC, Crair MC. Barrel map development relies on protein kinase A regulatory subunit II beta-mediated cAMP signaling. J Neurosci. 2006;26(16):4338–4349. doi: 10.1523/JNEUROSCI.3745-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Lee JA, Lee C, et al. An Aplysia type 4 phosphodiesterase homolog localizes at the presynaptic terminals of Aplysia neuron and regulates synaptic facilitation. J Neurosci. 2005;25(39):9037–9045. doi: 10.1523/JNEUROSCI.1989-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci U S A. 2004;101(23):8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse DD, Pereira FC, Marcillo AE, et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10(6):610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, et al. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34(6):895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- Qiu J, Cai D, Filbin MT. A role for cAMP in regeneration during development and after injury. Prog Brain Res. 2002;137:381–387. doi: 10.1016/s0079-6123(02)37029-8. [DOI] [PubMed] [Google Scholar]
- Kajana S, Goshgarian HG. Administration of phosphodiesterase inhibitors and an adenosine A1 receptor antagonist induces phrenic nerve recovery in high cervical spinal cord injured rats. Exp Neurol. 2008;210(2):671–680. doi: 10.1016/j.expneurol.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruangkittisakul A, Ballanyi K. Reversal by phosphodiesterase-4 blockers of in vitro apnea in the isolated brainstem-spinal cord preparation from newborn rats. Neurosci Lett. 2006;401((1–2)):194–198. doi: 10.1016/j.neulet.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Lalley PM, Hoch B, Richter DW. cAMP-dependent reversal of opioid- and prostaglandin-mediated depression of the isolated respiratory network in newborn rats. J Physiol. 1997;504((Pt 1)):127–134. doi: 10.1111/j.1469-7793.1997.127bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoletti A, Cancedda L, Reid SW, et al. Heterozygous knock-out mice for brain-derived neurotrophic factor show a pathway-specific impairment of long-term potentiation but normal critical period for monocular deprivation. J Neurosci. 2002;22(23):10072–10077. doi: 10.1523/JNEUROSCI.22-23-10072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy R, Pitts FW, Stauffer ES. Respiratory complications in traumatic quadriplegia. Analysis of 20 years' experience. J Neurosurg. 1973;39(5):596–600. doi: 10.3171/jns.1973.39.5.0596. [DOI] [PubMed] [Google Scholar]
- Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil. 2003;82(10):803–814. doi: 10.1097/01.PHM.0000078184.08835.01. [DOI] [PubMed] [Google Scholar]
- Jackson AB, Groomes TE. Incidence of respiratory complications following spinal cord injury. Arch Phys Med Rehabil. 1994;75(3):270–275. doi: 10.1016/0003-9993(94)90027-2. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Black KJ, Stover SL. Causes of death during the first 12 years after spinal cord injury. Arch Phys Med Rehabil. 1993;74(3):248–254. [PubMed] [Google Scholar]
- Nantwi KD, Goshgarian HG. Actions of specific adenosine receptor A1 and A2 agonists and antagonists in recovery of phrenic motor output following upper cervical spinal cord injury in adult rats. Clin Exp Pharmacol Physiol. 2002;29(10):915–923. doi: 10.1046/j.1440-1681.2002.03750.x. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy AA, Schrimsher GW, Reier PJ, Goshgarian HG. Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehabil Neural Repair. 1999;13:225–234. [Google Scholar]
- Basura GJ, Nantwi KD, Goshgarian HG. Theophylline-induced respiratory recovery following cervical spinal cord hemisection is augmented by serotonin 2 receptor stimulation. Brain Res. 2002;956(1):1–13. doi: 10.1016/s0006-8993(02)03097-4. [DOI] [PubMed] [Google Scholar]
- Zimmer MB, Goshgarian HG. GABA, not glycine, mediates inhibition of latent respiratory motor pathways after spinal cord injury. Exp Neurol. 2007;203(2):493–501. doi: 10.1016/j.expneurol.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol. 2008;211(1):97–106. doi: 10.1016/j.expneurol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara TE, Jr, Goshgarian HG. Quantitative assessment of phrenic nerve functional recovery mediated by the crossed phrenic reflex at various time intervals after spinal cord injury. Exp Neurol. 1991;111(2):244–250. doi: 10.1016/0014-4886(91)90012-2. [DOI] [PubMed] [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405(6789):955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Woo NH. Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog Neurobiol. 2003;71(6):401–437. doi: 10.1016/j.pneurobio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Wiseman SL, Olausson P, Taylor JR. Bidirectional behavioral plasticity of memory reconsolidation depends on amygdalar protein kinase A. Nat Neurosci. 2006;9(2):167–169. doi: 10.1038/nn1628. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Crissman AM, Dorairaj NR, Chandler LJ, O'Donnell JM. Inhibition of cyclic AMP phosphodiesterase (PDE4) reverses memory deficits associated with NMDA receptor antagonism. Neuropsychopharmacology. 2000;23(2):198–204. doi: 10.1016/S0893-133X(00)00108-1. [DOI] [PubMed] [Google Scholar]
- Zhang HT, O'Donnell JM. Effects of rolipram on scopolamine-induced impairment of working and reference memory in the radial-arm maze tests in rats. Psychopharmacology (Berl). 2000;150(3):311–316. doi: 10.1007/s002130000414. [DOI] [PubMed] [Google Scholar]
- Kato H, Araki T, Itoyama Y, Kogure K. Rolipram, a cyclic AMP-selective phosphodiesterase inhibitor, reduces neuronal damage following cerebral ischemia in the gerbil. Eur J Pharmacol. 1995;272(1):107–110. doi: 10.1016/0014-2999(94)00694-3. [DOI] [PubMed] [Google Scholar]
- Block F, Schmidt W, Nolden-Koch M, Schwarz M. Rolipram reduces excitotoxic neuronal damage. Neuroreport. 2001;12(7):1507–1511. doi: 10.1097/00001756-200105250-00041. [DOI] [PubMed] [Google Scholar]