Abstract
Background/Objective:
To describe a case of fatigue associated with cardiomyopathy in a man with spinal cord injury.
Study Design:
Case report.
Subject:
An obese 35-year-old man with long-term ASIA A L2 paraplegia, accompanied by a 2-year history of progressive severe fatigue.
Methods:
Physical examination showed obesity, hypertension, tachycardia, and pitting edema. An echocardiogram showed a 20% ejection fraction, severe dilation, and global hypokinesis in the left ventricle and mild to moderate mitral regurgitation.
Results:
Symptoms improved after treatment with furosemide, warfarin, ramipril, and continuous positive airway pressure for obstructive sleep apnea.
Conclusion:
Severe progressive fatigue in a patient with chronic SCI may signal cardiomyopathy. Diagnostic studies may be warranted in patients with progressive fatigue.
Keywords: Cardiomyopathy, Spinal cord injuries, Paraplegia, Congestive heart failure, Mitral regurgitation, Fatigue, Chronic obstructive pulmonary disease, Obstructive sleep apnea, Obesity
INTRODUCTION
Fatigue is a common symptom reported by patients with spinal cord injuries (SCIs) in the postacute and postdischarge phases (1). The prevalence of fatigue may be as high as 57% and may result in functional impairment (2). Intrinsic fatigue is the result of histologic and metabolic changes in complete or partially denervated muscles at the level of or below the lesion (3). In addition, contributing factors may include metabolic and endocrine disturbances, obesity, heart or respiratory failure, sleep apnea, pain from muscle overuse, rehospitalization, and difficulties coping with chronic injury (3–5) (Table 1). Even though heart failure is one cause of chronic fatigue in patients with chronic SCI, a paucity of research exists regarding the causes of heart failure in this population.
Table 1.
Differential Diagnosis of Fatigue After SCI3,32,33
Currently, coronary artery disease (CAD) is a leading cause of morbidity and mortality in individuals with SCI because of their elevated percentage of body fat and sedentary lifestyle (6–9). A study examining the prevalence of CAD in 47 clinically asymptomatic patients with chronic SCI also reported that those individuals with combined tetraplegia and complete injuries experienced an increased risk for CAD. In this study, 63.8% had abnormal results on cardiac stress testing with thallium-201 myocardial perfusion single photon emission computed tomography (Tl-201 SPECT) (10).
The majority of human and animal research studies in the area of SCI and cardiovascular diseases are related to the acute phase of SCI, especially in individuals with higher levels of SCI (ie, cervical to mid-thoracic) (11–14). Individuals with cervical and/or upper thoracic spinal injuries tend to have cardiovascular instability, including unstable arterial pressure and heart rate. As a result, they are at a higher risk for hypertensive cardiovascular diseases (15). With respect to lower level SCI (ie, below T12), one animal study showed that a dog developed symptoms of neurogenic cardiomyopathy only a few days after an acute SCI in the lumbosacral region (16). Pahl et al (17) examined cardiovascular complications in 20 men (age range, 39–63 years) with SCI (levels of injury, C4–T12) of 6- to 32-year duration who were also on hemodialysis. Relevant postmortem data indicated that all subjects exhibited cardiovascular abnormalities: pericarditis (45%); left and right ventricular hypertrophy (45% and 20%); left and right ventricular dilation (40% and 30%); cardiac amyloidosis (25%); and myocardial fibrosis (45%). In this study, 1 case of aortic stenosis and 2 cases of mitral valve dilatation were reported, whereas no evidence of vegetation, endocarditis, or acute myocardial infarction was noted. The authors speculated that the high prevalence of ventricular dilation was possibly from long-term elevated cardiac output secondary to chronic fluid overload in this population with end-stage renal disease.
PubMed/MEDLINE was searched, without limitations, in December 2007. Search terms included cardiomyopathy, spinal cord injury, fatigue, heart failure, obesity, dilated cardiomyopathy (DCM), spinal cord injury comorbidity, cardiac complication(s), sleep apnea, and myocardial changes. All pertinent literature was reviewed to identify other similar cases, but no cases of cardiomyopathy and long-term SCI were found.
CASE DESCRIPTION
A 35-year-old man with L2 ASIA A paraplegia presented with a history of fatigue severe enough to interfere with his ability to work full time. He was injured in a motor vehicle crash 19 years before admission, resulting in a fracture/dislocation of T12 to L1 with immediate paraplegia. With mobilization and increased activities, he reached independence at the wheelchair level. He sporadically attended follow-up appointments, citing lethargy, and his last appointment was 4 years ago. He was admitted to our rehabilitation hospital for further study.
On admission, he reported lethargy, daytime somnolence, and a tendency to snore excessively at night. A gradual gain in weight of 35 lb over 2 years was accompanied by a history of low affect over the previous 3 months and disinterest in daily activities and socialization. However, no recent psychosocial stressors could be identified. He also reported nocturnal panic attacks that began with shortness of breath and were associated with nausea, sweating, hot flushes, and tremor.
Medications before admission included Combivent inhaler (2 puffs; 4 times/d) and the NicoDerm patch. He was a 19-pack-year smoker and a social drinker. Complications since the injury included recurrent urinary tract infections, constipation, neurogenic bladder, neurogenic bowel, and erectile dysfunction. His history was significant for childhood asthma, which has been in remission since the age of 12 years. The patient was divorced and lived alone in a condominium apartment. Before admission, he had been employed as a seed analyst supervisor for a private company. He used a wheelchair on a full-time basis and he was independent with activities of daily living (ADLs) and instrumental activities of daily living (IADLs), except for requiring assistance with bathing. He reported that he had poor eating habits and often ordered take-out food.
Physical Examination
At admission, he was morbidly obese; body weight was 129 kg (body mass index [BMI], 40.8 kg/m2). He was alert, oriented, and in no apparent distress. Pulse was 124/min and regular; blood pressure was 160/102 mmHg. Jugular venous pulse was not raised, and heart sounds were normal with no audible murmurs. Pitting edema was observed in both lower limbs. He had grade 5 strength in his upper limbs bilaterally and grade 2 to 3/5 strength in his hip abductors/adductors bilaterally.
Laboratory Work-up
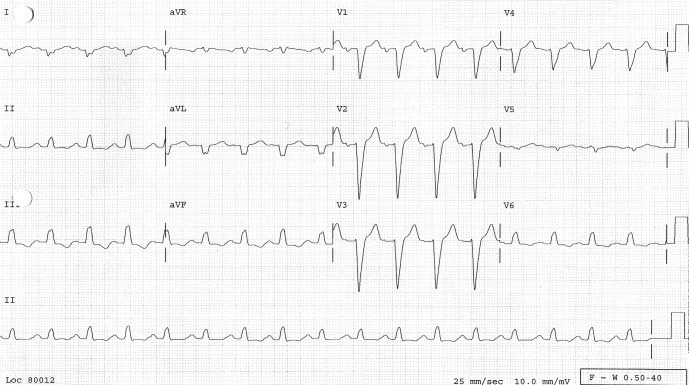
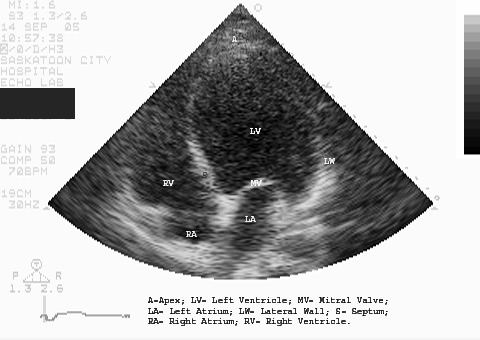
A 12-lead electrocardiogram (ECG) showed sinus tachycardia, right axis deviation, nonspecific intraventricular conduction delay, left atrial enlargement, poor R-wave progression, long QT interval, and inferior ST-T abnormalities, indicating possible ischemia (Figure 1). A chest radiograph showed marked left ventricular enlargement. Peribronchial cuffing (arrow) indicated some degree of early perivascular interstitial edema (Figure 2). Transthoracic echocardiogram showed a severely dilated left ventricle with severe global hypokinesis. Left ventricular wall thickness was normal. Left ventricular systolic function was severely reduced (Figure 3). Left ventricular ejection fraction was less than 20%. The right ventricle was moderate to severely dilated, and systolic function was moderately reduced. The left and right atria were moderately dilated. Mild to moderate mitral regurgitation and tricuspid regurgitation were also present.
Figure 1. ECG showed sinus tachycardia, right axis deviation, intraventricular conduction delay, poor R-wave progression, long QT interval, and inferior ST-T abnormalities.
Figure 2. Chest radiograph showed marked left ventricular enlargement. Peribronchial cuffing (arrow) indicated perivascular interstitial edema.
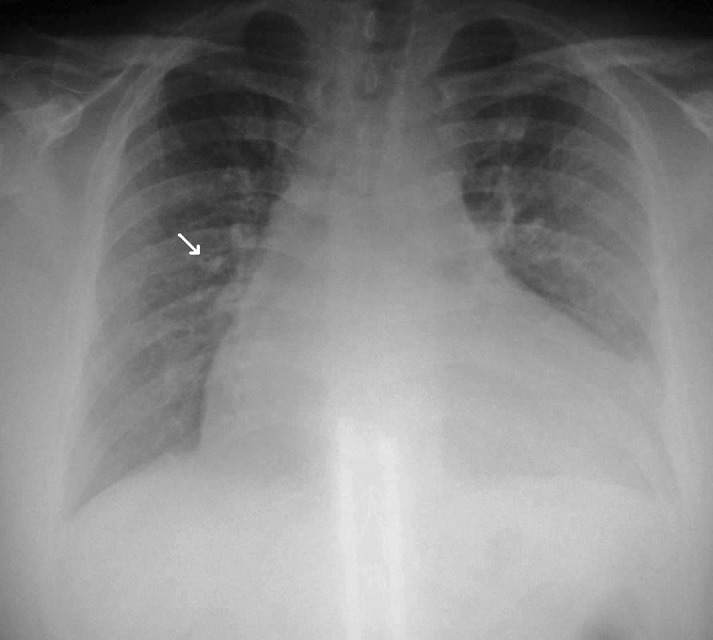
Figure 3. Transthoracic echocardiogram showed severe dilation of the left ventricle with marked global hypokinesis.
Overnight pulse oxygen monitoring showed 217 episodes of desaturation with the lowest reading at 76%. The total duration of oxygen saturation less than 90% was 1 hour and 39 minutes. History and subsequent studies were consistent with a diagnosis of chronic obstructive pulmonary disease, obstructive sleep apnea (OSA), and severe cardiomyopathy resulting in congestive heart failure. After furosemide administration, the patient immediately became less dyspneic and more involved in ADL activities. Treatment with warfarin and ramipril was also initiated. Two days later, he was prescribed continuous positive airway pressure (CPAP) for OSA. At discharge, cardiovascular and respiratory symptoms, as well as strength and endurance, had improved, and he was able to manage wheelchair transfers independently.
Approximately 1 year after discharge, his weight had decreased to 94 kg (BMI, 29.7 kg/m2), his ADL functioning and endurance had improved, and his fatigue level had decreased. He was able to propel his wheelchair for short distances without experiencing shortness of breath, and he had been able to return to work. A referral for a sleep study was also made. He remained on anticoagulant therapy. A repeat transthoracic echocardiography showed that the ejection fraction was still low (<20%). Severe left ventricular enlargement with severe global left ventricular systolic dysfunction was noted. Trace mitral regurgitation was present. These findings were compatible with the diagnosis of dilated cardiomyopathy.
DISCUSSION
Cardiomyopathy, especially dilated cardiomyopathy, is recognized as a significant cause of heart failure in the normal population (18). Signs and symptoms of cardiomyopathy, which are similar to those of heart failure, include shortness of breath, progressive dyspnea on exertion, orthopnea, leg edema, paroxysmal nocturnal dyspnea, jugular venous distension, systolic murmurs of mitral and tricuspid valve regurgitation, enlarged and pulsatile liver, S3 or S4 gallop, and displaced apical impulse (19). Individuals with dilated cardiomyopathy often present with symptoms of congestive heart failure, systemic or pulmonary emboli, fatigue, and weakness (20). Dilated cardiomyopathy can be subdivided into 9 subcategories based on the cause: ischemic cardiomyopathy, idiopathic dilated cardiomyopathy, toxic cardiomyopathy (alcohol or illicit drugs), hypertensive heart disease, infective cardiomyopathy, valvular heart disease, peripartum cardiomyopathy, tachycardia-induced cardiomyopathy, and cardiomyopathy secondary to metabolic conditions (19).
Typically, individuals with lumbar SCI do not present with cardiovascular compromise. Blocker et al (21) studied the resting ECG of 98 individuals with chronic SCI and reported that individuals with lumbar SCI, unlike those with cervical and thoracic SCI, had no ECG abnormalities. This finding was surprising, considering that patients with chronic SCI are often overweight or obese (22) and certainly at a higher risk for various cardiovascular diseases. The prevalence of overweight (proportion of patients with BMI of 25–29.99 kg/m2) and obesity (proportion of patients with BMI ≥ 30 kg/m2) among individuals with paraplegia and ASIA score C was 70.83% and 50%, respectively. According to the American Heart Association scientific statement, obesity is an independent risk factor for cardiovascular diseases (23). Excessive body weight increases metabolic demand and results in increases in total blood volume, cardiac output, and stroke volume. An increase in left ventricular filling pressure and volume may eventually lead to chamber dilation or hypertrophy.
In addition, sleep-related breathing disorder is a potential risk factor for cardiovascular disease (24–27). Shamsuzzaman et al (28) completed a systematic review regarding the interactions of OSA with cardiovascular pathophysiology and disease. They concluded that repetitive apneic events in patients with OSA may disrupt the normal physiologic interactions between sleep and the cardiovascular system. The activation of a number of neural, humoral, thrombotic, metabolic, and inflammatory disease mechanisms (ie, chronic sympathetic activation, endothelial dysfunction, increased levels of C-reactive protein and inflammatory cytokines, elevated leptin level, hypercoagulability, as well as increased production of endothelin and catecholamines) may induce structural cardiac changes that may be implicated in the initiation and progression of arrhythmia, cardiac ischemia, congestive heart failure, and stroke (28).
Various studies (29–32) have documented a high prevalence of sleep apnea among individuals with tetraplegia. Bach and Wang (29) found that at 5 years after injury, 5 of the 10 individuals with stable traumatic tetraplegia had an increased frequency of transient nocturnal oxyhemoglobin desaturations. Hypercapnia was also more common in this study (29). Burns et al (30) examined the prevalence of sleep apnea among 20 men with various levels and degrees of chronic SCI and found a greater prevalence of sleep apnea with tetraplegia. In a cross-sectional study, Leduc et al (31) reported that 22 of 41 individuals with chronic cervical SCI had OSA-hypopnea syndrome. Berlowitz et al (32) also studied the incidence of sleep disordered breathing among 13 subjects in the first year after cervical SCI and concluded that 62% of the subjects had sleep-disordered breathing within 4 weeks of the injury.
Because of other comorbidities, differentiating the underlying cause of cardiomyopathy was difficult in this case. Heart failure could have been precipitated by a combination of causes because he had many risk factors including smoking, hypertension, poor diet, chronic obstructive pulmonary disease, and long-term obesity (33). All these factors are also well-known risks for cardiovascular diseases in the general population.
OSA likely also contributed to his fatigue. However, the primary causes for his symptoms was felt to be cardiomyopathy and congestive heart failure, because his fatigue levels and shortness of breath improved immediately after administration of furosemide, even before his use of CPAP. However, in the long term, CPAP also had a positive effect on his symptoms.
CONCLUSION
This report illustrates an obese patient with chronic SCI and severe, progressive fatigue that interfered with his ability to work and perform ADLs. Diagnostic work-up showed severe dilated cardiomyopathy. Treatment of cardiomyopathy, congestive heart failure, and sleep apnea resulted in an improvement of symptoms and in ADL function.
REFERENCES
- Levi R, Hultling C, Nash MS, Seiger A. The Stockholm spinal cord injury study: 1. Medical problems in a regional SCI population. Paraplegia. 1995;33(6):308–315. doi: 10.1038/sc.1995.70. [DOI] [PubMed] [Google Scholar]
- Fawkes-Kirby TM, Wheeler MA, Anton HA, Miller WC, Townson AF, Weeks CAO. Clinical correlates of fatigue in spinal cord injury. Spinal Cord. 2008;46:21–25. doi: 10.1038/sj.sc.3102053. [DOI] [PubMed] [Google Scholar]
- Barat M, Dehail P, de Seze M. Fatigue after spinal cord injury. Ann Readapt Med Phys. 2006;49(6):365–369. doi: 10.1016/j.annrmp.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Saultz JW. Fatigue. In: Taylor RB, editor. Manual of Family Practice. 2nd ed. Boston, MA: Lippincott William & Wilkins; 1997. pp. 43–45. [Google Scholar]
- Kasper DL, Fauci AS, Eugene Braunwald E, editors. Harrison's Principles of Internal Medicine. 16th ed., online edition. New York: McGraw-Hill Health Professions Division; 2004. [Google Scholar]
- Levi R, Hultling C, Seiger A. The Stockholm Spinal Cord Injury Study. Health-related issues of the Swedish annual level-of-living survey in SCI subjects and controls. Paraplegia. 1995;33(12):726–730. doi: 10.1038/sc.1995.152. [DOI] [PubMed] [Google Scholar]
- Frankel HL, Coll JR, Charlifue SW, et al. Long-term survival in spinal cord injury: a fifty-year investigation. Spinal Cord. 1998;36(4):266–274. doi: 10.1038/sj.sc.3100638. [DOI] [PubMed] [Google Scholar]
- Le CT, Price M. Survival from spinal cord injury. J Chronic Dis. 1982;35(6):487–492. doi: 10.1016/0021-9681(82)90063-7. [DOI] [PubMed] [Google Scholar]
- Soden RJ, Walsh J, Middleton JW, Craven ML, Rutkowski SB, Yeo JD. Causes of death after spinal cord injury. Spinal Cord. 2000;38(10):604–610. doi: 10.1038/sj.sc.3101080. [DOI] [PubMed] [Google Scholar]
- Lee CS, Lu YH, Lee ST, Lin CC, Ding HJ. Evaluating the prevalence of silent coronary artery disease in asymptomatic patients with spinal cord injury. Int Heart J. 2006;47(3):325–330. doi: 10.1536/ihj.47.325. [DOI] [PubMed] [Google Scholar]
- Franga DL, Hawkins ML, Medeiros RS, Adewumi D. Recurrent asystole resulting from high cervical spinal cord injuries. Am Surg. 2006;72(6):525–529. [PubMed] [Google Scholar]
- Gilgoff IS, Ward SL, Hohn AR. Cardiac pacemaker in high spinal cord injury. Arch Phys Med Rehabil. 1991;72(8):601–603. [PubMed] [Google Scholar]
- Macintire DK, Snider TG., III Cardiac arrhythmias associated with multiple trauma in dogs. J Am Vet Med Assoc. 1984;184(5):541–545. [PubMed] [Google Scholar]
- Jacobs PL, Mahoney ET, Robbins A, Nash M. Hypokinetic circulation in persons with paraplegia. Med Sci Sports Exerc. 2002;34(9):1401–1407. doi: 10.1097/00005768-200209000-00001. [DOI] [PubMed] [Google Scholar]
- Rodenbaugh DW, Collins HL, DiCarlo SE. Increased susceptibility to ventricular arrhythmias in hypertensive paraplegic rats. Clin Exp Hypertens. 2003;25(6):349–358. doi: 10.1081/ceh-120023544. [DOI] [PubMed] [Google Scholar]
- King JM, Roth L, Haschek WM. Myocardial necrosis secondary to neural lesions in domestic animals. J Am Vet Med Assoc. 1982;180(2):144–148. [PubMed] [Google Scholar]
- Pahl MV, Vaziri ND, Gordon S, Tuero S. Cardiovascular pathology in dialysis patients with spinal cord injury. Artif Organs. 1983;7(4):416–419. doi: 10.1111/j.1525-1594.1983.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Mazzone M, La Sala M, Portale G, et al. Review of dilated cardiomyopathies. Dilated cardiomyopathies and altered prothrombotic state: a point of view of the literature. Panminerva Med. 2005;47(3):157–167. [PubMed] [Google Scholar]
- Sanchez Torres RJ, Calderon R. The cardiomyopathies, a review for the primary physician. P R Health Sci J. 2004;23(4):285–292. [PubMed] [Google Scholar]
- Zipes DP, Braunwald E. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 7th ed. Philadelphia, PA: WB Saunders; 2005. [Google Scholar]
- Blocker WP, Merrill JM, Krebs MA, Cardus DP, Ostermann HJ. An electrocardiographic survey of patients with chronic spinal cord injury. Am Correct Ther J. 1983;37(4):101–104. [PubMed] [Google Scholar]
- Gupta N, White KT, Sandford PR. Body mass index in spinal cord injury—a retrospective study. Spinal Cord. 2006;44(2):92–94. doi: 10.1038/sj.sc.3101790. [DOI] [PubMed] [Google Scholar]
- Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- Phillipson EA. Sleep apnea—a major public health problem. N Engl J Med. 1993;328:1271–1273. doi: 10.1056/NEJM199304293281712. [DOI] [PubMed] [Google Scholar]
- Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- Dyken ME, Somers VK, Yamada T, Ren ZY, Zimmerman MB. Investigating the relationship between stroke and obstructive sleep apnea. Stroke. 1996;27:401–407. doi: 10.1161/01.str.27.3.401. [DOI] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- Shamsuzzaman ASM, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- Bach JR, Wang TG. Pulmonary function and sleep disordered breathing in patients with traumatic tetraplegia: a longitudinal study. Arch Phys Med Rehabil. 1994;75(3):279–284. doi: 10.1016/0003-9993(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Burns SP, Little JW, Hussey JD, Lyman P, Lakshminarayanan S. Sleep apnea syndrome in chronic spinal cord injury: associated factors and treatment. Arch Phys Med Rehabil. 2000;81(10):1334–1339. doi: 10.1053/apmr.2000.9398. [DOI] [PubMed] [Google Scholar]
- Leduc BE, Dagher JH, Mayer P, Bellemare F, Lepage Y. Estimated prevalence of obstructive sleep apnea-hypopnea syndrome after cervical cord injury. Arch Phys Med Rehabil. 2007;88(3):333–337. doi: 10.1016/j.apmr.2006.12.025. [DOI] [PubMed] [Google Scholar]
- Berlowitz DJ, Brown DJ, Campbell DA, Pierce RJ. A longitudinal evaluation of sleep and breathing in the first year after cervical spinal cord injury. Arch Phys Med Rehabil. 2005;86(6):1193–1199. doi: 10.1016/j.apmr.2004.11.033. [DOI] [PubMed] [Google Scholar]
- Taylor AJ, Arora NS, Bindeman J, Bhattari S, Feuerstein IM, O'Malley PG. Conventional, emerging, heredity, lifestyle, and psychosocial coronary risk factors: relationships to subclinical atherosclerosis. Prev Cardiol. 2006;9(1):25–32. doi: 10.1111/j.1520-037x.2006.04301.x. [DOI] [PubMed] [Google Scholar]