Abstract
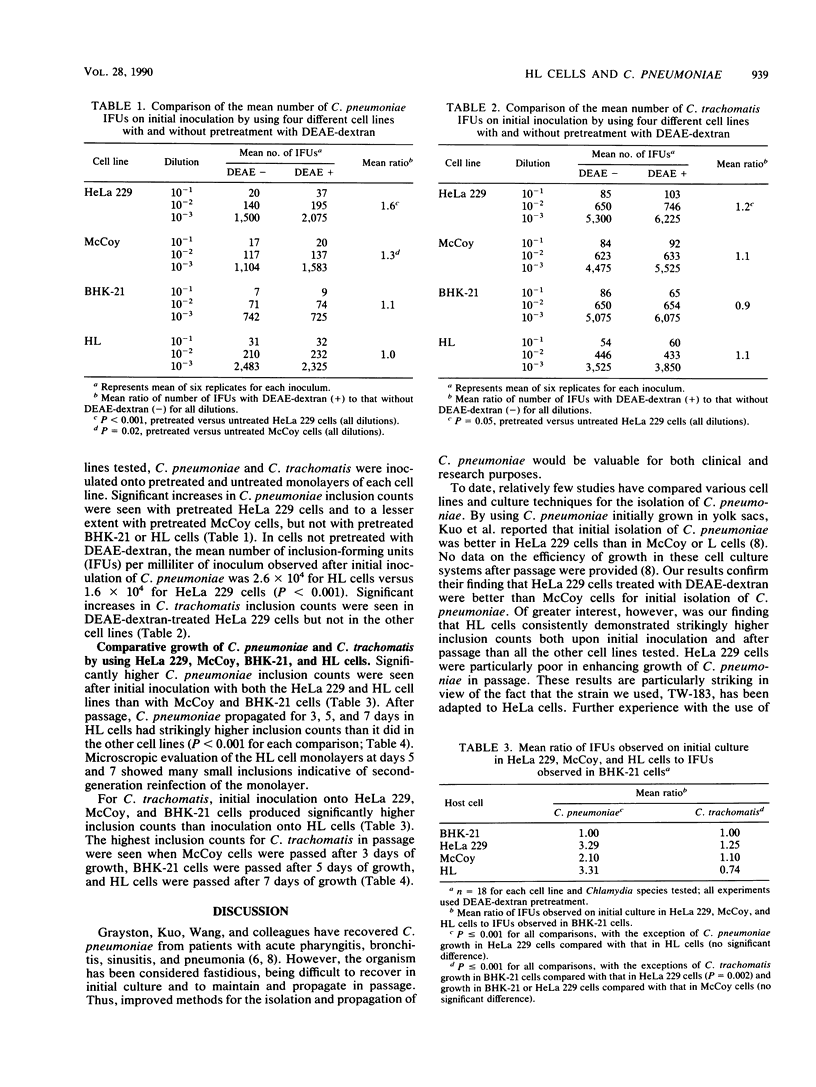
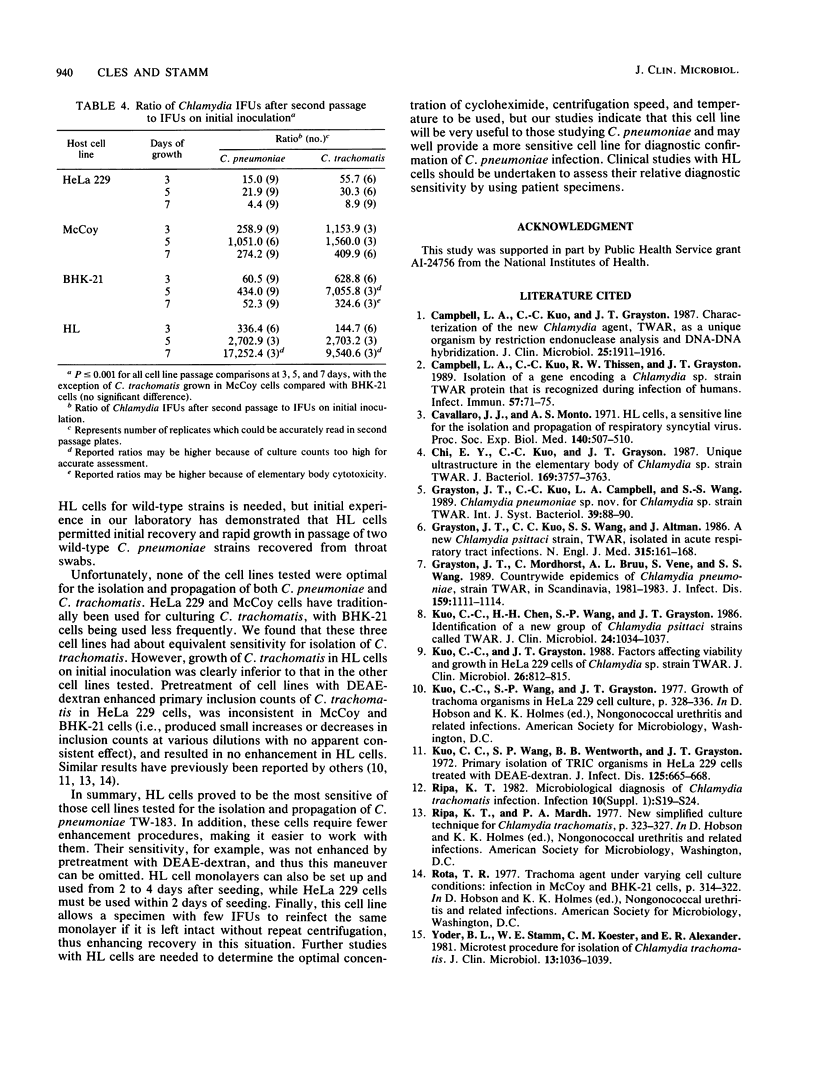
We compared growth of the recently discovered respiratory pathogen Chlamydia pneumoniae in McCoy, HeLa 229, BHK-21, and HL cells. When cells were not pretreated with DEAE-dextran, HL cells had significantly higher mean numbers of inclusion-forming units (IFUs) on initial inoculation than the other cell lines. When cells were pretreated with DEAE-dextran, HeLa 229 and HL cells had equivalent mean numbers of IFUs on initial inoculation. HL cells had strikingly higher mean numbers of IFUs in passage than HeLa 229, BHK-21, or McCoy cells. In addition, HL cells did not require pretreatment with DEAE-dextran and could be used from 2 to 4 days after seeding. We conclude that HL cells are an excellent cell culture system for laboratory propagation of C. pneumoniae and may be a more sensitive cell line for initial isolation.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell L. A., Kuo C. C., Grayston J. T. Characterization of the new Chlamydia agent, TWAR, as a unique organism by restriction endonuclease analysis and DNA-DNA hybridization. J Clin Microbiol. 1987 Oct;25(10):1911–1916. doi: 10.1128/jcm.25.10.1911-1916.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L. A., Kuo C. C., Thissen R. W., Grayston J. T. Isolation of a gene encoding a Chlamydia sp. strain TWAR protein that is recognized during infection of humans. Infect Immun. 1989 Jan;57(1):71–75. doi: 10.1128/iai.57.1.71-75.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallaro J. J., Monto A. S. HL cells, a sensitive line for the isolation and propagation of respiratory syncytial virus. Proc Soc Exp Biol Med. 1972 Jun;140(2):507–510. doi: 10.3181/00379727-140-36491. [DOI] [PubMed] [Google Scholar]
- Chi E. Y., Kuo C. C., Grayston J. T. Unique ultrastructure in the elementary body of Chlamydia sp. strain TWAR. J Bacteriol. 1987 Aug;169(8):3757–3763. doi: 10.1128/jb.169.8.3757-3763.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayston J. T., Kuo C. C., Wang S. P., Altman J. A new Chlamydia psittaci strain, TWAR, isolated in acute respiratory tract infections. N Engl J Med. 1986 Jul 17;315(3):161–168. doi: 10.1056/NEJM198607173150305. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Mordhorst C., Bruu A. L., Vene S., Wang S. P. Countrywide epidemics of Chlamydia pneumoniae, strain TWAR, in Scandinavia, 1981-1983. J Infect Dis. 1989 Jun;159(6):1111–1114. doi: 10.1093/infdis/159.6.1111. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Chen H. H., Wang S. P., Grayston J. T. Identification of a new group of Chlamydia psittaci strains called TWAR. J Clin Microbiol. 1986 Dec;24(6):1034–1037. doi: 10.1128/jcm.24.6.1034-1037.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Grayston J. T. Factors affecting viability and growth in HeLa 229 cells of Chlamydia sp. strain TWAR. J Clin Microbiol. 1988 May;26(5):812–815. doi: 10.1128/jcm.26.5.812-815.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C., Wang S., Wentworth B. B., Grayston J. T. Primary isolation of TRIC organisms in HeLa 229 cells treated with DEAE-dextran. J Infect Dis. 1972 Jun;125(6):665–668. doi: 10.1093/infdis/125.6.665. [DOI] [PubMed] [Google Scholar]
- Ripa K. T. Microbiology Diagnosis of chlamydia trachomatis infection. Infection. 1982;10 (Suppl 1):S19–S24. doi: 10.1007/BF01640710. [DOI] [PubMed] [Google Scholar]
- Yoder B. L., Stamm W. E., Koester C. M., Alexander E. R. Microtest procedure for isolation of Chlamydia trachomatis. J Clin Microbiol. 1981 Jun;13(6):1036–1039. doi: 10.1128/jcm.13.6.1036-1039.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


