Abstract
Large-scale biogeographical shifts in vegetation are predicted in response to the altered precipitation and temperature regimes associated with global climate change. Vegetation shifts have profound ecological impacts and are an important climate-ecosystem feedback through their alteration of carbon, water, and energy exchanges of the land surface. Of particular concern is the potential for warmer temperatures to compound the effects of increasingly severe droughts by triggering widespread vegetation shifts via woody plant mortality. The sensitivity of tree mortality to temperature is dependent on which of 2 non-mutually-exclusive mechanisms predominates—temperature-sensitive carbon starvation in response to a period of protracted water stress or temperature-insensitive sudden hydraulic failure under extreme water stress (cavitation). Here we show that experimentally induced warmer temperatures (≈4 °C) shortened the time to drought-induced mortality in Pinus edulis (piñon shortened pine) trees by nearly a third, with temperature-dependent differences in cumulative respiration costs implicating carbon starvation as the primary mechanism of mortality. Extrapolating this temperature effect to the historic frequency of water deficit in the southwestern United States predicts a 5-fold increase in the frequency of regional-scale tree die-off events for this species due to temperature alone. Projected increases in drought frequency due to changes in precipitation and increases in stress from biotic agents (e.g., bark beetles) would further exacerbate mortality. Our results demonstrate the mechanism by which warmer temperatures have exacerbated recent regional die-off events and background mortality rates. Because of pervasive projected increases in temperature, our results portend widespread increases in the extent and frequency of vegetation die-off.
Keywords: biosphere–atmosphere feedbacks, drought impacts, global-change ecology, Pinus edulis, carbon starvation
Global change assessments and supporting research have largely focused on how vegetation will respond to incremental changes in the central tendency of climate variables, but the most dramatic vegetation shifts are likely to result from changes in climate extremes altering patterns of disturbance events arising from hurricanes, freezes, fires, and droughts (1, 2). The effects of drought on vegetation under warmer conditions can be severe, as highlighted by recent regional-scale woody-plant die-off across the southwestern United States (3–6) and around the globe (7–10). Worldwide, many coniferous tree species are experiencing widespread, historically unprecedented mortality, mainly as a result of drought and the eruption of tree pests, such as bark beetles (1, 3, 7–9, 11–16). Consequent impacts of regional tree die-off could include reduction in habitat for wildlife, enhanced opportunities for invasion by exotic species, formation of novel communities, alterations to the hydrologic cycle, and temporal disruptions in ecosystem goods and services (2, 3, 17, 18). In addition, extensive tree die-off could impact regional carbon (C) budgets, reducing ecosystem potential to sequester C and increasing C losses through enhanced soil respiration rates (19–22). Drought-induced tree mortality not only alters C fluxes but also modifies water and energy fluxes between the atmosphere and land surface (3, 20, 23, 24). The consequences of potentially large releases of C from the biosphere to the atmosphere due to widespread mortality could contribute to further warming (19–21, 25). Small drought-induced increases solely in background mortality rates may even be sufficient to alter regional C budgets (13, 16, 26).
Drought-induced tree mortality is a pivotal vulnerability of vegetation to climate change, yet our understanding of, and ability to predict, tree mortality is astonishingly poor. Drought-induced tree mortality is difficult to predict because it is a nonlinear threshold process (1, 19, 27). Recent observational studies have raised concern that warmer temperatures could be amplifying the effects of drought on tree mortality both for background rates of mortality and for regional die-off events (3, 16). Yet experimental assessment of whether warmer temperatures associated with drought exacerbate tree mortality is lacking for any tree species, and therefore tree mortality has only been predicted in response to a simple metric of accumulated dry conditions (28, 29). The sensitivity of tree mortality to temperature is dependent on which of 2 non-mutually-exclusive mechanisms predominates: (i) carbon starvation, whereby trees close stomata to keep safe levels of xylem pressure, stopping most photosynthesis, and rely on stored carbohydrates to support the metabolic costs of maintaining tissue; or (ii) catastrophic hydraulic failure, whereby trees maintain stomatal conductance during drought to continue photosynthesizing, but run the risk of xylem pressures suddenly exceeding cavitation thresholds beyond which air bubbles block transport of stem water (30–32). Both hypotheses are interrelated with biotic agents, such as bark beetles and associated fungi, and carbon starvation would preclude production of the photosynthate necessary for tree defense, thereby increasing susceptibility to biotic agents (9, 33). Because respiration rates increase with temperature (34), carbon starvation should be highly sensitive to temperature, whereas hydraulic failure should not.
We experimentally investigated the temperature sensitivity of drought-induced mortality in Pinus edulis, a piñon pine tree that has exhibited regional-scale die-off in response to recent drought (3, 4) and which has been evaluated in observational and modeling studies (30, 31, 35). We transplanted small, reproductively mature piñon pines into the environmentally controlled Biosphere 2 facility to grow them in either near-ambient or warmer (+4.3 °C) temperatures, and then imposed a drought treatment that completely excluded water from selected trees until all “drought” trees in both temperature treatments died.
Results and Discussion
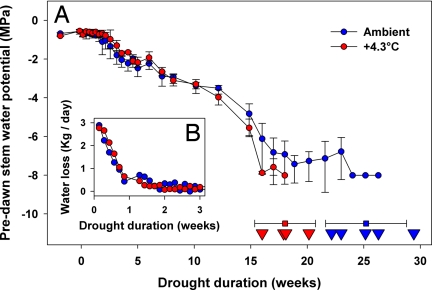
All drought trees in the warmer treatment died before any of the drought trees in the ambient treatment (on average 18.0 vs. 25.1 weeks, P <0.01; Fig. 1A). This 28% shortening in time to mortality was not reflected in a water balance difference (Fig. 1B). Indeed, predawn stem water potential measurements documented no differences in xylem pressure between drought treatments at any time during the experiment (P = 0.88, Fig. 1). Because water potential is highly correlated with the level of air embolism occluding water transport in the xylem (30, 32, 36), we saw no evidence of catastrophic loss of hydraulic transport as a primary driver of differences between treatments. Catastrophic loss in this species has been consistently observed at a stem water potential of −6 MPa (36). Therefore, we suggest that higher respiratory loads associated with warmer temperatures incited differences in mortality, reflecting carbon starvation, not sudden hydraulic failure as the causal mechanism required to predict tree mortality differences in a future warmer world. Such results are consistent with inferences from recent observational and modeling assessments (30, 31, 35).
Fig. 1.
Water relations progression and death dates. (A) Predawn stem water potential (circles), death dates (triangles), and death date means (squares) of piñon pines during simulated drought under ambient (blue) and elevated (red) temperatures. Error bars are standard errors. (B) Water loss from a subset of trees in A.
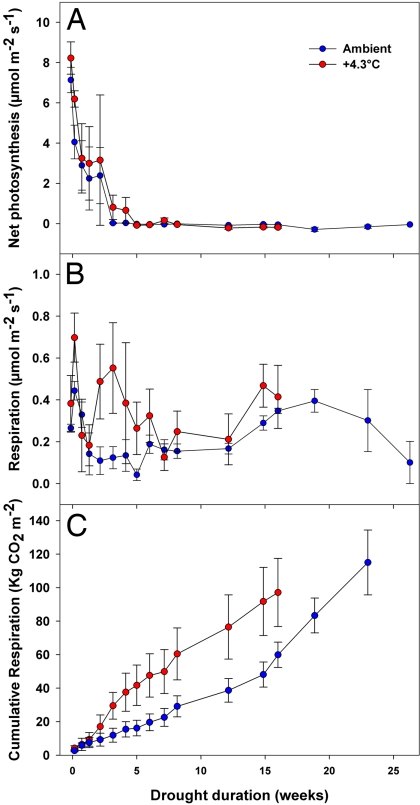
During the experiment, we also measured leaf-level exchange of CO2 before dawn to estimate respiratory load and during the middle of the day to follow photosynthetic patterns. After the start of drought treatment, photosynthesis declined rapidly and similarly in both temperature treatments, approaching zero by the third week of the experiment in drought trees (Fig. 2A). Initially, instantaneous rates of respiration were similar among drought trees in both temperature treatments, but they diverged during the third and fourth weeks of drought (Fig. 2B). An analysis of time-integrated respiration revealed that trees in the elevated-temperature drought treatment consumed C reserves faster than trees in the ambient drought treatment (Fig. 2C), reflecting the increased C cost for maintenance of tissue under warmer temperatures (34). Mean time-integrated cumulative respiration just before mortality for drought trees did not differ significantly between temperature treatments (P = 0.57). Combined, our results provide experimental evidence that piñon pines attempted to avoid drought-induced mortality by regulating stomata and foregoing further photosynthesis but subsequently succumbed to drought due to carbon starvation, not sudden hydraulic failure. Importantly, we isolate the effect of temperature from other climate variables and biotic agents and show that the effect of warmer temperature in conjunction with drought can be substantial.
Fig. 2.
Leaf carbon exchange progression. (A and B) Instantaneous midday net photosynthesis (A) and predawn respiration (B) of piñon pines during simulated drought under ambient (blue) and elevated (red) temperatures. Error bars are standard errors. (C) Cumulative time-integrated respiration costs of piñon pines during simulated drought under ambient (blue) and elevated (red) temperatures. Error bars are standard errors calculated by following standard methods for summation.
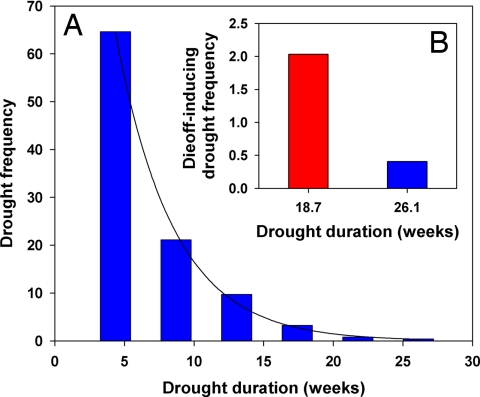
Our results imply that future warmer temperatures will not only increase background rates of tree mortality (13, 16), but also result in more frequent widespread vegetation die-off events (3, 35) through an exacerbation of metabolic stress associated with drought. With warmer temperatures, droughts of shorter duration—which occur more frequently—would be sufficient to cause widespread die-off. In our calculation of a 103-year record of regional drought duration for piñon, widespread mortality occurred only once, during a 6-month (26.1-week) drought in 2002 (Fig. 3A). By fitting a curve to the frequency distribution of regional drought duration, we estimated that our observed 28% acceleration in mortality with warmer temperatures indicated that a shorter, ≈4-month (18.7-week) drought would cause widespread mortality. Therefore we estimated that a 4.3 °C increase in temperature corresponded to a 5-fold increase in the frequency of mortality-inducing events (Fig. 3B). This projection is conservative because it is based on the historical drought record and therefore does not include changes in drought frequency, which is predicted to increase concurrently with warming (2, 37–39). In addition, populations of tree pests, such as bark beetles, which are often the proximal cause of mortality in this species and others, are also expected to increase with future warming (7, 9, 38).
Fig. 3.
Drought frequency and die-off projections. (A) Duration and frequency of drought events from a 103-year record of regional climate. (B) A comparison of the frequency of a widespread die-off causing drought from the 103-year record (26.1-week regional drought, blue), and under a warmer-temperature, accelerated mortality scenario (18.7-week regional drought, red) for the Four Corners region.
Our results demonstrate that future warming will exacerbate regional die-off (3, 35) and elevate background mortality rates (13, 16) independently of other changes in ecosystem water balance. The high degree of temperature sensitivity we have documented in drought-induced mortality for piñon pine needs to be assessed for other widespread, dominant tree species. The temperature sensitivity we document highlights the need to improve model predictions and could profoundly alter assessments of climate change impacts, which continue to reveal increasingly dangerous risks (40), including those for ecosystem function, species distributions, energy fluxes, hydrological processes, and perhaps most importantly C fluxes (17, 19–22, 25, 35, 39, 41, 42). Our results underscore the critical importance of understanding temperature sensitivities associated with the mechanisms that trigger plant mortality and drive vegetation change and their implications for assessments of climate change impacts and consequent land surface–atmospheric feedbacks. Most importantly, because increased temperature is among the most widespread and least uncertain climate projections (37–39), our results portend widespread increases in the extent and frequency of vegetation die-off.
Materials and Methods
We selected reproductively mature piñon pines (P. edulis) from a ranch near Ojitos Frios, NM (35.5177°N, 105.3337°W, 2,050 m above sea level) with similar allometry, an average height of 1.7 m (range: 1.3–2.4 m tall), and an average root-collar-diameter of 6.5 cm (range: 3.5–11 cm) that were isolated (nearest-neighbor canopy-to-canopy distances >1 m) and were not in rocky areas or eroded rills. After transport to Biosphere 2, we placed trees in 0.5-m-diameter, 100-L containers. Where minor soil additions were needed, we added a soil with similar texture, organic C, and nitrogen content. Twenty trees were randomly distributed into 2 conditions at Biosphere 2: temperatures that approximately tracked mean ambient conditions for piñon pine (weekly mean minima of 10.9 to 20.8 °C and maxima of 22.8 to 34.2 °C), vs. those elevated consistently by an average of 4.3 °C. Mean weekly relative humidity was kept constant between treatments and varied from 34% to 78%, resulting in mean vapor pressure deficits of 1.18 kPa for the ambient treatment (weekly mean range: 0.35–1.85 kPa) and 1.51 kPa for the warmer treatment (weekly mean range: 0.56–2.64 kPa).
Initially high volumetric soil water content (20–30%) was maintained for all trees by daily watering (confirmed with 20-cm ECH2O probes, Decagon Devices). Irrigation was curtailed for 5 randomly selected trees on February 9, 2008, in each temperature treatment, while the remaining 5 trees in each continued to be watered. Weighing scales (Industrial Commercial Scales) were placed under 3 drought trees in each temperature treatment to record water loss gravimetrically. We measured predawn plant water potentials before and during the experiment on excised twigs from the south side of the tree canopy by using a pressure chamber (PMS Instruments). We curtailed water potential measurements at −8 MPa because previous research documented complete loss of hydraulic conductivity in P. edulis at branch water potential of −6 MPa (36). We measured midday photosynthesis and predawn respiration by using an LI-6400 portable infrared gas analyzer (LI-6400, LI-COR Biosciences). Time-integrated cumulative respiration costs were calculated by multiplying instantaneous predawn rates by the period in seconds each measurement represented. These time periods began and ended at the halfway point in time between each sampling date. Trees were checked weekly for signs of needle browning and declared dead when 90% of their canopy foliage turned brown.
We estimated a relevant regional drought distribution with climate data (Western Regional Climate Center, www.wrcc.dri.edu) by using 1 station each from Arizona, Colorado, New Mexico, and Utah with an ≈100-year record and in an elevation range which includes P. edulis (1,300–2,100 m) (Table S1). We averaged monthly precipitation totals (January 1905 to July 2008, excluding months with >10 days missing data) and defined drought months as those where total precipitation was <50% of the long-term monthly mean, consistent with the recent die-off (3). To project our temperature sensitivity on the historical record we fit a negative exponential function to the probability distribution of regional drought, yielding: drought frequency = 183.5 × e−0.2408×(drought duration). The recent, widespread mortality-causing drought was the only 6-month regional drought (26.1-week) on the record, whereas during the same period, there were more than 5 drought events exceeding 4.3 months (18.7 weeks) in duration.
Supplementary Material
Acknowledgments.
We thank John Adams and the Biosphere 2 staff, interns, and volunteers for assistance with the experiment; and C. Allen, L. Graumlich, D. Law, and S. Saleska for comments on the analysis and manuscript. Research was supported by Biosphere 2 (B2 Earthscience via Philecology Foundation) and U.S. Department of Agriculture Cooperative State Research, Education, and Extension Service Grant 2005-38420-15809 in collaboration with Department of Energy Grant DE-FC02-06ER64159 and National Science Foundation Grant DEB-043526.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901438106/DCSupplemental.
References
- 1.Allen CD, Breshears DD. Drought-induced shift of a forest-woodland ecotone: Rapid landscape response to climate variation. Proc Natl Acad Sci USA. 1998;95:14839–14842. doi: 10.1073/pnas.95.25.14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jentsch A, Kreyling J, Beierkuhnlein C. A new generation of climate-change experiments: Events, not trends. Front Ecol Environ. 2007:65–374. [Google Scholar]
- 3.Breshears DD, et al. Regional vegetation die-off in response to global-change-type drought. Proc Natl Acad Sci USA. 2005;102:15144–15148. doi: 10.1073/pnas.0505734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw JD, Steed BE, DeBlander LT. Forest inventory and analysis (FIA) annual inventory answers the question: What is happening to pinyon-juniper woodlands? J Forest. 2005;103:280–285. [Google Scholar]
- 5.Gitlin AR, et al. Mortality gradients within and among dominant plant populations as barometers of ecosystem change during extreme drought. Conserv Biol. 2006;20:1477–1486. doi: 10.1111/j.1523-1739.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 6.Mueller RC, et al. Differential tree mortality in response to severe drought: Evidence for long-term vegetation shifts. J Ecol. 2005;93:1085–1093. [Google Scholar]
- 7.Logan JA, Regniere J, Powell JA. Assessing the impacts of global warming on forest pest dynamics. Front Ecol Environ. 2003;1:130–137. [Google Scholar]
- 8.Allen CD, Breshears DD. Climate-induced forest dieback as an emergent global phenomenon. Eos Trans Am Geophys Union. 2007;88:504. [Google Scholar]
- 9.Raffa KF, et al. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: The dynamics of bark beetle eruptions. Bioscience. 2008;58:501–517. [Google Scholar]
- 10.Fensham RJ, Fairfax RJ, Ward DP. Drought-induced tree death in savanna. Global Change Biol. 2009;15:380–387. [Google Scholar]
- 11.Bigler C, Braker OU, Bugmann H, Dobbertin M, Rigling A. Drought as an inciting mortality factor in Scots pine stands of the Valais, Switzerland. Ecosystems. 2006;9:330–343. [Google Scholar]
- 12.Dobbertin M, et al. Linking increasing drought stress to Scots pine mortality and bark beetle infestations. Sci World J. 2007;7:231–239. doi: 10.1100/tsw.2007.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Mantgem PJ, Stephenson NL. Apparent climatically induced increase of tree mortality rates in a temperate forest. Ecol Lett. 2007;10:909–916. doi: 10.1111/j.1461-0248.2007.01080.x. [DOI] [PubMed] [Google Scholar]
- 14.Reich PB, Oleksyn J. Climate warming will reduce growth and survival of Scots pine except in the far north. Ecol Lett. 2008;11:588–597. doi: 10.1111/j.1461-0248.2008.01172.x. [DOI] [PubMed] [Google Scholar]
- 15.Salle A, Ye H, Yart A, Lieutier F. Seasonal water stress and the resistance of Pinus yunnanensis to a bark-beetle-associated fungus. Tree Physiol. 2008;28:679–687. doi: 10.1093/treephys/28.5.679. [DOI] [PubMed] [Google Scholar]
- 16.van Mantgem PJ, et al. Widespread increase of tree mortality rates in the western United States. Science. 2009;323:521–524. doi: 10.1126/science.1165000. [DOI] [PubMed] [Google Scholar]
- 17.Engelbrecht BMJ, et al. Drought sensitivity shapes species distribution patterns in tropical forests. Nature. 2007;447:80–82. doi: 10.1038/nature05747. [DOI] [PubMed] [Google Scholar]
- 18.Williams JW, Jackson ST, Kutzbacht JE. Projected distributions of novel and disappearing climates by 2100 AD. Proc Natl Acad Sci USA. 2007;104:5738–5742. doi: 10.1073/pnas.0606292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moorcroft PR, Hurtt GC, Pacala SW. A method for scaling vegetation dynamics: The ecosystem demography model (ED) Ecol Monogr. 2001;71:557–585. [Google Scholar]
- 20.Breshears DD, Allen CD. The importance of rapid, disturbance-induced losses in carbon management and sequestration. Global Ecol and Biogeogr. 2002;11:1–5. [Google Scholar]
- 21.Luo YQ. Terrestrial carbon-cycle feedback to climate warming. Annu Rev Ecol Evol Syst. 2007;38:683–712. [Google Scholar]
- 22.Luyssaert S, et al. Old-growth forests as global carbon sinks. Nature. 2008;455:213–215. doi: 10.1038/nature07276. [DOI] [PubMed] [Google Scholar]
- 23.Ford CR, Vose JM. Tsuga canadensis (L.) Carr. mortality will impact hydrologic processes in southern Appalachian forest ecosystems. Ecol Appl. 2007;17:1156–1167. doi: 10.1890/06-0027. [DOI] [PubMed] [Google Scholar]
- 24.Chapin FS, Randerson JT, McGuire AD, Foley JA, Field CB. Changing feedbacks in the climate-biosphere system. Front Ecol Environ. 2008;6:313–320. [Google Scholar]
- 25.Running SW. Climate change: Ecosystem disturbance, carbon, and climate. Science. 2008;321:652–653. doi: 10.1126/science.1159607. [DOI] [PubMed] [Google Scholar]
- 26.Lewis SL, Malhi Y, Phillips OL. Fingerprinting the impacts of global change on tropical forests. Philos Trans R Soc London Ser B. 2004;359:437–462. doi: 10.1098/rstb.2003.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franklin JF, Shugart HH, Harmon ME. Tree death as an ecological process. Bioscience. 1987;37:550–556. [Google Scholar]
- 28.Sitch S, et al. Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model. Global Change Biol. 2003;9:161–185. [Google Scholar]
- 29.Gerten D, et al. Modelled effects of precipitation on ecosystem carbon and water dynamics in different climatic zones. Global Change Biol. 2008;14:2365–2379. [Google Scholar]
- 30.McDowell N, et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008;178:719–739. doi: 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- 31.West AG, Hultine KR, Sperry JS, Bush SE, Ehleringer JR. Transpiration and hydraulic strategies in a piñon-juniper woodland. Ecol Appl. 2008;18:911–927. doi: 10.1890/06-2094.1. [DOI] [PubMed] [Google Scholar]
- 32.Brodribb TJ, Cochard H. Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol. 2009;149:575–584. doi: 10.1104/pp.108.129783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christiansen E, Waring RH, Berryman AA. Resistance of conifers to bark beetle attack: Searching for general relationships. Forest Ecol Manage. 1987;22:89–106. [Google Scholar]
- 34.Hartley IP, et al. The dependence of respiration on photosynthetic substrate supply and temperature: Integrating leaf, soil and ecosystem measurements. Global Change Biol. 2006;12:1954–1968. [Google Scholar]
- 35.Breshears DD, et al. Tree die-off in response to global-change type drought: Mortality insights from a decade of plant water-potential measurements. Front Ecol Environ. 2009 in press. [Google Scholar]
- 36.Linton MJ, Sperry JS, Williams DG. Limits to water transport in Juniperus osteosperma and Pinus edulis: Implications for drought tolerance and regulation of transpiration. Funct Ecol. 1998;12:906–911. [Google Scholar]
- 37.Weltzin JF, et al. Assessing the response of terrestrial ecosystems to potential changes in precipitation. Bioscience. 2003;53:941–952. [Google Scholar]
- 38.Intergovernmental Panel on Climate Change. London: Cambridge Univ Press; 2007. Climate Change 2007—Impacts, Adaptation and Vulnerability: Contribution of Working Group II to the Fourth Assessment Report of the IPCC. [Google Scholar]
- 39.U.S. Climate Change Science Program. Washington, DC: U.S. Environmental Protection Agency; 2008. The Effects of Climate Change on Agriculture, Land Resources, Water Resources, and Biodiversity in the United States. Synthesis and Assessment Product 4.3. Report by the USCCSP and the Subcommittee on Global Change Research. [Google Scholar]
- 40.Smith JB, et al. Assessing dangerous climate change though an update on the International Panel on Climate Change (IPCC) “reasons for concern.”. Proc Natl Acad Sci USA. 2009;106:4133–4137. doi: 10.1073/pnas.0812355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niinemets U, Valladares F. Tolerance to shade, drought, and waterlogging of temperate Northern Hemisphere trees and shrubs. Ecol Monogr. 2006;76:521–547. [Google Scholar]
- 42.Valladares F, et al. Is shade beneficial for Mediterranean shrubs experiencing periods of extreme drought and late-winter frosts? Ann Bot (London) 2008;102:923–933. doi: 10.1093/aob/mcn182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.