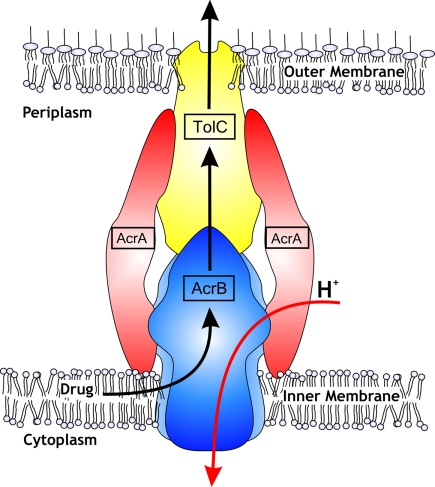
The capacity of bacteria to expel from themselves a broad diversity of compounds confers upon them resistance to drugs and other bacteriocidal agents (1). In Gram-negative bacteria such as Escherichia coli and Pseudomonas aeruginosa multidrug efflux is achieved by energy-powered macromolecular machinery that extrudes cytotoxic substances like multiple anti biotics, dyes, bile salts, and detergents from the inner membrane of the cell directly into the medium, bypassing the periplasm and the outer membrane. Known complexes of such multidrug efflux pumps are composed for three types of components: an outer membrane channel, a periplasmic bridge, and an inner membrane energy-transducer. There are a variety of possible inner membrane components, which can include members of the ATP binding cassette transporter superfamily, the major facilitator superfamily, or the resistance nodulation cell division (RND) superfamily. These inner membrane-located units are the major sites for substrate recognition and energy transduction of the entire tripartite system and function in conjunction with the 2 other pump components. The periplasmic bridge component often goes by the name of “membrane fusion protein” (MFP) for historical reasons and is represented by the AcrA protein. Well-characterized representatives of the outer membrane factor (OMF) are channel-like proteins such as TolC. Both the outer membrane and tripartite efflux pumps are considered to be the main barrier for drugs on their way to the inside of the bacterial cell (2). Importantly, only assembly of all 3 components into a trinity results in an active and effective drug efflux phenotype. In this issue of PNAS, Symmons et al. (3) complete the picture of an E. coli 3-component multidrug efflux system with a well-tested and insightful model.
The most extensively-studied 3-component systems are AcrAB–TolC of E. coli and the homologous MexAB-OprM of P. aeruginosa, which are generally referred to as RND efflux systems. The substrate range of these pumps includes dyes, bile salts, organic solvents, and antibiotics of different chemical classes; molecules that are anionic, cationic, zwitterionic, aromatic, or just aliphatic; and chemicals of bulky or planar geometry. The energy source for drug transport is derived from the proton motive force and these transporters function as drug/proton antiporters. For all 3 individual components, structural information based on X-ray crystallography is available (4–11) and has led to several suggestions of tripartite pump assemblies (10–13) (Fig. 1). Both TolC and AcrB are known homotrimers. The interaction between AcrB and TolC has been shown to be weak in vitro (14), and the AcrA adapter consolidates this interaction by forming a complex between both the inner membrane component and outer membrane channel.
Fig. 1.
Schematic drawing of tripartite RND multidrug efflux system AcrAB–TolC of the Gram-negative bacterium Escherichia coli (courtesy of Andrea Eberle, Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany). Suggestions on the stoichiometry of the adaptor AcrA to inner membrane RND component AcrA (or to outer membrane channel TolC) vary between 1 and 4.
However, for the AcrAB–TolC efflux pump, the interaction between periplasmic adapter AcrA, especially its C-terminal domain (15), and the inner membrane component AcrB have remained largely obscure. The available crystal structures have not previously revealed large portions of the N and C termini of the adapter because of apparent disorder, and these are key to the protein-to-protein interactions with the inner membrane partner. By reexamining the existing crystallographic data of MexA (11), Symmons et al. (3) provide a re-refined model of MexA that reveals the previously invisible domain. From that data they completed the homologous AcrA structure that now contains an additional β-roll membrane proximal (MP) domain with a distinctive concave face including 2 exposed helical turns. The MP domain adds ≈45 Å to the height of AcrA and completes the structure of this membrane adapter molecule to a successive stretch of α-helical hairpin, lipoyl, β-barrel, and MP domains.
On the basis of the completed AcrA model, Symmons et al. (3) conducted 74 cross-linking assays by introducing cysteines in both AcrA and AcrB and using hetero-bifunctional cross-linkers. From the cross-linking results, a data-driven docking was conducted with the experimental cross-linking data as restraints. Most intriguingly, the docking procedure produced only 1 solution that was consistent with all experimental data. This solution predicts stoichiometric interaction between AcrA and AcrB and therefore reveals a complete trinity within the heterotrimeric complex of homotrimers.
The whole pump was finally assembled by reoptimization of the previously determined interaction between TolC and AcrA.
Does this model provide a general paradigm for all known tripartite systems? Recent crystallographic studies with the adapter MacA of the ATP-driven MacAB–TolC system suggests a MacA:TolC stoichiometry of 2, and the interactions appear to be much more stable compared with the AcrAB–TolC RND system in vitro and possibly also in vivo (16, 17). Whether this stoichiometry is exclusive to this particular type of pump remains to be tested as well, because another very recent study (18) provides evidence for yet another 2:1 ratio between the MexA/OrpM subunits of the RND-type MexAB–OprM tripartite efflux pump from P. aeruginosa. Previous studies on AcrA, however, seem to be in accordance with its trimeric appearance (19).
The role of the adapter protein within the tripartite system has been and is a heavily debated issue (19). Formerly thought as a rigid adapter “just” linking the inner membrane component and the outer membrane channel, AcrA has advanced to a much more dynamic molecule with multiple tasks. It is believed to have a crucial role in not only recruiting but it is also thought of as the key to open the locked TolC by means of the extensive coil-coil interactions also displayed by the current model (3). Mutations in AcrA also had an effect on the substrate specificity of the entire pump, a feature that was thought to be solely attributed to the RND part (20). Furthermore, it has been speculated that the linker molecule might be involved in signal transduction from the functionally (not physically) rotating AcrB component toward TolC (6, 7). The model by Symmons et al. (3) might give us insights for designing experiments to address these important questions.
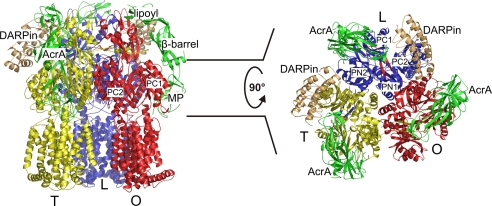
The impact of the pumps on antibiotic resistance of Gram-negative pathogens is another important and hotly-debated issue. Several inhibitors of the pumps have been reported (1), one being a designed ankyrin repeat protein (DARPin), which fortunately also happened to facilitate crystallization of the AcrB component, leading to good resolution (2.5 Å) data (8). The model by Symmons et al. (3) suggests that the DARPin molecule might be inhibiting the function of AcrB rather than hampering the assembly of the pump, because the attachment sides of these (in)voluntary partners appear to be markedly different (Fig. 2).
Fig. 2.
DARPins interact with the AcrB L and T monomers only (8). (Left) Side view of the AcrB trimer (with monomers L (loose, access) in blue, T (tight, binding) in yellow, and O (open, extrusion) in red, in complex with DARPins (light orange) and AcrA (green). Only the membrane-proximal (MP), the β-barrel, and the lipoyl domains of AcrA are shown, whereas the α-helical hairpin following the lipoyl domain has been omitted. (Right) Top view on the AcrB periplasmic porter domain with its subdomains PN1, PN2, PC1, and PC2. Shown is the interaction of AcrA with the AcrB PN2 and PC1 porter domains.
The β-barrel domain of the docked AcrA adapter interacts with the upper part of the PN2 and PC1 periplasmic porter subdomains of AcrB and extends toward the TolC docking domain while making an interaction with an intermonomeric loop (Fig. 2). The newly-assigned MP domain of AcrA snugly interacts with the PN2 and PC1 subdomains of AcrB, which are the cradle of the hydrophobic binding pocket involved in substrate binding. During transport of drugs and consecutive changes of the AcrB monomers through different conformations that have been designated access, binding, and extrusion (or loose, tight and open), the PN2 and to a lesser degree the PC1 subdomains undergo substantial changes upon binding and releasing the drug molecule (6–8). In principle, these changes might be leading to changes in conformation of the adapter molecule as well and might lead to a signaling cascade all up to TolC. One outstanding puzzle is how hydrophobic molecules are encouraged to enter and get transported through the electronegative interior of TolC. Perhaps other mechanisms rather than simple diffusion might be at work, including peristaltic-like motions that might follow those of AcrB (21). For this, TolC needs to be nudged at times to provide the pulse waves leading the drugs to the outside. The AcrAB–TolC trinity would then represent machinery.
Footnotes
The author declares no conflict of interest.
See companion article on page 7173.
References
- 1.Piddock LJ. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev. 2006;19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem. 2009 doi: 10.1146/annurev.biochem.78.082907.145923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Symmons MF, Bokma E, Koronakis E, Hughes C, Koronakis V. The assembled structure of a complete tripartite bacterial multidrug efflux pump. Proc Natl Acad Sci USA. 2009;106:7173–7178. doi: 10.1073/pnas.0900693106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koronakis V, Sharff A, Koronakis E, Luisi B, Hughes C. Crystal structure of the bacterial membrane protein TolC central to multidrug efflux and protein export. Nature. 2000;405:914–919. doi: 10.1038/35016007. [DOI] [PubMed] [Google Scholar]
- 5.Murakami S, Nakashima R, Yamashita E, Yamaguchi A. Crystal structure of bacterial multidrug efflux transporter AcrB. Nature. 2002;419:587–593. doi: 10.1038/nature01050. [DOI] [PubMed] [Google Scholar]
- 6.Murakami S, Nakashima R, Yamashita E, Matsumoto T, Yamaguchi A. Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature. 2006;443:173–179. doi: 10.1038/nature05076. [DOI] [PubMed] [Google Scholar]
- 7.Seeger MA, et al. Structural asymmetry of AcrB trimer suggests a peristaltic pump mechanism. Science. 2006;313:1295–1298. doi: 10.1126/science.1131542. [DOI] [PubMed] [Google Scholar]
- 8.Sennhauser G, Amstutz P, Briand C, Storchenegger O, Grutter MG. Drug export pathway of multidrug exporter AcrB revealed by DARPin inhibitors. PLoS Biol. 2007;5:e7. doi: 10.1371/journal.pbio.0050007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikolosko J, Bobyk K, Zgurskaya HI, Ghosh P. Conformational flexibility in the multidrug efflux system protein AcrA. Structure (London) 2006;14:577–587. doi: 10.1016/j.str.2005.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akama H, et al. Crystal structure of the membrane fusion protein, MexA, of the multidrug transporter in Pseudomonas aeruginosa. J Biol Chem. 2004;279:25939–25942. doi: 10.1074/jbc.C400164200. [DOI] [PubMed] [Google Scholar]
- 11.Higgins MK, Bokma E, Koronakis E, Hughes C, Koronakis V. Structure of the periplasmic component of a bacterial drug efflux pump. Proc Natl Acad Sci USA. 2004;101:9994–9999. doi: 10.1073/pnas.0400375101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Recio J, et al. A model of a transmembrane drug-efflux pump from Gram-negative bacteria. FEBS Lett. 2004;578:5–9. doi: 10.1016/j.febslet.2004.10.097. [DOI] [PubMed] [Google Scholar]
- 13.Misra R, Bavro VN. Assembly and transport mechanism of tripartite drug efflux systems. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbapap.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Touze T, et al. Interactions underlying assembly of the Escherichia coli AcrAB–TolC multidrug efflux system. Mol Microbiol. 2004;53:697–706. doi: 10.1111/j.1365-2958.2004.04158.x. [DOI] [PubMed] [Google Scholar]
- 15.Elkins CA, Nikaido H. Chimeric analysis of AcrA function reveals the importance of its C-terminal do-main in its interaction with the AcrB multidrug efflux pump. J Bacteriol. 2003;185:5349–5356. doi: 10.1128/JB.185.18.5349-5356.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yum S, et al. Crystal structure of the periplasmic component of a tripartite macrolide-specific efflux pump. J Mol Biol. 2009;16:1286–1297. doi: 10.1016/j.jmb.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 17.Tikhonova EB, Devroy VK, Lau SY, Zgurskaya HI. Reconstitution of the Escherichia coli macrolide transporter: The periplasmic membrane fusion protein MacA stimulates the ATPase activity of MacB. Mol Microbiol. 2007;63:895–910. doi: 10.1111/j.1365-2958.2006.05549.x. [DOI] [PubMed] [Google Scholar]
- 18.Reffay M, et al. Tracking membrane protein association in model membranes. PLoS One. 2009;4:e5035. doi: 10.1371/journal.pone.0005035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zgurskaya HI, Yamada Y, Tikhonova EB, Ge Q, Krishnamoorthy G. Structural and functional diversity of bacterial membrane fusion proteins. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbapap.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Krishnamoorthy G, Tikhonova EB, Zgurskaya HI. Fitting periplasmic membrane fusion proteins to inner membrane transporters: Mutations that enable Escherichia coli AcrA to function with Pseudomonas aeruginosa MexB. J Bacteriol. 2008;190:691–698. doi: 10.1128/JB.01276-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaccaro L, Scott KA, Sansom MS. Gating at both ends and breathing in the middle: Conformational dynamics of TolC. Biophys J. 2008;95:5681–5691. doi: 10.1529/biophysj.108.136028. [DOI] [PMC free article] [PubMed] [Google Scholar]