Abstract
Many behavioral traits and most brain disorders are common to males and females but are more evident in one sex than the other. The control of these subtle sex-linked biases is largely unstudied and has been presumed to mirror that of the highly dimorphic reproductive nuclei. Sexual dimorphism in the reproductive tract is a product of Müllerian inhibiting substance (MIS), as well as the sex steroids. Males with a genetic deficiency in MIS signaling are sexually males, leading to the presumption that MIS is not a neural regulator. We challenge this presumption by reporting that most immature neurons in mice express the MIS-specific receptor (MISRII) and that male Mis−/− and Misrii−/− mice exhibit subtle feminization of their spinal motor neurons and of their exploratory behavior. Consequently, MIS may be a broad regulator of the subtle sex-linked biases in the nervous system.
Keywords: anti-Müllerian hormone, exploratory behavior, motor neuron, sexual dimorphism
Many behavioral traits are more evident in one sex than the other, but they do not define the biological sex of an individual. Empathy, for example, has a female bias, but some of the greatest men are empathetic. Likewise, girls engage in less rough-and-tumble play than boys, but a boy who shuns rough-and-tumble play is still a boy. With traits such as these, the characteristics of individual males and females overlap, with the sex-linked bias existing only within the population as a whole. This subtle form of sexual dimorphism is pervasive throughout the nervous system and involves nuclei whose function is not directly related to reproduction. In this paper, we refer to this form of sexual dimorphism as sex-linked bias, to distinguish it from the sexual dimorphism of the primary reproductive tissues, which is binary in nature.
The nervous system also contains nuclei where the male and female forms do not overlap. Such nuclei control primary sexual function, with their dimorphic nature being generated by testosterone, or its active metabolites (1). The regulation of sex-linked bias in the brain has been widely assumed to mirror that of the sexually dimorphic nuclei. However, testosterone is only episodically present during male development, with third-trimester male fetuses and boys older than 1 year having minimal levels of testosterone (2–4). Extensive brain development occurs during these periods, with some regions having increased anatomical dimorphism during the period when testosterone is minimal (5, 6). Thus, although the sex steroids contribute to sex-linked biases, other factors must also be important. We present evidence here that Müllerian inhibiting substance (MIS) (synonym, anti-Müllerian hormone) is one of these factors.
MIS is a testicular hormone that triggers the regression of the uterine precursor (Müllerian ducts) in males around the ninth week of gestation (7). However, the level of MIS in the blood of males does not decline after the loss of the Müllerian ducts, remaining high until puberty, at which stage it falls by an order of magnitude (8–10). The low level of ovarian production of MIS, in contrast, begins only postnatally (8–10). High levels of plasma MIS are thus a unique feature of developing males. This hormonal MIS may affect the maturation of the fetal lung (11), but there is currently no explanation for why MIS is present in male blood for such an extended period of development. We report here that most developing neurons express the MIS-specific receptor, MISRII, and that male mice with null mutations in either Mis or Misrii have partial feminization of their sex-linked biases, even though they are phenotypically and sexually male. This finding implicates MIS as a regulator of sex-linked bias.
Results
The Testes Is the Sole Embryonic Source of MIS.
The gonads have historically been considered to be the sole source of MIS, but we have recently shown that mature motor neurons in both sexes also produce it (12). We therefore determined whether production of MIS also occurred in the developing brain. In confirmation of the historic understanding, only trace levels of MIS mRNA were detected in the embryonic head, which by mass is predominantly the brain. The only proportion of the embryo that contained significant levels of MIS mRNA was the urogenital region (Fig. S1A). Similarly, MIS protein was detected by immunohistochemistry in the testes (Fig. S1B), but not in the developing brain (Fig. S1C). This finding contrasts with the mature brain, where motor (12) and various other neurons contain readily detectable levels of MIS.
MIS Regulates the Number of Spinal Motor Neurons.
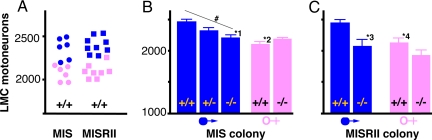
The numbers of motor neurons that innervate the limbs have been extensively studied, but the possibility that they exhibit subtle sex-linked bias has only rarely been considered (13). Our recent observation that MIS promotes the survival of embryonic motor neurons in vitro (12) suggests that they should be. Simply, over one-half of the motor neurons present in the spinal cords of mouse embryos die between the 13th day of gestation and birth (14), with the extent of this cell death being controlled by multiple survival factors. MIS is produced in the embryonic testes from the 12th day of gestation onward (15), which should attenuate motor neuron loss in male fetuses, provided that MIS is a neuronal survival factor in vivo. Consistent with this prediction, the number of motor neurons in the lumbar lateral motor column (LMC) of wild-type male and female mice had almost no overlap in both the Mis and Misrii colonies (Fig. 1A). The magnitude of the male bias was 16–17% in both colonies. This sex-linked difference depended on MIS, because MIS-deficient (Mis−/−) males had female numbers of motor neurons (Fig. 1B), with heterozygous males (Mis+/−) being intermediate between wild-type males and females (Fig. 1B). The effect of Mis gene dose on motor neuron number was significant (P = 0.03) by regression analysis.
Fig. 1.
The LMC of the lumbar spinal cord has MIS-dependent dimorphism. (A) Number of motor neurons in male (blue) and female (pink) wild-type mice derived from matings of either Mis+/− (circles) or Misrii+/− parents (squares). (B and C) Effect of Mis (B) or Misrii (C) genotype on the sexual dimorphism illustrated in A. The bars are the mean ± standard error of the mean of 6–9 mice. *, Significant difference (Student's t) from the wild-type males: 1, P = 0.002; 2, P < 0.001; 3, P = 0.013; 4, P = 0.010. #, A significant effect (P = 0.030) of gene dose by regression analysis.
MIS is a member of the TGFβ superfamily, which signals through a complex consisting of type I and type II receptors. MISRII is thought to be a unique and obligatory receptor for MIS, because null mutations of Mis and Misrii both lead to persistent Müllerian duct syndrome (retention of the uterine precursor in males) (7). MISRII mRNA in neurons is abundant compared with the mRNA for other growth factor receptors but is an order of magnitude less abundant than that present within the classical MIS targets (Müllerian ducts, testes) (12, 16). This observation raises the issue of whether MISRII is essential for MIS signaling in neurons. Consistent with this possibility, male Misrii−/− mice had feminized numbers of LMC motor neurons (Fig. 1C), a malformation that mirrored that seen in the Mis−/− male mice (Fig. 1B). Female Mis−/− and Misrii−/− mice both had wild-type numbers of LMC motor neurons (Fig. 1 B and C), which was expected given that female embryos do not produce MIS (15, 17). Consequently, MISRII is a useful marker for MIS-sensitive neurons (see below).
Androgen-Dependent Dimorphism Is Independent of MIS.
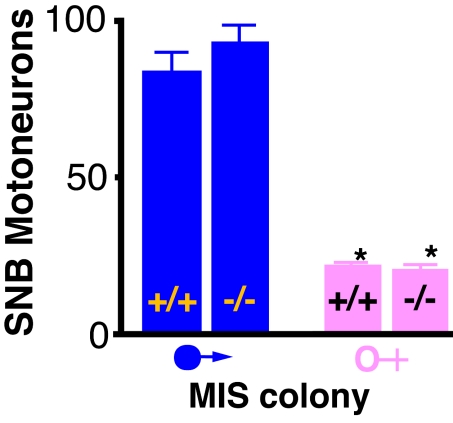
The motor neurons involved in primary sexual function are highly dimorphic, under the control of testosterone acting through the androgen receptor (18, 19). Some LMC motor neurons express androgen receptors (18), creating the possibility that the effect of MIS on LMC neurons is secondary to perturbation of testosterone function. If this was occurring, then testosterone-dependent dimorphism should be diminished in the Mis−/− mice. We therefore examined the spinal bulbocavernosus motor nucleus, which is involved in the function of the penis (18, 19). In marked contrast to the LMC motor neurons, the numbers of motor neurons in the bulbocavernosus nucleus of male Mis−/− mice were similar to those of a wild-type male and were overtly different from the female number (Fig. 2).
Fig. 2.
The dimorphism of the spinal bulbocavernosus (SNB) motor neurons is normal in mice that lack the Mis gene. The bars are the mean ± standard error of the mean of 4–6 mice. *, Both female groups are significantly different from both of the male groups, P < 0.001.
Most Neurons Express MIS Receptors.
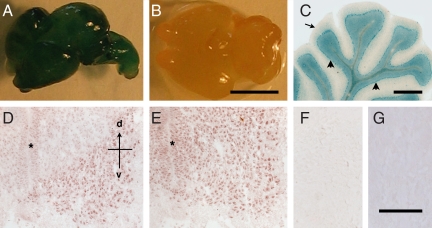
All of the classical neuronal survival factors affect multiple types of neurons. We therefore examined whether MIS was a specific regulator of limb-innervating motor neurons or a broad regulator of the nervous system. Consistent with the latter, the levels of mRNA for the MIS-specific receptor in the embryonic brain and spinal cord were similar. The cellular lineages that produce MISRII were identified by using genetically modified mice that produce lacZ in MISRII-expressing cells (MISRII-Cre-lacZ) (Fig. S2). Most neurons in the brain, spinal cord, and peripheral nervous system of adult MISRII-Cre-lacZ mice were lacZ-positive, whereas the glia, blood vessels, and fibroblasts were lacZ-negative (Fig. 3 A–C). Once lacZ is induced in a cell in the MISRII-Cre-lacZ mice, all daughter cells will express it (Fig. S2). The immediate precursor of neurons (neuroepithelia) undergo a series of stem cell-like divisions, which give rise to postmitotic neurons during the early stages of the lineage and various glia in the terminal divisions. The absence of lacZ in the glia thus indicates that postmitotic neurons are the only cells within the neuronal lineage to express significant levels of MISRII. This contention was verified by examining the brains of MISRII-Cre-lacZ embryos. The neuroepithelium of the spinal cord and brain were lacZ-negative, whereas lacZ-positive neurons were abundant in the brains of embryonic day 12 (E12) MISRII-Cre-lacZ embryos. This finding indicates that lacZ reporter is activated within 24 h of neurons becoming postmitotic and beginning to differentiate.
Fig. 3.
Localization of MISRII in the nervous system. (A–C) MISRII-Cre-lacZ lineage tracing. (A) Whole brain of a MISRII-Cre-lacZ E16 embryo. (B) Littermate control (Misrii+/+, ROSA26-lacZ Cre). (C) Section of the cerebellum of a MISRII-Cre-lacZ adult mouse showing lacZ stain in neurons (arrowheads) but not the white matter (arrow). (D–G) Immunohistochemical detection of MISRII. Sections of lumbar spinal cord of E12 (D) and E14 (E) embryos stained with an anti-MISRII antibody. The asterisk indicates the neuroepithelium, with the dorsal–ventral (d–v) axis indicated. (F) Section of E14 spinal cord stained with control IgG. (G) Section of the cerebellum from a Misrii−/− mouse stained with the anti-MISRII antibody. (Scale bars: B, 10 mm; C, 1 mm; F, 100 μm.)
Sections of embryonic brains were then stained with an antibody to MISRII to determine whether the expression of MISRII in neurons is continuous during development and whether all developing neurons expressed MISRII at a similar level. The antibody is specific to MISRII as it immunoprecipitates a single protein from the spinal cord (12) and does not stain sections of Misrii−/− brains (Fig. 3G). The anti-MISRII antibody stained most neurons in the developing brain and spinal cord but with differing intensities. The motor neurons in the spinal cord, for example, were more intensely stained than adjacent interneurons, although this may merely be because motor neurons are comparatively large and are the most mature neurons in the developing brain. This differential staining of motor and interneurons persisted throughout development and is also evident in the mature spinal cord (12). The staining of neurons in the ventral spinal cord preceded that of the dorsal cord, with dorsal spinal neurons having only trace levels of MISRII protein at E12 (Fig. 3D). This dorsal–ventral difference was lost by E14 (Fig. 3E).
Exploratory Behavior Is MIS Dependent.
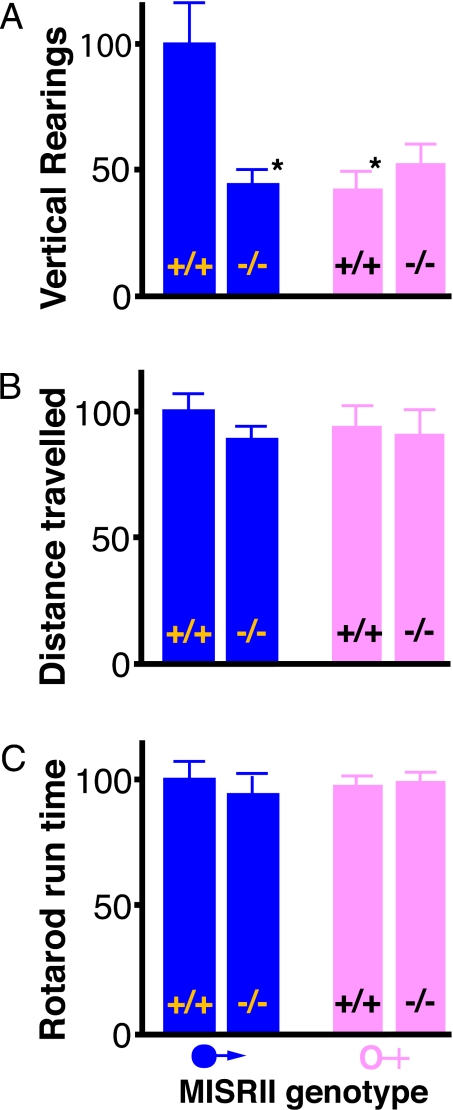
We then examined whether the expression of MISRII in the brain leads to a male bias in a behavioral trait. Male mice have larger territories and a greater tendency to explore than females. When mice were placed in a nocturnal open-field chamber, the males and female traversed the floor to similar extents (Fig. 4A), but the wild-type males reared twice as often as the wild-type females, in an attempt to move beyond the confines of the chamber (Fig. 4B). In marked contrast to the wild-type males, the behavior of Misrii−/− males was indistinguishable from that of the Misrii+/+ and Misrii−/− females (Fig. 4 A and B). This is consistent with the male bias in this behavior being generated by MIS. In other tests, such as running on a rotarod, Misrii+/+ and Misrii−/− male mice were indistinguishable (Fig. 4C). This is also consistent with MIS regulating the subtle sex-linked differences in the brain, while being entirely dispensable for acquisition of the nondimorphic features of the brain.
Fig. 4.
MISRII-dependent behavior. Mice were placed in an open-field apparatus under nocturnal conditions for 20 min and their movement was recorded by sensors. (A) Rearing: the data are the number of times each mouse reared during the second 10 min of the test, to explore beyond the confines of the apparatus. (B) Distance traveled: the data are the distance traveled by the mouse in the first 10 min of the test. (C) Rotarod run time: the data are the maximum length of time that the mouse ran on an accelerating rotarod, from 3 trials. The results are the mean ± standard error of the mean and are presented as a percentage of the mean for Misrii+/+ male mice. The Misrii+/+ and Misrii−/− mice from 11 litters were tested (40 mice; group size, 9–11) on the open field. Ten of the litters were also tested on the rotarod (34 mice; group size, 7–10). *, Significant difference (Student's t) from the Misrii+/+ males, P < 0.01.
Discussion
MIS Contributes to Sex-Linked Biases in the Brain.
Sex-linked biases are pervasive throughout the nervous system, as evidenced by quantitative anatomical, pharmacological, fMRI, and behavioral studies (6, 20, 21). The current report provides evidence that the testicular hormone MIS contributes to these sex-linked biases.
The natural expression of MIS in the current study was sufficient to increase the number of neurons in the LMC in male mice to beyond the female range (Fig. 1) and to produce male exploratory behavior that was overtly different than that of female mice (Fig. 4). The clarity of these sex-linked differences reflects the fact that the mice had minimal genetic variation and had been raised in controlled conditions. In human populations and outbred colonies, the size of motor nuclei varies greatly between individuals (22). In such populations, a 16% male–female difference (Fig. 1) would present as a subtle male bias, with the male and female populations having extensive overlap.
MIS May Contribute to Variation in the Male Population.
The expression of MIS varies between individual males, which may contribute to diversity within the male population. In 1-year-old boys, for instance, the plasma concentration of MIS ranges from 10 to 444 ng/ml (n = 40; girl range, 1–4 ng/ml) (ref. 8; see also ref. 23). The effect of MIS on neurons is dose dependent, both in vitro (12) and in vivo (Fig. 1B). Hence, MIS would be expected to produce a population of males that contains individuals who are little different than females for a particular trait and other individuals who exhibit an extreme male phenotype for that trait.
The incidences and/or severities of most brain disorders exhibit a sex-linked bias (24). This observation suggests that the sex-linked biases in the brain are able to either attenuate or amplify the consequences of underlying brain pathology. If so, then individuals with a high expression of MIS may be predisposed to develop certain conditions with a male bias (e.g., attention-deficit hyperactivity disorder, autism, motor neuron disease) and to be relatively protected against some female-bias conditions (e.g., anorexia).
MIS May Be a Broad Regulator of Neurons.
The neural basis of the sex-linked difference in the exploratory behavior of mice is unknown. However, the initiation of movement is controlled in the brain proper, indicating that the observed MIS-dependent bias in exploratory behavior is unlikely to be secondary to the effect of MIS on spinal motor neurons. The MIS receptor is broadly present in brain neurons (Fig. 3) (25), thus providing multiple theoretical pathways by which MIS could regulate exploratory and other behaviors. Furthermore, the physiological actions of neural regulators are often context dependent, variably regulating neuronal number, pattern of synaptic connection, neurotransmitter type, and neurotransmitter expression in a neuron-specific and stage-specific manner (26). Thus, although the current data implicate MIS as a regulator of neural networks, the definition of the breadth of its action will require detailed investigations of many different types of neurons.
The Neural Actions of MIS Are Cryptic.
Mis−/− and Misrii−/− boys and male mice have been extensively studied with respect to the persistence of their Müllerian ducts. This fact raises the issue of why MIS has not previously been recognized as a regulator of the nervous system. Two issues may be relevant here.
First, the role of MIS in the brain appears to relate to sex-linked biases, rather than to binary sexual dimorphism. Sex-linked biases are evident only at the level of the population and would not be apparent unless groups of MIS-deficient individuals were compared with their peers. In humans, Mis−/− and Misrii−/− males have a persistent Müllerian duct, which prevents the descent of their testes (7). This malformation is readily detected, but Mis−/− and Misrii−/− men are very rare and have not been systematically studied. MIS has both positive and negative effects on the production of testosterone, with the consequence that Mis−/− mice have grossly normal levels of testosterone (27), and are sexually male (28). Hence, the partial feminization of Mis−/− males may have been overlooked because of their overt male features, which have been generated by the normality of their sex steroids and sex chromosomes.
Second, MIS signaling in neurons may be partially different than that which occurs in the classical target of MIS (Müllerian ducts and the gonads). The neural and reproductive forms of MIS signaling are both dependent on MISRII, because the phenotype of Mis−/− and Misrii−/− exhibit the same neural (Fig. 1) and reproductive defects (28). The abundance of MISRII in the different cell types, however, is quite different: MISRII is one of the most abundant growth factor receptors produced by motor neurons, but, paradoxically, the level of MISRII mRNA and protein in neurons is <1% of that in the testes (12, 16). Consequently, historic studies of MISRII have overlooked the presence of MISRII in the brain, because the levels of MISRII in nonreproductive tissues were normalized to that of the testes. The reason for the extraordinary abundance of MISRII in reproductive tissues is unknown. In this context, however, it is worth noting that the MIS-induced regression of the Müllerian ducts is insensitive to the abundance of MIS, with partial retention of the ducts occurring only if the level of MIS falls below a low threshold (29). In contrast, Mis+/− male mice exhibit a diminished sex bias in their lateral motor column (Fig. 1), and MIS-induced survival of motor neurons in vitro has a log-linear dose–response curve (12) that spans the entire physiological range for MIS (8).
The Neural Actions of MIS May Be Hormonal.
MIS is generally considered to be a hormone during development, because MIS from a twin male in some species can remove the uterus from his twin sister, if their placental blood supplies anastomose (7). The neural actions of MIS described here also appear to be hormonal. First, the embryonic brain lacked significant levels of MIS mRNA and none of its cells was MIS immunoreactive (Fig. S1C). The only detectable source of MIS was the testes. Hence, the neural actions of MIS must be either hormonal or indirect. Second, purified embryonic motor neurons respond to MIS in vitro, indicating their MIS receptors are functional (12). Furthermore, the observed actions of MIS in vitro (neuronal survival) (12) and in vivo (increased neuronal number) (Fig. 1) are analogous, which is consistent with MIS acting directly on neurons. Third, the normality of the testosterone-dependent dimorphism in Mis−/− mice (Fig. 2 and above discussion) indicates that the phenotype of Mis−/− mice is not secondary to altered production or function of testosterone.
If testicular MIS acts directly on neurons, then it must be able to reach the brain and spinal cord. MIS is a protein and will therefore be unable to passively pass through the blood–cerebrospinal fluid (CSF) barrier. The blood–CSF barrier develops early, but its embryonic and postnatal characteristics differ from each other and from the mature state (30, 31). Many protein hormones are actively transported into the CSF (32, 33), and it will be important to determine whether MIS is so transported. If it is, then the testes may be directly affecting the brain of boys, via MIS, and male puberty may involve the diminishment of one male signal (MIS) as well as the reemergence of another (testosterone).
In conclusion, the primary reproductive tissues have binary sexual dimorphism, because the roles of males and females in reproduction are fundamentally different. Binary sex differences are predominantly generated via testosterone and its active metabolites, with the role of MIS being limited to preventing males from developing a uterus. However, there are many traits that are variable in both male and female populations, but which are more common in one sex or the other. Sex-linked biases in nonreproductive tissues appear to result from multiple mechanisms, including sex chromosomes, gonadal hormones, and the environment (1). Consequently, the various traits with sex-linked biases are not coordinately regulated, creating rich diversity within the male and female populations. The current study implicates MIS as one of the factors that generates sex-linked biases and variability within the male lineage.
Materials and Methods
Animals.
The University of Otago's Animal Ethics Committee approved all experiments. The Misrii+/− mice have Cre recombinase knocked into the Misrii coding region, leading to a null Misrii allele and to the expression of Cre recombinase under the control of the endogenous Misrii promoter (34) (Fig. S2). The Misrii+/− mice were generated from AB-1 embryonic stem cells and were initially maintained on a C57BL/6 × 129/SvEv background (34). These mice were a generous gift from Richard R. Behringer (University of Texas, Houston, TX). The Mis+/− mice were also generated from AB-1 stem cells (35) and backcrossed to C57BL/6 by The Jackson Laboratory, from whom they were purchased. Both the Mis and Misrii colonies have been maintained by brother–sister matings at the University of Otago. The ROS26-lacZ Cre reporter mice (36) were obtained from a colony at the University of Otago. The housing of the mice was as described in ref. 37, with the genotype determined by PCRs using the primers listed in Table S1.
Neuronal Counts.
Wild-type, heterozygous, and null-mutant neonates from Mis+/− × Mis+/− and Misrii+/− × Misrii+/− matings were killed at birth by decapitation and fixed by immersion in 4% paraformaldehyde in 0.01 M sodium phosphate buffer, pH 7.4 (PB). The caudal spinal cords from the midthoracic region were removed for analysis of the lumbar LMC. Cell death in the murine LMC is complete by birth (14), and this age is the standard for estimation of LMC motor neurons (38). The spinal bulbocavernosus (SNB) motor neurons develop later than LMC motor neurons and may be dually influenced by prenatal and postnatal testosterone (18). The numbers of motor neurons in this nucleus were therefore determined by using spinal cords from 6-week-old mice that have been fixed by cardiac perfusion with 4% paraformaldehyde. The spinal cords were embedded in Technovit resin, serially sectioned at either 40 μm (LMC) or 30 μm (SNB) and stained with cresyl violet. The numbers of motor neurons were estimated by using an optical dissector (39), with the counting particle being the nucleolus. Every fifth section was examined through the entire LMC, whereas all sections containing the SNB were analyzed. Examples of LMC and bulbocavernosus motor neurons are illustrated in Fig. S3. The cell counts were done blind with respect to the sex and the genotype of the mice.
MISRII-Cre-lacZ.
Misrii+/− dams were time mated with a ROSA26-lacZ Cre reporter stud, and the brains from the resulting MISRII-Cre-lacZ fetuses (Fig. S2) were collected at 13, 14, 16, or 20 days of gestation, or after the pups had matured to adulthood (6–8 weeks). The fetal brains were fixed on ice for 2 h in a solution containing 2% paraformaldehyde, 0.2% glutaraldehyde, 0.02% Nonidet P-40, and 0.01% sodium deoxycholate, and after washing they were stained overnight in 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 1 mM MgCl2, 0.02% Nonidet P-40, 0.01% sodium deoxycholate, and 0.1 M PB (40). The tails of the fetuses were used for genotyping, with the ROSA26-lacZ Cre, Misrii+/+ pups serving as controls. The adult brains were snap-frozen in isopentane and serially sectioned in a cryostat, and the resulting sections were fixed for 10 min at 4 °C in 2% paraformaldehyde in 0.1 M PB containing 2 mM MgCl2, pH 7.3, after which they were washed in PBS containing 2 mM MgCl2, and stained overnight at room temperature with 2 mM MgCl2, 20 mM K3Fe(CN)6, 20 mM K4Fe(CN)6, and 4 mg/ml X-Gal in PBS.
Immunohistochemistry.
Transverse sections of lumbar spinal cords and testes from E12, E14, E16, and E18 embryos, neonates, and adult wild-type mice were cut in a cryostat at a thickness of 10 μm. The sections were stained by immunohistochemistry as described in ref. 41, using either goat anti-MISRII or goat anti-MIS (R&D Systems), with nonimmune IgG (Sigma) used as a control antibody. Sections of the brains of adult Misrii−/− mice were used as an additional negative control. The immunoreactivity was visualized by using 3-amino-9-ethylcarbamide (Sigma).
Real-Time PCR.
Five 12-day-old C57BL/6 fetuses were dissected into the portions listed in Fig. S1, and total RNA fractions were prepared for each portion by using TRIzol reagent (Invitrogen). The isolated RNA fractions were treated with DNase I (Promega) to remove genomic DNA contamination and cDNA was synthesized by using SuperScript II RNase H− (Invitrogen) and oligo(dT)20. The real-time PCRs were performed by using an ABI Prism 7000 (Applied Biosystems), SYBR Green Master Mix (Applied Biosystems), and gene-specific primers (12). A 2-step PCR was carried out with denaturation at 95 °C for 15 s, annealing and extension combined at 60 °C for 1 min in a total of 40–50 cycles. The uniqueness of amplicons was analyzed by using dissociation curves and by sequencing. Standard curves were generated for each gene, and the copy number of the mRNA transcripts was calculated and normalized relative to the level of GAPDH.
Behavioral Analysis.
The 9-week-old wild-type and null-mutant mice from 11 litters from Misrii+/− studs and dams were placed in an open-field apparatus (Opto-varimex; Columbus Instruments) under nocturnal lighting conditions (37) (40 mice; 10 male and 9 female Misrii+/+, plus 10 male and 11 female Misrii−/−). The mice from 10 of the 11 litters were subsequently tested on a Rotamex rotarod (Columbus Instruments) when 10–12 weeks of age (10 male and 7 female Misrii+/+, plus 8 male and 7 female Misrii−/−). The mice were tested by their regular caregiver in an anteroom attached to the room that housed the mice. The apparatus was cleaned after each mouse.
With the open-field test, each mouse was placed in the center of the 43 × 43 cm chamber and its movements were tracked for 20 min by an array of infrared beams. The movement of the mice across the floor of the apparatus was recorded by a computer, along with a record of the number of times the mice reared on their hindlimbs. As the test progressed, the mice spent less time traversing the floor of the apparatus and were more likely to rear in an attempt to move beyond the chamber. The data are presented as the distance traveled in the first 10 min and the number of rearings in the second 10 min of the test, because these correspond to the periods when the behavior was predominantly occurring. The observed effects of sex and genotype were similar when the data for the entire 20 min were analyzed.
Mice were trained to run on the rotarod for 3 consecutive days and tested on the fourth day. The initial speed of the rotarod was 5 rpm, with an acceleration of 0.5 rpm every 5 s, and with the mouse's presence on the rod being automatically detected by infrared beams. Each mouse was tested 3 times, and the average and maximum performances of the mice were analyzed. Both methods of analysis gave comparable results and only the maximum performance of the mice is presented here.
Statistical Analysis.
The data were examined by 1-way ANOVA for sex differences (Fig. 1A) or by 2-way ANOVA for sex and genotype (Fig. 1 B and C) (2, 4). Significant effects were confirmed by Student's t tests. The effect of gene dose on LMC motor neuron number was examined by linear regression (Fig. 1B).
Supplementary Material
Acknowledgments.
We thank Mrs. N. Batchelor and J. McLay for expert technical assistance. The work was supported by The Marsden Fund (Royal Society, New Zealand) and by an Otago Research Grant. A.N.C. was supported by a postdoctoral fellowship for the Neurological Foundation (New Zealand).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902253106/DCSupplemental.
References
- 1.Arnold A. Sex chromosomes and brain gender. Nat Rev Neurosci. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- 2.Takagi S, et al. Sex differences in fetal gonadotropins and androgens. J Steroid Biochem. 1977;8:609–620. doi: 10.1016/0022-4731(77)90270-9. [DOI] [PubMed] [Google Scholar]
- 3.Wudy SA, Wachter UA, Homoki J, Teller WM. 17alpha-hydroxyprogesterone, 4-androstenedione and testosterone profiled by routine stable isotope dilution/gas chromatography-mass spectrometry of children. Pediatr Res. 1995;38:76–80. doi: 10.1203/00006450-199507000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Wudy SA, Dorr HG, Solleder C, Djalali M, Homoki J. Profiling steroid hormones in amniotic fluid of midpregnancy by routine stable isotope dilution/gas chromatography-mass spectrometry: Reference values and concentrations in fetuses at risk for 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1999;84:2724–2728. doi: 10.1210/jcem.84.8.5870. [DOI] [PubMed] [Google Scholar]
- 5.Witelson S. Neural sexual mosaicism: Sexual differentiation of the human temporo-parietal region for functional asymmetry. Psychoneuroendocrinology. 1991;16:131–153. doi: 10.1016/0306-4530(91)90075-5. [DOI] [PubMed] [Google Scholar]
- 6.Giedd JN, et al. Puberty-related influences on brain development. Mol Cell Endocrinol. 2006;254:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Teixeira J, Maheswaran S, Donahoe PK. Mullerian inhibiting substance: An instructive developmental hormone with diagnostic and possible therapeutic applications. Endocr Rev. 2001;22:657–674. doi: 10.1210/edrv.22.5.0445. [DOI] [PubMed] [Google Scholar]
- 8.Lee MM, et al. Mullerian inhibiting substance in humans: Normal levels from infancy to adulthood. J Clin Endocrinol Metab. 1996;81:571–576. doi: 10.1210/jcem.81.2.8636269. [DOI] [PubMed] [Google Scholar]
- 9.Rajpert-De Meyts E, et al. Expression of anti-Mullerian hormone during normal and pathological gonadal development: Association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab. 1999;84:3836–3844. doi: 10.1210/jcem.84.10.6047. [DOI] [PubMed] [Google Scholar]
- 10.Schwindt B, Doyle LW, Hutson JM. Serum levels of Mullerian inhibiting substance in preterm and term male neonates. J Urol. 1997;158:610–612. [PubMed] [Google Scholar]
- 11.Catlin EA, et al. Sex-specific fetal lung development and mullerian inhibiting substance. Am Rev Respir Dis. 1990;141:466–470. doi: 10.1164/ajrccm/141.2.466. [DOI] [PubMed] [Google Scholar]
- 12.Wang PY, et al. Mullerian inhibiting substance acts as a motor neuron survival factor in vitro. Proc Natl Acad Sci USA. 2005;102:16421–16425. doi: 10.1073/pnas.0508304102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Celichowski J, Drzymala H. Differences between properties of male and female motor units in the rat medial gastrocnemius muscle. J Physiol Pharmacol. 2006;57:83–93. [PubMed] [Google Scholar]
- 14.Lance-Jones C. Motoneuron cell death in the developing lumbar spinal cord of the mouse. Brain Res. 1982;256:473–479. doi: 10.1016/0165-3806(82)90192-4. [DOI] [PubMed] [Google Scholar]
- 15.Munsterberg A, Lovell-Badge R. Expression of the mouse anti-mullerian hormone gene suggests a role in both male and female sexual differentiation. Development. 1991;113:613–624. doi: 10.1242/dev.113.2.613. [DOI] [PubMed] [Google Scholar]
- 16.Wang PY, Koishi K, McLennan IS. BMP6 is axonally transported by motoneurons and supports their survival in vitro. Mol Cell Neurosci. 2007;34:653–661. doi: 10.1016/j.mcn.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Josso N, et al. Anti-mullerian hormone in early human development. Early Hum Dev. 1993;33:91–99. doi: 10.1016/0378-3782(93)90204-8. [DOI] [PubMed] [Google Scholar]
- 18.Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- 19.Breedlove S, Arnold A. Hormonal control of a developing neuromuscular system. II. Sensitive periods for the androgen-induced masculinization of the rat spinal nucleus of the bulbocavernosus. J Neurosci. 1983;3:424–432. doi: 10.1523/JNEUROSCI.03-02-00424.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galanopoulou AS. GABA receptors as broadcasters of sexually differentiating signals in the brain. Epilepsia. 2005;46(Suppl 5):107–112. doi: 10.1111/j.1528-1167.2005.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62:847–855. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell M, McComas A, Petito F. Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry. 1973;36:174–182. doi: 10.1136/jnnp.36.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guibourdenche J, et al. Anti-Mullerian hormone levels in serum from human foetuses and children: Pattern and clinical interest. Mol Cell Endocrinol. 2003;211:55–63. doi: 10.1016/j.mce.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Holden C. Sex and the suffering brain. Science. 2005;308:1574–1577. doi: 10.1126/science.308.5728.1574. [DOI] [PubMed] [Google Scholar]
- 25.Kow LM, et al. Development of a sexually differentiated behavior and its underlying CNS arousal functions. Curr Top Dev Biol. 2007;79:37–59. doi: 10.1016/S0070-2153(06)79002-0. [DOI] [PubMed] [Google Scholar]
- 26.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc London Ser B. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu X, Arumugam R, Baker SP, Lee MM. Pubertal and adult Leydig cell function in Mullerian inhibiting substance-deficient mice. Endocrinology. 2005;146:589–595. doi: 10.1210/en.2004-0646. [DOI] [PubMed] [Google Scholar]
- 28.Mishina Y, et al. Genetic analysis of the Mullerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev. 1996;10:2577–2587. doi: 10.1101/gad.10.20.2577. [DOI] [PubMed] [Google Scholar]
- 29.Arango NA, Lovell-Badge R, Behringer RR. Targeted mutagenesis of the endogenous mouse Mis gene promoter: In vivo definition of genetic pathways of vertebrate sexual development. Cell. 1999;99:409–419. doi: 10.1016/s0092-8674(00)81527-5. [DOI] [PubMed] [Google Scholar]
- 30.Habgood MD, Knott GW, Dziegielewska KM, Saunders NR. The nature of the decrease in blood-cerebrospinal fluid barrier exchange during postnatal brain development in the rat. J Physiol. 1993;468:73–83. doi: 10.1113/jphysiol.1993.sp019760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansson PA, Dziegielewska KM, Liddelow SA, Saunders NR. The blood-CSF barrier explained: When development is not immaturity. BioEssays. 2008;30:237–248. doi: 10.1002/bies.20718. [DOI] [PubMed] [Google Scholar]
- 32.McLennan IS, Weible MW, II, Hendry IA, Koishi K. Transport of transforming growth factor-β2 across the blood-brain barrier. Neuropharmacology. 2005;48:274–282. doi: 10.1016/j.neuropharm.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Smith DE, Johanson CE, Keep RF. Peptide and peptide analog transport systems at the blood-CSF barrier. Adv Drug Deliv Rev. 2004;56:1765–1791. doi: 10.1016/j.addr.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet. 2002;32:408–410. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- 35.Behringer RR, Finegold MJ, Cate RL. Mullerian-inhibiting substance function during mammalian sexual development. Cell. 1994;79:415–425. doi: 10.1016/0092-8674(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 36.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 37.McLennan IS, Taylor-Jeffs J. The use of sodium lamps to brightly illuminate mouse houses during their dark phases. Lab Anim. 2004;38:1–9. doi: 10.1258/0023677041958927. [DOI] [PubMed] [Google Scholar]
- 38.Oppenheim RW, et al. Glial cell line-derived neurotrophic factor and developing mammalian motoneurons: Regulation of programmed cell death among motoneuron subtypes. J Neurosci. 2000;20:5001–5011. doi: 10.1523/JNEUROSCI.20-13-05001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gundersen HJ, et al. The new stereological tools: Disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. Acta Pathol Microbiol Immunol Scand. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 40.Phippard D, Lu L, Lee D, Saunders JC, Crenshaw EB., III Targeted mutagenesis of the POU-domain gene Brn4/Pou3f4 causes developmental defects in the inner ear. J Neurosci. 1999;19:5980–5989. doi: 10.1523/JNEUROSCI.19-14-05980.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell FD, Koishi K, Jiang Y, McLennan IS. Anterograde axonal transport of glial cell line-derived neurotrophic factor and its receptors in rat hypoglossal nerve. Neuroscience. 2000;97:575–580. doi: 10.1016/s0306-4522(00)00079-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.