Abstract
Oligosaccharyltransferase (OT) transfers high mannose-type glycans to the nascent polypeptides that are translated by the membrane-bound ribosome and translocated into the lumen of the endoplasmic reticulum through the Sec61 translocon complex. In this article, we show that purified ribosomes and OT can form a binary complex with a stoichiometry of ≈1 to 1 in the presence of detergent. We present evidence that OT may bind to the large ribosomal subunit near the site where nascent polypeptides exit. We further show that OT and the Sec61 complex can simultaneously bind to ribosomes in vitro. Based on existing data and our findings, we propose that cotranslational translocation and N-glycosylation of nascent polypeptides are mediated by a ternary supramolecular complex consisting of OT, the Sec61 complex, and ribosomes.
Keywords: electron microscopy, glycoprotein biosynthesis, multicomponent complexes
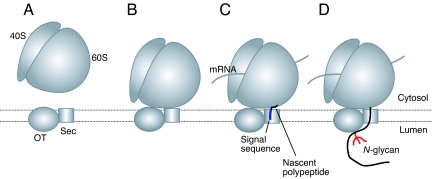
Glycoprotein biosynthesis on and in the endoplasmic reticulum (ER) is a sequential process mediated by several macromolecular complexes (1–3). At an early stage of translation, the ribosome–nascent polypeptide complex bearing a signal sequence is recognized by the signal recognition particle followed by recruitment of the ribosome–nascent polypeptide to the signal recognition particle receptor on the ER membrane (1). Next, the nascent polypeptide is transferred to the Sec61 complex on the ER membrane. The polypeptide is then cotranslationally translocated into the ER lumen through the Sec61 complex (4–7), which is composed of 3 membrane proteins (Sec61, Sbh1, and Sss1 in yeast) that form a pore structure in the ER membrane (8). During this process, if the polypeptide contains the sequence Asn-Xaa-Ser/Thr (where Xaa denotes any amino acid except Pro), it may be N-glycosylated (9). The enzyme complex catalyzing this process is the multimembrane protein complex oligosaccharyltransferase (OT). In yeast, OT is composed of 8 different membrane protein subunits: Stt3, Wbp1, Swp1, Ost1, Ost2, Ost4, Ost5, and either Ost3 or Ost6 (2). Of these 9, 5 subunits (Stt3, Wbp1, Swp1, Ost1, and Ost2) are encoded by essential genes (10–14). Although the other genes are dispensable, deletion of any one causes an N-glycosylation defect (15–18). Therefore, OT requires all subunits for maximal activity.
On the ER membrane ribosomes and the Sec61 complex form a complex functioning as the cotranslational translocation apparatus (19). It has been proposed that binding of the Sec61 complex to membrane-bound ribosomes ensures that nascent polypeptides are properly translocated into the ER lumen or membrane (1). The fact that OT cotranslationally transfers N-glycan to the nascent polypeptides in the ER lumen (20) and OT can be chemically cross-linked to the ribosomes (21) and the Sec61 complex (3) has suggested that the N-glycosylation may be functionally coupled with the protein translation and translocation. However, whereas structural basis of the cotranslational translocation is well understood, that of the cotranslational N-glycosylation is not clear.
In this study, we report the reconstitution of a binary complex containing OT and ribosomes and its electron microscopic images. We further show that OT, the Sec61 complex, and ribosomes form a ternary complex in vitro. Initially, we found that purified OT bound to ribosomes with a stoichiometry of ≈1 to 1 in vitro. This binding occurred on the 60S ribosomal subunit isolated from puromycin-treated ribosomes. In both binary and ternary complexes the OT complex was catalytically active. Electron microscopic analysis of the OT–ribosome binary complex revealed that OT bound in proximity to the tunnel where nascent polypeptides emerge from ribosomes. These biochemical and structural studies of a supramolecular complex involved in cotranslational N-glycosylation provide insight into important aspects of the molecular mechanism of glycoprotein biosynthesis.
Results
Purified and Catalytically-Active OT Binds to Ribosomes in Vitro.
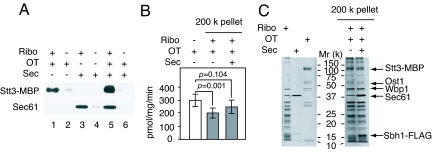
To assess the binding of OT to ribosomes, we used purified yeast OT and ribosomes (SI Text) Fig. S1 A and B, and Fig. S2A). During affinity purification of OT we successively exchanged detergent from digitonin to deoxy N,N′-bis(3-D-gluconamidopropyl) deoxycholamide CHAP, which preserved the OT activity. The ribosomal preparation showed the presence of 25S and 18S rRNA (Fig. S2A), indicating that the ribosomes maintained intact during our purification procedure. Cosedimentation and linear sucrose density-gradient centrifugation were used to analyze binding of OT to ribosomes, under conditions that sedimented ribosomes, as demonstrated by analysis of the rRNA (Fig. S3). As shown in Fig. 1A, lane 1, when excess amount of OT was incubated with ribosomes followed by sedimentation through a 20% sucrose cushion containing 0.1% deoxy Big CHAP at 200,000 × g, Stt3-maltose-binding protein was found in the pellet; however, in the absence of ribosomes, little Stt3-MBP was detected in the pellet (Fig. 1A, lane 2), indicating that the OT found in the pellet fraction was not a result of aggregation during centrifugation. The endogenous Sec61 complex was not found in the pellet as demonstrated by Western blot analysis with anti-Sec61 antibody (Fig. 1A, lane 1). These results clearly indicate that OT forms a binary complex with ribosomes. The Sec61 complex that have been shown to bind to ribosomes (19, 22–24) was purified (Fig. S1 A and B) and used for the sedimentation assay. When the Sec61 complex was incubated with ribosomes, the Sec61 complex was also found in the pellet in the presence (Fig. 1A, lane 3), but not in the absence of ribosomes (Fig. 1A, lane 4). Interestingly, when excess amounts of OT and the Sec61 complex together were incubated with ribosomes both cosedimented with ribosomes (Fig. 1A, lane 5).
Fig. 1.
Catalytically-active OT binds to ribosomes. (A) OT, the Sec61 complex, and ribosomes (3.2, 9.5, and 1.7 pmol, respectively) were incubated in the indicated combinations, followed by sedimentation through a 20% sucrose cushion containing 0.1% deoxy Big CHAP at 200,000 × g for 2 h. The pellet (30%) was analyzed by SDS/PAGE followed by Western blot analysis with anti-MBP and anti-Sec61 antibodies. (B) The OT activities in the original OT (n = 3) and the pellet (n = 3) that was sedimented through a 20% sucrose cushion containing 0.125% digitonin and 25 μg/mL phosphatidylcholine at 200,000 × g for 2 h are shown. The pellet fractions were prepared from OT (7.2 pmol of Stt3-MBP), the Sec61 complex (36 pmol of Sec61), and 1.7 pmol of ribosomes in the indicated combinations. The protein amount of OT in the pellet fraction was estimated by Western blot analysis with anti-MBP antibody. The intensity of Stt3-MBP in the purified OT (320 fmol) was compared with that in the pellet fraction. (C) The pellet in B was analyzed by SDS/PAGE followed by silver staining (Right). The purified OT (100 ng), Sec61 (100 ng), and ribosomes (800 ng) were analyzed on the same gel (Left).
Next, the OT activity in the pellet was examined by using fluorescence-labeled peptide (25). The ribosomal preparation showed no detectable endogenous OT activity (Fig. S4). The OT activity of OT–ribosome pellet was detectable but it was reduced to 30% of the original OT activity after the ultracentrifugation step, suggesting that OT was partially denatured during centrifugation. It has been reported that OT is stable in a buffer containing digitonin and phosphatidylcholine during a glycerol-density gradient centrifugation (26). For this reason, we changed deoxy Big CHAP to digitonin in a sucrose cushion and supplemented it with phosphatidylcholine. As shown in Fig. 1B, ≈65% of the original OT activity was preserved in the pellets, indicating that the ribosome-bound OT complex was catalytically active. We note that there is statistically-significant reduction of OT activity in the OT–ribosome complex, which might be caused by ribosome affecting the catalytic efficiency of OT in the in vitro system, although further analysis is required to clarify the reduction. It should be noted that after ultracentrifugation ≈1 pmol of Stt3-MBP was found in the pellet in the presence of either deoxy Big CHAP or digitonin as estimated by comparisons with the purified OT, suggesting that there is no significant difference between these 2 detergents in the binding of OT to ribosomes.
The pellet fraction was analyzed by SDS/PAGE followed by silver staining (Fig. 1C). By comparison with the purified OT, the Sec61 complex, and ribosomes (Fig. 1C Left), Stt3-MBP, Ost1, and Wbp1 were unambiguously identified in the OT–ribosome pair based on their molecular mass (Fig. 1C Right). In addition, Sec61 and Sbh1-FLAG, another component of the Sec61 complex, were found in the OT–Sec61–ribosome pair (Fig. 1C Right).
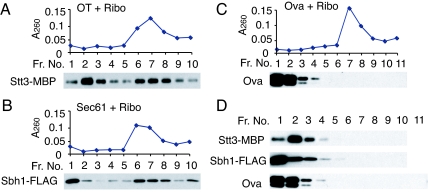
The binding of OT and the Sec61 complex to ribosomes was further confirmed by a linear sucrose density-gradient centrifugation. The rRNA profile of ribosomes was found to be consistent with the A260 profile (Fig. S3B). Therefore, we used the A260 profile to determine ribosomes in the gradient. OT and the Sec61 complex incubated without ribosomes remained in fractions 1–4 of the gradient (Fig. 2D). In the presence of ribosomes, however, OT and the Sec61 complex comigrated with ribosomes in fractions 6–9 (Figs. 2 A and B and 3A). The soluble protein ovalbumin (Ova) did not comigrate with ribosomes (Fig. 2C). These results indicate that OT and the Sec61 complex specifically bind to ribosomes.
Fig. 2.
Analysis of the interaction between OT, Sec61, and ribosome by linear sucrose density-gradient centrifugation. OT (3.2 pmol of Stt3-MBP), the Sec61 complex (9.5 pmol of Sec61), or Ova (22 pmol) were incubated with (A–C) or without (D) ribosomes (1.7 pmol) for 30 min on ice in the indicated combinations, followed by a further incubation for 30 min at room temperature. The mixture was centrifuged through a 10–40% linear sucrose density-gradient. After manually fractionating the gradient from top to bottom, a small volume (3% of each fraction) was analyzed without additional concentration by Western blot analysis using anti-MBP, anti-FLAG, or anti-Ova antibodies.
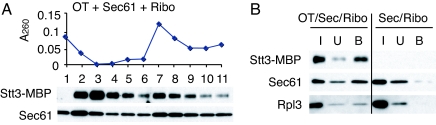
Fig. 3.
OT, the Sec61 complex, and ribosomes form a ternary complex. (A) OT (7.2 pmol of Stt3-MBP) was incubated with the Sec61 complex (36 pmol of Sec61) and ribosomes (1.7 pmol), and then the mixture was centrifuged through a 10–40% linear sucrose density-gradient. Fractions were recovered from top to bottom of the gradient and then analyzed for A260 to detect ribosomes. Each fraction (3%) was analyzed by a Western blot using anti-MBP and anti-Sec61 antibodies. (B) Ribosomal fractions containing OT, the Sec61 complex, and ribosomes (fractions 7–9 in A) were pooled, sedimented (OT/Sec/Ribo), and then subjected to the Con A pull-down assay (see SI Text). Ten percent of the bound (B) and unbound (U) to Con A beads, and the input (I) was analyzed by SDS/PAGE/Western blot with anti-MBP, anti-Sec61, and anti-Rpl3 antibodies. The ribosomal fractions containing the Sec61 complex and ribosomes (Sec/Ribo) were prepared essentially the same as with OT/Sec/Ribo, and then subjected to the Con A pull-down assay.
OT, Sec61 Complex, and Ribosomes Form a Ternary Complex.
Next, to determine whether OT, the Sec61 complex, and ribosomes form a ternary complex, the ribosomal fractions (fractions 7–9 in Fig. 3A) were pooled, diluted, sedimented and then subjected to a pull-down assay using Con A beads (see detail methods in SI Text) because 3 N-glycosylated OT subunits, Stt3, Ost1, and Wbp1, are shown to bind to ConA lectin, whereas the Sec61 complex and ribosomes do not (Fig. S1 C and D). After binding, the beads were analyzed by Western blot analysis. Fig. 3B shows that almost all of the Stt3 bound to Con A beads. When the ribosomal fraction was prepared in the presence of OT (OT/Sec/Ribo), much more Sec61 and Rpl3, a ribosomal protein, were found in the Con A beads-bound fraction than in the absence of OT (Sec/Ribo). All of these observations shown in Fig. 3B suggest that OT forms a ternary supramolecular complex with the Sec61 complex and ribosomes.
OT Binds to the 60S Subunit of Yeast 80S Ribosome.
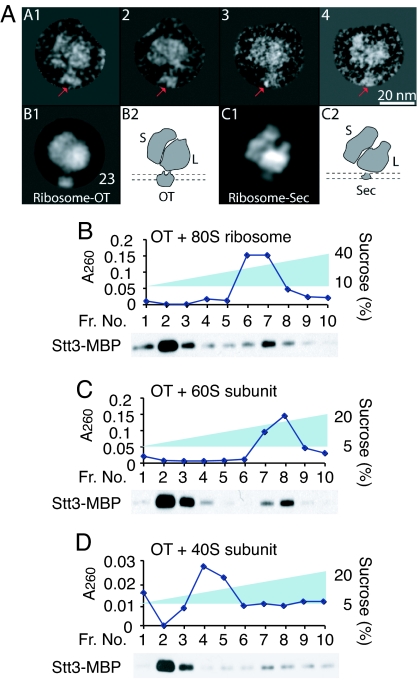
Finally, to identify the binding site of OT on ribosomes, we analyzed the binary complex by negative stain electron microscopy (EM) (Fig. 4A). We manually selected the ribosome particles with clear OT density from electron micrographs. The selected particle images were computationally classified or sorted according to their views. Particles belonging to a same view were grouped, aligned to each other rotationally and translationally, and subsequently averaged to produce an average or class image. Both the raw images and class averages revealed that OT binds to the large ribosomal subunit. The physical size of OT, at ≈10 nm, is estimated to be ≈2 times larger than that of the translocon (27) and is consistent with the size of the monomeric yeast OT in detergent (28). This result indicates that a single OT complex binds to a single ribosome. We also tested the finding by assaying the binding OT to ribosomes by linear sucrose density-gradient centrifugation/quantitative Western blot analysis (Fig. S5). Incubation of a fixed amount of ribosomes with increasing amounts of OT resulted in a near-saturation of binding of Stt3-MBP to ribosomes (Fig. S5C). On the basis of the amount of the ribosome-bound Stt3-MBP and the ribosomes at a near-saturation, we estimated that ≈1 OT complex was bound per ribosome. When taken together, the EM analysis and the binding assay both support the idea that OT and ribosomes are present in a 1:1 stoichiometry in the OT–ribosome binary complex.
Fig. 4.
OT directly binds to the 60S subunit near the nascent polypeptide-exiting site. (A) Negative stain electron micrograph of the OT–ribosome binary complex. (Upper) Shown are 4 raw OT-ribosome particle images. The particles are orientated with the 40S subunit on the left, and OT, as indicated by red arrows, on the bottom. (Lower) B1 shows a class image calculated by averaging 23 similar particles. B2 illustrates the likely orientation of ribosome–OT with respect to the ER membrane, which is shown by 2 dashed parallel lines. C1 is a reprojection of a previously-reported 3D cryoEM map of the yeast ribosome-translocon (19), and C2 shows the membrane orientation of the binary complex. (B–D) OT (3.2 pmol) was incubated with a fixed amount of either 80S ribosomes (B), the 60S (C), or the 40S subunit (D) (3.0 pmol each) and then analyzed by either a 10–40% or a 5–20% linear sucrose density-gradient centrifugation.
When compared with a projection image of the previously-reported cryoEM 3D map of the yeast ribosome-translocon (19), it appears that OT binds to the ribosome at a location near to the translocon-binding site. Because it is known that the translocon is located at the nascent peptide exit tunnel of the ribosome (19, 22–24, 27, 29), our observation suggests that OT also binds near the peptide exit site. This finding is consistent with the knowledge that N-glycosylation is carried out by OT on nascent polypeptide chains.
We also determined whether OT binds to the 60S subunit. The 40S and 60S subunits were purified by treatment of 80S ribosomes with puromycin/high salt followed by sucrose linear gradient ultracentrifugation. Puromycin is known to release nascent polypeptide, resulting in dissociation of the 40S and 60S subunits (30). When OT was incubated with the 60S subunit, fractions of Stt3-MBP (20% of total Stt3-MBP) comigrated with the A260 peak of the 60S subunit, which was essentially the same as with the 80S subunit (consisting of the 40S and 60S ribosomal subunits) (Fig. 4 B and C). These biochemical data agree with the EM observation that OT binds to the 60S subunit. In addition, puromycin is known to release nascent polypeptides (30).
Therefore, it is suggested that the nascent polypeptide is not necessary for the interaction of OT and the ribosomes. However, we also found that ≈6% of OT comigrated with the 40S preparation in fractions with predicted masses larger than 40S (Fig. 4D). This comigration of OT-40S did not result in cross-contamination of the 40S with the 60S subunit because the 40S subunit did not contain the 25S rRNA that consists of the 60S subunit (Fig. S2B). It has been reported that the 40S subunit can dimerize during preparation (31); it is possible that OT may bind weakly to the dimerized 40S subunit, in a situation comparable to the nonphysiological binding of the translocon to ribosomes (32).
Discussion
Based on the fact that OT has been demonstrated to be chemically cross-linked to the 60S ribosomal subunit (21) and photo-cross-linked to N-glycosylation sequences (33–36), we first hypothesized that OT physically binds to ribosomes and binding of OT to ribosomes was mediated by nascent polypeptides bound to ribosomes. Interestingly, we have now found that OT binds to ribosomes in vitro and binding is observed in the puromycin-treated 60S ribosomal subunit that lacks nascent polypeptide chains. All of these findings strongly indicate that OT physically binds to the 60S ribosomal subunit and its interaction with ribosomes is independent of nascent polypeptides.
EM studies of the binary OT–ribosome complex revealed that the extra density feature bound to the ribosomes is ≈10 nm in width, in agreement with the size of the purified and monomeric OT complex in detergent as determined by our previous cryoEM structural analysis (28). Thus, 1 complex of OT appears to bind to 1 ribosome complex in vitro. EM analysis of the OT–ribosome complex clearly indicated that the OT binds near the polypeptide-exiting site in the 60S ribosomal subunit. However, biochemical analysis of binding of OT to the 40S/60S ribosomal subunits raises the possibility that OT may make contacts not only with the 60S subunit, but also with the 40S subunit. Further EM analysis and chemical cross-linking experiments will be needed to identify in more detail the exact binding site for OT on ribosomes.
During translation in mammals ER membrane-bound ribosomes are associated with membrane proteins including OT, the Sec61 complex, signal peptidase, and translocon-associated protein in the ER membrane (37). We assumed that in yeast the supracomplex involved in the cotranslational translocation and N-glycosylation would consist of only 3 components (ribosomes, OT, and the Sec61 complex), because there is no evidence for the yeast homologue of TRAP. Also, in yeast N-glycosylation can occur without the action of signal peptidase (38). In fact, we found that the purified OT, Sec61 complex, and ribosomes together formed a ternary complex without any other components. Regarding the interaction between OT and the Sec61 complex, we have reported that OT is chemically cross-linked to the Sec61 complex in yeast rough membranes (3). In addition, OT and the Sec61 complex remained cross-linked in puromycin/0.5 M potassium acetate-treated rough membranes. These results suggest that the 2 protein complexes interact independently of ribosomes in the ER membrane.
We postulate in the model shown in Fig. 5 that monomeric OT forms a platform with the Sec61 complex in the ER membrane to receive the ribosomes (Fig. 5A). The nontranslating ribosome can interact with OT and the Sec61 complex together, and this interaction occurs on the 60S ribosomal subunit (Fig. 5B). Nascent polypeptides are not required for the interaction of OT and the ribosomes. Then, during translation and translocation of nascent polypeptides into the ER lumen (Fig. 5C), OT may be able to scan and N-glycosylate the glycosylatable sequences (Asn-Xaa-Ser/Thr) in nascent polypeptides as they emerge from the channel (Fig. 5D). We speculated that the channel formed by the Sec61 complex may be in proximity to the long groove in the large luminal domain of OT that is suggested to contain the catalytic site (28).
Fig. 5.
Hypothetical model for cotranslational N-glycosylation. (A) The monomeric OT forms a platform with the Sec61 complex (Sec) to receive the ribosomes on the ER membrane. (B) The ribosomes bind to the OT–Sec61 complex, forming a supramolecular complex. This binding occurs mainly at the 60S ribosomal subunit. (C and D) During translocation of a nascent polypeptide (C), OT cotranslationally scans it and then transfers N-glycan to glycosylatable sequences in the polypeptide as shown (D).
Materials and Methods
Materials.
EZ-view red anti-FLAG M2 affinity gel, 3×FLAG peptide, Triton X-100 (Tx-100), puromycin, Ova, Con A and egg yolk phosphatidylcholine were purchased from Sigma. Digitonin and deoxy Big CHAP were purchased from CalBiochem, and agarose-bound Con A was from Vector. Immobilon Western chemiluminescent horseradish peroxidase substrate was purchased from Millipore.
Antibodies.
Anti-FLAG M2 and anti-Con A antibodies were purchased from Sigma, and anti-Ova antibody was from Millipore. Anti-Sec61 and anti-Rpl3 antibodies were provided by T. A. Rapoport (Harvard Medical School, Boston) and J. R. Warner (Albert Einstein College of Medicine, New York), respectively.
Preparation of the OT Complex, Sec61 Complex, and Ribosomes.
Purification of OT from yeast microsomes was carried out according to our previous report (39). The Sec61 complex was purified from yeast microsomes containing Sbh1-FLAG (see SI Text). Briefly, the microsomes were solubilized in buffer A [100 mM Hepes·NaOH (pH 7.8), 1 M NaCl, 15 mM MgOAc2, and 2 mM MnCl2] containing 1.5% digitonin, diluted 15-fold with buffer A and then incubated with anti-FLAG beads. After incubation, the beads were washed twice with buffer A containing 0.1% digitonin and 0.25 mg/mL phosphatidylcholine, followed by washing 3 times with buffer B [20 mM Hepes·NaOH (pH 7.8), 100 mM NaCl, 1 mM MnCl2, and 3 mM MgCl2] containing 0.5% Tx-100 and 0.25 mg/ml phosphatidylcholine. The beads were further washed twice with buffer B containing 0.1% deoxy Big CHAP, and the Sec61 complex was eluted from the beads by incubation with 3×FLAG peptide. Yeast ribosomes were purified by conventional centrifugation methods (see SI Text) (40, 41). The 60S and 40S ribosomal subunits were purified from puromycin/0.5 M KCl-treated ribosomes by a 5–20% linear sucrose density-gradient centrifugation (42). Ribosomes and the subunits were resuspended in 10 mM Tris·HCl (pH 7.4), 50 mM KCl, 10 mM MgCl2, 1 mM DTT, 0.1 mM EDTA, and 1 mM phenylmethylsulfonylfluoride.
Reconstitution of OT, the Sec61 Complex, and Ribosomes.
OT, the Sec61 complex, and ribosomes were incubated in combinations and amounts as indicated in the figure legends for 30 min on ice, followed by further incubation for 30 min at room temperature. The mixture was layered onto 3 mL of a 20% sucrose cushion in either buffer C [20 mM Hepes·NaOH (pH 7.8), 100 mM NaCl, 0.1% deoxy Big CHAP, 2 mM MnCl2, and 2 mM MgCl2] or buffer D [20 mM Tris·HCl (pH 7.4), 50 mM NaCl, 0.125% digitonin, 1 mM MnCl2 1 mM MgCl2, and 25 μg/mL phosphatidylcholine], and then centrifuged at 200,000 × g for 2 h. The pellet was analyzed by SDS/PAGE followed by either Western blot analysis using anti-MBP and anti-Sec61 antibodies or silver staining. Alternatively, the mixture was centrifuged through a 10–40% linear sucrose density-gradient in buffer C at 200,000 × g for 2 h. For the binding of OT to the ribosomal subunits, a 5–20% linear sucrose density-gradient was used. Centrifugation was carried out at 200,000 × g for 100 min and then fractions were taken from top to bottom and analyzed by measuring the A260 and by SDS/PAGE followed by Western blot analysis with anti-MBP, anti-FLAG, and anti-Sec61 antibodies.
OT Activity Assay.
The OT activity assay was performed by using the tagged glycosylatable hexapeptide, tetramethylrhodamine (TAMRA)-Arg-Asn-Ala-Thr-Ala-Arg-COOH (Anaspec) (25) with some modifications (see SI Text).
Definitions.
The protein concentration of the purified OT and Sec61 complex was determined by Coomasie Plus Protein Assay Reagent (Thermo Scientific) using BSA as a standard. The amount of Stt3-MBP and Sec61 was estimated by SDS/PAGE/silver staining using BSA as a standard. Ribosome concentration was determined by absorbance (1.0 A260/mL = 18 nM for 80S, 28 nM for 60S, and 63 nM for 40S) (43).
EM and Image Processing.
EM analysis was carried out as described (28) (see SI Text).
Supplementary Material
Acknowledgments.
We thank Dr. Daisuke Kohda (Kyushu University, Fukuoka, Japan) for providing TAMRA-Arg-Asn-Ala-Thr-Ala-Arg-COOH peptide; Drs. Hermann Schindelin (University of Wüzburg, Wüzburg, Germany), Neta Dean, Gang Zhao, Guangtao Li, and Hideyuki Takeuchi (Stony Brook University, New York) for useful discussions; and Dr. Toshi Tsukiyama (Fred Hutchinson Cancer Research Center, Seattle) for 3FLAG-KANMX6 plasmid. This work was partially supported by National Institute of Health Grant GM33185 (to W.J.L), Brookhaven National Laboratory Laboratory-Directed Research and Development Grant 06-60 (to Huilin Li), and National Institutes of Health Grant GM74985 (to Huilin Li).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812489106/DCSupplemental.
References
- 1.Rapoport TA. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature. 2007;450:663–669. doi: 10.1038/nature06384. [DOI] [PubMed] [Google Scholar]
- 2.Lennarz WJ. Studies on oligosaccharyl transferase in yeast. Acta Biochim Pol. 2007;54:673–677. [PubMed] [Google Scholar]
- 3.Chavan M, Yan A, Lennarz WJ. Subunits of the translocon interact with components of the oligosaccharyl transferase complex. J Biol Chem. 2005;280:22917–22924. doi: 10.1074/jbc.M502858200. [DOI] [PubMed] [Google Scholar]
- 4.Gorlich D, Rapoport TA. Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell. 1993;75:615–630. doi: 10.1016/0092-8674(93)90483-7. [DOI] [PubMed] [Google Scholar]
- 5.Gorlich D, Prehn S, Hartmann E, Kalies KU, Rapoport TA. A mammalian homolog of SEC61p and SECYp is associated with ribosomes and nascent polypeptides during translocation. Cell. 1992;71:489–503. doi: 10.1016/0092-8674(92)90517-g. [DOI] [PubMed] [Google Scholar]
- 6.Musch A, Wiedmann M, Rapoport TA. Yeast Sec proteins interact with polypeptides traversing the endoplasmic reticulum membrane. Cell. 1992;69:343–352. doi: 10.1016/0092-8674(92)90414-8. [DOI] [PubMed] [Google Scholar]
- 7.Jungnickel B, Rapoport TA, Hartmann E. Protein translocation: Common themes from bacteria to man. FEBS Lett. 1994;346:73–77. doi: 10.1016/0014-5793(94)00367-x. [DOI] [PubMed] [Google Scholar]
- 8.Van den Berg B, et al. X-ray structure of a protein-conducting channel. Nature. 2004;427:36–44. doi: 10.1038/nature02218. [DOI] [PubMed] [Google Scholar]
- 9.Struck DK, Lennarz WJ, Brew K. Primary structural requirements for the enzymatic formation of the N-glycosidic bond in glycoproteins. Studies with α-lactalbumin. J Biol Chem. 1978;253:5786–5794. [PubMed] [Google Scholar]
- 10.te Heesen S, Knauer R, Lehle L, Aebi M. Yeast Wbp1p and Swp1p form a protein complex essential for oligosaccharyl transferase activity. EMBO J. 1993;12:279–284. doi: 10.1002/j.1460-2075.1993.tb05654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silberstein S, Collins PG, Kelleher DJ, Rapiejko PJ, Gilmore R. The α-subunit of the Saccharomyces cerevisiae oligosaccharyltransferase complex is essential for vegetative growth of yeast and is homologous to mammalian ribophorin I. J Cell Biol. 1995;128:525–536. doi: 10.1083/jcb.128.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silberstein S, Collins PG, Kelleher DJ, Gilmore R. The essential OST2 gene encodes the 16-kDa subunit of the yeast oligosaccharyltransferase, a highly conserved protein expressed in diverse eukaryotic organisms. J Cell Biol. 1995;131:371–383. doi: 10.1083/jcb.131.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zufferey R, et al. STT3, a highly conserved protein required for yeast oligosaccharyl transferase activity in vivo. EMBO J. 1995;14:4949–4960. doi: 10.1002/j.1460-2075.1995.tb00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.te Heesen S, Janetzky B, Lehle L, Aebi M. The yeast WBP1 is essential for oligosaccharyl transferase activity in vivo and in vitro. EMBO J. 1992;11:2071–2075. doi: 10.1002/j.1460-2075.1992.tb05265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chi JH, Roos J, Dean N. The OST4 gene of Saccharomyces cerevisiae encodes an unusually small protein required for normal levels of oligosaccharyltransferase activity. J Biol Chem. 1996;271:3132–3140. doi: 10.1074/jbc.271.6.3132. [DOI] [PubMed] [Google Scholar]
- 16.Karaoglu D, Kelleher DJ, Gilmore R. Functional characterization of Ost3p. Loss of the 34-kDa subunit of the Saccharomyces cerevisiae oligosaccharyltransferase results in biased underglycosylation of acceptor substrates. J Cell Biol. 1995;130:567–577. doi: 10.1083/jcb.130.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiss G, te Heesen S, Gilmore R, Zufferey R, Aebi M. A specific screen for oligosaccharyltransferase mutations identifies the 9-kDa OST5 protein required for optimal activity in vivo and in vitro. EMBO J. 1997;16:1164–1172. doi: 10.1093/emboj/16.6.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knauer R, Lehle L. The oligosaccharyltransferase complex from Saccharomyces cerevisiae. Isolation of the OST6 gene, its synthetic interaction with OST3, and analysis of the native complex. J Biol Chem. 1999;274:17249–17256. doi: 10.1074/jbc.274.24.17249. [DOI] [PubMed] [Google Scholar]
- 19.Beckmann R, et al. Architecture of the protein-conducting channel associated with the translating 80S ribosome. Cell. 2001;107:361–372. doi: 10.1016/s0092-8674(01)00541-4. [DOI] [PubMed] [Google Scholar]
- 20.Glabe CG, Hanover JA, Lennarz WJ. Glycosylation of ovalbumin nascent chains. The spatial relationship between translation and glycosylation. J Biol Chem. 1980;255:9236–9242. [PubMed] [Google Scholar]
- 21.Kreibich G, Freienstein CM, Pereyra BN, Ulrich BL, Sabatini DD. Proteins of rough microsomal membranes related to ribosome binding. II. Cross-linking of bound ribosomes to specific membrane proteins exposed at the binding sites. J Cell Biol. 1978;77:488–506. doi: 10.1083/jcb.77.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beckmann R, et al. Alignment of conduits for the nascent polypeptide chain in the ribosome–Sec61 complex. Science. 1997;278:2123–2126. doi: 10.1126/science.278.5346.2123. [DOI] [PubMed] [Google Scholar]
- 23.Menetret JF, et al. The structure of ribosome–channel complexes engaged in protein translocation. Mol Cell. 2000;6:1219–1232. doi: 10.1016/s1097-2765(00)00118-0. [DOI] [PubMed] [Google Scholar]
- 24.Morgan DG, Menetret JF, Neuhof A, Rapoport TA, Akey CW. Structure of the mammalian ribosome-channel complex at 17-Å resolution. J Mol Biol. 2002;324:871–886. doi: 10.1016/s0022-2836(02)01111-7. [DOI] [PubMed] [Google Scholar]
- 25.Kohda D, Yamada M, Igura M, Kamishikiryo J, Maenaka K. New oligosaccharyltransferase assay method. Glycobiology. 2007;17:1175–1182. doi: 10.1093/glycob/cwm087. [DOI] [PubMed] [Google Scholar]
- 26.Kelleher DJ, Kreibich G, Gilmore R. Oligosaccharyltransferase activity is associated with a protein complex composed of ribophorins I and II and a 48-kDa protein. Cell. 1992;69:55–65. doi: 10.1016/0092-8674(92)90118-v. [DOI] [PubMed] [Google Scholar]
- 27.Menetret JF, et al. Single copies of Sec61 and TRAP associate with a nontranslating mammalian ribosome. Structure (London) 2008;16:1126–1137. doi: 10.1016/j.str.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Chavan M, Schindelin H, Lennarz WJ, Li H. Structure of the oligosaccharyl transferase complex at 12-Å resolution. Structure (London) 2008;16:432–440. doi: 10.1016/j.str.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 29.Menetret JF, et al. Architecture of the ribosome–channel complex derived from native membranes. J Mol Biol. 2005;348:445–457. doi: 10.1016/j.jmb.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 30.Mast CA, Bloemers HPJ. The puromycin reaction mediated by yeast ribosomes in high salt. Mol Biol Rep. 1973;1:225–231. doi: 10.1007/BF00357646. [DOI] [PubMed] [Google Scholar]
- 31.Goumans H, Thomas A, Verhoeven A, Voorma HO, Benne R. The role of eIF-4C in protein synthesis initiation complex formation. Biochim Biophys Acta. 1980;608:39–46. doi: 10.1016/0005-2787(80)90131-8. [DOI] [PubMed] [Google Scholar]
- 32.Mitra K, et al. Structure of the E. coli protein-conducting channel bound to a translating ribosome. Nature. 2005;438:318–324. doi: 10.1038/nature04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan Q, Prestwich GD, Lennarz WJ. The Ost1p subunit of yeast oligosaccharyl transferase recognizes the peptide glycosylation site sequence, -Asn-X-Ser/Thr. J Biol Chem. 1999;274:5021–5025. doi: 10.1074/jbc.274.8.5021. [DOI] [PubMed] [Google Scholar]
- 34.Yan Q, Lennarz WJ. Studies on the function of oligosaccharyl transferase subunits: A glycosylatable photoprobe binds to the luminal domain of Ost1p. Proc Natl Acad Sci USA. 2002;99:15994–15999. doi: 10.1073/pnas.212637999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan Q, Lennarz WJ. Studies on the function of oligosaccharyl transferase subunits. Stt3p is directly involved in the glycosylation process. J Biol Chem. 2002;277:47692–47700. doi: 10.1074/jbc.M208136200. [DOI] [PubMed] [Google Scholar]
- 36.Nilsson I, et al. Photocross-linking of nascent chains to the STT3 subunit of the oligosaccharyltransferase complex. J Cell Biol. 2003;161:715–725. doi: 10.1083/jcb.200301043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibatani T, David LL, McCormack AL, Frueh K, Skach WR. Proteomic analysis of mammalian oligosaccharyltransferase reveals multiple subcomplexes that contain Sec61, TRAP, and two potential new subunits. Biochemistry. 2005;44:5982–5992. doi: 10.1021/bi047328f. [DOI] [PubMed] [Google Scholar]
- 38.Chen X, VanValkenburgh C, Liang H, Fang H, Green N. Signal peptidase and oligosaccharyltransferase interact in a sequential and dependent manner within the endoplasmic reticulum. J Biol Chem. 2001;276:2411–2416. doi: 10.1074/jbc.M007723200. [DOI] [PubMed] [Google Scholar]
- 39.Chavan M, et al. Dimeric organization of the yeast oligosaccharyl transferase complex. Proc Natl Acad Sci USA. 2006;103:8947–8952. doi: 10.1073/pnas.0603262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inada T, et al. One-step affinity purification of the yeast ribosome and its associated proteins and mRNAs. RNA. 2002;8:948–958. doi: 10.1017/s1355838202026018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgan DG, et al. A comparison of the yeast and rabbit 80 S ribosome reveals the topology of the nascent chain exit tunnel, intersubunit bridges, and mammalian rRNA expansion segments. J Mol Biol. 2000;301:301–321. doi: 10.1006/jmbi.2000.3947. [DOI] [PubMed] [Google Scholar]
- 42.Levy R, Wiedmann M, Kreibich G. In vitro binding of ribosomes to the β-subunit of the Sec61p protein translocation complex. J Biol Chem. 2001;276:2340–2346. doi: 10.1074/jbc.M004867200. [DOI] [PubMed] [Google Scholar]
- 43.Smith RL, Baca O, Gordon J. Coordinate synthesis of ribosomes and elongation factors in the liver of immature chicks following a metabolic shift up. J Mol Biol. 1976;100:115–126. doi: 10.1016/s0022-2836(76)80143-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.