Abstract
Androgen receptor (AR) inhibitors are used to treat multiple human diseases, including hirsutism, benign prostatic hypertrophy, and prostate cancer, but all available anti-androgens target only ligand binding, either by reduction of available hormone or by competitive antagonism. New strategies are needed, and could have an important impact on therapy. One approach could be to target other cellular mechanisms required for receptor activation. In prior work, we used a cell-based assay of AR conformation change to identify non-ligand inhibitors of AR activity. Here, we characterize 2 compounds identified in this screen: pyrvinium pamoate, a Food and Drug Administration-approved drug, and harmol hydrochloride, a natural product. Each compound functions by a unique, non-competitive mechanism and synergizes with competitive antagonists to disrupt AR activity. Harmol blocks DNA occupancy by AR, whereas pyrvinium does not. Pyrvinium inhibits AR-dependent gene expression in the prostate gland in vivo, and induces prostate atrophy. These results highlight new therapeutic strategies to inhibit AR activity.
Keywords: antagonist, harmol, pyrvinium
The androgen receptor (AR) is a member of the nuclear hormone receptor superfamily, which consists of a large group of ligand-regulated transcription factors. AR is expressed in many tissues and influences an enormous range of physiologic processes such as cognition, muscle hypertrophy, bone density, and prostate growth and differentiation (1). However, AR signaling is directly linked to numerous diseases, including benign prostatic hyperplasia, alopecia, and hirsutism (2). AR signaling also drives the proliferation of prostate cancer, even in the setting of therapies that reduce systemic hormone ligand levels, making AR the major therapeutic target for this malignancy (3).
Before ligand binding, AR associates with a complex of cytoplasmic factors and molecular chaperones that maintain the receptor in a high-affinity ligand binding conformation (4). AR signaling is initiated by binding of testosterone or the more potent dihydrotestosterone (DHT). This induces an intramolecular conformation change in AR that brings the amino (N) and carboxy (C) termini into close proximity. This occurs with a t1/2 of approximately 3.5 min in cells treated with DHT (5), and does not occur in cell lysates (6), suggesting that the induced conformation change is not protein autonomous, but depends on additional cellular factors. Activated AR accumulates in the nucleus, where it binds to DNA as a homodimer at specific androgen response elements to regulate gene expression. Transcriptional control by AR results from complex interactions with positive (i.e., co-activator) and negative (i.e., co-repressor) factors (1). These co-regulatory factors fine-tune AR activity, and AR can even be activated in the absence of ligand by certain cross-talk pathways (7).
Although AR activity is highly regulated, with many possible points for intervention, all existing approaches to block AR signaling ultimately target ligand binding to AR. This includes direct competition with competitive antagonists, and reduction of systemic ligand levels with chemical castration agents. We hypothesized that, by targeting AR signaling via alternative means, we could identify non-ligand AR inhibitors. These inhibitors might lead to new therapeutic approaches to treat androgen-dependent diseases, and new insights into the molecular mechanisms that control AR activity. We previously used a cell-based assay of ligand-induced conformational change in AR to identify multiple non-competitive antagonists (6). Here, we evaluate the 2 most potent AR inhibitors, pyrvinium pamoate (PP), a Food and Drug Administration-approved drug (8, 9), and harmol hydrochloride (HH), a natural product (10).
Results
PP and HH Are Potent, Synergistic Anti-Androgens in Cultured Prostate Carcinoma Cells.
We began by testing the ability of PP and HH (Fig. 1A) to inhibit endogenous AR activity in 2 prostate cancer-derived cell lines: LNCaP, which expresses an AR mutant with reduced specificity for DHT, and which responds to hydroxy-flutamide (OH-F) as an agonist (11), and LAPC4, which expresses WT AR (12). These cells were transfected with androgen-responsive firefly luciferase reporter and androgen-unresponsive renilla luciferase reporter plasmids. PP and HH inhibited AR signaling more potently than the competitive antagonists OH-F or bicalutamide (BiC) (Fig. 1B). Neither drug induced reporter activity in the absence of DHT. When the pamoate counter ion of PP was replaced with chloride, the resultant compound was equally potent, confirming pyrvinium as the active component (Fig. 1B).
Fig. 1.
(A) Structures of pyrvinium and harmol. (B and C) LAPC4 or LNCaP cells were transfected with luciferase reporter constructs and treated with titrations of the indicated compounds for 24 h. IC50 values and SDs were calculated from the renilla-normalized PSA-luciferase reporter activities from 4 independent experiments with single (B) or combination (C) treatments. Expected and actual IC50 and combinatorial index values (CI at IC50) were calculated from mean-effect plots. A CI50 of 1 indicates an additive effect, a CI <1 indicates synergy, and a CI >1 indicates antagonism. (PCl, pyrvinium chloride.) (D) LNCaP cells were treated with 1 nM DHT and the indicated compounds for 24 h and androgen-responsive transcript levels were quantified relative to vehicle-treated cells. Averaged results from 4 independent experiments indicate that PP and HH inhibit both AR transcriptional activation and repression. (Error bars indicate SEM.)
The potencies of various combinations of PP, HH, and BiC were examined using this assay (Fig. 1C). A 1:1 combination of PP and BiC was synergistic in LAPC4 cells, using combination indices as a gauge (13). The same combination appeared synergistic in LNCaP cells as well, although a 1:10 combination was more powerful. A 1:1 combination of HH and BiC displayed strong synergy in both LNCaP and LAPC4 cells, as did a 1:10 combination of HH and PP. The synergy observed between these compounds suggests that BiC, HH, and PP each regulate AR signaling by distinct mechanisms.
Next we quantified the transcript levels of 6 endogenous androgen-responsive genes (14–18) in LNCaP cells treated with AR inhibitors. DHT induced the transcription of transmembrane protease serine 2 (TMPRSS2), kallikrein 3 [or prostate specific antigen (PSA)], NK homeobox family member 3 (Nkx3.1), and FK506-binding immunophilin 51 (FKBP51). BiC, PP, and HH reduced the expression of most genes by more than 50% (Fig. 1D). DHT suppressed the transcription of matrix metalloproteinase 16 (MMP16) and G protein-coupled receptor RDC1 homologue, which was blocked to varying degrees by each inhibitor. Although reaching statistical significance with such a small number of samples is difficult, the trend for each gene was identical in all 4 experiments, and PP and HH were more potent than BiC at most genes, corroborating results with the luciferase reporter. The concentrations of drugs used in this study were not necessarily matched to IC50 values, thus limiting a direct comparison of potencies.
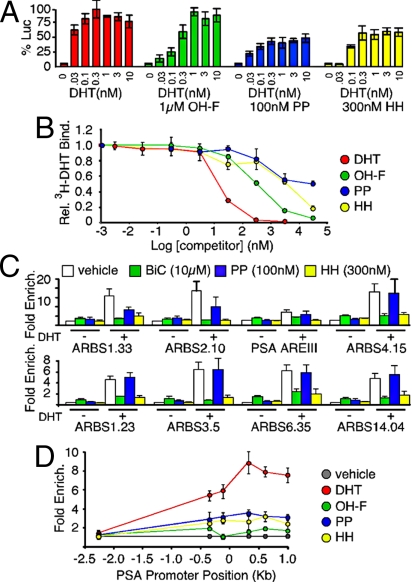
Neither PP nor HH have chemical structures similar to known AR ligands (Fig. 1A). Nonetheless, we carried out 2 experiments to search for patterns of inhibition characteristic of competitive antagonists. First, we titrated DHT in LAPC4 cells transfected with an androgen-responsive firefly luciferase reporter in the presence or absence of 1 μM OH-F, 100 nM PP, or 300 nM HH (Fig. 2A). As expected, the competitive antagonist OH-F shifted the DHT dose–response curve to the right, without preventing the maximal DHT response. In contrast, PP and HH blocked maximal AR activity, and excess DHT could not overcome this inhibition. These responses are most consistent with PP and HH being non-competitive inhibitors. To rule out effects on DHT binding to AR, LAPC4 cells were incubated with 1 nM [3H]DHT and unlabeled inhibitors (Fig. 2B). Unlabeled DHT and the competitive antagonist OH-F effectively competed [3H]DHT binding. PP did not display any significant competition with [3H]DHT, demonstrating its non-competitive nature. HH competed for ligand binding, but only at concentrations ≈30 times higher than its EC50, suggesting that this is not its mechanism of action. Results were similar in LNCaP cells (data not shown). Taken together, these data indicate that PP and HH are not competitive antagonists at their effective concentrations.
Fig. 2.
Non-competitive inhibition of AR. (A) LAPC4 cells were transfected with luciferase reporter constructs and treated with a DHT dose titration in the presence or absence of 1 μM OH-F, 100 nM PP, or 300 nM HH. Renilla-normalized PSA-luciferase activity was measured 24 h later. OH-F shifted the response to the right, indicating competitive antagonism. PP and HH did not shift the curve, but prevented the maximum response, consistent with non-competitive inhibition. (B) LAPC4 cells were incubated with 1 nM [3H]DHT and the indicated compounds. Inhibition of [3H]DHT binding by each compound is expressed relative to the no-competition value, which was set to 1. Neither PP nor HH competed for binding at their fully effective concentrations (100 nM and 300 nM, respectively). (C and D) Chromatin immunoprecipitation: LNCaP cells were treated with the indicated compounds for 4 h, followed by cross-linking, sonication, and immunoprecipitation with anti-AR or anti-RNA pol II antibodies. AR- (C) or RNA pol II- (D) bound chromatin was isolated and occupancy was quantified by quantitative PCR using primers specific to known AR-binding sites (C) or sites −2257 to +995 bp from the PSA transcription start site (D). Primers specific to the HSPA1A promoter were used to control for non-specific occupancy (31). BiC and HH displaced AR from regulated promoters, whereas PP had no effect, although all 3 AR inhibitors prevented recruitment of RNA pol II to the PSA promoter. Error bars indicate SEM (8 biological replicates).
Subtle changes in AR conformation are thought to translate to discrete downstream AR activities (19, 20). The synergy between PP and HH suggested that, although both drugs inhibit ligand-induced conformation change, they function by distinct mechanisms to prevent AR transcriptional activity. Neither inhibitor affected AR expression levels or nuclear localization following DHT treatment (data not shown). Thus, we used chromatin immunoprecipitation (ChIP) to test for effects of each compound on AR occupancy at AR binding sites (ARBSs) located near androgen-responsive genes. The androgen-unresponsive heat shock protein 70 (HSPA1A) promoter was a negative control. In the absence of DHT, neither PP nor HH affected AR promoter occupancy (Fig. 2C). DHT stimulated AR occupancy of binding sites near androgen-induced genes (ARBSs 2.10, 3.25, 6.35, 12.13, 14.04, and androgen response element III) and androgen-repressed genes (ARBSs 4.15, 8.04) in LNCaP cells. In the presence of DHT, HH and BiC each reduced receptor occupancy of ARBSs. In contrast, PP did not appreciably affect DHT-induced AR occupancy of ARBSs. BiC treatment alone modestly recruited AR to ARBSs, in accordance with previous findings (21–23). To assess the effect of each compound on RNA polymerase II (RNA pol II) recruitment, we carried out ChIP experiments to evaluate occupancy at various points along the PSA promoter. LNCaP cells were treated with each compound, followed by immunoprecipitation with a monoclonal antibody against RNA pol II. We used a series of primers to test for RNA pol II occupancy from −2,257 to +995 bp from the PSA transcription start site. DHT treatment resulted in accumulation of RNA pol II at the start site, whereas treatment with PP, HH, or BiC reduced RNA pol II occupancy (Fig. 2D). In conjunction with their synergy in transcription assays, these results indicate that PP and HH inhibit AR signaling by independent mechanisms. Whereas HH blocks AR promoter occupancy, PP permits promoter occupancy, but blocks subsequent RNA pol II recruitment.
The ability to inhibit the androgen dependent growth of cells is a hallmark of anti-androgen activity. LNCaP cells depend on androgens to proliferate in culture, so we tested for growth inhibition by PP and HH. PP was more effective than either HH or BiC, although all inhibited the growth of LNCaP cells (Fig. 3A). LNCaP cells over-expressing WT AR (LN-AR) do not require androgens for proliferation (3). As previously described (3), BiC did not inhibit the proliferation of these cells (Fig. 3B). HH reduced the growth, but not significantly, whereas PP potently blocked LN-AR proliferation. The control HEK293 cells were not significantly affected by any of the compounds (Fig. 3C). Taken together, these results indicate that both PP and HH can inhibit AR-dependent cell proliferation, although PP is more potent.
Fig. 3.
Inhibition of androgen-dependent and independent growth. The proliferation of LNCaP, LN-AR, and HEK293 cells in the presence or absence of 3 nM DHT and indicated drugs was determined, using DAPI staining to measure the DNA content of the cells in 12 independent replicates for each condition. PP significantly inhibited the growth of both LNCaP (i.e., androgen-dependent) and LN-AR (i.e., androgen-independent) cells at day 7 (ANOVA, P < 0.0004), whereas BiC and HH inhibited the growth of only LNCaP cells (ANOVA, P < 0.03). Control HEK293 cells were unaffected.
Our next step was to determine whether the anti-androgen activities observed in vitro would extend into animals. We first determined the approximate half-life and toxicity of PP and HH. A single 5-mg/kg dose of PP or 2-mg/kg dose of HH was administered to male FVB mice by i.p. injection or oral gavage. The plasma concentration of PP was much greater in mice after i.p. administration (Fig. 4A). HH was too rapidly metabolized to be evaluated further in vivo. PP was toxic at i.p. doses of 5 mg/kg or greater when administered for 2 weeks. PP at 1 mg/kg caused a mild reduction in body weight in the first 3 days of treatment, whereas mice given less than 1 mg/kg PP showed no adverse effects (data not shown). Weight loss was averted by escalating the dose of PP from 0.1 mg/kg to 1 mg/kg over the first week of treatment. These data were consistent with the results of the National Cancer Institute's in vivo tumor screen (http://dtp.nci.nih.gov). PP serum levels 24 h after the final dosing were approximately 5 ng/mL, indicating that the pharmacokinetics of PP given chronically were similar to those of a single dose (Fig. 4A), and that PP did not accumulate during the course of this experiment. The concentration of PP in the plasma ranged from approximately 150 nM to 20 nM using this once-daily dosing regimen—well within its efficacious range. These experiments indicated it was feasible to carry out a study of in vivo efficacy of PP as an anti-androgen.
Fig. 4.
Properties and efficacy of PP and HH in vivo. (A) Mice were administered 5 mg/kg PP or 2 mg/kg HH by the indicated methods. Plasma concentrations were determined by MS, and reported in ng/mL. (nd, compound not detected.) (B and C) Litter-mate-controlled FVB male mice were treated for 4 weeks with 100 mg/kg oral BiC (n = 9), an escalating dose of i.p. PP to 1 mg/kg (n = 9), or a combination of these treatments (n = 9). Cohorts of mice were castrated at the onset of the study (n = 9), or were sham-treated with i.p. and oral vehicles (n = 10). (B) Prostate wet weight was measured as a proximal marker of anti-androgen potency. (C) Quantitative PCR was performed on reverse-transcribed RNA isolated from mouse prostate tissues. Transcripts for each gene were normalized to RPL19, an androgen-unresponsive gene. (ANOVA, *P < 0.05; **P < 0.01; ***P < 0.001; ns, no significant difference between indicated populations; error bars indicate SEM.) (D) Representative sections of dorsal prostate from each treatment group were stained using hematoxylin and eosin. Substantial prostate atrophy is present in castrated and combination treatment samples. Signs of androgen deprivation are also visible in the samples of mice treated with BiC and PP alone. Note the loss of columnar epithelial architecture (white arrows), decreased secretory protein, increased nuclear staining, a relative thickening of the stromal layer, and dead cell accumulation in the lumen (black arrows).
Androgen deprivation in vivo causes global prostate atrophy, and castrated mice have reduced prostate size accompanied by characteristic changes at the cellular level (24). Thus, we examined the effects of PP in mice, using prostate size, gene expression, and morphologic changes as indicators of anti-androgen activity. Cohorts of 9 to 10 litter-controlled FVB male mice were treated for 4 weeks with PP (escalated over 10 d to 1 mg/kg), BiC (100 mg/kg), a combination of the two, or vehicle controls. An additional cohort was castrated at the onset of the study as a positive control. Weights of the mice did not vary significantly among the treatment populations (data not shown). After 4 weeks, animals were killed, and the prostates were dissected and weighed, including equal portions of each lobe for histochemical analysis and RNA extraction. We used ANOVA to test for differences among treatment groups. PP alone caused a 9% reduction in prostate weight compared with the vehicle-treated group, which was not statistically significant (P = 0.3). The remaining treatments all caused significant changes in prostate weight. BiC caused a 35% reduction, and combining BiC with PP caused a 63% reduction in prostate weight. This was not significantly different from the 74% reduction observed in the castrated group (Fig. 4B), suggesting that combination treatment was as effective as castration.
We measured AR-regulated transcript levels in the prostate via quantitative PCR. We included the probasin gene (25) instead of PSA, which is not expressed in mice. All 4 treatments significantly decreased the level of TMPRSS2, Nkx3.1, probasin, PSP94, and FKBP51 transcripts (Fig. 4C). PP and BiC had similar effects. In every case, their combination was more effective than either drug alone, and in most cases this difference was significant. Castration consistently resulted in the greatest decrease in androgen-responsive transcripts. Importantly, at 4 of 5 genes, this decrease was not significantly different from the combination treatment.
Hematoxylin and eosin staining of the prostate revealed changes characteristic of androgen deprivation in all prostate lobes, including loss of columnar architecture, decreased secretory protein, thickening of the stromal layer, and dead cell accumulation in the lumen (Fig. 4D). Taken together, these data indicate that combination treatment with a competitive (i.e., BiC) and non-competitive (i.e., PP) AR inhibitor is nearly as powerful as castration, using the prostate as a physiologically relevant biomarker.
Discussion
Our prior work has indicated that ligand-induced conformational change in AR is separable from ligand binding. Here we have characterized 2 potent, non-ligand AR inhibitors, PP and HH, each of which synergizes with a competitive antagonist in vitro. We have used these inhibitors to demonstrate AR antagonism in vitro and in vivo that is not based on competition for ligand binding. Synergy between PP and BiC appears to be present in vivo, as PP causes morphologic changes consistent with androgen deprivation, reduces AR-dependent gene expression, and augments the inhibitory activity of BiC at the prostate gland in experimental mice. Although PP alone did not have a statistically significant effect on mouse prostate size, it had significant effects on prostate gene transcription and altered the histology of the prostate comparable to BiC alone.
Several lines of evidence suggest that HH, PP, and BiC each function by a unique mechanism, including their synergy with each other, the lack of effect of PP and HH on ligand binding, and the different effects of PP and HH on AR promoter occupancy (Fig. 5). BiC binds the ligand binding pocket of AR, thus preventing DHT binding. HH and PP do not prevent DHT binding, yet they block ligand-induced conformation change and inhibit subsequent AR activity. Neither drug affects AR protein stability or nuclear accumulation. ChIP experiments suggest that HH blocks AR binding to response elements. In contrast, PP does not block DNA binding by AR, but prevents the subsequent recruitment of RNA pol II to initiate transcription of AR-regulated genes (Fig. 5).
Fig. 5.
Model of mechanisms of non-competitive AR antagonism. Apo-AR is in a distinct conformation before ligand binding. Ligand binding induces conformation change, nuclear accumulation, dimerization, and assembly of a transcriptional complex at regulated promoters. BiC directly competes for DHT binding, blocking AR activation at a proximal step. HH prevents DNA binding by nuclear-localized AR. PP permits AR promoter binding, but interferes with assembly of a productive transcription initiation complex.
The targets of these novel anti-androgens remain to be identified. We predict either that PP and HH directly bind AR to prevent normal conformation change, or that they target co-regulator proteins or cross-talk pathways that are necessary for ligand-induced conformation change. Of note, a recent study has found that harmine, a compound that is structurally very similar to harmol, and which also inhibits AR in our assays (data not shown), regulates PPAR-γ activity by an indirect mechanism, perhaps via effects on Wnt signaling (26). HH may inhibit AR in a similar fashion.
A clear implication of our work, and that of others, is that there are multiple intracellular targets to inhibit AR, and potentially other NRs. It is known that small molecules that target heat shock proteins (27, 28), histone deacetylases (29), and several kinases, including HER2/neu kinase (30), can influence AR activity. The pattern of inhibition we have found is not consistent with Hsp90 blockade, and we have observed potency that exceeds that reported for other inhibitors. Thus, we suspect that PP and HH do not function via previously identified mechanisms. Identification of intracellular factors that mediate the effects of these compounds could vastly improve our understanding of nuclear receptor biology. Our work also indicates that it may be possible to develop effective, non-competitive AR antagonists that could have a significant impact in many diseases.
Materials and Methods
Cell Culture.
LNCaP and LN-AR cells (a gift from Charles Sawyers, Los Angeles, CA) were maintained in RPMI 1640 media supplemented with sodium bicarbonate, glutamine, Hepes, antibiotics, and 10% FBS. LAPC4 cells were maintained in phenol red-free RPMI 1640 media supplemented with antibiotics and 10% FBS. BiC was a gift of Ingo Mellinghoff (Los Angeles, CA), PP was purchased from MP Biochemicals, and all other compounds were purchased from Sigma.
Cell Proliferation Assays.
For growth curves, cells were transferred to charcoal-stripped (C/S) media 3 days before they were split and plated at a density of approximately 20,000 cells/well in 48-well plates, in quadruplicate. The assay was repeated 3 times. The following day, medium with or without 3 nM DHT and with or without PP, HH, or BiC was added to the cells. Media were changed daily. Proliferation was determined by measuring the DNA content of the cells in each well. Each day, the cells were fixed in 100% cold methanol, followed by staining for 5 min at room temperature with 0.2 ng/mL DAPI in PBS solution. The cells were washed with PBS solution, then read on a fluorescence plate reader using 365/439 excitation/emission wavelengths. A Student t test was used to determine significant differences among populations.
Dowex Ion Exchange.
Dowex 1 × 8 200–400 Mesh Cl (Sigma) was washed in ethanol followed by 0.5M NaOH to remove ions and water to neutralize. The Dowex was then washed with 0.5M HCl to charge the matrix and water to neutralize. PP (100 mg) in a 2% DMSO solution was incubated with the Dowex for 1 h at room temperature. The resultant supernatant was retrieved by filtration (2 μm). Pyrvinium with chloride counterion was lyophilized and the powder resuspended in water. The purity was examined by MS and found to be >99%.
Transcription Assays and Statistics.
For all transfections, pools of cells were transfected using Lipofectamine Plus (Invitrogen) with pRL-SV40 (Promega) and PSA-luciferase (The region from −4,882 to +12 relative to the transcription start site of PSA was amplified from human genomic DNA by PCR and inserted into pGL4.10 (Promega). This region has been shown to induce expression of a similar luciferase reporter gene upon treatment with androgen (14). The following day, the cells were re-plated, drugs were added, and 24 h later luciferase production was measured (Dual luciferase assay kit; Promega). Mean-effect plots (log[compound] vs. log[fractional effect]) were generated to determine the IC50 values for each compound or combinations of compounds at constant ratios. Microsoft Excel was used to calculate the statistics for a line using the least-squares method. The F statistic was used to determine whether the observed relationship between the dependent and independent variables occurred by chance. Only data with an r2 value greater than 0.95 and an F value that was greater than that indicated by the F table for an α of 0.05 were used for analysis. The methods of Chou and Talalay (13) were used to determine whether 2 compounds had antagonistic, additive, or synergistic reactions toward each other. Briefly, a combination index (CI) was established for a range of fractional effects, where a CI of approximately 1 indicates additivity, a CI >1 indicates antagonism, and a CI <1 indicates synergy. The CIs were based upon a non-exclusive assumption, which was indicated by the slope of the line of the combination of drugs from the mean-effect plot. However, CIs based upon an exclusive assumption were similar.
RT-PCR and Statistics.
LNCaP and LAPC4 cells were grown in media containing C/S FBS in the presence or absence of test compounds for 24 h. Total RNA was isolated from cells in culture plates using an RNAeasy kit (Qiagen) and reverse-transcribed (MMLV-RT; Invitrogen). Primers used to detect human transcripts in these studies are as follows: RPL19, sense 5′-ATGTATCACAGCCTGTACCTG-3′; antisense, 5′-TTCTTGGTCTCTTCCTCCTTG-3′; kallikrein 3, sense 5′-CCCGAGCAGGTGCTTTTG-3′; antisense, 5′-GGAGTTCTTGACCCCAAA-3′; Nxk3.1, sense 5′-TTCTCCCACACTCAGGTGATC-3′; antisense, 5′-GTGAGCTTGAGGTTCTTGGC-3′; FKBP51, sense 5′-CTGTGACAAGGCCCTTGGA-3′; antisense, 5′-CTGGGCTTCACCCCTCCTA-3′; TMPRSS2, sense 5′-cagcaagtgctccaactctg-3′; antisense, 5′-ACACACCGATTCTCGTCCTC-3′; MMP16, sense 5′-TCCTTGAGGATGGATCTTGG-3′; antisense, 5′-TCTCCTCAGGGAGCATTTGT-3′; and RDC1, sense 5′-CCAGTCTGGGTGGTCAGTCT-3′; antisense, 5′-CTCATGCACGTGAGGAAGAA-3′. Primers used to detect mouse transcripts were as follows: probasin, sense 5′-ATCATCCTCCTGCTCACACTGCATG-3′; antisense, 5′-ACAGTTGTCCGTGTCCATGATACGC-3′; RPL19, sense 5′-ATGTATCACAGCCTGTACCTG-3′; antisense, 5′-TTCTTGGTCTCCTCCTCCTTG-3′; MMP16, sense 5′-CCTGAATCACCTCAGGGAGC-3′; antisense, 5′-ATCACAGCCCATAAAGTCCT-3′; Nkx3.1, sense 5′-TTCTCTCACACTCAGGTGATT-3′; antisense, 5′-GTGAGTTT GAGGTTCTTGGC-3′; FKBP51, sense 5′-CTGCGACAAGGCCCTTGGA-3′; antisense, 5′-CTGGGCCTCGCCCCTTCTG-3′; RDC1, sense 5′-CCCGTCTGGGTGGTCAGTCT-3′; antisense, 5′-CTCATGCAGGCGAGGAAGAA-3′; and TMPRSS2, sense 5′-GAACCCAGGCATGATGCTAGA-3′; antisense, 5′-CACCCCGAAATCCAGCATT-3′. For studies on mouse prostates, tissue was frozen in RNA Later (Qiagen) and mechanically homogenized before RNA isolation. Qiagen Taq and reagents were used for amplification, and each sample was measured in triplicate in 96-well plates. Real-time PCR was carried out on a 7300 Real Time PCR System (Applied Biosystems), using SYBR green (Invitrogen) as the detecting dye and Rox (Invitrogen) as the reference dye. Differences between experimental (x) and no-DHT control (y) samples were normalized to RPL19 transcript levels (i.e., androgen-unresponsive) and determined with the following calculation: (2[Ctxgene1 − Ctygene1])/(2[CtxRPL19 − CtyRPL19]). To test for differences in means, ANOVA methods were used for planned comparisons between treatment groups that were defined by linear contrast statements.
Radioligand Competition Binding Assay.
LAPC4 or LNCaP cells (5 × 105) were seeded in 12-well plates in phenol red-free media containing 5% C/S FBS. After 3 days, media were replaced with serum-free media containing 1 nM [3H]DHT in the absence or presence of 0.1–1,000-fold molar excess of unlabeled competitor ligands for 90 min at 37 °C. Cells were washed with phosphate buffer, and bound ligand was extracted in ethanol for 30 min at room temperature and detected using a scintillation counter. Curve fitting and IC50 calculations were performed using Prism software (GraphPad).
ChIP.
ChIP assays were performed as described (31) with the following modifications. LNCaP cells in medium containing C/S FBS were treated with 0.1% DMSO vehicle, 10 nM DHT, 10 nM DHT plus 10 μM BiC, 10 nM DHT plus 300 nM PP, or 10 nM DHT plus 300 nM HH for 4 h at 37 °C, 1% formaldehyde for 3 min as the dishes cooled from 37 °C to 22 °C, and 125 mM glycine for 10 min as the dishes cooled from 22 °C to 4 °C. Cells were lysed in immunoprecipitation lysis buffer (50 mM Hepes-KOH, pH 7.4, 1 mM EDTA, 150 mM NaCl, 10% glycerol, 0.5% Triton X-100, supplemented with protease inhibitors) and harvested by scraping. Nuclei were collected by centrifugation (500 × g for 5 min at 4 °C), resuspended in 2 mL of radioimmunoprecipitation buffer (10 mM Tris-HCl, pH 8.0, 1 mM EDTA, 150 mM NaCl, 5% glycerol, 0.1% sodium deoxycholate, 0.1% SDS, 1% Triton X-100, supplemented with protease inhibitors), and sonicated until an average DNA fragment size of 100–500 bp was achieved (assessed by agarose gel electrophoresis). For ≈107 LNCaP nuclei, 6 μg of anti-AR antibody (PG-21; Upstate) or 10 μg of anti-RNA pol II (clone 8WG16; Covance) was used for immunoprecipitation. Immunoprecipitated material was washed with radioimmunoprecipitation buffer containing 300 mM NaCl plus 100 μg/mL yeast tRNA and resuspended in 80 μL of proteinase K solution (pH 8.0, 0.7% SDS, 200 μg/mL proteinase K). After reversing cross-links, DNA fragments were purified using QIAquick PCR Purification kit (Qiagen). Real-time PCR was carried out as described earlier, and ChIP data were normalized to a region 140 bp upstream of the HSPA1A gene, which is not occupied by AR. Differences between experimental (x) and no-DHT control (y) samples were determined with the following calculation: (2[Ctxgene1 − Ctygene1])/(2[CtxHSP1A1 − CtyHSP1A1]).
Mouse Studies.
All experiments were performed with institutional review board regulatory approval in collaboration with the preclinical therapeutics and mouse pathology core facilities at the University of California, San Francisco. All mice used in these studies were male, FVB-background mice, greater than 10 weeks of age. For studies measuring the availability of compounds in plasma, mice were given compounds in 100-μL doses of saline solution with 1% DMSO, either by mouth or i.p., and plasma samples taken at the indicated times. PP and HH were measured by LC/MS/MS method. Briefly, mouse plasma (20 μL) was added to 100 μL of 70% CH3CN and vortexed for 1 min, then centrifuged at 10,000 rpm for 5 min. The supernatant was transferred to an auto-sampler vial and 10 μL was injected into LC/MS/MS. The LC/MS/MS conditions were set as follows: column, C8, 4.6 × 50 mm; mobile phase, 60% CH3CN, 0.05% acetic acid and 5 mM ammonium acetate for PP and 14% CH3CN, 1.8% methanol, 0.1% formic acid for HH. The flow rate was set at 1.2 mL/min and 1/4 of elution from the column was split to the mass system. Compounds were monitored in electrospray ionization-positive multiple reaction monitoring mode at 382.2 > 352.3 m/z for PP and 199.1 > 171.3 m/z for HH (Quattro Ultima; Micromass). The cone voltage and collision energy were set at 40 V and 30 eV for PP and 40 V and 25 eV for HH.
For efficacy studies, mice were administered PP by IP injection or BiC by PO (capsule crushed and re-suspended in saline solution) once daily (M-F) for 4 weeks. The dose of PP was escalated over the course of the study (0.1 mg/kg for the first week, 0.3 mg/kg for the second week, 1.0 mg/kg for the final 2 weeks). Two mice died from fighting at the onset of the study, and one mouse each from the BiC- and PP-treated groups died from unrelated causes during the study, leaving 10 mice in the untreated group and 9 mice in the others. At the end of the study, mouse liver and prostate were dissected, extraneous tissue was removed, and the wet weights were recorded. ANOVA was used to detect differences among treatment groups. The prostate was dissected along an axis such that each of the lobes was equally represented in each half, and one half was fixed in formalin and paraffin-embedded. Tissue sections were stained with hematoxylin and eosin. The other half was frozen in RNA-later (Qiagen) for RT-PCR.
Acknowledgments.
The authors thank Anne Donjacour for help with tissue histology and Vivian Weinberg for statistical consultation; Dr. Anthony Gerber, the Preclinical Therapeutics Core Facility, and Adam Renslo and the Small Molecule Discovery Center at University of California San Francisco. This work was supported by a research fellowship from the National Institutes of Health (NIH) (J.O.J.), research grants from the American Lebanese Syrian Associated Charities (C.F.), St. Jude Children's Research Hospital (R.K.G.), Department of Defense Prostate Cancer Research Program Grant PC060344-W81XWH-07–1-0073 (to R.K.G.), the NIH/National Cancer Institute (M.I.D., K.R.Y.), Prostate Cancer Foundation (M.I.D.), and Sandler Family Supporting Foundation (M.I.D.).
Footnotes
Conflict of interest statement: Given the potential utility of pyrvinium and harmol as therapeutic agents, the University of California, San Francisco, has filed a novel use patent that claims these compounds. J.O.J. and M.I.D. are co-inventors on this patent, and will stand to profit if it is issued. To the extent that publication of this manuscript will increase the value of this patent, or the likelihood that it will be out-licensed, J.O.J. and M.I.D. have a potential conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001–3015. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Keller ET, Ershler WB, Chang C. The androgen receptor: a mediator of diverse responses. Front Biosci. 1996;1:d59–71. doi: 10.2741/a116. [DOI] [PubMed] [Google Scholar]
- 3.Chen CD, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 4.Georget V, Terouanne B, Nicolas JC, Sultan C. Mechanism of antiandrogen action: key role of hsp90 in conformational change and transcriptional activity of the androgen receptor. Biochemistry. 2002;41:11824–11831. doi: 10.1021/bi0259150. [DOI] [PubMed] [Google Scholar]
- 5.Schaufele F, et al. The structural basis of androgen receptor activation: intramolecular and intermolecular amino-carboxy interactions. Proc Natl Acad Sci USA. 2005;102:9802–9807. doi: 10.1073/pnas.0408819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones JO, Diamond MI. A cellular conformation-based screen for androgen receptor inhibitors. ACS Chem Biol. 2008;3:412–418. doi: 10.1021/cb800054w. [DOI] [PubMed] [Google Scholar]
- 7.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 8.Beck JW, Saavedra D, Antell GJ, Tejeiro B. The treatment of pinworm infections in humans (enterobiasis) with pyrvinium chloride and pyrvinium pamoate. Am J Trop Med Hyg. 1959;8:349–352. doi: 10.4269/ajtmh.1959.8.349. [DOI] [PubMed] [Google Scholar]
- 9.Thompson PE, Worley DE, Meisenhelder JE. Anthelmintic studies on pyrvinium pamoate (Povan) and other drugs in rodents, dogs, and monkeys. Am J Trop Med Hyg. 1962;11:89–95. doi: 10.4269/ajtmh.1962.11.89. [DOI] [PubMed] [Google Scholar]
- 10.Picada JN, da Silva KV, Erdtmann B, Henriques AT, Henriques JA. Genotoxic effects of structurally related beta-carboline alkaloids. Mutat Res. 1997;379:135–149. doi: 10.1016/s0027-5107(97)00116-4. [DOI] [PubMed] [Google Scholar]
- 11.Horoszewicz JS, et al. The LNCaP cell line–a new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–132. [PubMed] [Google Scholar]
- 12.Klein KA, et al. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat Med. 1997;3:402–408. doi: 10.1038/nm0497-402. [DOI] [PubMed] [Google Scholar]
- 13.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 14.Cleutjens KB, van Eekelen CC, van der Korput HA, Brinkmann AO, Trapman J. Two androgen response regions cooperate in steroid hormone regulated activity of the prostate-specific antigen promoter. J Biol Chem. 1996;271:6379–6388. doi: 10.1074/jbc.271.11.6379. [DOI] [PubMed] [Google Scholar]
- 15.Nelson PS, et al. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci USA. 2002;99:11890–11895. doi: 10.1073/pnas.182376299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin B, et al. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res. 1999;59:4180–4184. [PubMed] [Google Scholar]
- 17.Amler LC, et al. Dysregulated expression of androgen-responsive and nonresponsive genes in the androgen-independent prostate cancer xenograft model CWR22–R1. Cancer Res. 2000;60:6134–6141. [PubMed] [Google Scholar]
- 18.Bieberich CJ, Fujita K, He WW, Jay G. Prostate-specific and androgen-dependent expression of a novel homeobox gene. J Biol Chem. 1996;271:31779–31782. doi: 10.1074/jbc.271.50.31779. [DOI] [PubMed] [Google Scholar]
- 19.Kazmin D, et al. Linking ligand-induced alterations in androgen receptor structure to differential gene expression: a first step in the rational design of selective androgen receptor modulators. Mol Endocrinol. 2006;20:1201–1217. doi: 10.1210/me.2005-0309. [DOI] [PubMed] [Google Scholar]
- 20.Smith CL, O'Malley BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25:45–71. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- 21.Kang Z, Janne OA, Palvimo JJ. Coregulator recruitment and histone modifications in transcriptional regulation by the androgen receptor. Mol Endocrinol. 2004;18:2633–2648. doi: 10.1210/me.2004-0245. [DOI] [PubMed] [Google Scholar]
- 22.Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9:601–610. doi: 10.1016/s1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- 23.Hodgson MC, Astapova I, Hollenberg AN, Balk SP. Activity of androgen receptor antagonist bicalutamide in prostate cancer cells is independent of NCoR and SMRT corepressors. Cancer Res. 2007;67:8388–8395. doi: 10.1158/0008-5472.CAN-07-0617. [DOI] [PubMed] [Google Scholar]
- 24.Luo S, et al. Daily dosing with flutamide or Casodex exerts maximal antiandrogenic activity. Urology. 1997;50:913–919. doi: 10.1016/s0090-4295(97)00393-2. [DOI] [PubMed] [Google Scholar]
- 25.Matuo Y, Nishi N, Negi T, Tanaka Y, Wada F. Isolation and characterization of androgen-dependent non-histone chromosomal protein from dorsolateral prostate of rats. Biochem Biophys Res Commun. 1982;109:334–340. doi: 10.1016/0006-291x(82)91725-9. [DOI] [PubMed] [Google Scholar]
- 26.Waki H, et al. The small molecule harmine is an antidiabetic cell-type-specific regulator of PPARgamma expression. Cell Metab. 2007;5:357–370. doi: 10.1016/j.cmet.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Solit DB, Scher HI, Rosen N. Hsp90 as a therapeutic target in prostate cancer. Semin Oncol. 2003;30:709–716. doi: 10.1016/s0093-7754(03)00346-4. [DOI] [PubMed] [Google Scholar]
- 28.Hieronymus H, et al. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell. 2006;10:321–330. doi: 10.1016/j.ccr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Dobosy JR, Roberts JL, Fu VX, Jarrard DF. The expanding role of epigenetics in the development, diagnosis and treatment of prostate cancer and benign prostatic hyperplasia. J Urol. 2007;177:822–831. doi: 10.1016/j.juro.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 30.Mellinghoff IK, et al. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 31.Bolton EC, et al. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev. 2007;21:2005–2017. doi: 10.1101/gad.1564207. [DOI] [PMC free article] [PubMed] [Google Scholar]