Abstract
The mouse Ly49 and human killer cell immunoglobulin-like receptors (KIR) gene clusters encode activating and inhibitory class I MHC receptors on natural killer (NK) cells. A direct correlation between the presence of multiple activating KIR and various human autoimmune diseases including diabetes has been shown. Previous studies have implicated NK cell receptors in the development of diabetes in the non-obese diabetic (NOD) inbred mouse strain. To assess the contribution of Ly49 to NOD disease acceleration the Ly49 gene cluster of these mice was sequenced. Remarkably, the NOD Ly49 haplotype encodes the largest haplotype and the most functional activating Ly49 of any known mouse strain. These activating Ly49 include three Ly49p-related and two Ly49h-related genes. The NOD cluster contains large regions highly homologous to both C57BL/6 and 129 haplotypes, suggesting unequal crossing over as a mechanism of Ly49 haplotype evolution. Interestingly, the 129-like region has duplicated in the NOD genome. Thus, the NOD Ly49 cluster is a unique mix of elements seen in previously characterized Ly49 haplotypes resulting in a disproportionately large number of functional activating Ly49 genes. Finally, the functionality of activating Ly49 in NOD mice was confirmed in cytotoxicity assays.
Keywords: Ly49, Diabetes, Natural Killer Cells, Comparative Immunology/Evolution, Cell Surface Molecules
Introduction
Type 1, insulin-dependant, diabetes (T1D) is an autoimmune disease characterized by targeted destruction of insulin-producing β-cell islets of the pancreas by infiltrating lymphocytes.1 Much of our understanding of T1D has come from the study of disease in the non-obese diabetic (NOD) mouse.2,3 In addition to being a model of spontaneous T1D, NOD mice spontaneously develop other tissue-related autoimmune responses.2,4 Several genetic loci have been associated with susceptibility and development of diabetes in both humans and NOD mice.5 Multiple candidate genes have been identified that contribute to the genetic susceptibility of NOD mice to autoimmune diseases, including MHC class II, Il2 and Ctla4.2,3
NK cells are large granular lymphocytes that are able to lyse virally-infected and transformed cells.6 Interestingly, several NK anomalies have been linked with diabetes. An early study demonstrated that diabetic patients have lower NK activity compared to healthy controls,7 while a second group found that NK cells isolated from diabetic patients showed an activated phenotype compared to NK cells from healthy controls.8 Several recent studies have reported abnormal NOD NK cell functions and may be indicative of a possible association between diabetes development and NK cell dysfunction.9–11 Cytotoxicity of NK cells from NOD mice against NK-sensitive tumor cell targets is lower than the killing activity from NK cells of non-diabetic mice.12 In particular, Johansson et. al. showed defective killing triggered by FcR and Ly49D activating receptors in NOD mice.9 Similarly, impaired NKG2D modulation in NOD mice has recently been demonstrated. Ogasawara et al. suggested that NKG2D, an activating receptor, may become desensitized to its ligands as they are over-expressed in autoimmune, inflammatory environments resulting in decreased NKG2D expression and defective cytotoxicity and cytokine secretion by NOD NK cells.10 Collectively, these studies suggest that multiple NK cell activation pathways may be affected in NOD mice.
Cytokine production and cytotoxicity in mouse NK cells is regulated to a large extent by Ly49 family members through their binding to MHC class I and related molecules.13 Ly49 proteins are disulfide-linked homodimeric type II transmembrane receptors belonging to the C-type lectin receptor superfamily.6 Ly49 genes are grouped in a gene cluster as part of the Natural Killer Gene Complex (NKC) on chromosome 6. There are large differences in the gene content of the Ly49 haplotypes found in different mouse strains. To date, the 129S6 Ly49 gene cluster is the largest known with 20 genes and BALB/c is the smallest with 9 genes.14,15 The Ly49 receptor family is composed of two major groups: 1) Inhibitory Ly49 receptors possess an immunoreceptor tyrosine-based inhibitory motif (ITIM; I/VxYxxL/V) in the intracellular domain that recruits SHP-1 upon phosphorylation,16,17 and 2) activating Ly49 molecules lack an intact ITIM sequence but possess an arginine in their transmembrane domain for association with the signal-transducing protein DAP12.18,19 The binding of inhibitory Ly49 receptors to MHC class I ligands results in an inhibition of cytotoxicity.13,20 In contrast, NK killing can be triggered via activating Ly49 recognition of MHC class I expressed on target cells, suggesting a possible mechanism of NK cell-induced autoimmunity.21,22 Furthermore, cross-linking of activating Ly49 molecules by specific mAb or MHC ligand results in cytokine production and intracellular calcium ion mobilization.21,23,24
KIRs are the human functional equivalents of murine Ly49 molecules.6 Diabetic patients tend to have KIR haplotypes that differ from those of healthy controls. These individuals generally have an increased number of activating KIR in their genome compared to healthy controls or are more likely to possess KIR haplotypes containing specific activating KIR.8,25 Mapping studies in the NOD mouse model have shown that a diabetes susceptibility locus (idd 6) is present on chromosome 6 near the Ly49 gene cluster.26 In agreement with this finding, NOD mice congenic for C57BL/6 (B6) NK1.1, a marker near the Ly49 cluster, manifested reduced disease incidence and improved NK and NKT cell performance as compared to wild-type NOD mice.11
In order to better understand the role activating Ly49 receptors and NK cells may play in the development of diabetes, we analyzed the Ly49 gene cluster of the NOD/ShiLtJ (NOD) inbred mouse. Initial sequencing of Ly49 cDNAs revealed the presence of closely related duplicated genes. Subsequent sequence analysis of the NOD Ly49 gene cluster revealed a significantly increased number of activating Ly49 genes compared to other known Ly49 haplotypes. This is due in part to a large-scale duplication from a 129-related Ly49 region. A separate region of the NOD Ly49 cluster also shows great similarity to a part of the B6 haplotype. Furthermore, the identification of novel Ly49 genes in the NOD mouse is confirmed via flow cytometry. Elucidation of the MHC receptors regulating NOD NK cells will facilitate linking potential NK cell dysfunction to the induction of autoimmunity in the NOD mouse.
Results
New and unusual Ly49 genes detected in NOD mice
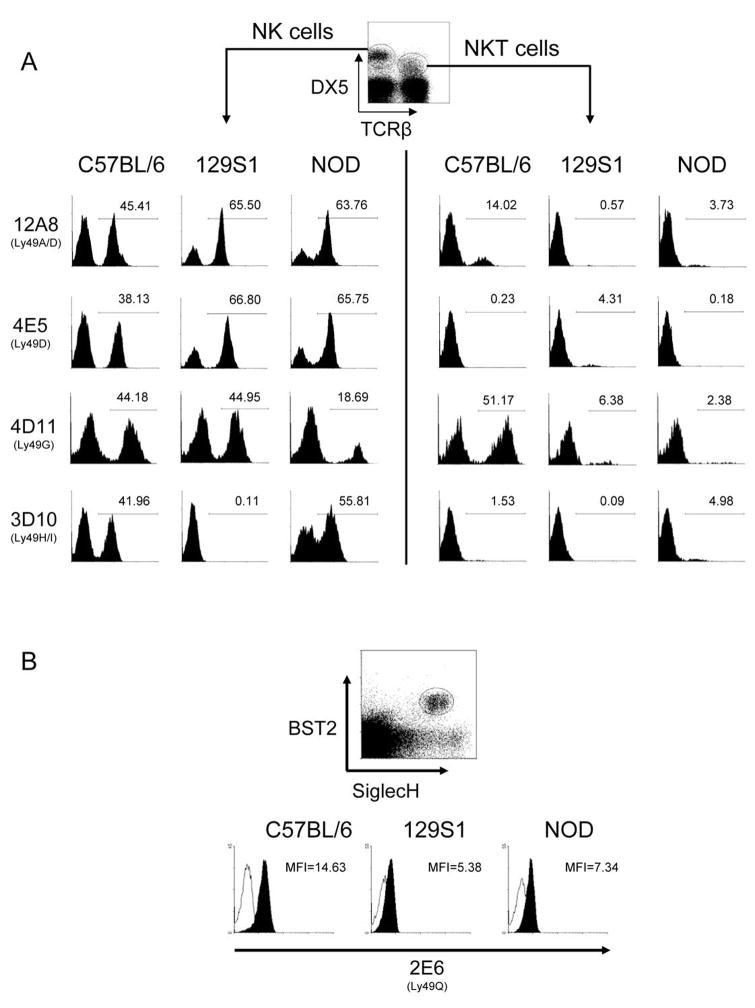
Previous in-depth studies of the Ly49 expressed by NOD mice by the Kane laboratory reported allelic variations of Ly49 known to exist in multiple mouse strains (Ly49A, G, D, E), in 129 mice (Ly49P), in B6 mice (Ly49M), and the NOD-specific Ly49W.21,22 As the above combination of Ly49 genes is unique, it appears that NOD inbred mice possess a haplotype different from the three sequenced mouse haplotypes (B6, 129, and BALB/c). To better understand the Ly49 expressed by NOD mice, flow cytometry was performed with anti-Ly49-specific mAb on NK cells present in fresh splenocyte isolations. Fresh ex vivo spleen NK cells from NOD mice showed reactivity with 12A8, 4E5, 4D11, and 3D10 mAbs (Figure 1a), which in B6 mice indicate the presence of Ly49A, D, G, and H/I.20,27,28 However, no binding with Cwy-3 (Ly49GB6), AT8 (Ly49GBALB/129), 5E6 (Ly49C/IB6), or 14B11 (Ly49C/I/F/HB6) was detected (data not shown), possibly due to differences in allele epitopes or missing genes. Interestingly, the activating Ly49D receptor appears to be expressed on a majority of NOD NK cells as evidenced by mAb 4E5 staining of NK cells. However, it is possible that these mAbs bind to additional NOD Ly49 as seen for 129 NK cells.29
Figure 1.
Ly49-specific mAb reactivity of NOD, 129S1 and B6 NK and NKT cells. (a) Splenocyte suspensions of the indicated mouse strains were stained with anti-TCRβ, DX5 and the indicated Ly49-specific mAb. The three left and three right columns show Ly49 surface expression of gated NK (DX5+ TCRβ-) and NKT (DX5+ TCRβ+) cells, respectively. The B6 specificities for each of the mAb used are indicated in parentheses. (b) Collagenase-liberated splenocytes were stained for BST2, SiglecH, and Ly49Q. Plasmacytoid DC were analyzed by gating on BST2+SiglecH+ events. The empty and filled histograms indicate pDC binding of isotype control and anti-Ly49Q mAb, respectively.
Ly49 expression by splenic NKT cells of NOD mice was also evaluated. Relative to B6 NKT cells, very few NOD NKT cells expressed Ly49. Ly49 expression on NOD NKT was detected using 12A8, 4D11, and 3D10 mAb. The small but significant percentage of 3D10-specific NKT cells suggests that either Ly49H is expressed on NKT cells or, more likely, that a closely related inhibitory receptor, such as Ly49I, cross-reacts with this mAb in NOD mice. Similarly, the 12A8 binding to NOD NKT cells is likely due to Ly49A expression. NOD plasmacytoid dendritic cells (pDC) bound to the 2E6 mAb specific for Ly49Q, but the mean fluorescence intensity was significantly lower compared to B6-derived pDC (Figure 1b). In summary, mAb staining suggests that Ly49A, D, G, H, I, and Q-like proteins are encoded by genes present in the NOD genome.
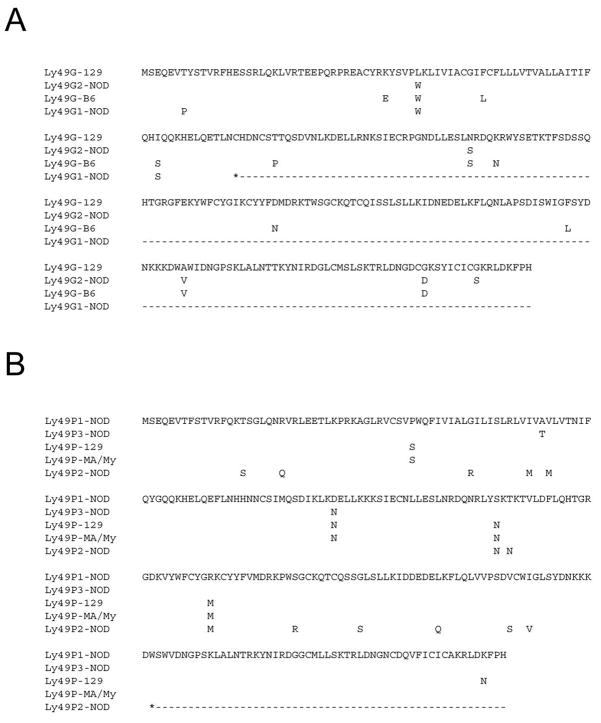
RT-PCR on NOD ALAK cDNA using Ly49-specific primers was then employed to identify novel Ly49 expressed by NOD NK cells. A cDNA closely matching the already described Ly49gNOD was isolated (98.5% identity). Interestingly, the novel Ly49g-related cDNA appears to be a pseudogene as it contains an early in-frame stop codon in exon 4 (Figure 2a). In addition, a cDNA corresponding to the Ly49gNOD allele (now termed Ly49g2NOD due to its position in the cluster) previously reported by Kane was also isolated.22 Thus, the Ly49 cluster of NOD mice is unique in that there appears to be a duplication of the Ly49g gene. To completely elucidate the complement of Ly49 in the NOD mouse a request was put forward and approved by the NOD Genome Sequencing Project at The Wellcome Trust Sanger Institute to completely sequence the NOD Ly49 region.
Figure 2.
NOD mice have two Ly49g and three Ly49p genes. Alignment of the putative amino acid translation of the novel NOD (a) Ly49G and (b) Ly49P alleles is shown along with their 129, B6, and MA/My counterparts. NOD Ly49g1 cDNA was isolated by RT-PCR of NOD ALAK mRNA. NOD Ly49p1 cDNA was deduced from genomic sequence, while Ly49p2 cDNA was previously submitted to GenBank (AK172530). The NOD Ly49g2 and Ly49p3 sequences were previously reported 21,22, but renumbered here due to their location in the genome. Dashes indicate non-existing amino acids due to an early stop.
NOD Ly49 cluster sequence
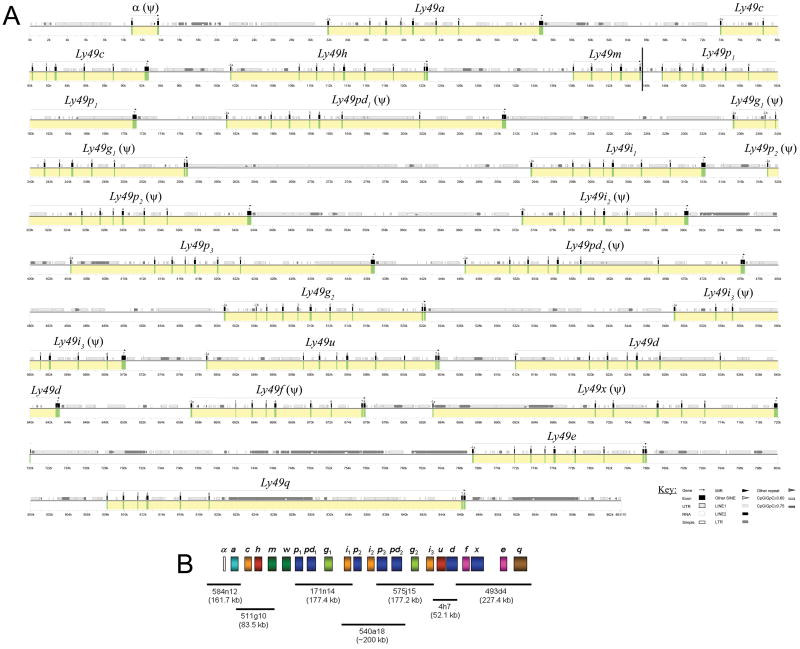
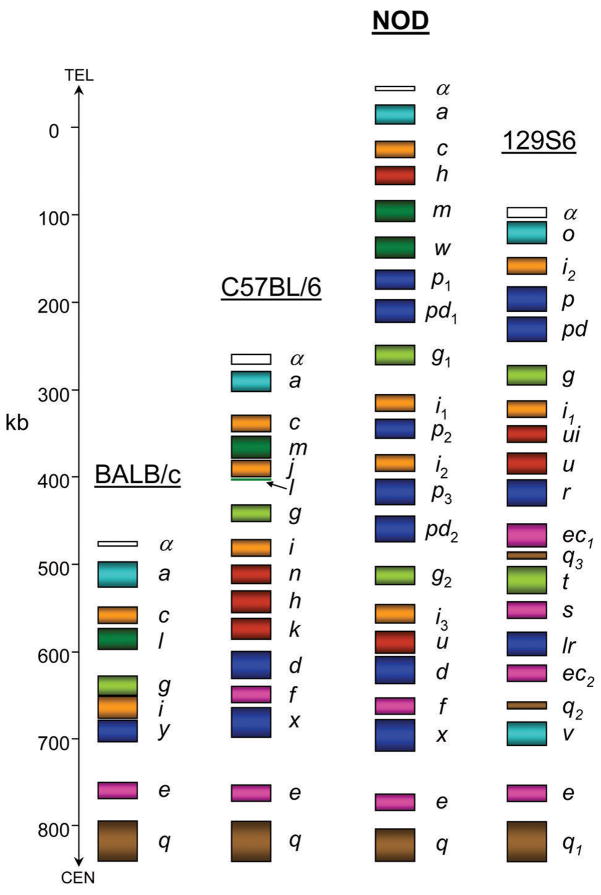
NOD BACs containing Ly49 genes were identified by end-sequencing and comparison to the relevant region of the B6 genome. A total of seven BACs covering the entire Ly49 cluster from the NOD genomic library (CHORI-29) were fully sequenced using the shotgun approach. BAC and gene order was assured by significant overlap (2 kb minimum) between neighboring BACs. The Ly49 cluster of NOD mice contains 22 genes as deduced by BLAST analysis of the genomic sequence using known Ly49 cDNAs for similarity searches. The new NOD genes were named based on their best BLAST matches (Table 1), and the allele relationships implied therein are supported by phylogenetic analysis (Figure 3). All new Ly49 genes fit into the Ly49 subfamilies defined in previous sequence alignment/grouping analyses. The NOD Ly49 gene order is: Ly49a, c, h, m, w, p1, pd1, g1, i1, p2, i2, p3, pd2, g2, i3, u, d, f, x, e, and q. The framework Ly49 genes (a, c, g, i, e, and q) previously identified in B6, BALB/c, and 129 mice are maintained in this haplotype.30 The relative spacing and size of each gene along with location of exons and repetitive elements is shown in the PIP diagram of Figure 4a. The NOD Ly49 cluster is approximately 850 kb in length with a single gap between BACs CH29-511G10 and CH29-171N14 (Figure 4b). This gap is between the Ly49m and Ly49p1 genes. The previously reported,22 but missing, Ly49w gene has been tentatively placed here for the following reasons: 1) the entire cluster is covered by overlapping BACs except for this gap, 2) there is sufficient additional BAC sequence upstream and downstream of the cluster to know that the Ly49w gene cannot be immediately present outside the main cluster, and 3) the placement beside Ly49m would be logical as these genes are closely related and most likely arose as a tandem duplication similar to Ly49p and pd in 129 mice and Ly49n, h, and k in B6 mice.
Table 1.
Characteristics of NOD/ShiLtJ Ly49 genes
| Ly49 | Arg/ITIMa | Observed sequence anomalies | Best cDNA match (B6 or 129) | Accession numbers |
|---|---|---|---|---|
| α | NAb | Exons 2 and 4 only | NA | |
| a | ITIM | None | 98.23% B6 Ly49a | AF218077 |
| c | ITIM | Extra 97 nt in exon 7 (early stop) | 97.08% B6 Ly49c | |
| h | Arg | None | 96.12% B6 Ly49h | |
| m | Arg | None | 98.75% B6 Ly49m | AF283252 |
| w | Arg | None | 96.65% B6 Ly49m | AF283250 |
| p1 | Arg | None | 99.42% 129 Ly49p | |
| pd1 | NA | Early stop in exon 2 | 99.62% 129 Ly49pd | |
| g1 | NA | Early stop in exon 4 | 98.51% B6 Ly49g | |
| i1 | ITIM | None | 97.75% B6 Ly49i | |
| p2 | NA | Early stop in exon 6 | 96.59% 129 Ly49p | AK172530 |
| i2 | NA | Early stop in exon 4 | 97.25% B6 Ly49i | |
| p3 | Arg | None | 99.24% 129 Ly49p | AF218080 |
| pd2 | NA | Early stop in exon 2 | 99.62% 129 Ly49pd | |
| g2 | ITIM | None | 99.00% 129 Ly49g | AF283249 |
| i3 | NA | Early stops in exons 2 and 5 | 97.63% B6 Ly49i | |
| u | Arg | None | 98.13% 129 Ly49u | |
| d | Arg | None | 98.48% B6 Ly49d | AF218078 |
| f | NA | No splice exon 6, no exon 7 | 98.32% B6 Ly49f | |
| x | NA | Early stop in exon 4, no exon 3 | 97.35% B6 Ly49x | |
| e | ITIM | None | 100% B6 Ly49e | |
| q | ITIM | None | 99.15% B6 Ly49q |
The predicted presence of an arginine (Arg) in the transmembrane region or an ITIM in the cytoplasmic domain is indicated. Complementary DNA for new genes were deduced from the genomic sequence.
Not applicable.
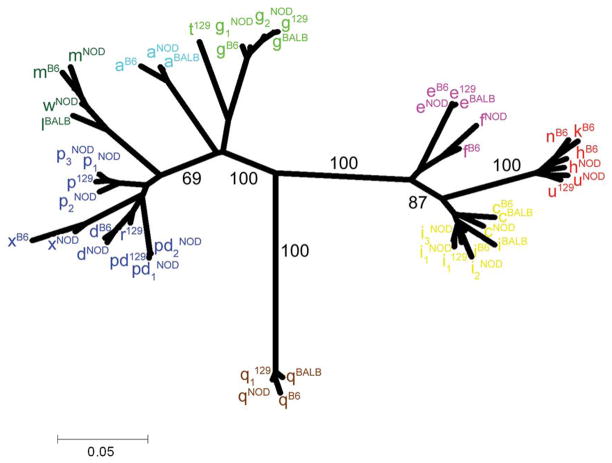
Figure 3.
Phylogenetic analysis of all NOD Ly49. Ly49 cDNA sequences from NOD, B6, BALB/c, and 129S6 mice, including artificially spliced cDNAs from non-transcribed pseudogenes, were aligned using ClustalX. Bootstrap analysis was performed on 1000 data sets with PHYLIP and the final consensus phylogram was visualized with TreeExplorer. The percentage bootstrap values are given for major branchpoints. The Ly49 family of genes can be subdivided into the D, L, A, G, Q, E, H, and C-related groups. The scale bar indicates the percentage of divergence between cDNAs.
Figure 4.
Organization of the NOD Ly49 gene cluster. (a) A 862 kb sequence of the NOD Ly49 cluster is represented in graphical form using Pipmaker. The sequence is demarcated below the plot in kilobases. Regions that contain genes are marked in yellow and exons in green. The name of the gene is given above the yellow area and predicted pseudogenes are denoted with a (ψ). A vertical bar between the Ly49m and p1 genes indicates the gap believed to contain Ly49w. See key for symbols of different types of repeats and other sequence elements identified using Repeatmasker. (b) An inset (bottom) displaying the location, name, and size of the BACs used for sequencing the cluster.
Evidence for intra-Ly49 cluster recombination in the NOD genome
The NOD Ly49 cluster is composed of a unique combination of six regions (Figure 5). The cluster begins with Ly49a and c, which is common to all Ly49 haplotypes. Next, the unique combination of Ly49h, m, and w genes are present. As mentioned above, Ly49w and Ly49m are likely the result of a tandem duplication event. The residual Ly49l exon 7 in the B6 cluster may be the remains of a Ly49w gene that was destroyed by the creation of Ly49j, which itself is presumably a duplication of a Ly49c/i-like gene. Remarkably, Ly49hNOD, which is closely related to the MCMV-m157 binding receptor encoded by Ly49hB6 and the 129 allele Ly49u, has been transposed from its normal position 5′ of Ly49d and r in the B6 and 129 clusters, respectively. This may be the result of a duplication event, since a similar gene (Ly49uNOD) is found in the typical location, but it is more likely that the unusual location of Ly49hNOD may be a result of an unequal crossing-over event. This finding highlights the great plasticity in Ly49 cluster evolution. Following Ly49h, m, and w is a cluster of genes first identified in 129 mice: Ly49p1, pd1, g1 and i1.15 This region is in turn followed by duplications of Ly49p and i-related genes: Ly49p2 and i2. Following these two genes a large duplication of the Ly49p1-i1 region is present: Ly49p3, pd2, g2, and i3. This large-scale gene duplication explains the initial identification of two Ly49g-related cDNAs from NOD NK cDNA (Figure 2a). It should be noted that the Ly49p and Ly49g cDNAs first identified by Kane in NOD mice correspond to Ly49p3 and Ly49g2, respectively, in the present study. Finally, a region closely resembling the centromeric end of the B6 Ly49 cluster is found: Ly49u, d, f, x, e, and q.
Figure 5.
Comparison of the NOD Ly49 cluster to known murine Ly49 haplotypes. The location and number of Ly49 genes of BALB/c, B6, NOD and 129 are compared graphically. The figure is drawn to scale and the scale in kilobases is shown on the left along with the relative locations of the chromosome 6 telomere (TEL) and centromere (CEN). The colors of the various Ly49 genes follow the scheme of Figure 3. For each gene, the rectangle covers the first known exon to the last. The location of the last exon of Ly49mNOD is inferred from the B6 haplotype. The length of Ly49w is unknown and has been tentatively placed in the only gap between Ly49m and p1.
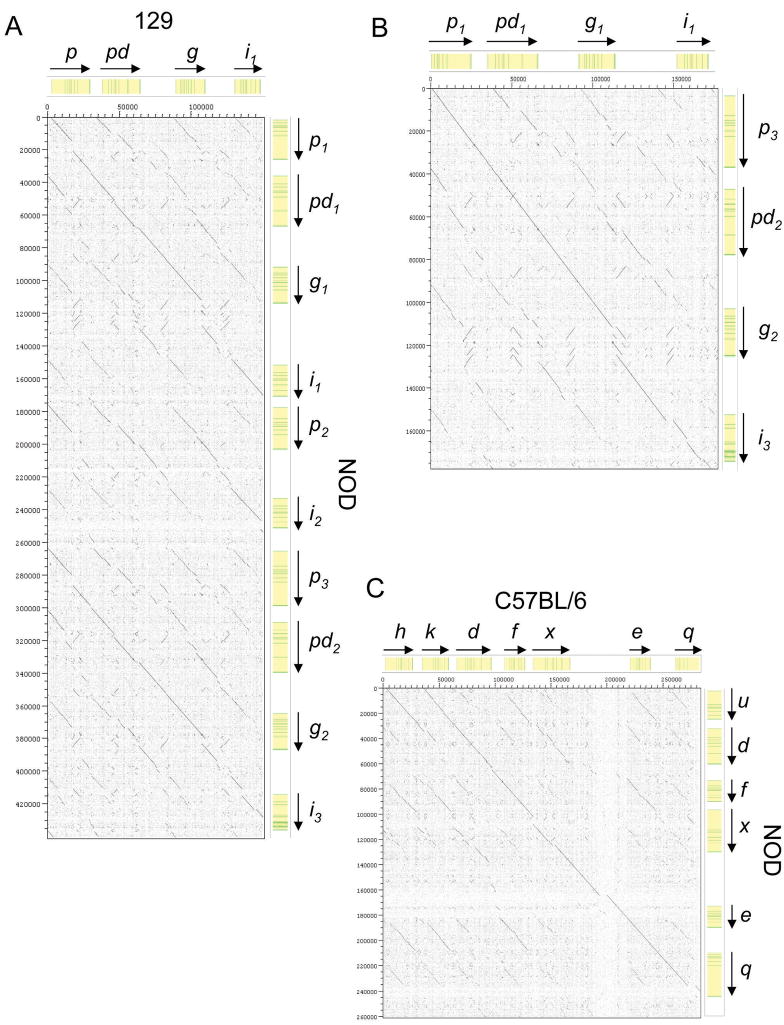
DOTTER analysis was employed to better assess the similarity of the various regions of the NOD Ly49 cluster to that of B6 and 129 mice. DOTTER genomic sequence comparison displays identity between two sequences as an uninterrupted diagonal line. Comparison of the NOD and the 129 Ly49 clusters shows that the NOD Ly49p1-i1 and Ly49p3-i3 regions are closely related to the 129 Ly49p-i region (Figure 6a). However, in both cases there are breaks in the diagonal indicative of insertions/deletions as a result of divergence over time. DOTTER analysis of the NOD Ly49p1-i1 and Ly49p3-i3 regions reveals that they are more closely related to each other than to the homologous region in the 129 cluster, suggesting their likely origin as a large duplication (Figure 6b). Sequence comparison between the NOD and B6 Ly49 clusters reveals that the 3′ ends stretching from Ly49h/u to q, although not identical, are closely related (Figure 6c). Otherwise, comparison of the total NOD cluster to that of either 129 or B6 only reveals homology to the beginning and end of the clusters, due to Ly49α, a, c and Ly49e, q regions being highly conserved in all haplotypes thus far sequenced (data not shown). Thus, DOTTER analysis strongly supports the possibility that the NOD Ly49 cluster was, in large part, the result of an unequal cross-over between 129-like and B6-like haplotypes present in an ancestral mouse heterozygous for these Ly49 haplotypes. However, whether the duplication of the NOD Ly49p-i region occurred before, during, or after the B6/129 cross-over is not clear.
Figure 6.
Direct sequence comparison between the B6, 129S6, and NOD Ly49 gene clusters. Specific regions of B6, 129S1 and NOD Ly49 gene clusters were directly compared at the nucleotide level using the Dotter program. Sequence comparisons are shown for (a) the Ly49p-i regions of 129 vs NOD, (b) the NOD Ly49p1-i1 and Ly49p3-i3 regions, and (c) the centromeric portion of the NOD vs. B6 haplotypes. Diagonal lines represent regions of sequence homology. The location of genes (yellow) and exons (green) is shown above and on the right side of the plot. The scale on both axes is in base pairs. Breaks in continuity of the homology lines indicate the locations of haplotype-specific deletions/insertions such as repetitive elements.
NOD Ly49 cluster open reading frames
Putative NOD Ly49 cDNAs were constructed after artificially splicing together the predicted exons of the new genes. The NOD haplotype has seven Ly49 genes that are predicted to produce functional activating receptors: Ly49h, m, w, p1, p3, u, and d. The Ly49h, p1, and u genes are newly identified in the current study and their surface expression needs to be confirmed by the production of specific monoclonal antibodies. Like Ly49H, Ly49P has been implicated in the resistance of MA/My mice to MCMV,31 thus the finding of three Ly49p-related genes in one haplotype is especially intriguing. The close identity between the functional Ly49p1, p3, and the pseudogene Ly49p2 cDNAs (and previously identified Ly49p alleles) is shown in Figure 2b.
Similar to 129 and B6, but not BALB/c inbred mice, the NOD Ly49 cluster contains a large number of pseudogenes with Ly49α, pd1, g1, p2, i2, pd2, i3, f, and x predicted to be non-functional for various reasons including missing exons, early in-frame stop codons, and single or multiple insertions leading to frame-shift stop codons (Table 1). As with their 129 or B6 counterparts, the Ly49α, pd1, pd2, and x genes are nonfunctional in NOD mice. The NOD Ly49c allele, unlike its B6 counterpart, has an early stop in the last exon. However, Ly49cNOD is not necessarily a pseudogene, as the majority of the protein including the carbohydrate recognition domain should be properly translated and folded. There are four members of the Ly49c/i subfamily in NOD mice (Ly49c, i1, i2, and i3), the most known in any one haplotype. The Ly49i1 gene may be the only functional member of this group in NOD; Ly49i2 and i3 are definitely pseudogenes and the status of Ly49c is unclear. Similarly, Ly49g1 and g2 is the second known instance of Ly49g duplicating within a haplotype; Ly49t is closely related to Ly49g in 129 mice. However, in NOD mice the Ly49g duplication is more recent, but one gene is nonfunctional due to an early in-frame stop codon in exon 4 of Ly49g1. Repetitive element-related ORFs are the only additional open reading frames found in the NOD Ly49 cluster. The incidence of SINE, LINE, LTR, and other simple repeats in the NOD Ly49 region was similar to that seen for 129, B6, and BALB/c Ly49 regions (data not shown).
NOD Pro1 promoter polymorphisms
Previous studies have indicated that the Ly49 Pro1 promoter is active in immature NK cells and plays an important role in Ly49 gene activation and the control of variegated expression.32,33 Figure 7 shows a comparison of the Pro1 regions of the activating and inhibitory NOD Ly49 genes. The activating genes have promoters that are nearly identical to the previously characterized Pro1 promoters of the B6, 129 and BALB/c Ly49 genes. The Ly49p2 gene has a deletion that selectively removes the TATA element associated with Pro1 antisense transcription, similar to the 1640 bp deletion observed for the BALB/c Ly49y gene,14 and it was suggested that this deletion may allow expression of the gene since in vitro studies have shown that the forward Pro1 promoter is active in reporter constructs lacking the reverse TATA box.33 Although the NOD Ly49p2 gene contains a premature stop codon in exon 6, it is transcribed, since a NOD Ly49p-related cDNA reported in GenBank (AK172530) is identical to the predicted Ly49p2 transcript. The Ly49p3 gene has a typical Pro1 element, and the predicted Ly49p3 transcript corresponds to the NOD Ly49P-coding cDNAs previously identified (GenBank AF074458, AF218080). No sequences identical to the predicted Ly49p1 transcript were found in GenBank; however the sequence of the Ly49p1 Pro1 element is not available due to a gap in the sequence assembly.
Figure 7.
Promoter region analysis of NOD Ly49 genes. The Pro1 regions of the NOD Ly49 genes were aligned and separated into activating or inhibitory subgroups. The important transcription factor binding sites are boxed. A dash (-) indicates identity and a period (.) indicates absent nucleotides.
The forward Pro1 TATA elements of the inhibitory Ly49a and Ly49g2 genes are distinct from the Pro1 promoters observed for these genes in the B6, 129 and BALB/c Ly49 gene clusters. The Ly49g Pro1 forward TATA element is “TATAAAT” in the three previously characterized strains; however, it has the sequence “TGTAAAT” in NOD. Conversely, the Ly49a Pro1 TATA element is “TATAAAT” in NOD, whereas it is “TGTAAAT” in the other three strains. Characterization of the functional activity of the B6 and 129 Pro1 promoters demonstrated that Ly49g Pro1 had significantly higher forward transcriptional activity than the Ly49a Pro1 promoter,33 correlating with a higher frequency of Ly49G expression than Ly49A in these mouse strains. These observations predict that the frequency of Ly49A expression should be higher than Ly49G expression in NOD NK cells if Pro1 is the major element controlling the degree to which each gene is expressed. In agreement with this prediction previous studies have shown that anti-Ly49A-specific mAbs YE1/48 and A1 stain the majority of NOD NK cells in contrast to anti-Ly49G mAb (Figure 1).21 It should be noted that Ly49P-related proteins may have also been detected on NOD NK cells with these mAb.
Activating Ly49 function in NOD mice
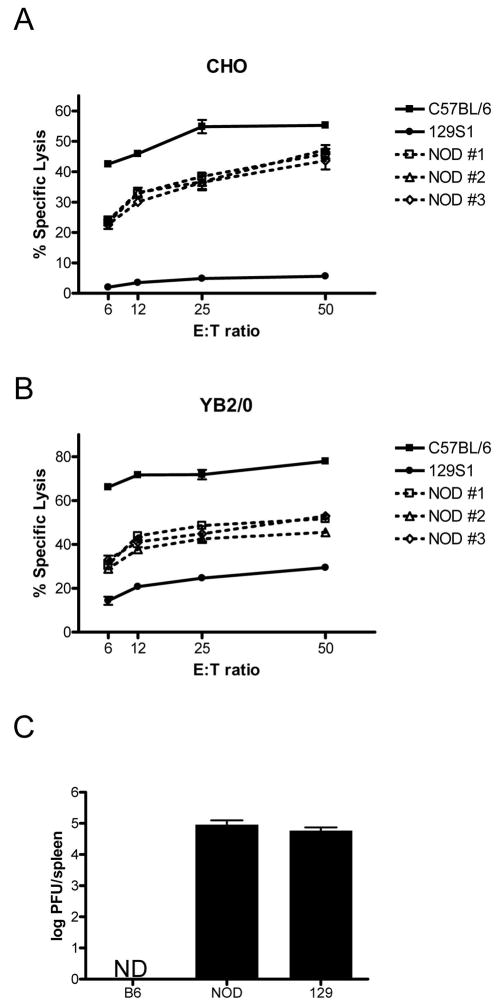
The presence of multiple and specific activating class I MHC receptors in humans has been correlated with various autoimmune diseases including diabetes.25,34 However, activating Ly49 do not signal normally in certain mouse strains such as 129X1.35 To further explore the possibility that the activating Ly49 in NOD NK cells contribute to the onset of diabetes, their functional activity in CHO cell cytotoxicity and MCMV infection assays was assessed. CHO cell killing was tested since NK cells from B6 mice preferentially lyse these tumor cells due to a fortuitous affinity of the activating Ly49D receptor for the hamster MHC Hm1-C4 expressed by CHO cells.36 To determine if Ly49D present on NOD NK cells is functional, ALAK from B6, NOD, and 129S1 mice were used as effectors in cytotoxicity assays with CHO as target cells. B6 ALAK displayed high killing ability in agreement with previous reports.37 Also in agreement with previous publications, 129S1 ALAK displayed low cytotoxicity towards CHO cells,35 which may be due to defects in the DAP12 signaling pathway of 129-background mice (Figure 8a). NOD NK cells from individual mice behaved similarly to B6 mouse NK cells, consistently showing significant cytotoxicity towards CHO cells. Note that this killing was typically lower than that displayed by B6 NK cells; the reasons for this are unclear. To determine if the lower cytotoxicity displayed by NOD NK cells is due to a defect in DAP12 signal transduction similar to that seen in 129 NK cells, the YB2/0 target cell that is killed by an Ly49D-independent interaction was tested. Interestingly, the pattern of NK cell cytotoxicity observed for the three mouse strain was similar to the CHO killing: B6 > NOD > 129S1 (Figure 8b). This suggests that the lower CHO killing displayed by NOD NK cells is due to non-Ly49D-related factors and is in agreement with previous studies of NOD NK cell cytotoxic function.9,38
Figure 8.
NOD activating Ly49 function. Splenic NK cells cultured in the presence of IL-2 from B6, 129S1, and NOD mice were prepared and used in chromium release assays against (a) CHO, and (b) YB2/0 target cells. Killing of CHO cells is largely dependent on competent signaling through the activating Ly49D receptor. Each line on the cytotoxicity graph indicates the killing achieved from ALAK cultures of different individual mice. (c) Three mice each of the indicated strains were injected i.p. with 5000 PFU MCMV and after three days spleen homogenates were prepared and used to infect BALB/c MEF monolayers to assess plaque forming potential. Data are shown in log scale with standard deviation. ND, not detectable.
The ability of B6 mice to resist MCMV infection is due to the activating Ly49H gene, which has been shown to bind to the MHC-like m157 protein on the surface of infected cells, thus triggering cytotoxicity and cytokine production by Ly49H+ NK cells.39 Similarly, Ly49P provides resistance to MCMV infection in MA/My mice although the mechanism is distinct from Ly49H.31 The current study reveals that NOD mice may have up to four functional anti-MCMV activating Ly49 (Ly49h, u, p1, and p3). Thus, NOD, like B6 and MA/My, may be resistant to MCMV. To test this possibility, NOD mice along with 129S1 and B6 controls were infected with MCMV and after three days spleen viral titer was assessed. B6 and 129S1 mice had low and high viral titers, respectively, as previously reported. Interestingly, NOD mice had high splenic MCMV levels similar to 129S1 mice (Figure 8c). Thus, NOD, like 129S1, is an MCMV-susceptible mouse strain despite the four Ly49h and p-related genes in its genome.
Discussion
The NOD mouse strain has been used extensively in studies identifying genes that contribute to diabetes development and severity. There is strong evidence that NK cells or NK cell receptors play an important role in the induction of autoimmune disease in this mouse strain.40–42 NK cell function is dependent on the types of class I MHC receptors that are expressed on their surface. It has long been realized that in vitro NK cell function determined by cytotoxicity assays against specific tumor cell targets and in vivo responses such as bone marrow or skin graft rejection is highly dependent on mouse strain background. This is due not only to differing MHC haplotypes, but equally to variable receptors for MHC (such as Ly49 and KIR) expressed by NK cells.
The Ly49 repertoire is highly variable among mouse strains. The number of genes can vary from 9 to 20, including six framework genes that are always present.30 All of the framework genes are inhibitory in nature. However, each of the mouse Ly49 haplotypes previously characterized also contain at least one functional activating Ly49, with a maximum of three in the 129 mouse. The NOD mouse now is known to have the largest known mouse Ly49 haplotype, with 22 genes. This includes up to seven genes coding for activating Ly49, but the functional status of these proteins is uncertain. The role of activating Ly49 has been the subject of much debate. Various ligands have been found for activating Ly49 and all are MHC or MHC-like. Ly49H has been shown to bind the MCMV-m157 gene product on infected target cells.39,43 Other activating Ly49 have been found to bind normal MHC class I from mouse and other rodent species. For example, Ly49D+ NK cells preferentially lyse H-2Dd target cells, especially when the NK cells are also negative for inhibitory receptors of H-2Dd, such as Ly49G.44,45 Ly49D has also been shown to recognize other types of rodent class I MHC including hamster Hm1-C4 and ligands encoded by the rat MHC.36,37
Similarly, the activating Ly49P and Ly49W of NOD mice were shown to specifically activate NK cell cytotoxicity toward target cells expressing H-2Dd and H-2Dk class I MHC alleles.21,22 The outcome of an activating NK cell receptor having affinity for a ubiquitous self MHC molecule could be either autoimmunity or anergy. Inhibitory Ly49 guard against NK cell autoimmunity and thus provide tolerance by binding to self MHC on normal target cells. However, the physiological significance and functional role for activating NK cell receptors binding to self MHC still needs to be addressed. At least two activating Ly49 (Ly49P and H) have been shown to be necessary for resistance to MCMV infection; however the two mechanisms are very different. In contrast to Ly49H, Ly49P recognizes MCMV-infected cells in the context of H-2Dk31 Thus, it is not surprising that Ly49P has some affinity for MHC of non-infected cells. As the Ly49D subfamily is closely related to Ly49P (Figure 3), it is logical that Ly49D also has affinity for normal MHC. Perhaps, Ly49D has an in vivo function similar to Ly49P. The role of the Ly49W group is unknown, but it is more similar to Ly49P than to Ly49H, both in terms of evidence for MHC binding and in sequence identity.
Thus, the protective effect of an anti-viral activating Ly49 may come at the price of an increased chance for autoimmunity. This may be the reason why most murine Ly49 haplotypes have relatively few activating Ly49. Such a hypothesis can be tested directly in the NOD mouse. The present study has found that NOD mice may express up to a maximum of seven activating Ly49. The analogy to observations seen in human diabetic patients is intriguing. Human diabetics have on average more activating KIR (the analogues of activating Ly49 on human NK cells) than non-diabetic individuals.25 The presence of specific activating KIR in combination with certain HLA alleles is also positively correlated with other types of autoimmune disease incidence such as psoriatic arthritis and rheumatoid arthritis.34,46 However, activating KIR also appear to have positive protective effects against viruses similar to the observations with activating Ly49 in mice. Individuals with HIV or HCV that express KIR3DS1 and HLA–B Bw4–80Ile are protected from disease progression.47,48
While the high number of activating Ly49 in NOD mice is consistent with the hypothesis that activating class I MHC receptors contribute to autoimmune disease incidence, the assumed strong anti-viral protective effect of many activating Ly49 is not found when NOD mice are challenged with MCMV (Figure 8c). This is despite the presence of two Ly49h- and two Ly49p-related genes. The reason for the lack of function of these genes is not clear, but there are several possibilities. First, it is unknown if Ly49P1 and P3 are expressed on the surface of NOD NK cells due to a lack of specific mAb. Second, Ly49P resistance in MA/My mice is dependent on the presence of H-2Dk,31 and NOD mice instead have the non-protective H-2Db allele. Third, the NOD Ly49h and u cDNAs are not identical to B6 Ly49h and the amino acid differences may result in loss of binding to m157, similar to Ly49U of 129 mice.39 This is supported by a recent report that Y146 and G151 of Ly49HB6 are critical for functional recognition of m157.49 In contrast to G151 of Ly49HB6, Ly49UNOD possesses S151 and partially explains the MCMV-susceptibility of NOD mice. Ly49HNOD has both necessary residues, but it is unclear whether the 3D10 staining of Figure 1 reveals surface expression of Ly49HNOD, Ly49UNOD, or both. Interestingly, the inhibitory Ly49I1NOD, like Ly49I1129, also share the m157 binding residues making it possible that this subset of NOD NK cells are inhibited by MCMV-infected target cells as speculated for Ly49I1+ 129 NK cells.39
While the MCMV infection assays are not informative, the CHO killing results suggest that the activating Ly49D is functional in NOD NK cells although NOD ALAK cytotoxicity was lower than that seen for B6 ALAKs (Figure 8a and b). The reasons for the lower cytotoxicity displayed by NOD NK cells are likely multifactorial, but previous studies of NK cells from human type 1 diabetic patients show significantly lower cytotoxicity and decreased expression of activating receptors.8,50 The observed cytotoxicity against CHO targets and the unusually large number of activating Ly49 in NOD mice, is especially intriguing in light of recent reported correlations between diabetes incidence and the number of activating KIR expressed by an individual’s NK cells.8,25 Supporting evidence for this hypothesis is found in the observation that disease incidence is reduced in NOD mice congenic for the B6 NKC,11 which encompasses the B6 Ly49 region and contains only two functional activating Ly49 in contrast to the seven predicted for NOD.
In conclusion, this study reveals the full panel of MHC class I receptors that may be expressed by NOD NK cells through analysis of the genomic sequence of the NOD Ly49 region. In addition to finding that the NOD mouse has the largest known haplotype in terms of total genes and total length, NOD mice also possess the most activating Ly49 receptors. Furthermore, these activating Ly49 are functional as determined by cytotoxicity assays. Thus, the NOD inbred mouse is a suitable model to study the contribution of activating MHC class I receptors expressed by NK cells to diabetes induction.
Materials and Methods
Mice, cells, and viruses
B6, 129S1 and NOD/ShiLtJ mice were purchased from the Jackson Laboratory and then bred and maintained at the IRCM animal care facility in accordance with institutional guidelines. Animal studies were reviewed and approved by the IRCM Animal Ethics Committee. ALAK preparation and cytotoxicity assays were performed as previously described.51 Spleens were first treated with collagenase for flow cytometry of pDC. MCMV plaque assay was performed as previously described.31 BALB/c MEF used for plaque assays were a kind gift of Dr. Silvia Vidal (McGill University, Montréal).
cDNA cloning
Total RNA was extracted from ALAK cultures using TRIzol reagent (Invitrogen). cDNA was synthesized using the Superscript First Strand cDNA synthesis kit (Invitrogen). Full length Ly49g1 and Ly49g2 coding sequences were amplified with the following primers: forward 5′ CTTCATACATCATTCCCAAG-3′ and reverse 5′ ATTTTACACTCGTTGGAGAG-3′ PCR products were cloned with the pCR2.1 TOPO cloning kit (Invitrogen) and sequenced.
Flow cytometry
Splenocytes were isolated from mice and stained for various cell surface markers with the following mAb: APC-CD49b (DX5), PE-TCRβ (H57-597) (eBioscience), FITC-Ly49D (4E5), (BD Biosciences), APC-mPDCA-1/BST2 (JF05-1C2.4.1) (Miltenyi Biotech), FITC-Ly49Q (2E6) (MBL), and purified SiglecH (440c) (HyCult). MAb to Ly49A/D (12A8), Ly49G (4D11), Ly49H (3D10) and SiglecH were biotinylated using a kit (Roche) and detected using FITC/PE-Streptavidin (BD Biosciences). Fc receptors were blocked with rat serum (Sigma) and dead cells were excluded with propidium iodide (BD Biosciences). Flow cytometry and analysis was performed using a FACsCalibur (BD Biosciences) with CellQuest software (BD Biosciences).
NOD Ly49 BAC sequencing
Funds were made available to The Wellcome Trust Sanger Institute (Cambridge, UK) by The National Institute of Allergy and Infectious Diseases (NIAID), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and the Juvenile Diabetes Research Foundation International (JDRF) for sequencing Idd regions in the NOD mouse. The Wellcome Trust Sanger Institute generated initial sequences from the ends of BACs from a NOD BAC library (CHORI-29; Pieter de Jong, Research Genetics), which was developed from the NOD/ShiLtJ mouse strain. An application was made to the Sanger Institute by A.P.M. and Christophe Benoist (Harvard Medical School, Boston, MA) for the sequencing of the Ly49 cluster (IDD6.3). CHORI-29 BACs with end-sequence homologous to the Ly49 region of the B6 genome were shotgun subcloned and sequenced. Finished Ly49-containing BAC sequence is publicly available online (http://www.sanger.ac.uk/Projects/M_musculus-NOD/) and through GenBank with the following accession numbers: CH29-584N12 (CU442703), CH29-511G10 (CU467659), CH29-575J15 (CU463286), CH29-171N14 (CU424478), CH29-540A18 (CU570803), CH29-4H7 (CU469450), and CH29-493D4 (CU207332).
Sequence analysis
The NCBI BLAST program was used to identify the location of Ly49 exons with known Ly49 cDNAs as queries on individual BAC sequences. This was followed by Lasergene DNASTAR program analysis to map and annotate the exons on the sequence. Putative cDNA sequences were constructed manually and putative amino acid sequences were determined with Sequencher 4.6 software. cDNA and amino acid sequences were compared using the BLAST 2 sequence analysis tool (http://www.ncbi.nlm.nih.gov/blast/bl2seq/wblast2.cgi). Putative cDNA and amino acid sequences were aligned using ClustalX version 1.83. Dotplot comparison of different sequences was performed using Dotter (available from http://www.cgb.ki.se/groups/sonnhammer/Dotter.html). Repetitive elements were identified with Repeatmasker version 3.1.8 (A.F.A. Smit and P. Green unpublished; available at http://www.repeatmasker.org). A PIP of the repeat-masked NOD/ShiLtJ Ly49 sequence versus itself was constructed using Advanced Pipmaker with a setting of single coverage (available at http://pipmaker.bx.psu.edu/pipmaker). Bootstrap analysis of 1000 replicates was performed on aligned cDNA sequences using PHYLIP (Phylogeny Inference Package) version 3.5c (available at http://evolution.genetics.washington.edu/phylip.html). Phylograms were constructed with TreeExplorer version 2.12 (K. Tamura, unpublished; available from http://evolgen.biol.metrou/ac.jp/TE/TE_man.html).
Acknowledgments
We thank Christophe Benoist for his essential aid in initiating the sequencing of the NOD Ly49 region. We also thank Dr. Silvia Vidal for her help with MCMV plaque assays and reagents. We gratefully acknowledge the genome sequencing efforts of The Wellcome Trust Sanger Institute.
Abbreviations
- B6
C57BL/6
- KIR
killer cell Ig-like receptor
- NOD
non-obese diabetic
- NK
natural killer
- pDC
plasmacytoid dendritic cell
Footnotes
By acceptance of this article, the publisher or recipient acknowledges the right of the U.S. Government to retain a non-exclusive, royalty-free license in and to any copyright covering the article.
This work was supported by an Operating Grant from the Canadian Institutes of Health Research (CIHR MOP 62841). This Research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This project has also been funded in part with Federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. NO1-CO-12400. S.B. is supported by a scholarship from the Fonds de la recherche en santé du Québec. L-H.T. is supported by a CIHR Cancer Training Program scholarship. A.P.M. is supported by a New Investigator Award from the CIHR.
Animal care was provided in accordance with the procedures approved by the IRCM Animal Care Committee.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Babaya N, Nakayama M, Eisenbarth GS. The stages of type 1A diabetes. Ann N Y Acad Sci. 2005;1051:194–204. doi: 10.1196/annals.1361.061. [DOI] [PubMed] [Google Scholar]
- 2.Aoki CA, Borchers AT, Ridgway WM, Keen CL, Ansari AA, Gershwin ME. NOD mice and autoimmunity. Autoimmun Rev. 2005;4:373–379. doi: 10.1016/j.autrev.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Wicker LS, Clark J, Fraser HI, Garner VE, Gonzalez-Munoz A, Healy B, et al. Type 1 diabetes genes and pathways shared by humans and NOD mice. J Autoimmun. 2005;25 Suppl:29–33. doi: 10.1016/j.jaut.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Alyanakian MA, You S, Damotte D, Gouarin C, Esling A, Garcia C, et al. Diversity of regulatory CD4+T cells controlling distinct organ-specific autoimmune diseases. Proc Natl Acad Sci U S A. 2003;100:15806–15811. doi: 10.1073/pnas.2636971100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–297. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 6.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 7.Nair MP, Lewis EW, Schwartz SA. Immunoregulatory dysfunctions in type I diabetes: natural and antibody-dependent cellular cytotoxic activities. J Clin Immunol. 1986;6:363–372. doi: 10.1007/BF00915375. [DOI] [PubMed] [Google Scholar]
- 8.Rodacki M, Svoren B, Butty V, Besse W, Laffel L, Benoist C, et al. Altered natural killer cells in type 1 diabetic patients. Diabetes. 2007;56:177–185. doi: 10.2337/db06-0493. [DOI] [PubMed] [Google Scholar]
- 9.Johansson SE, Hall H, Bjorklund J, Hoglund P. Broadly impaired NK cell function in non-obese diabetic mice is partially restored by NK cell activation in vivo and by IL-12/IL-18 in vitro. Int Immunol. 2004;16:1–11. doi: 10.1093/intimm/dxh002. [DOI] [PubMed] [Google Scholar]
- 10.Ogasawara K, Hamerman JA, Hsin H, Chikuma S, Bour-Jordan H, Chen T, et al. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity. 2003;18:41–51. doi: 10.1016/s1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- 11.Carnaud C, Gombert J, Donnars O, Garchon H, Herbelin A. Protection against diabetes and improved NK/NKT cell performance in NOD.NK1.1 mice congenic at the NK complex. J Immunol. 2001;166:2404–2411. doi: 10.4049/jimmunol.166.4.2404. [DOI] [PubMed] [Google Scholar]
- 12.Johansson S, Berg L, Hall H, Hoglund P. NK cells: elusive players in autoimmunity. Trends Immunol. 2005;26:613–618. doi: 10.1016/j.it.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 14.Anderson SK, Dewar K, Goulet ML, Leveque G, Makrigiannis AP. Complete elucidation of a minimal class I MHC natural killer cell receptor haplotype. Genes Immun. 2005;6:481–492. doi: 10.1038/sj.gene.6364232. [DOI] [PubMed] [Google Scholar]
- 15.Makrigiannis AP, Patel D, Goulet ML, Dewar K, Anderson SK. Direct sequence comparison of two divergent class I MHC natural killer cell receptor haplotypes. Genes Immun. 2005;6:71–83. doi: 10.1038/sj.gene.6364154. [DOI] [PubMed] [Google Scholar]
- 16.Mason LH, Gosselin P, Anderson SK, Fogler WE, Ortaldo JR, McVicar DW. Differential tyrosine phosphorylation of inhibitory versus activating Ly-49 receptor proteins and their recruitment of SHP-1 phosphatase. J Immunol. 1997;159:4187–4196. [PubMed] [Google Scholar]
- 17.Nakamura MC, Niemi EC, Fisher MJ, Shultz LD, Seaman WE, Ryan JC. Mouse Ly-49A interrupts early signaling events in natural killer cell cytotoxicity and functionally associates with the SHP-1 tyrosine phosphatase. J Exp Med. 1997;185:673–684. doi: 10.1084/jem.185.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason LH, Willette-Brown J, Anderson SK, Gosselin P, Shores EW, Love PE, et al. Characterization of an associated 16-kDa tyrosine phosphoprotein required for Ly-49D signal transduction. J Immunol. 1998;160:4148–4152. [PubMed] [Google Scholar]
- 19.Smith KM, Wu J, Bakker AB, Phillips JH, Lanier LL. Ly-49D and Ly-49H associate with mouse DAP12 and form activating receptors. J Immunol. 1998;161:7–10. [PubMed] [Google Scholar]
- 20.Mason LH, Ortaldo JR, Young HA, Kumar V, Bennett M, Anderson SK. Cloning and functional characteristics of murine large granular lymphocyte-1: a member of the Ly-49 gene family (Ly-49G2) J Exp Med. 1995;182:293–303. doi: 10.1084/jem.182.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silver ET, Gong DE, Chang CS, Amrani A, Santamaria P, Kane KP. Ly-49P activates NK-mediated lysis by recognizing H-2Dd. J Immunol. 2000;165:1771–1781. doi: 10.4049/jimmunol.165.4.1771. [DOI] [PubMed] [Google Scholar]
- 22.Silver ET, Gong DE, Hazes B, Kane KP. Ly-49W, an activating receptor of nonobese diabetic mice with close homology to the inhibitory receptor Ly-49G, recognizes H-2Dk and H-2Dd. J Immunol. 2001;166:2333–2341. doi: 10.4049/jimmunol.166.4.2333. [DOI] [PubMed] [Google Scholar]
- 23.Makrigiannis AP, Gosselin P, Mason LH, Taylor LS, McVicar DW, Ortaldo JR, et al. Cloning and characterization of a novel activating Ly49 closely related to Ly49A. J Immunol. 1999;163:4931–4938. [PubMed] [Google Scholar]
- 24.Gosselin P, Mason LH, Willette-Brown J, Ortaldo JR, McVicar DW, Anderson SK. Induction of DAP12 phosphorylation, calcium mobilization, and cytokine secretion by Ly49H. J Leukoc Biol. 1999;66:165–171. doi: 10.1002/jlb.66.1.165. [DOI] [PubMed] [Google Scholar]
- 25.van der Slik AR, Koeleman BP, Verduijn W, Bruining GJ, Roep BO, Giphart MJ. KIR in type 1 diabetes: disparate distribution of activating and inhibitory natural killer cell receptors in patients versus HLA-matched control subjects. Diabetes. 2003;52:2639–2642. doi: 10.2337/diabetes.52.10.2639. [DOI] [PubMed] [Google Scholar]
- 26.Rogner UC, Boitard C, Morin J, Melanitou E, Avner P. Three loci on mouse chromosome 6 influence onset and final incidence of type I diabetes in NOD.C3H congenic strains. Genomics. 2001;74:163–171. doi: 10.1006/geno.2001.6508. [DOI] [PubMed] [Google Scholar]
- 27.Mason LH, Anderson SK, Yokoyama WM, Smith HR, Winkler-Pickett R, Ortaldo JR. The Ly-49D receptor activates murine natural killer cells. J Exp Med. 1996;184:2119–2128. doi: 10.1084/jem.184.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith HR, Chuang HH, Wang LL, Salcedo M, Heusel JW, Yokoyama WM. Nonstochastic coexpression of activation receptors on murine natural killer cells. J Exp Med. 2000;191:1341–1354. doi: 10.1084/jem.191.8.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makrigiannis AP, Pau AT, Saleh A, Winkler-Pickett R, Ortaldo JR, Anderson SK. Class I MHC-binding characteristics of the 129/J Ly49 repertoire. J Immunol. 2001;166:5034–5043. doi: 10.4049/jimmunol.166.8.5034. [DOI] [PubMed] [Google Scholar]
- 30.Proteau M-F, Rousselle E, Makrigiannis AP. Mapping of the BALB/c Ly49 cluster defines a minimal natural killer cell receptor gene repertoire. Genomics. 2004;84:669–677. doi: 10.1016/j.ygeno.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Desrosiers MP, Kielczewska A, Loredo-Osti JC, Adam SG, Makrigiannis AP, Lemieux S, et al. Epistasis between mouse Klra and major histocompatibility complex class I loci is associated with a new mechanism of natural killer cell-mediated innate resistance to cytomegalovirus infection. Nat Genet. 2005;37:593–599. doi: 10.1038/ng1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saleh A, Makrigiannis AP, Hodge DL, Anderson SK. Identification of a novel Ly49 promoter that is active in bone marrow and fetal thymus. J Immunol. 2002;168:5163–5169. doi: 10.4049/jimmunol.168.10.5163. [DOI] [PubMed] [Google Scholar]
- 33.Saleh A, Davies GE, Pascal V, Wright PW, Hodge DL, Cho EH, et al. Identification of probabilistic transcriptional switches in the Ly49 gene cluster: a eukaryotic mechanism for selective gene activation. Immunity. 2004;21:55–66. doi: 10.1016/j.immuni.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Martin MP, Nelson G, Lee JH, Pellett F, Gao X, Wade J, et al. Cutting edge: susceptibility to psoriatic arthritis: influence of activating killer Ig-like receptor genes in the absence of specific HLA-C alleles. J Immunol. 2002;169:2818–2822. doi: 10.4049/jimmunol.169.6.2818. [DOI] [PubMed] [Google Scholar]
- 35.McVicar DW, Winkler-Pickett R, Taylor LS, Makrigiannis A, Bennett M, Anderson SK, et al. Aberrant DAP12 signaling in the 129 strain of mice: implications for the analysis of gene-targeted mice. J Immunol. 2002;169:1721–1728. doi: 10.4049/jimmunol.169.4.1721. [DOI] [PubMed] [Google Scholar]
- 36.Furukawa H, Iizuka K, Poursine-Laurent J, Shastri N, Yokoyama WM. A ligand for the murine NK activation receptor Ly-49D: activation of tolerized NK cells from beta 2-microglobulin-deficient mice. J Immunol. 2002;169:126–136. doi: 10.4049/jimmunol.169.1.126. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura MC, Naper C, Niemi EC, Spusta SC, Rolstad B, Butcher GW, et al. Natural killing of xenogeneic cells mediated by the mouse Ly-49D receptor. J Immunol. 1999;163:4694–4700. [PubMed] [Google Scholar]
- 38.Poulton LD, Smyth MJ, Hawke CG, Silveira P, Shepherd D, Naidenko OV, et al. Cytometric and functional analyses of NK and NKT cell deficiencies in NOD mice. Int Immunol. 2001;13:887–896. doi: 10.1093/intimm/13.7.887. [DOI] [PubMed] [Google Scholar]
- 39.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 40.Ogasawara K, Hamerman JA, Ehrlich LR, Bour-Jordan H, Santamaria P, Bluestone JA, et al. NKG2D blockade prevents autoimmune diabetes in NOD mice. Immunity. 2004;20:757–767. doi: 10.1016/j.immuni.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 41.Alba A, Planas R, Clemente X, Carrillo J, Ampudia R, Puertas MC, et al. Natural killer cells are required for accelerated type 1 diabetes driven by interferon-beta. Clin Exp Immunol. 2008 doi: 10.1111/j.1365-2249.2007.03580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inoue Y, Kaifu T, Sugahara-Tobinai A, Nakamura A, Miyazaki J, Takai T. Activating Fc gamma receptors participate in the development of autoimmune diabetes in NOD mice. J Immunol. 2007;179:764–774. doi: 10.4049/jimmunol.179.2.764. [DOI] [PubMed] [Google Scholar]
- 43.Smith HR, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci USA. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakamura MC, Linnemeyer PA, Niemi EC, Mason LH, Ortaldo JR, Ryan JC, et al. Mouse Ly-49D recognizes H-2Dd and activates natural killer cell cytotoxicity. J Exp Med. 1999;189:493–500. doi: 10.1084/jem.189.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mason LH, Willette-Brown J, Mason AT, McVicar D, Ortaldo JR. Interaction of Ly-49D+ NK cells with H-2Dd target cells leads to Dap-12 phosphorylation and IFN-gamma secretion. J Immunol. 2000;164:603–611. doi: 10.4049/jimmunol.164.2.603. [DOI] [PubMed] [Google Scholar]
- 46.Yen JH, Moore BE, Nakajima T, Scholl D, Schaid DJ, Weyand CM, et al. Major histocompatibility complex class I-recognizing receptors are disease risk genes in rheumatoid arthritis. J Exp Med. 2001;193:1159–1167. doi: 10.1084/jem.193.10.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin MP, Gao X, Lee JH, Nelson GW, Detels R, Goedert JJ, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 48.Lopez-Vazquez A, Rodrigo L, Martinez-Borra J, Perez R, Rodriguez M, Fdez-Morera JL, et al. Protective effect of the HLA-Bw4I80 epitope and the killer cell immunoglobulin-like receptor 3DS1 gene against the development of hepatocellular carcinoma in patients with hepatitis C virus infection. J Infect Dis. 2005;192:162–165. doi: 10.1086/430351. [DOI] [PubMed] [Google Scholar]
- 49.Kielczewska A, Kim HS, Lanier LL, Dimasi N, Vidal SM. Critical residues at the Ly49 natural killer receptor’s homodimer interface determine functional recognition of m157, a mouse cytomegalovirus MHC class I-like protein. J Immunol. 2007;178:369–377. doi: 10.4049/jimmunol.178.1.369. [DOI] [PubMed] [Google Scholar]
- 50.Negishi K, Waldeck N, Chandy G, Buckingham B, Kershnar A, Fisher L, et al. Natural killer cell and islet killer cell activities in human type 1 diabetes. Exp Clin Endocrinol. 1987;89:345–353. doi: 10.1055/s-0029-1210661. [DOI] [PubMed] [Google Scholar]
- 51.Makrigiannis AP, Rousselle E, Anderson SK. Independent control of Ly49g alleles: implications for NK cell repertoire selection and tumor cell killing. J Immunol. 2004;172:1414–1425. doi: 10.4049/jimmunol.172.3.1414. [DOI] [PubMed] [Google Scholar]