Abstract
OBJECTIVE
Mast cells are found in large numbers in atherosclerotic plaques. The present study was conducted to determine whether tryptase stimulation of human coronary artery endothelial cells (HCAEC) would lead to an increase in transmigration of CD133 positive cells (CD133+). In vitro these cells can differentiate into mast cells under the influence of specific cytokines and growth factors.
METHODS AND RESULTS
CD133+ cells were isolated from umbilical cord blood. They express mRNA for several adhesion molecules that are also utilized in neutrophil migration and can migrate across an HCAEC monolayer. Migration increased significantly when HCAEC were stimulated with tryptase and decreased when CD133+ cells were pretreated with CV3988, a platelet activating factor receptor (PTAFR) antagonist. Following long-term cell culture, these cells stained positively for the presence of tryptase, a mast cell enzyme.
CONCLUSION
CD133+ cells can be utilized as a mast cell precursor population. The transendothelial migration is facilitated by the presence of tryptase and may utilize the PAF/PTAFR interaction in a manner similar to that involved in neutrophil transmigration. Following transmigration, a subset of these progenitor cells may mature into mast cells in the subendothelial space and play a role in propagation of the inflammatory process in atherosclerosis.
Keywords: atherosclerosis, endothelial cells, CD133 positive cells, tryptase, transmigration
The presence of mast cells, activated T lymphocytes and macrophages in atherosclerotic lesions suggests the involvement of immune and inflammatory processes in the pathogenesis of atherosclerosis [1–3]. Leukocyte recruitment and the expression of pro-inflammatory cytokines are prevalent characteristics of early atherogenesis. Recently, several inflammatory mediators have been linked to atheroma formation and inflammatory pathways have been shown to promote thrombosis [4].
Mature mast cells are not found circulating within peripheral blood. Instead they have been shown to undergo the final stages of maturation following the migration of the precursor cell into the tissue space in which they reside [5]. The identification of increased mast cell numbers in atherosclerotic plaques suggests that recruitment of precursor cells and their eventual maturation into mast cells may play an important role in the progression of atherosclerosis [6–8]. To date, the exact mechanism of precursor cell recruitment and migration has not been well characterized. We hypothesized that precursor cells utilize a mechanism of migration similar to one that has been well characterized for neutrophils.
The process of migration of neutrophils from the bloodstream to inflamed tissues involves three major steps [9]. The initial transient adhesion of the leukocyte to the endothelial cell (EC) is mediated by P-selectin, stored in EC within Weibel Palade bodies [10]. P-selectin binds to leukocytes via its P-selectin ligand (PSGL-1), thus tethering them to the activated EC. The neutrophils now scan the EC monolayer for the presence of activating signals, such as integrins. If a signal is detected, the leukocyte is activated and expresses corresponding integrins to augment the adhesive response [11]. In addition platelet-activating factor, expressed on the surface of stimulated EC, promotes the adherence of neutrophils to the endothelium [12].
The integrins expressed on surface of leukocytes interact with endothelial counter receptors belonging to the Ig superfamily, such as, intracellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM). Integrins, such as the β1-integrins (binds to VCAM), on the neutrophil are upregulated by the presence of PAF and are critically involved in PAF-induced leukocyte locomotion in extravascular tissue [13]. Finally, chemoattractants stimulate the transmigration of leukocytes across the vessel wall [13].
In this study, we used CD133 + cells isolated from human umbilical cord blood as our model for undifferentiated cells. CD133 is a marker for hematopoietic and progenitor stem cells. CD133 + cells have been isolated from umbilical cord blood and adult peripheral blood [14, 15] and are capable of differentiation into various cells, including mast cells [16], when cultured in the presence of specific factors.
We hypothesize that CD133 + cells can be recruited to areas of EC damage and, in a process similar to that of leukocyte adherence to the endothelium, these cells may be able to adhere to, and subsequently migrate, across an EC monolayer. Under the influence of appropriate growth factors and cytokines a subset of these multipotent precursors acquire phenotypic characteristics typical of mature mast cell such as tryptase and histamine in the granules and expression of FcεR1 on the cell surface. As they mature into mast cells these can play a role in the inflammatory component of atherosclerosis.
MATERIALS AND METHODS
Reagents
Human tryptase (Promega), Rabbit anti-PTAFR antibody (Cayman Chemicals), Horse radish peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology, Santa Cruz, CA. All other reagents were purchased from Sigma Chemical, St. Louis, MO.
Cell Culture of Human Coronary Artery Endothelial Cells (HCAEC)
HCAEC were obtained from Lonza (Walkersville, MD) and grown in EGM-2MV medium. Cells were passaged in a 1:3 dilution and cells from passages 3–4 used for experiments.
Isolation of CD133 Positive Cells from Human Umbilical Cord Blood
Units of human umbilical cord blood were obtained from the St. Louis Cord Blood Bank. 33 ml of blood was placed on 17 ml of Histopaque 1077 (Sigma Diagnostics, Inc., St. Louis, MO) and centrifuged without braking at 450 × g for 30 minutes at room temperature. The opaque layer of cells located at the interface between the plasma and Histopaque layers and containing the mononuclear cells was removed with a Pasteur pipette to a clean tube. Following 4 washes with PBS cells were resuspended in 300 µl of buffer containing PBS, 0.5% FBS and 2mM EDTA. 100 µl of FcR blocking reagent and 100 µl of CD133 MicroBeads were added for every 108 cells and incubated at 4°C for 30 minutes. Cells were then washed, resuspended in 500 µl of running buffer. Cells were separated utilizing the positive selection for rare cells program (PSSELDS) on an AutoMACS (Miltenyi). Cells were pelleted, then used for experiment.
CD133 positive cell migration
An HCAEC monolayer was grown to confluence on a 3.0 µm pore polyester Transwell membrane (Corning Inc, Corning, NY). HCAEC were left unstimulated or were stimulated with 20 ng/ml of tryptase placed in the lower chamber for 10 minutes prior to the addition of CD133+ cells. Freshly isolated CD133 + cells (200,000 cells /well) were placed in the upper chamber of a Transwell membrane and incubated at 37°C for 18 hours. For PTAFR pretreatment experiments, freshly isolated CD133+ cells were left untreated or were treated with 10 µM CV3988 (Sigma, St. Louis, MO) for 10 minutes prior to their addition to the upper chamber.
Detection of mRNA by PCR Analysis
Total RNA was isolated from cells utilizing the PureScript RNA Isolation Kit (Gentra, Minneapolis, MN). cDNA was synthesized using random hexamer priming of RNA and amplified with isoform-specific oligonucleotide primers flanking 100–1200 base pairs of GC-rich cDNA for each gene of interest. Amplicons recovered from each PCR reaction mixture were separated by agarose gel electrophoresis and visualized with ethidium bromide. PCR products were excised from the gel and purified utilizing MinElute Gel Extraction columns (Qiagen, Valencia, CA) or isolated from the PCR reaction mixture and purified utilizing MinElute PCR Purification columns (Qiagen, Valencia CA), subjected to automated sequencing (Applied Biosystems 377 sequencer) and compared to the cDNA sequences published in the GeneBank database.
Flow Cytometry Analysis
CD133 positive cells isolated from umbilical cord blood were incubated with PerCP-Cy5.5 conjugated CD34, PE conjugated CD117, FITC conjugated CD18 and APC conjugated CD29 antibodies respectively for 30 minutes at 4°C. Appropriate fluorophore conjugated mouse IgG1 were used as negative control (All from BD Biosciences). 50,000 cells per sample were used and at least 20,000 events were acquired in each case. Cells were processed using BD FACSCalibur system . Data was analyzed using Flowjo software. (TreeStar Inc. OR). APC conjugated CD133 antibody (Miltenyi) was also used to verify the purity of isolate from autoMACS.
Immunoblot Analysis
CD133+ cells isolated from umbilical cord blood were lysed. The protein was loaded onto a 10% polyacrylamide gel, separated by SDS/PAGE at 200 V for 40 minutes and electrophoretically transferred to PVDF-Plus membranes (Micron Separations Inc., Westborough, MA) at 100 V for 1 hour. After blocking the non specific binding sites the membranes were probed with rabbit anti-PTAFR primary antibody (Cayman Chemical Company, Ann Arbor, Michigan), washed with TBST and incubated with a 1:10,000 goat anti-rabbit HRP conjugated secondary antibody for 1 hour. Regions of antibody binding were detected using enhanced chemiluminescence (SuperSignal, Pierce, Rockford, IL) and exposure to film (Hyperfilm, Amersham).
Preparation of Transwell membranes for SEM viewing
Following incubation to allow for migration, non-adherent CD133+ cells were removed from the upper and lower chambers. The membrane was gently rinsed with 500 µl PBS. CD133+ cells were allowed to transmigrate and samples removed from the upper and lower chambers. The Transwell membrane was fixed in 2.5% glutaraldehyde, dehydrated in 100% ethanol followed by critical point drying. After drying, the samples were mounted on aluminum stubs and sputter coated with gold/palladium.
Quantitation of adherent CD133 positive cells
Transwell membranes were viewed utilizing a Joel 5800 SEM at 400×. Adherent CD133+ cells in twenty random fields on both the apical and basolateral surface were counted. One field at 400× measures 8.724 µm2. Adherent cells were expressed as the percent of total cells counted found on that surface of the membrane.
Tryptase staining of cultured CD133 positive cells
CD133+ cells from human umbilical cord blood were incubated on a lysine coated glass slides in Dulbecco’s minimal essential medium containing IL-6 (50 ng/ml) and stem cell factor (80 ng/ml) for four weeks. Cells were fixed with 3.7% formaldehyde and stained for the presence of tryptase utilizing a DakoCytomation EnVision+ System-HRP (DAB) (DakoCytomation, Inc., Carpinteria, CA). Light microscopy was utilized to observe and image staining.
Statistical Analysis
Data were analyzed using the Student's t-test. p-values of <0.05 were considered statistically significant (shown as * or +); p-values of <0.001 were considered highly statistically significant (shown as ** or ++).
RESULTS
CD133+ cells express mRNA for adhesion molecules and receptors involved in transmigration
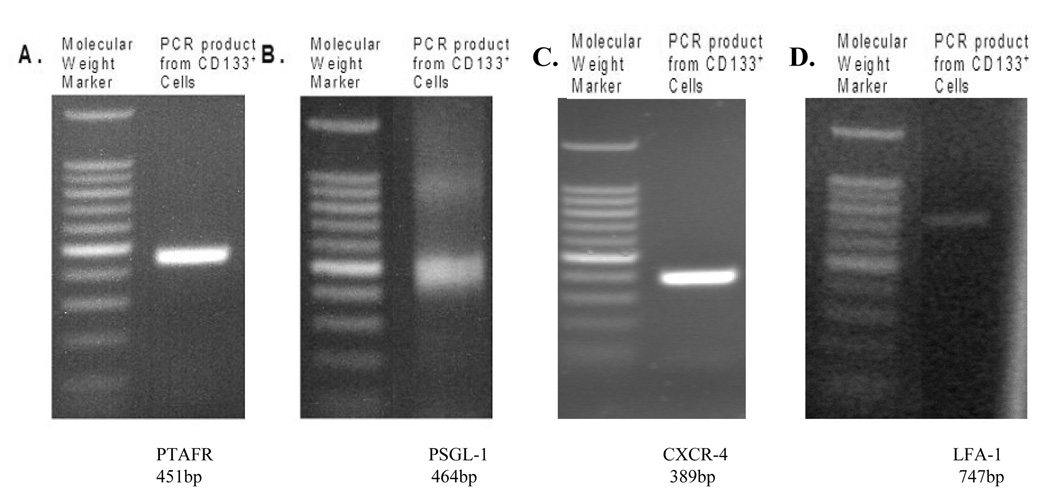
We explored the potential of CD133+ cells to migrate across the endothelium utilizing a mechanism similar to that of neutrophils, involving the interaction of ligands and receptors on the circulating cell with corresponding molecules on the EC surface. We performed RT-PCR analysis to determine whether mRNA for various ligands, receptors and adhesion molecules known to play a role in neutrophil migration were present. We detected mRNA for E-selectin ligand (ESL)-1, β-2 integrin lymphocyte function associated antigen (LFA-1), β-1 integrin very late activating antigen (VLA-4-α and-β), stromal derived factor receptor (CXCR-4), platelet/endothelial cell adhesion molecule (PECAM)-1, and platelet activating factor receptor (PTAFR) in freshly isolated CD133+ cells (Table 1). Following four weeks in culture, mRNA for LFA-1, VLA-4-α and -β, CXCR-4, and PTAFR was still detected. Figure 1 shows representative gels for the presence of PTAFR (451bp) and PSGL-1 (464 bp), CXCR-4 (389 bp) and LFA-1 (747 bp) mRNA in freshly isolated CD133+ cells. PAF is known to be responsible for the up regulation of β1-integrin another important adhesion molecule [13]. The presence of mRNA for various adhesion molecules in fresh and long term cultured CD133+ cells indicates that these cells may be able to utilize the cell surface adhesion molecules that are responsible for neutrophil adhesion and transmigration.
TABLE 1.
Adhesion molecules, their functions and the presence of mRNA in freshly isolated or long term cultured cells.
| Adhesion Molecules | Known Actions of Molecule | mRNA Detected by PCR | |
|---|---|---|---|
| Freshly isolated CD133 positive cells | Cultured CD133 positive cells | ||
| E-selectin Ligand (ESL)-1 | Involved in adhesion of leukocytes to endothelium via the ESL-1 E-selectin interaction | Present | Not detected |
| β-2 integrin lymphocyte function associated antigen (LFA-1) | Ligand of intracellular adhesion molecule (ICAM)-1, involved in adhesion of leukocytes to endothelium | Present | Present |
| β-1 integrin very late activating antigen (VLA-4 α & β) | Ligand of vascular cell adhesion molecule (VCAM), involved in arresting rolling of inflammatory cell | Present | Present |
| Stromal derived factor receptor (CXCR-4) | Aids in migration of cells stimulated by stromal derived factor | Present | Present |
| Platelet/endothelial cell adhesion molecule (PECAM)-1 | Involved in transendothelial migration and endothelial-endothelial cell adhesion | Present | Not detected |
| Platelet activating factor (PAF) receptor | Receptor for PAF, involved in transmigration of cells | Present | Present |
| Mast Cell characteristics of CD133 | Known Actions of Molecule | Freshly isolated CD133 positive cells | Cultured CD133 positive cells |
| CD133 | Membrane glycoprotein found on cord blood precursor cells | Present | Present |
| Fcε | High affinity receptor on surface of cells responsible for IgE-mediated aggregation | Present | Present |
| c-kit | Tyrosine kinase receptor, binds stem cell factor | Present | Present |
| Tryptase | An enzyme, cleaves PAR-2 | Present | Present |
FIGURE 1.
PCR results demonstrating PTAFR (Panel A), PSGL-1 (Panel B), CXCR-4 (Panel C) and LFA-1 (Panel D) mRNA in freshly isolated CD133+ cells. Water and no template controls failed to produce a PCR product (data not shown).
CD133+ cells express cell surface proteins for adhesion molecules and receptors involved in transmigration
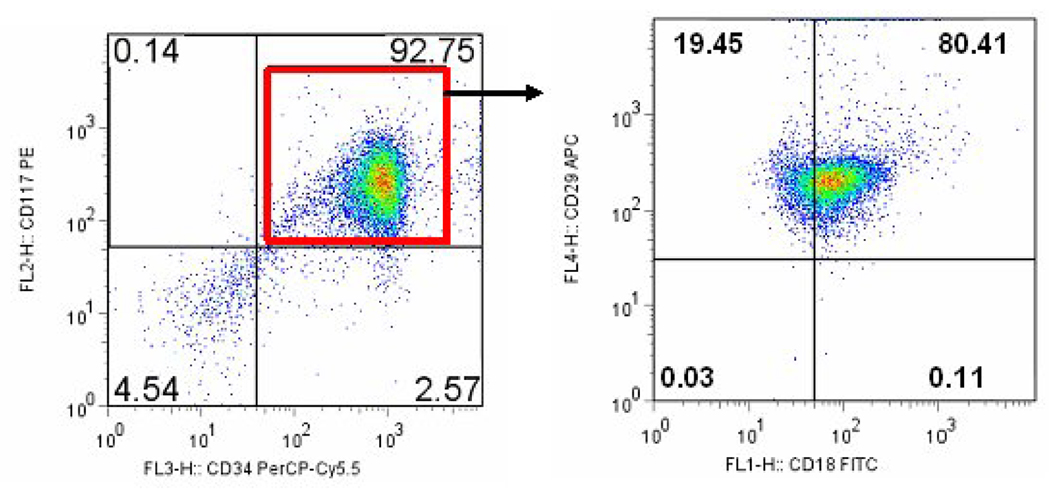
Cells were incubated with PerCP-Cy5.5 conjugated CD34, PE conjugated CD117, FITC conjugated CD18 and APC conjugated CD29 antibodies along with appropriate isotype controls. As shown in Figure 2 (left panel) 92.75% of the cells demonstrated both CD117 and CD34 on their surface. Upon further analysis 80.41% of the cells also stained for CD29 and CD18 (Figure 2, right panel). Thus 74.5% of the cells demonstrated presence of all four surface markers examined. Stem cell factor and its receptor c-kit (CD117) are prerequisites for the homing and subsequent differentiation of mast cells in the tissue. CD18 (integrin β2) is the beta subunit of lymphocyte function associated antigen -1 (LFA-1). It is involved in recruitment and adhesion of neutrophils and macrophages to the site of inflammation. CD29 (integrin β1) in conjunction with alpha subunit of very late antigen (VLA) family of adhesion molecules is involved in cell-cell and cell- extracellular matrix interactions.
FIGURE 2.
Isolated CD133+ cells were analyzed by flow cytometry. Left panel shows a dot plot gated on the CD117+CD34+ cells. 92.75% cells demonstrate both of these markers. The dual positive cells were further gated on FITC coupled CD18+ and APC coupled CD29+ cells (right panel). 80.41% of the dual positive cells from the left panel are also positive for CD18 and CD29 antibodies.
Freshly isolated CD133+ cells express PTAFR protein
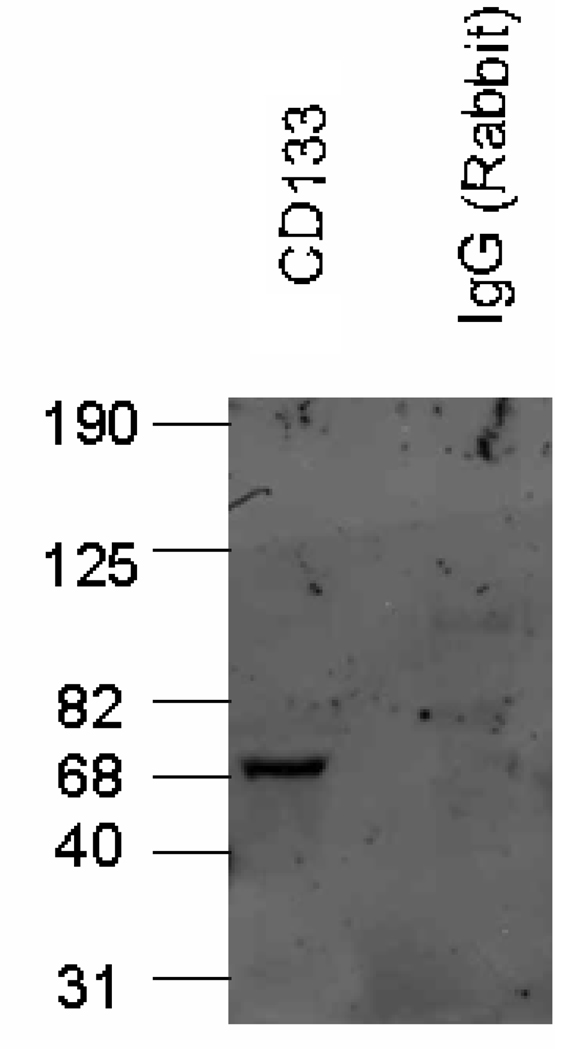
Immunoblot analysis demonstrated that CD133+ cells express the PTAFR protein (Figure 3). CD133+ cells have been shown by several labs [18] to express the P-selectin ligand, and this further supports our hypothesis that the processes of stem cell homing and leukocyte adherence may share common mechanisms. These results suggest CD133+ cells have the potential to adhere to an EC monolayer utilizing the interaction of specific ligands and receptors.
FIGURE 3.
Immunoblot analysis of CD133+ cells shows PAF receptor (PTAFR) protein (69kD). No band was detected when rabbit IgG was used (negative control).
CD133+ cells can migrate across an HCAEC monolayer and involve interaction between PAF and PTAFR
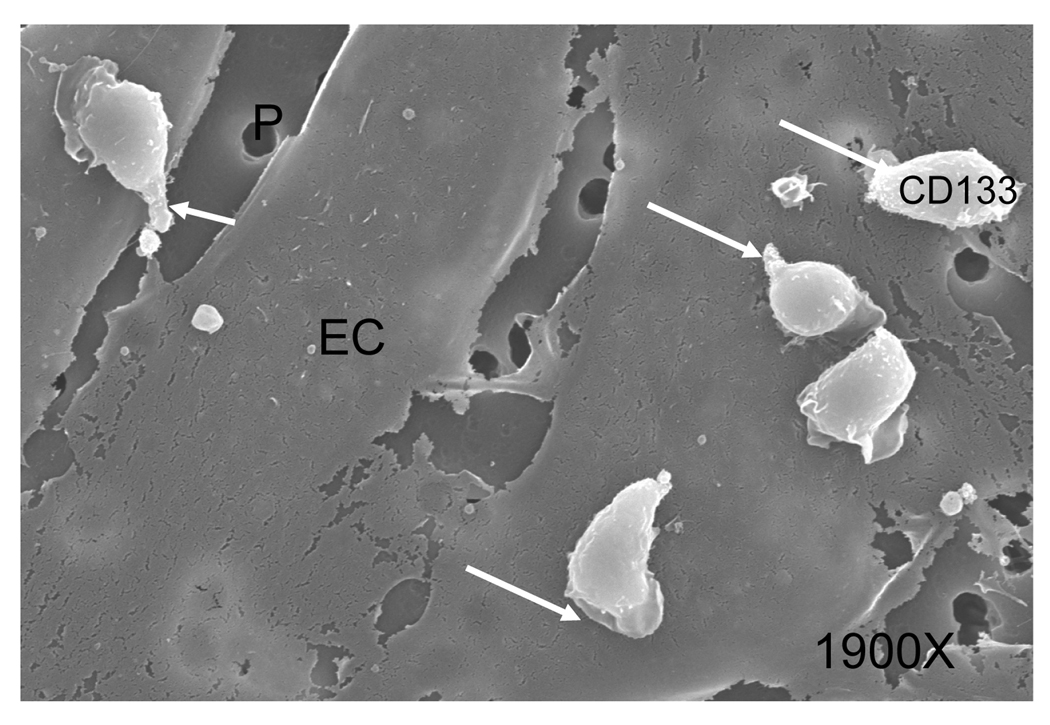
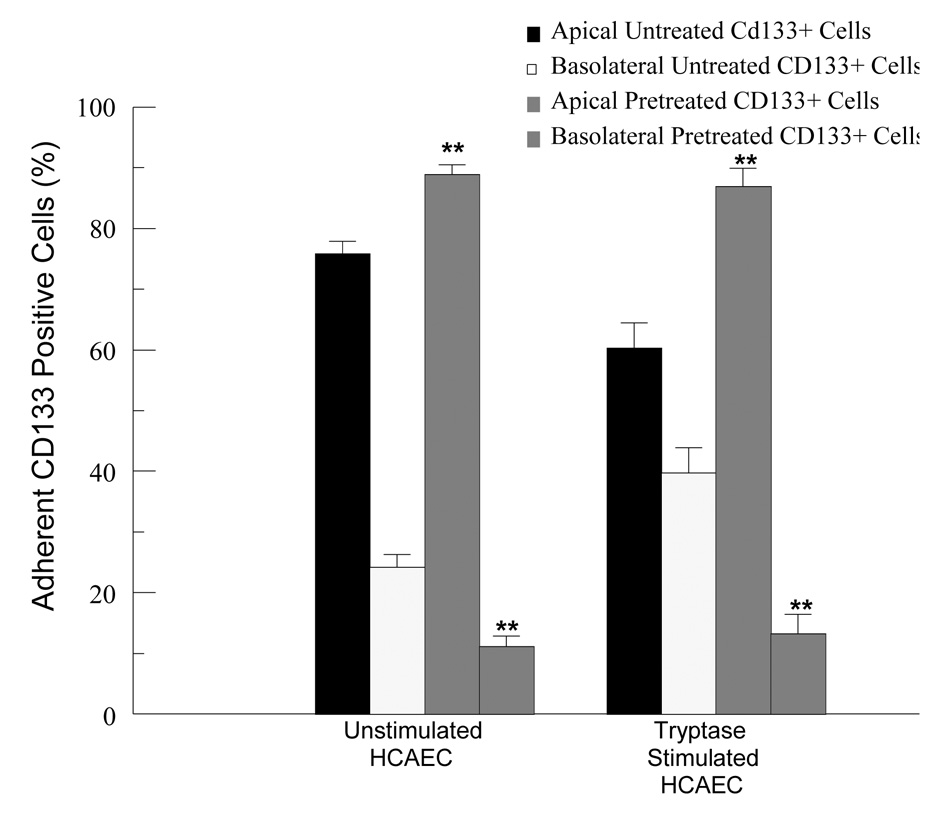
A limited number of CD133+ cells were found in the medium of the lower well, making quantitation of migration by counting difficult. Therefore, we utilized scanning electron microscopy to determine the number of cells adherent to both the apical and basolateral surface of an HCAEC monolayer, speculating that adherence to the apical surface directly precedes migration. Figure 4a displays a scanning electron micrograph of CD133+ cells adherent to the apical surface of HCAEC. CD133+ cells were found to be adherent to both the apical and basolateral surfaces in all treatment conditions. The appearance of the CD133+ cells did not vary among treatment conditions. Kuijpers et al.[19] demonstrated that PTAFR antagonists prevented neutrophil migration across cytokine pretreated EC monolayer by approximately 60 percent. To determine whether PTAFR-PAF interaction plays a role in CD133+ migration, we pretreated these cells with a PTAFR specific antagonist, CV3988, prior to their addition to the apical well of the Transwell system. Tryptase stimulation of HCAEC induces almost a 2-fold increase over unstimulated HCAEC in the percentage of CD133+ cells found on the basolateral surface but pretreatment with CV3988 (10 µM, 10 minutes) caused a 3-fold reduction in the percentage of CD133+ cells adherent to the basolateral surface. These results suggest that CD133+ cell transendothelial migration is dependent, at least in part, on the PAF-PTAFR interaction. (Figure 4b).
FIGURE 4.
FIGURE 4a. Scanning electron microscope images of adherent CD133+ cells transmigrating across HCAEC on transwell membranes. Arrows indicate pseudopodia and membrane ruffling (white arrows) present on adherent CD133+ cells.(Mag.1900×.). EC-endothelial cells, P-pores in the transwell membrane.
FIGURE 4b. Effect of pretreatment of CD133+ cells with a PTAFR antagonist (CV3988, 10 µM, 10 minutes) on their adherence to unstimulated or tryptase stimulated (20 ng/ml, 10 minutes) HCAEC. Results represent mean ± SEM for at least 4 separate cell cultures. **p<0.01 when comparing corresponding data sets with or without pretreatment.
Cultured CD133+ cells have granules and produce tryptase
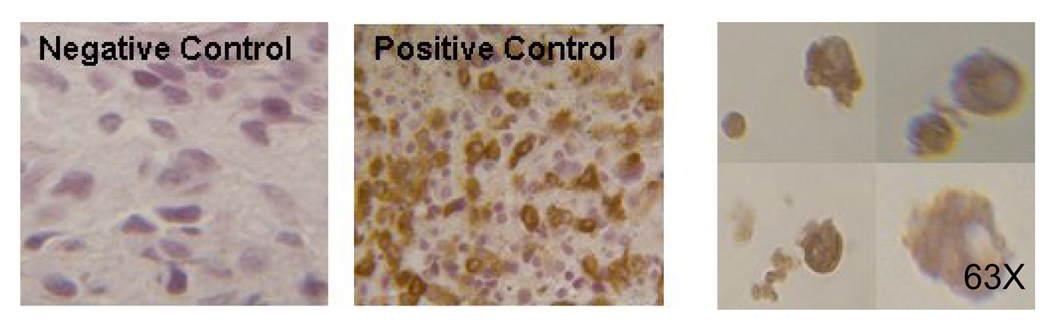
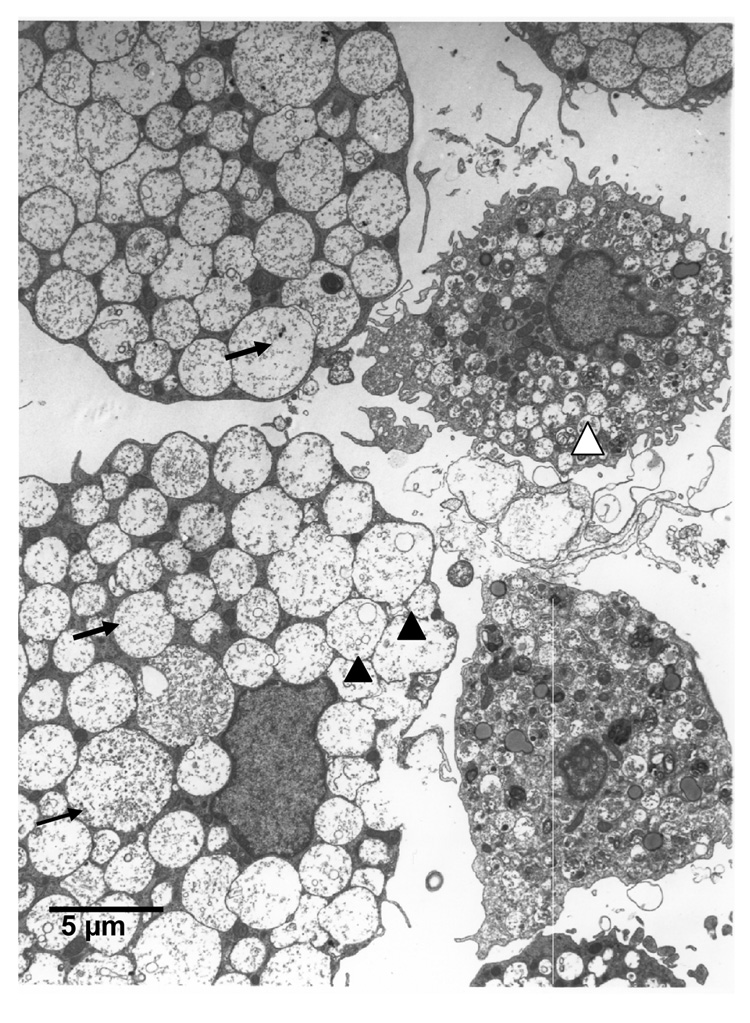
Dense granules contain various enzymes, including tryptase, which are a characteristic feature of mast cells. As seen in Figure 5a, following seven weeks of culture, CD133+ cells stain positively for tryptase, demonstrating their ability to produce tryptase. Additionally by electron microscopy (Figure 5b) we were able to observe cells having round or indented nuclei, finely condensed nuclear chromatin around the nuclear membrane and thin cytoplasmic projections. The cytoplasm was filled with many vesicles and pleomorphic granules closely resembling those seen in mast cells in vivo [20]. The granules were particulated, vesicular, coiled lamellar or scroll like. Some granules also had additional myelin figures.
FIGURE 5.
5a. Tryptase staining in cultured CD133+ cells. Tryptase positive cells appear brown.(63×). A section of mastocytosis tumor was used as positive control.
5b. Transmission electron microscope images of CD133+, CD34+ cells in culture for 10 weeks. Cells demonstrate many pleomorphic granules in the cytoplasm, vesicular granules (black arrowhead), particulate granules (black arrow), and granules with scroll like figures (white arrowhead). (Mag. Bar- 5µm).
DISCUSSION
Mast cells have been detected in all stages of atherosclerotic plaque development, showing both increases in numbers and focal accumulations [7]. The presence of mast cells in the intima of early atherosclerotic lesions strongly supports a role for these cells in early plaque development. Mast cell accumulation is seen in the shoulder regions of the atheroma in the later stages of plaque development, correlating with increased foam cell accumulation and activation of matrix metalloproteinases in these areas [6].
Mast cells undergo the final stages of maturation following the migration of the precursor cell into the tissue space in which it will reside [5]. We utilized CD133+ cells isolated from human umbilical cord blood to generate mast cells. CD133+ cells can also be isolated from adult peripheral blood [14, 15] and have been shown to be capable of differentiation into various cells [15], including mast cells [16], when cultured in the presence of specific factors. It has been shown that CD133+ cells are mobilized into peripheral blood in cases of heart failure [21] and vascular trauma [22] making these cells appropriate candidates to utilize as mast cell precursors.
Tryptase, an enzyme unique to human mast cell granules [23, 24], activates endothelial cell protease-activated receptor (PAR)-2 expressed on the endothelial cell surface [25]. Previous data from our laboratory has shown that tryptase stimulation of human coronary artery endothelial cells (HCAEC) leads to an increase in calcium-independent phospholipase A2 (iPLA2) activity, and accumulation of biologically active membrane phospholipid-derived metabolites, such as platelet activating factor (PAF) and arachidonic acid, that play a role in propagating the inflammatory response [26].
PAF promotes the aggregation, chemotaxis, granule secretion and oxygen radical generation from leukocytes and the adherence of leukocytes to the endothelium [27]. The enhanced expression of endothelial cell-associated PAF has been shown to cause transient adherence of neutrophils to the endothelium [28–30]. Additionally, PAF has been shown to activate neutrophils that are tethered by P-selectin to the endothelial cell surface [31]. Prescott et al. [31] have correlated the adhesion of neutrophils to thrombin-activated endothelium with PAF synthesis and expression on the EC surface.
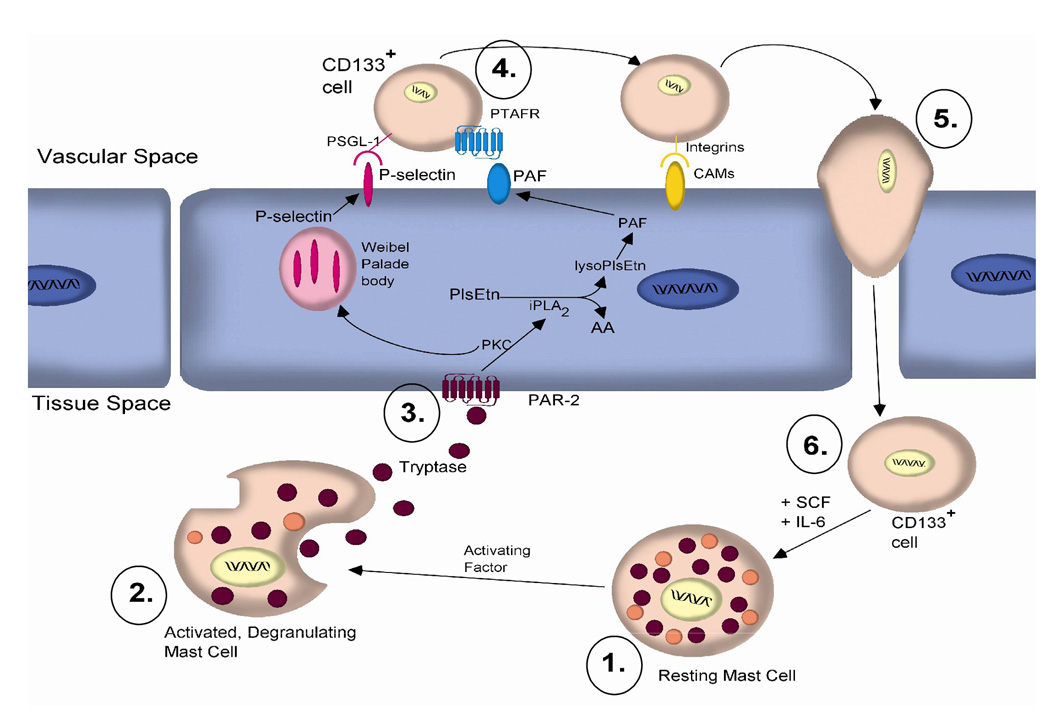
Based on our results we propose the following mechanism for the recruitment of mast cell precursors across HCAEC monolayer. Resident mast cells (➀ in Figure 6) underlying the endothelium are activated by various factors such as IgE, bacteria or tryptase [32]. The activated mast cell releases its granular contents (➁ in Figure 6), which include the protease tryptase (6). Tryptase then cleaves its receptor, PAR-2, on the surface of the HCAEC (➂ in Figure 6. Cleavage of PAR-2 initiates an intracellular signaling cascade leading to the activation of iPLA2. iPLA2 hydrolyzes membrane plasmalogen phospholipids at the sn-2 position leading to downstream production of inflammatory mediators such as PAF, which is expressed at the cell surface [33]. Experiments in our laboratory have described a role for PKC in the activation of iPLA2 [26]. Interestingly, PKC is responsible for the translocation of Weibel-Palade bodies to the cell membrane and the subsequent presentation of P-selectin at the cell surface [34]. Circulating CD133+ cells expressing the P-selectin ligand (PSGL)-1 tentatively bind to the HCAEC during “rolling.” The tethered cell is then further activated by the interaction of its PAF receptor (PTAFR) with PAF present on the cell surface (➃ in Figure 6). The attached CD133 + cell then cross the endothelial cell membrane during diapedesis (➄ in Figure 6). After transmigration a subset of these cells, (➅ in Figure 6), in the presence of growth factors such as stem cell factor (SCF) and IL-6, can potentially mature into mast cells (➀ in Figure 6) capable of tryptase production. Tryptase has also been shown to activate mast cells, demonstrating a possible amplification cascade [32]. By this mechanism, recruitment of CD133+ mast cell precursors and their subsequent maturation would result in the activation of the HCAEC (➂ in Figure 6) and the continued recruitment of CD133+ cells (➃ in Figure 6), accounting for both the propagation of the inflammatory response [35] and increase in mast cell numbers in the atherosclerotic plaque [6].
FIGURE 6.
Proposed mechanism of CD133+ mast cell precursor migration. (1.) Activation of mast cells. (2.) Release of tryptase from their granules. (3.) Tryptase cleaves its receptor, PAR-2, on the surface of the HCAEC and activates iPLA2, leading to production of PAF. Experiments in our laboratory have described a role for PKC in the activation of iPLA2. (4.) Circulating CD133+ cells are tethered to HCAEC by P-selectin ligand (PSGL)-1 and the interaction of its PAF receptor (PTAFR) with PAF present on the cell surface. (5.) The attached CD133+ cell then crosses the endothelium during diapedesis. (6.) In the presence of stem cell factor (SCF) and IL-6, it has the potential to mature into a mast cell (1.) capable of tryptase production. By this series of events, we propose that propagation of the inflammatory response may, in part, contribute to the progression of atherosclerosis.
The results presented here demonstrate the transendothelial migration of CD133+ cell is facilitated by the presence of tryptase (Figure 4b). Pretreatment of CD133+ cells with a PTAFR specific antagonist prior to transmigration significantly reduces the percentage of CD133+ cells adherent to the basolateral surface (Figure 4b). These results suggest that CD133+ cell migration also depends on the PAF/PTAFR interaction. Once transmigrated, a subset of CD133 + cells may have the potential to mature into mast cells becoming capable of tryptase production and upon stimulation may release tryptase, contributing to the potentiation of the inflammatory response seen in atherosclerosis.
TABLE 2.
Primers used to amplify various mRNA of interest
| Primer for: | Sense Primer | Anti-sense Primer |
|---|---|---|
| PSGL-1 | 5’-CCTGCCAGAAACGGAGCCTCC-3’ | 5’-AAGCACAGACCACTCCACCAGCAG-3’ |
| ESL-1 | 5’-GACCTGATGGAGTGTCTGATACA-3’ | 5’-GGGTGTCCCTGAAGTGCCGCA-3’ |
| LFA-1 | 5’-CAGAGCTGCTGCTGATTGGTGCCC-3’ | 5’-GGGAGATGAGGTCTTGAACACATACCGGG-3’ |
| VLA-4α | 5’-GTCGGAGCTGGTCATTTTCGGAGCC-3’ | 5-’CAATGGCCGTGCAGATGGGATCTCG-3’ |
| VLA-4β | 5-’CAAGAGAGCTGAAGACTATCCCATTGACCTC-3’ | 5’-CTTTGCTGGAGATGGGAAACTTGGTGGCA-3’ |
| CXCR-4 | 5’-CGTCCACGCCACCAACAGTCAG-3’ | 5’-CAGCATCGACTCCTTCATCCTCCTGG-3’ |
| PECAM-1 | 5’-GCAGACCTCAGAATCTACCAAGAGTGAAC-3’ | 5’-GCATCATAAGAAATCCTGGGCTGGGAGAGC-3’ |
| CD133 | 5’-CTCTATGTGGTACAGCCGCGTG-3’ | 5’-AGACCTAAGATTACAGTTTCTGG-3’ |
| Fcε | 5’-CGCAGAAATCCCAGTCCACGCAGATTTC-3’ | 5’-GTCCAAGTTCCGAAGGCCAATCCAG-3’ |
| c-kit | 5’-CTGGGTGCTGGAGCTTTCGGGAAGG-3’ | 5’-CATGGAGGATGACGAGTTGGCCCTA-3’ |
| Tryptase | 5-’GCAAAATACCACCTTGGCGCCTACACGG-3’ | 5’-GTGACACGGGTGTAGATGCCAGGC-3’ |
ACKNOWLEDGEMENT
This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-68588 (JM) and the American Heart Association-Heartland Affiliate (PR, MCW). The authors would like to thank Dr. Jan Ryerse for the preparation of samples for viewing by electron microscopy and Dr. Michael H. Creer and the St. Louis Cord Blood Bank for the units of umbilical cord blood.
Abbreviations
- HCAEC
Human coronary artery endothelial cells
- PAF
Platelet activating factor
- EC
Endothelial cells
- PSGL
P-selectin glycoprotein ligand
- ICAM
Intracellular adhesion molecule
- VCAM
Vascular cell adhesion molecule
- ESL
E-selectin ligand
- LFA
Lymphocyte function associated antigen
- VLA
Very late activating antigen
- CXCR-4
Stromal derived factor receptor
- PTAFR
Platelet activating factor receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989;135:169–175. [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P, Hansson GK. Involvement of the immune system in human atherogenesis: current knowledge and unanswered questions. Lab Invest. 1991;64:5–15. [PubMed] [Google Scholar]
- 3.Wick G, Schett G, Amberger A, Kleindienst R, Xu Q. Is atherosclerosis an immunologically mediated disease? Immunol Today. 1995;16:27–33. doi: 10.1016/0167-5699(95)80067-0. [DOI] [PubMed] [Google Scholar]
- 4.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 5.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nature Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 6.Jeziorska M, McCollum C, Woolley DE. Mast cell distribution, activation, and phenotype in atherosclerotic lesions of human carotid arteries. J Pathol. 1997;182:115–122. doi: 10.1002/(SICI)1096-9896(199705)182:1<115::AID-PATH806>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Kaartinen M, Penttila A, Kovanen PT. Mast cells of two types differing in neutral protease composition in the human aortic intima. Demonstration of tryptase- and tryptase/chymase-containing mast cells in normal intimas, fatty streaks, and the shoulder region of atheromas. Arterioscler Thromb. 1994;14:966–974. doi: 10.1161/01.atv.14.6.966. [DOI] [PubMed] [Google Scholar]
- 8.Laine P, Kaartinen M, Penttilä A, Panula P, Paavonen T, Kovanen P. Association between myocardial infarction and the mast cells in the adventitia of the infarct-related coronary artery. Circulation. 1999;99:361–369. doi: 10.1161/01.cir.99.3.361. [DOI] [PubMed] [Google Scholar]
- 9.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 10.Burns AR, Bowden RA, Abe Y, Walker DC, Simon SI, Entman ML, Smith CW. P-selectin mediates neutrophil adhesion to endothelial cell borders. J Leukoc Biol. 1999;65:299–306. doi: 10.1002/jlb.65.3.299. [DOI] [PubMed] [Google Scholar]
- 11.Prescott SM, McIntyre TM, Zimmerman GA, Stafforini DM. Sol Sherry lecture in thrombosis: molecular events in acute inflammation. Arterioscer Thromb Vasc Biol. 2002;22:727–233. doi: 10.1161/01.atv.0000016153.47693.b2. [DOI] [PubMed] [Google Scholar]
- 12.Kilgore KS, Ward PA, Warren JS. Neutrophil adhesion to human endothelial cells is induced by the membrane attack complex: the roles of P-selectin and platelet activating factor. Inflammation. 1998;22:583–598. doi: 10.1023/a:1022362413939. [DOI] [PubMed] [Google Scholar]
- 13.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 14.Piechaczek C, Widera D, Micheli D, Schwamborn E, Voelkel T, Schmitz J. Differentiation of adult CD133+ cells isolated from peripheral blood into cells with a neural phenotype. MACS & more. 2003;7:9–10. [Google Scholar]
- 15.Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 16.Dahl C, Hoffmann HJ, Saito H, Schiotz PO. Human mast cells express receptors for IL-3, IL-5 and GM-CSF; a partial map of receptors on human mast cells cultured in vitro. Allergy. 2004;59:1087–1096. doi: 10.1111/j.1398-9995.2004.00606.x. [DOI] [PubMed] [Google Scholar]
- 17.Stein B, Khew-Goodall Y, Gamble J, Vadas MA. The Endothelium in clinical practice: source and target of novel therapies. New York, NY: Marcel Dekker; 1977. Transmigration of leukocytes. [Google Scholar]
- 18.Levesque J-P, Zannettino ACW, Pudney M, Niutta S, Haylock DN, Snapp KR, Kansas GS, Berndt MC, Simmons PJ. PSGL-1-mediated adhesion of human hematopoietic progenitors to P-selectin results in suppression of hematopoiesis. Immunity. 1999;11:369–378. doi: 10.1016/s1074-7613(00)80112-0. [DOI] [PubMed] [Google Scholar]
- 19.Kuijpers TW, Hakkert BC, Hart MHL, Roos D. Neutrophil migration across monolayers of cytokine-prestimulated endothelial cells: a role for platelet-activating factor and IL-8. J Cell Biol. 1992;117:565–572. doi: 10.1083/jcb.117.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eguchi M, Nakahata T, Tsuji K, Furukawa T. Morphological and cytochemical changes in human mast cells during culture. Med Electron Microsc. 1997;30:25–30. [Google Scholar]
- 21.Valgimigli M, Rigolin GM, Fucili A, Porta MD, Soukhomovskaia O, Malagutti P, Bugli AM, Bragotti LZ, Francolini G, Mauro E, Castoldi G, Ferrari R. CD34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation. 2002;110:1209–1212. doi: 10.1161/01.CIR.0000136813.89036.21. [DOI] [PubMed] [Google Scholar]
- 22.Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, Girardi L, Yurt R, Himel H, Rafii S. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001;88:167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 23.Church MK, Caulfield JP. Mast cell and basophil functions. Allergy, London, England: Gower Medical Publishing; 1993. [Google Scholar]
- 24.Galli SJ. New insights into “the riddle of the mast cells”: microenvironmental regulation of mast cell development and phenotypic heterogeneity. Lab Invest. 1990;62:5–33. [PubMed] [Google Scholar]
- 25.Molino M, Barnathan ES, Numerof R, Clark J, Dreyer M, Cumashi A, Hoxie J, Schechter N, Woolkalis MJ, Brass LF. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- 26.Meyer MC, Kell PJ, Creer MH, McHowat J. Calcium-independent phospholipase A2 is regulated by a novel protein kinase C in human coronary artery endothelial cells. Am J Physiol. 2005;288:C475–C482. doi: 10.1152/ajpcell.00306.2004. [DOI] [PubMed] [Google Scholar]
- 27.Montrucchio G, Alloatti G, Camussi G. Role of platelet-activating factor in cardiovascular pathophysiology. Physiol Rev. 2000;80:1669–1699. doi: 10.1152/physrev.2000.80.4.1669. [DOI] [PubMed] [Google Scholar]
- 28.Gamble JR, Skinner MP, Berndt MC, Vadas MA. Prevention of activated neutrophil adhesion to endothelium by soluble adhesion protein GMP140. Science. 1990;249:414–417. doi: 10.1126/science.1696029. [DOI] [PubMed] [Google Scholar]
- 29.Lorant DE, Patel KD, McIntyre TM, McEver RP, Prescott SM, Zimmerman GA. Coexpression of GMP-140 and PAF by endothelium stimulated by histamine or thrombin: a juxtacrine system for adhesion and activation of neutrophils. J Cell Biol. 1991;115:223–234. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White MC, McHowat J. Protease activation of calcium-independent phospholipase A2 leads to neutrophil recruitment to coronary artery endothelial cells. Throm. Res. 2007;120:597–605. doi: 10.1016/j.thromres.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prescott SM, Zimmerman GA, McIntyre TM. Human endothelial cells in culture produce platelet-activating factor (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) when stimulated with thrombin. Proc Natl Acad Sci USA. 1984;81:3534–3538. doi: 10.1073/pnas.81.11.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson JL, Jackson CL, Angelini GD, George SJ. Activation of matrix-degrading metalloproteinases by mast cell proteases in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 1998;18:1707–1715. doi: 10.1161/01.atv.18.11.1707. [DOI] [PubMed] [Google Scholar]
- 33.Meyer MC, Creer MH, McHowat J. Potential role for mast cell tryptase in recruitment of inflammatory cells to endothelium. Am J Physiol. 2005;289:C1485–C1491. doi: 10.1152/ajpcell.00215.2005. [DOI] [PubMed] [Google Scholar]
- 34.McEver RP, Beckstead JH, Moore KL, Marshall-Carison L, Bainton DF. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989;84:92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross R. Atherosclerosis-an inflammatory disease. New Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]