Abstract
For many years it has been known that viral capsid proteins are capable of self-assembly, but increasing evidence over the past decade indicates that in cells HIV-1 capsid assembly occurs via a complex but transient series of steps requiring multiple viral-host interactions. To better understand the biochemistry of HIV assembly, our group established a cell-free system that faithfully reconstitutes HIV-1 Gag synthesis and post-translational events of capsid assembly using cellular extracts, albeit more slowly and less efficiently. This system allowed initial identification of interactions that occur very transiently in cells but can be tracked in the cell-free system. Analysis of the cell-free system revealed that Gag progresses sequentially through a step-wise, energy-dependent series of assembly intermediates containing cellular proteins. One of these cellular proteins, the ATPase ABCE1, has been shown to play a critical role in the assembly process. The existence of this energy-dependent assembly pathway was subsequently confirmed in cellular systems, further validating the cell-free HIV-1 capsid assembly system as an excellent tool for identifying mechanisms underlying HIV-1 capsid formation. Here we describe how to assemble immature HIV-1 capsids in a cell-free system and separate assembly intermediates by velocity sedimentation.
Keywords: capsid, assembly, cell-free system, wheat germ extract, in vitro translation, myristoylation, Gag, ABCE1, velocity sedimentation
1. Introduction
Immature HIV-1 capsids are protein shells that encapsidate the viral genome. These structures subsequently become enveloped by the host cell plasma membrane resulting in virus budding and release. Each immature HIV-1 capsid contains ∼ 5000 copies of the HIV-1 Gag polyprotein (1) that assemble into an irregular spherical capsid with a diameter of ∼100 nm. The events of assembly follow translation of the 55 kDa Gag polypeptide in the cytoplasm of the host cell. Gag undergoes myristoylation, in which a 14-carbon fatty acid that is required for membrane targeting (2-4) is added to the N-terminus of Gag. During capsid formation, Gag polypeptides target to the cytoplasmic aspect of the host plasma membrane, where they form the immature capsid. In addition, the viral genome becomes encapsidated during the assembly process. Formation of the immature capsid is followed by capsid maturation, budding, and release of enveloped capsids from the cell (reviewed in (5)).
A decade ago, it was assumed that assembly occurs without the involvement of cellular factors, since purified recombinant Gag polypeptides, at high concentrations, were found to self assemble into spherical particles, albeit ones that are much smaller than immature capsids produced in cells (6). Studies using the cell-free HIV-1 capsid assembly system have altered this view by revealing that in the context of cellular proteins, Gag assembly occurs through a stepwise, ordered, assembly pathway, utilizing energy as well as at least one cellular factor during post-translational events. Thus, the cell-free capsid assembly system has proven to be a powerful system for studying mechanisms of viral-host interactions during assembly, and has also contributed to an important paradigm shift in current thinking about HIV-1 capsid assembly.
The immature HIV-1 capsids produced in the cell-free system have been shown to closely resemble immature HIV-1 capsids produced in cells, by biochemical as well as electron microscopic criteria. In addition, key features of immature HIV-1 capsid formation that were initially described in cellular systems, such as the requirement for myristoylation and membranes, are faithfully reproduced in the cell-free HIV-1 capsid assembly system (7). During assembly in the cell-free system and in cells, Gag progresses through a series of assembly intermediates that can be distinguished by their sedimentation value (10S, 80S/150S, 500S; see Fig. 13.1), culminating in the formation of ∼ 750S completed immature HIV-1 capsids (7). The finding of an energy requirement during post-translational events of capsid assembly (7) led to identification of a cellular ATPase (ABCE1) that appears to be critical for proper formation of immature HIV-1 capsids in the cell-free system and in cells (8). ABCE1 is associated with the 80S/150S and 500S assembly intermediates but is not associated with the 10S form of Gag or with the completed immature capsid product (8), which has a sedimentation value of 750S (7). To date, the exact role of ABCE1 remains unclear, but it appears to chaperone events in capsid assembly, allowing efficient virus formation even when Gag is present at low concentrations in the hostile environment of the host cytoplasm.
Fig. 13.1. The HIV-1 assembly pathway.
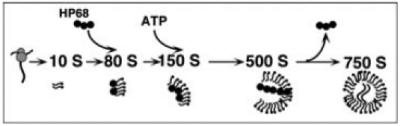
Studies of assembly, initially in a cell-free system and subsequently in cells, has revealed that newly synthesized HIV-1 Gag progresses through a series of post-translational assembly intermediates of increasing size (10S, 80S, 150S, and 500S intermediates) before producing the completely assembled 750S immature capsid. This pathway requires ATP at a discrete step as indicated and also requires the cellular ATPase ABCE1, which associates with selected assembly intermediates.
All the features of the HIV-1 capsid assembly pathway were initially identified using the cell-free HIV-1 translation and assembly reactions described in this chapter. However, each of the key findings from the cell-free system has subsequently been verified in infected and transfected primate cells producing the HIV-1 provirus (8--10). Moreover, studies suggest that all primate lentiviral Gag polypeptides assemble into immature capsids using a mechanism that closely resembles the mechanism underlying HIV-1 capsid assembly (9, 11).
The methods below describe production of immature HIV-1 capsids using the HIV-1 cell-free translation and assembly reaction. The products of this reaction can then be analyzed by velocity sedimentation (7, 8, 12) as described below, which separates protein complexes based on their size. The cell-free reaction products can also be analyzed by immunoprecipitation (7, 8, 13) and transmission electron microscopy (8). Notable variations of this method involve programming the cell-free translation and assembly reactions with HIV-1 Gag mutants (7, 12) or other primate lentiviral Gags (9,13). In addition, similar cell-free assembly reactions have been established for studying hepatitis B virus (14), hepatitis C virus (15, 16), and Venezuelan equine encephalitis virus (unpublished observations B. K. Thielen, K. C. Klein, and J. R. Lingappa).
2. Materials
2.1. In Vitro Transcription
5X Compensating Buffer for Transcription (CB5X): 200 mM TrisAcetate, pH 7.5 (pH with Acetic Acid; see Note 1), 30 mM MgAcetate, 10 mM spermidine.
Nucleotide triphosphate (4NTP) mix: 5 mM ATP, 5 mM CTP, 5 mM UTP, 1 mM GTP. Adust pH to ∼ 7.4 using 2 M Tris base. The NTPs are incorporated into the mRNA transcript (see Note 2).
10X (5 mM) Diguanosine triphosphate (GE Healthcare Bio-Sciences Corp, Piscataway, NJ). This modified nucleotide serves as the first nucleotide of the in vitro transcript and mimics the capped guanosine nucleotide that is produced by the 5′ capping reaction in vivo. To make 5 mM diguanosine triphosphate, dissolve 25 A250 units (1.2 mg) in 300 μL of 20 mM Tris Acetate pH 8.0.
10X Dithiothreitol (DTT; Roche, Basel, Switzerland), 0.1 M.
50X tRNA: Type XI bovine liver tRNA (Sigma, St. Louis, MO), 10 mg/mL.
25X RNase inhibitor (Promega, Madison, WI), 40 units/μL (see Note 3).
25X SP6 Polymerase, 10 units/μL (see Note 4).
Linearized DNA template. In the case of HIV Gag plasmid, this should be used at final concentration of 0.8-1.0 mg/mL. The DNA template for the in vitro transcription reaction is the SP6 HIV-1 Gag plasmid, which contains the SP6 promoter, followed by the 5′ untranslated region for xenopus oocytes and the coding region for HIV-1 Gag (from the SF 2 strain of HIV-1) (see Note 5).
2.2. Wheat Germ Translation
Wheat germ extract (WGE), made by the method of Erickson et al. (17) but without cyclohexane flotation (see Note 6). WGE made by this method should be subjected to centrifugation in a TLA 100 rotor (Beckman-Coulter, Fullerton, CA) at 50,000 rpm (96, 000 × g) for 15 min, and the supernatent removed and flash frozen in liquid nitrogen (see Note 7). Typically, WGE should constitute 20% of the final reaction volume (i.e., wheat germ stock is 5X), although this could vary with specific batch of WGE.
10X Compensating Buffer for Translation (10X CB) contains salts needed for the final reaction (adjusted for salts introduced by WGE used at a 20% volume in the final reaction): 40 mM Hepes, pH 7.6 (from a stock of 1.0 M Hepes, pH 7.6), 1.2 M potassium acetate (from a 4-M Potassium acetate stock), 2 mM EDTA.
Energy and Amino Acid Mix (5X Emix): 5 mM ATP, 5 mM GTP, 60 mM creatine phosphate, and 0.2 mM each of all amino acids except methionine, and 1 mCi of 35Smet/cys Translabel (MP Biomedicals, Solon, OH) (see Note 8).
Myristoyl Coenzyme A lithium salt (Sigma), made as a 125 μM stock in 20 mM Tris Acetate, pH 7.4 (see Note 9).
Creatine kinase (Roche), made as a 4 mg/mL stock in 20 mM Tris Acetate, pH 7.4 with 50% glycerol and stored at -20 °C. Creatine kinase allows for rapid regeneration of ATP by transferring a phosphate to ADP from creatine phosphate.
RNase inhibitor (Promega), 40 units/μL.
Type XI bovine liver tRNA (Sigma), 10 mg/mL.
In vitro transcription reaction, 20% of final in vitro translation reaction volume.
All stocks for transcription and translation except enzymes are best stored as aliquots at -80 °C. Thaw at room temperature, keep on ice for short times, and flash freeze in liquid nitrogen for storage as soon as possible. Most reagents can tolerate many flash freezes, but it is best to aliquot WGE so that each aliquot is thawed fewer than ∼ 20 times. The enzymes (SP6 polymerase, RNase inhibitor, and creatine kinase) should be stored at -20 °C in 50% glycerol.
2.3. Velocity Sedimentation Gradients
10X NP40 Buffer: 500 mM KAc, 0.1 M Tris Acetate 3.0% NP-40 (Roche), 1 M NaCl, 40 mM MgAc.
Sucrose solutions: 10%, 15%, 20%, 40%, 50%, 66%, and 80% wt/vol solutions in 1XNP40 buffer (can be stored at -20 °C).
Optima TL centrifuge and MLS 50 rotor (Beckman-Coulter)
5 mL polyallomer gradient tubes (Beckman-Coulter)
3. Methods
The cell-free HIV-1 capsid assembly system involves two linked reactions: in vitro transcription and cell-free translation. A plasmid encoding the SP6 polymerase and the Xenopus globin 5′ untranslated region (18) upstream from the HIV-1 Gag coding region constitutes the template used to program in vitro synthesis of the HIV Gag mRNA transcript (7). Components that are added to the in vitro transcription reaction include the ribonucleotide triphosphates (rNTPs) that form the mRNA transcript, as well as the modified rGTP diguanosine nucleotide triphosphate that forms the 5′ mRNA cap. Additionally, the reaction includes an RNase inhibitor, the SP6 polymerase enzyme, and bovine tRNA, which also acts to inhibit RNases. The buffers in this in vitro transcription reaction are adjusted to be compatible with the cell-free translation/assembly reaction, thereby eliminating the need to purify the mRNA transcript. Thus, the in vitro transcription reaction can be added directly to the cell-free translation/assembly reaction at a final volume of 20%. WGE is used in the cell-free translation/assembly reaction as a source of cellular factors, while amino acids and energy substrates (ATP and GTP) are all added exogenously (Fig. 13.2), since they are removed during a desalting step that occurs during preparation of WGE. Cellular factors present in the WGE include initiation and elongation factors required for translation (17), membrane vesicles required for Gag targeting (7,11), as well as cellular proteins required for assembly such as ABCE1 (8-10). In addition, the cellular extract contains membrane vesicles derived from various organelles during preparation of the WGE (see Note 10).
Fig. 13.2. Components of the cell-free HIV-1 capsid assembly system and events reconstituted by this system.
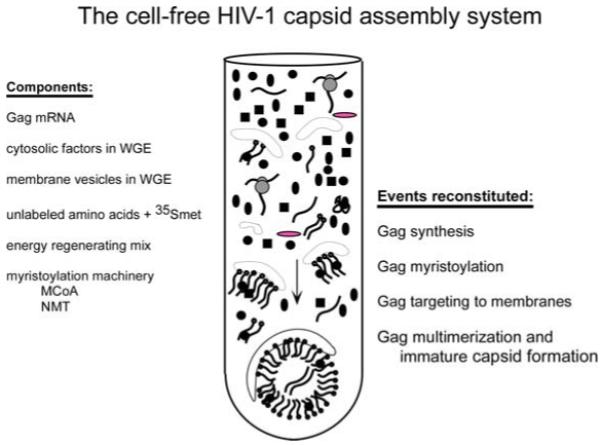
The system is programmed using an in vitro mRNA transcript that codes for HIV-1 Gag. Wheat germ extract (WGE) provides cytosolic factors that are critical for translation and assembly, as well as membrane vesicles important for Gag targeting during assembly. Unlabeled amino acids, a radiolabeled amino acid (mainly methionine), and energy substrates (ATP and GTP) are added exogenously since they are lost from wheat germ extract during buffer exchange. Creatine phosphate and creatine kinase are added to the system to allow regeneration of ATP and GTP. The enzyme N myristoyl transferase, which myristoylates Gag, is present in wheat germ extract, but myristoyl coA must be added since it is lost during preparation of wheat germ extract. The cell-free system reconstitutes translation of Gag, co-translational myristoylation of Gag, membrane targeting of Gag, and multimerization of Gag in a manner that closely resembles these events in infected cells.
While this cell-free system faithfully reconstitutes the events of Gag multimerization that occur in infected cells, it should be noted that there are a number of limitations to this system. First, the final product of the cell-free translation/assembly reaction is radiolabeled HIV-1 Gag (also called p55), while in infected cells, both Gag and GagPol are produced. Notably, the GagPol polyprotein encodes the HIV-1 protease which cleaves Gag and Pol during maturation, so in the absence of GagPol, Gag remains unprocessed and spherical immature HIV-1 capsids (rather than cone-shaped mature capsids) are produced. Additionally, in infected cells, many other viral proteins are produced, while in the cell-free system Gag is the only viral protein present. Moreover, only 20-40% of newly-synthesized Gag polypeptides assemble into completed immature HIV-1 capsids in the cell-free system. The remainder of newly synthesized radiolabeled Gag polypeptides are arrested in the form of various assembly intermediates. Finally, although immature capsid formation is reproduced with great fidelity in the cell-free translation/assembly reaction, neither budding nor envelopment is reconstituted in these cell-free reactions. Nevertheless, since production of immature capsids is a necessary prerequisite for proper budding, envelopment, release, and maturation of virus particles, this reaction can be used to study mechanisms involved in the critical first stage of virus formation. Moreover, by altering the plasmid used to generate the in vitro transcript, the reactions described here can also be used to study the effect of mutations in HIV-1 Gag on assembly (7, 9, 11, 12), or to study assembly of other primate lentivirus immature capsids (9, 11).
3.1. HIV-1 Gag in Vitro Transcription
For each translation, prepare the following 6. 0 μL in vitro transcription Master Mix from components listed in 2.1 above:
| 5X CB | 2μl |
| 4NTP mix | 1. 0μL |
| CAP | 1. 0μL |
| 1M DTT | 1. 0μL |
| SP6 polymerase | 4 units (0. 4μL) |
| RNase inhibitor | 10 units (0. 4μL) |
| bovine liver tRNA | 2μg (0. 2μL) |
To the 6. 0 μL Master Mix, add 4. 0 μL linearized HIV-1 Gag plasmid (2 or 2. 5 μg/μL).
Incubate the 10-μL reaction for 1.25 h at 40 °C. At 50 min into the incubation, add an additional 2 units of SP6 polymerase to replenish the enzyme in the reaction. Stop the reaction by placing it in an ice bath.
3.2. Cell-Free Translation/Assembly Reaction
For a standard velocity sedimentation reaction, set up the following 25 μL cell-free translation/assembly reaction (also called the final reaction):
| CB 10X | 2. 5μL |
| 5X EMIX containing 35S met/cys | 5. 0μL |
| WGE | 5. 0μL |
| Myristoyl CoA | 2. 0μL |
| RNase inhibitor | 0. 25μL |
| Creatine Kinase | 0. 25μL |
| tRNA | 0. 25μL |
| transcript (from 3.1) | 5. 0μL |
We have previously shown that capsid assembly in the cell-free system is highly concentration dependent (11). Thus, anything that reduces the amount of Gag protein synthesized will result in a dramatic reduction in capsid assembly (see Note 11).
3.3. Velocity Sedimentation Gradients
To make 10/15/20/40/50/66/80% sucrose step gradients, first make each sucrose solution by dissolving the appropriate amount of sucrose into 1X NP40 buffer. Form step gradients in 5-mL centrifuge tubes by using a pipetman to layer 675 μL of each sucrose/NP40 solution, starting with the 80% sucrose and over-layering with progressively lighter solutions (see Note 12).
Dilute the 25-μL cell-free reaction into a final volume of 100 μL using 1XNP 40 buffer. Layer this sample gently onto the 10% (top) sucrose layer of the gradient. Centrifuge in an MLS 50 rotor (Beckman-Coulter) for 45 min at 45,000 rpm (163, 000 × g). Fractionate into serial 200 μL fractions at the end of the spin (see Note 13).
3.4. SDS-PAGE and Autoradiography
Subaliquot 20 μL from each fraction into loading buffer containing SDS and DTT, and load serially into lanes of a 10% or 12% SDS-PAGE gel. When gel has finished running, fix the proteins into the gel by agitating in a solution of 30% methanol/10% acetic acid, and dry it onto filter paper using a gel dryer. The dried gel can be exposed to Biomax MR film (Kodak, Rochester, NY) to generate autoradiographs showing the amount of 35-S labeled HIV-1 Gag in different fractions of the velocity sedimentation gradients. Alternatively, a phosphorimager can be used to visualize radiolabeled Gag in gradient fractions. When using the gradients described above, the 10S Gag-containing complex (or assembly intermediate) is present in fractions 1 and 2; the 80S assembly intermediate is typically present in fractions 4-6; the 150S assembly intermediate is typically present in fractions 7-9; the 500S assembly intermediate is typically present in fraction 16-18; and the 750S completed capsid is typically present in fractions 21-24 (see Note 14). Approximately 20-40% of newly synthesized Gag is typically found in fractions 21-24. The pellet typically contains aggregates of virus particles or denatured Gag.
Acknowledgments
This work was supported by NIH R01AI48389 to JRL; BKT was supported by the Medical Scientist Training Program NIGMS 5 T32 GM07266, an STD/AIDS Research Training Fellowship NIH T32 AI007140, and a Poncin Award.
Footnotes
Chloride ions inhibit cell-free translation in the wheat germ system, thus acetic acid, rather than HCl, should be used to pH solutions.
10X 4NTP mix is made from separate 0.1 M stocks of each rNTP (sodium or lithium salts dissolved in water). These stocks can be kept frozen at -80 and thawed to make the 4NTP mix. Note that the amount of GTP is lower because of the presence of capped GTP in the reaction (see below). SP6 RNA polymerase incorporates capped GTP at the 5′ end of the newly synthesized transcripts, thereby mimicking the capping reaction that occurs post-transcriptionally in vivo.
RNase inhibitor is sufficient for inhibiting RNase A activity in these reactions, so use of DEPC treated water and other reagents for inactivating RNAse A are not required. However, use of glassware that has been baked for 6 h at 180 °C to inactivate RNase A is recommended while making solutions.
While other polymerases can be used for in vitro transcription, the system described here is set up as a linked transcription/translation system in which the transcription is performed using SP6 polymerase.
For best results, plasmid should be linearized at any restriction site that is downstream from the Gag coding region and creates 5′ overhangs, and linearized plasmid should be repurified using phenol/chloroform extraction and dissolved in water at concentration of 2.0-2.5 mg/mL (as determined by OD260), with an OD260/280 ratio ≥ 1. 5 indicating sufficient DNA purity. In the absence of linearization, somewhat less transcript may be produced. The 5′ UTR is important for optimizing translation.
Production of WGE has been described in detail previously (17). Our group always uses WGE that we produce from fresh wheat germ ourselves (as opposed to commercial WGE) because that allows us to control the quality of the WGE we use.
WGE contains an as yet unidentified inhibitory factor that is removed by the centrifugation described. In addition, some lots of WGE support translation and capsid assembly better than others, so multiple batches are usually made and tested for their quality. WGE should be kept thawed on ice for as short a time as possible. Aliquots can be repeatedly flash frozen.
The nonradioactive components of the Emix can be set up in advance as a mix of unlabeled components. The unlabeled component mix consists of: 10 μL of 01.M ATP (5 mM final concentration); 10 μL of 01.M GTP (5 mM final concentration); 30 μL 0.4 M creatine phosphate (60 mM final concentration); and 40 μL of a 19 amino acid mix in which each amino acid except methionine is at 0.4 mM. Bring this to a volume of 90 μL and store at -80. Just before the first use of this Emix, thaw the unlabeled component mix, add 1 mCi (∼ 100 μL) of 35Smet/cys Translabel, adjust the pH to ∼ 7. 5 with 2 M Tris base, bring final volume to 200 μL with water, mix, aliquot, flash freeze, and store at -80 °C.
Myristoyl CoA is the substrate for myristoylation and must be added exogenously because it is lost upon preparation of WGE during desalting. The enzyme responsible for myristoylation, N-myristoyl transferase, is present in WGE. Myristoylation is critical for proper targeting of Gag in the cell-free system (7) and in cells (2, 4). The percent of newly synthesized Gag that undergoes myristoylation is not known.
Historically, cell-free translations have been performed using WGE or rabbit reticulocyte lysate (RRL). However our group always uses WGE for cell-free translation/assembly because to date we have not found that assembly occurs efficiently in RRL. It has also been reported that RRL can be used for cell-free assembly of HIV-1 capsids (19), but the authors of this study did not comment on the percent of newly synthesized Gag that forms fully assembled capsids when RRL is used (i.e., reaction efficiency).
The efficiency of immature capsid assembly is dependent on the concentration of Gag produced in the cell-free assembly reaction. At maximal efficiency, up to 35% of HIV-1 Gag assembles into immature capsids in the cell-free translation/assembly reaction although more typically, ∼ 25% of radiolabeled Gag assembles (7). We demonstrated this by programming cell-free translation/assembly reactions with diluted in vitro mRNA transcript and demonstrating that this resulted in a proportional reduction in both the amount of radiolabeled Gag synthesized as well as the amount of capsid formation (11). On a practical level, this means that suboptimal in vitro transcription or poor quality WGE will result in low levels of capsid production.
Note that the gradients that are used to analyze these reactions are not linear. This is because the assembly pathway contains three relatively small complexes that are similar to each other in size (the 10S, 80S, and 150S assembly intermediates) and two large complexes (the 500S assembly intermediate and the 750S completed capsid) that are also similar in size, with a relatively large gap in between the 150S and 500S complexes. To optimally separate all of these complexes we use a step gradient that maximizes resolution in both the 10-150S and the 500-750S regions. S values of assembly intermediates are calculated S values (see reference 7 for details on how to calculate S values).
Our group uses either fractionation by hand from top to bottom, or fractionation using a Haake-Buchler automatic gradient fractionator (Labconco Corporation, Kansas City, MI) from top to bottom. However, there are many other approaches to gradient fractionation and the method described here can be adapted to any other approach including bottom to top fractionation.
To mark the position of immature capsids in this gradient, we collect 2.0 mL of medium containing virus from cells transfected to express Gag, concentrate the medium 10-fold using an Amicon concentrator, bring to 0.6% NP40 to remove virus envelopes, and layer these authentic HIV-1 capsids onto the sucrose gradients described in Section 3.3 above in parallel with cell-free reactions.
References
- 1.Briggs JA, Simon MN, Gross I, et al. The stoichiometry of Gag protein in HIV-1. Nat Struct Mol Biol. 2004;11:672–675. doi: 10.1038/nsmb785. [DOI] [PubMed] [Google Scholar]
- 2.Bryant M, Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc Natl Acad Sci USA. 1990;87:523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gheysen D, Jacobs E, de Foresta F, et al. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus - infected insect cells. Cell. 1989;59:103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- 4.Gottlinger HG, Sodroski JG, Haseltine WA. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein K, Reed JC, Lingappa JR. Intracellular destinies: degradation, targeting, assembly, and endocytosis of HIV Gag. AIDS Rev. 2007;9:150–161. [PubMed] [Google Scholar]
- 6.Campbell S, Rein A. In vitro assembly properties of human immunodeficiency virus type 1 Gag protein lacking the p6 domain. J Virol. 1999;73:2270–2279. doi: 10.1128/jvi.73.3.2270-2279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lingappa JR, Hill RL, Wong ML, et al. A multistep, ATP-dependent pathway for assembly of human immunodeficiency virus capsids in a cell - free system. J Cell Biol. 1997;136:567–581. doi: 10.1083/jcb.136.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmerman C, Klein KC, Kiser PK, et al. Identification of a host protein essential for assembly of immature HIV-1 capsids. Nature. 2002;415:88–92. doi: 10.1038/415088a. [DOI] [PubMed] [Google Scholar]
- 9.Dooher JE, Lingappa JR. Conservation of a step-wise, energy-sensitive pathway involving HP68 for assembly of primate lentiviral capsids in cells. J Virol. 2004;78:1645–56. doi: 10.1128/JVI.78.4.1645-1656.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dooher JE, Schneider BL, Reed JC, et al. Host ABCE1 is at plasma membrane HIV assembly sites and its dissociation from Gag is linked to subsequent events of virus production. Traffic. 2007;8:195–211. doi: 10.1111/j.1600-0854.2006.00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lingappa JR, Newman MA, Klein KC, et al. Comparing capsid assembly of primate lentiviruses and hepatitis B virus using cell-free systems. Virology. 2005;333:114–123. doi: 10.1016/j.virol.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 12.Singh AR, Hill RL, Lingappa JR. Effect of mutations in Gag on assembly of immature human immunodeficiency virus type 1 capsids in a cell-free system. Virology. 2001;279:257–270. doi: 10.1006/viro.2000.0706. [DOI] [PubMed] [Google Scholar]
- 13.Dooher JE, Lingappa JR. Cell-free capsid assembly of primate lentiviruses from three different lineages. J Med Primatol. 2004;33:272–280. doi: 10.1111/j.1600-0684.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- 14.Lingappa JR, Martin RL, Wong ML, et al. A eukaryotic cytosolic chaperonin is associated with a high molecular weight intermediate in the assembly of hepatitis B virus capsid, a multimeric particle. J Cell Biol. 1994;125:99–111. doi: 10.1083/jcb.125.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein KC, Dellos SR, Lingappa JR. Identification of residues in the hepatitis C virus core protein that are critical for capsid assembly in a cell-free system. J Virol. 2005;79:6814–6826. doi: 10.1128/JVI.79.11.6814-6826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein KC, Polyak SJ, Lingappa JR. Unique features of Hepatitis C Virus capsid formation revealed by de novo cell-free assembly. J Virol. 2004;78:9257–9269. doi: 10.1128/JVI.78.17.9257-9269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erickson AH, Blobel G. Cell-free translation of messenger RNA in a wheat germ system. Methods Enzymol. 1983;96:38–50. doi: 10.1016/s0076-6879(83)96007-x. [DOI] [PubMed] [Google Scholar]
- 18.Melton DA, Krieg PA, Rebagliati MR, et al. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984;12:7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spearman P, Ratner L. Human immunodeficiency virus type 1 capsid formation in reticulocyte lysates. J Virol. 1996;70:8187–8194. doi: 10.1128/jvi.70.11.8187-8194.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


