Abstract
Background
Psychotic symptoms in Alzheimer Disease (AD+P) identify a heritable phenotype associated with greater cognitive impairment. Knowing when the cognitive course of AD+P subjects diverges from that of subjects without psychosis would enhance understanding of how genetic variation results in AD+P and its associated cognitive burden. We specifically sought to determine if degree of cognitive impairment and cognitive decline in early AD predict subsequent AD+P onset.
Methods
361 Subjects with possible or probable AD or Mild Cognitive Impairment (MCI) without psychosis were evaluated every 6 months until psychosis onset.
Results
Severity of cognitive dysfunction was a strong predictor of AD+P up to two years prior to psychosis onset. Cognition did not decline more rapidly prior to onset of AD+P.
Conclusions
Individuals who will develop AD+P already demonstrate excess cognitive impairment during the mild stages of disease. Genetic variation and brain pathophysiology may lead to a cognitive risk phenotype which is present prior to dementia onset.
Keywords: [ 26 ] Alzheimer's disease, [ 39 ] MCI (mild cognitive impairment), [ 236 ] Psychosis, [ 37 ] Cognitive neuropsychology in dementia, [ 38 ] Assessment of cognitive disorders/dementia
Introduction
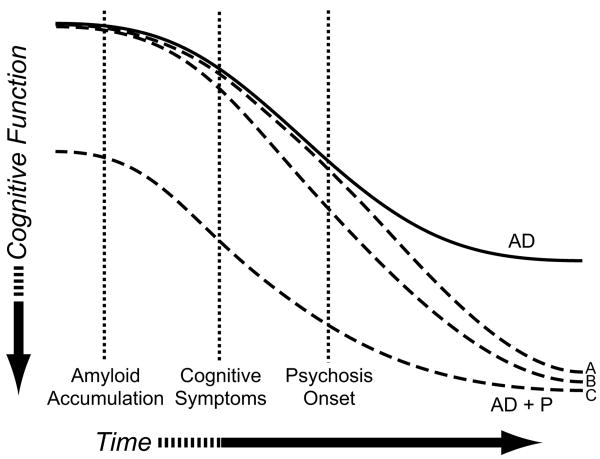
Psychotic symptoms in Alzheimer Disease (AD+P) identify a distinct subtype of AD characterized by a more rapid rate of cognitive decline, higher rates of institutionalization, and higher rates of cognitive impairment than in AD subjects without psychosis. There is evidence that genetic variation contributes to the development of AD+P. AD+P has been shown to aggregate within families and demonstrate linkage to chromosomal loci (Bacanu et al., 2002;Hollingworth et al., 2007;Sweet et al., 2002a). How genetic variation may result in AD+P and the associated excess cognitive burden is unknown, though several possibilities which can be differentiated by their time course are shown in Figure 1. Genetic variation may lead to onset of psychotic symptoms, with excess cognitive impairment only emerging as part of the psychotic state (Figure 1, Trajectory A). Alternatively, genetic variation may interact with neurodegeneration of the brain during AD to accelerate the accumulation of pathologic lesions, resulting in a more rapid cognitive decline and ultimately greater cognitive impairment in AD+P subjects (Figure 1, Trajectory B). Finally, AD+P might result from genetic variation creating a ‘cognitive risk phenotype’ which is present prior to AD onset (Figure 1, Trajectory C). As neurodegeneration progresses, this cognitive risk phenotype would manifest itself as AD+P. There is evidence for this latter trajectory, for example, in schizophrenic psychosis, in which cognitive deficits may be present during neurodevelopment, years prior to the onset of frank psychotic symptoms (Niendam et al., 2003). Moreover, cognitive deficits are present in non-affected 1st degree relatives of subjects with schizophrenia, suggesting that these deficits may result from genetic variation that contributes to a cognitive risk phenotype, in at least this disorder (Snitz et al., 2006).
Figure 1. Possible Trajectories of Greater Cognitive Impairment Relative to Onset of Psychosis in AD, and Implications for the Relationship of Genetic Variation to AD+P.
Genetic variation may contribute to the risk of psychosis onset, with greater cognitive burden only emerging as a result of the psychotic process, i.e. after the onset of psychotic symptoms (Trajectory A). Alternatively, genetic variation may accelerate the neurodegenerative process leading to greater cognitive decline and onset of AD+P (Trajectory B). Finally, genetic variants may act during neurodevelopment or normal aging to create a “cognitive risk” phenotype that will manifest as AD+P after the independent onset of neurodegeneration (Trajectory C).
Although numerous studies have demonstrated greater cognitive impairment after the onset of AD+P (Ballard et al., 1997;Drevets and Rubin, 1989;Jeste et al., 1992;Levy et al., 1996;Lopez et al., 1997;Lopez et al., 1999;Paulsen et al., 2000a;Rockwell et al., 1994;Scarmeas et al., 2005;Stern et al., 1987), there is limited information that would help differentiate these possible trajectories. Two prior studies have examined whether the risk of incident psychosis in subjects with AD is predicted by greater cognitive impairment. Paulsen et al (, 2000b) in subjects with AD, and Wilkosz et al (, 2006) in subjects with AD or Mild Cognitive Impairment (MCI), longitudinally examined individuals who were without psychosis at study entry. Significant associations were found between lower MMSE scores and an increased risk for the onset of psychosis in both studies. However, because neither of these two studies separately evaluated subjects with mild AD at study baseline, it is possible that the observed associations of cognitive impairment with increased AD+P risk were largely due to the occurrence of AD+P in more advanced disease stages (Trajectory A), rather than to a cognitive phenotype emerging prior to, and mediating, the subsequent onset of psychosis.
Thus, the primary objective of the current study was to prospectively and longitudinally examine subjects, stratified by degree of cognitive impairment, to determine the relationship of greater cognitive impairment to subsequent AD+P onset.
Methods
Subjects
All subjects participated in the University of Pittsburgh Alzheimer Disease Research Center (ADRC), using a protocol approved by the University of Pittsburgh Institutional Review Board. All subjects were assessed at baseline with standardized neurological, neuropsychological, psychiatric evaluations, cognitive testing, laboratory studies and brain imaging as previously described (Becker et al., 1994;Lopez et al., 1997;Sweet et al., 1998;Wilkosz et al., 2006). Subjects diagnosed with Possible or Probable AD (Lopez et al., 2000a;Lopez et al., 2000b;McKhann et al., 1984) or MCI (Lopez et al., 2003) without psychosis at baseline were included in the analyses.
All subjects underwent a neuropsychological battery assessing global cognitive functioning, immediate and delayed memory, attention, working memory, and visuospatial and language abilities as follows: the MMSE (Folstein et al., 1975); the Alzheimer's Disease Assessment Scale 10-item word-list learning test and 30-minute delayed recall (Rosen et al., 1984); the copy, immediate, and delayed recall of the modified Rey-Osterrieth Complex Figure (Becker et al., 1987)); the Benton Visual Discrimination Test (Benton et al., 1983); the Digit Span Forwards and Backwards test (Wechsler, 1987); the Category Fluency test (Benton, 1968), the Phonological Fluency test (Borkowski et al., 1967); the Boston Naming test (Kaplan et al., 1983); the Trail Making test A and B (Reitan and Wolfson, 1993). Neurological evaluation, cognitive testing, and diagnostic re-evaluation was conducted on an annual basis. Subjects were followed longitudinally until study completion, loss to follow-up, or until the subject became too impaired to return to clinic for assessments.
Assessment of Psychosis Status
Psychosis was assessed using the Consortium to Establish a Registry for Alzheimer Disease Behavioral Rating Scale (CBRS; (Tariot et al., 1995). The CBRS interview was designed to be administered to an informant. Informants for the CBRS were predominantly spouses (51%) or children (34%). Most (77%) informants had contact with the subject 5 or more days per week. The CBRS was completed at baseline and annual assessments, and between assessments at approximately six month intervals via telephone. Symptoms occurring during an episode of delirium were not rated on the CBRS. Psychosis at any assessment was considered present when any of the CBRS items #33 and #35-#45 were rated as occurring ≥3 times in the past month. The CBRS items include: misidentified people (#33), not recognized self in mirror (#34), misidentified things (#35), people harming or stealing (#36), spouse is unfaithful (#37), caregiver plotting to abandon (#38), caregiver is an imposter (#39), characters on TV are real (#40), phantom boarder in home (#41), dead person is alive (#42), house is not home (#43), auditory hallucinations (#44) visual hallucinations (#45). Interrater reliability on the CBRS was established using 10 CBRS interviews. Interrater agreement for the presence or absence of psychosis was high (mean ± SD of Kappa, .98 ±.08).
Statistical Analysis
Cox proportional hazard models with time-dependent covariates were used to test associations of time to psychosis onset with MMSE. The selected covariates were age, education, age of onset, gender, and Cognitive Enhancer (CE) use. In all models, age, MMSE, and CE use were included as time dependent variables. The same analyses were repeated twice with restricted samples of patients with early to middle stage disease (baseline MMSE scores were greater than or equal to 16, n=293) and early disease (baseline MMSE scores were greater than or equal to 20, n=224).
For testing the association of psychosis onset with MMSE score 1 year and 2 years prior, all subjects with a baseline visit and without onset of psychosis prior to one (or two) consecutive annual follow up assessments were identified, resulting in a 259 subjects available for analysis of the 1 year lag and 143 subjects available for analysis of the 2 year lag. For the 1 year lag analysis, the time to psychosis onset was calculated from the 1 year follow-up assessment date. As in the models described above, the MMSE scores were entered as a time dependent variable. In order to model the time to psychosis onset and MMSE 1 year prior, the baseline MMSE scores were entered in the model as starting values. Similarly, for the 2 years lag analysis, 2 year follow-up dates were used to calculate the time to psychosis onset, and the baseline MMSE scores were used as starting scores.
For the MMSE change analysis, the MMSE rate of change was calculated by taking the difference between two consecutive MMSE scores and dividing by the number of years between the two assessments. In addition to the covariates listed above, MMSE rate of change was included in the models as a time dependent variable. Analysis of neuropsychologic tests on time to psychosis onset used Cox proportional hazard models with time dependent covariates as described for the analyses of MMSE, above, with all of the tests entered as independent variables.
Results
A total of 361 subjects completed at least one follow-up assessment. Demographic and clinical information on these 361 subjects is presented in Table 1. Psychotic symptoms were observed in 122 subjects during the duration of the study. The only variable consistently associated with reduced time to onset of AD+P was (lower) MMSE (Table 2). The association of MMSE with onset of AD+P was strengthened when analyses were restricted to those with early to mid-stage disease (MMSE ≥ 16) or to those with early stage disease (MMSE ≥ 20).
Table 1. Demographic and Clinical Characteristics.
| Entire Group (N=361) Mean (SD) or N (%) |
|
|---|---|
| Age | 74.4 (8.8) |
| Education | 13.3 (3.1) |
| Onset Age | 71.0 (9.2) |
| Baseline MMSE | 20.1 (5.9) |
| Sex | |
| Female | 214 (59) |
| Male | 147 (41) |
| Race | |
| Caucasian | 342 (95) |
| African American | 17 (5) |
| Asian/Other | 2 (1) |
| Baseline Diagnosis | |
| MCI | 60 (17) |
| Possible AD | 37 (10) |
| Probable AD | 264 (73) |
| Psychosis Onset during follow-up | |
| Not Present | 239 (66) |
| Present | 122 (34) |
| Cognitive Enhancer Use | |
| At Baseline | 177 (49) |
| At Baseline or Follow-up | 288 (80) |
MMSE, Mini Mental Status Examination; MCI, Mild Cognitive Impairment; AD, Alzheimer Disease
Table 2. Association of Demographic Variables, Mini Mental Status Examination Score, and Cognitive Enhancer Use with Risk of Psychosis Onset.
| Variable | All subjects (N=361) | Baseline MMSE ≥ 16 (N=293) | Baseline MMSE ≥ 20 (N=224) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | χ2* | p | Hazard Ratio | χ2* | p | Hazard Ratio | χ2* | p | |
| Age | 1.081 | 3.62 | 0.06 | 1.150 | 5.98 | 0.01 | 1.094 | 1.79 | 0.21 |
| MMSE | 0.918 | 33.89 | <0.0001 | 0.865 | 34.59 | <0.0001 | 0.770 | 51.89 | <0.0001 |
| Education | 1.035 | 0.94 | 0.33 | 1.012 | 0.08 | 0.77 | 1.048 | 0.81 | 0.37 |
| Onset Age | 0.930 | 3.22 | 0.07 | 0.885 | 4.98 | 0.03 | 0.926 | 1.39 | 0.24 |
| Gender | 0.683 | 3.55 | 0.06 | 0.764 | 1.12 | 0.29 | 0.923 | 0.07 | 0.79 |
| CE Use | 1.331 | 1.67 | 0.20 | 1.239 | 0.55 | 0.46 | 1.047 | 0.02 | 0.89 |
Wald χ2 test with 1 df
MMSE, Mini Mental Status Examination; CE, Cognitive Enhancer
Cognitive Impairment 1 and 2 years prior and AD+P Risk
Subjects for whom the 1 year and 2 year lag analyses could be conducted did not differ significantly from the entire cohort in age, sex, race, education, age of onset, or baseline MMSE. Results of the lag analyses for the entire group, the subjects with early to middle stage disease, and the group with early stage disease are presented in Table 3. Lower MMSE 1 year prior remained a strong predictor of psychosis onset. The association between lower MMSE score and subsequent onset of AD+P remained significant when examining MMSE scores lagged by 2 years. Once again the association was strongest in those subjects with early stage disease.
Table 3. Association of Mini Mental Status Examination Scores One to Two Years Prior with Risk of Psychosis Onset.
| Analysis | Group | All subjects | Baseline MMSE ≥ 16 | Baseline MMSE ≥ 20 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Hazard Ratio | χ2* | p | N | Hazard Ratio | χ2* | p | N | Hazard Ratio | χ2* | p | ||
| No Lag | All Subjects | 361 | 0.918 | 33.89 | <0.0001 | 293 | 0.865 | 35.74 | <0.0001 | 224 | 0.770 | 51.89 | <0.0001 |
| 1 Year Lag | All Subjects | 259 | 0.924 | 13.78 | 0.0002 | 217 | 0.853 | 18.99 | <0.0001 | 169 | 0.756 | 21.33 | <0.0001 |
| 2 Year Lag | All Subjects | 143 | 0.923 | 5.089 | 0.02 | 124 | 0.844 | 7.69 | 0.0056 | 96 | 0.680 | 15.39 | <0.0001 |
Wald χ2 test with 1 df
MMSE, Mini Mental Status Examination
Rate of Cognitive Decline and AD+P Risk
There was no association of rate of change in MMSE score with onset of psychosis in the entire group, the subjects with early to middle stage disease, and the group with early stage disease (Table 4).
Table 4. Association of Annualized Rate of Change in Mini Mental Status Examination Scores with Risk of Psychosis Onset.
| Variable | All Subjects (N=239) | Baseline MMSE ≥ 16 (N=201) | Baseline MMSE ≥ 20 (N=157) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | χ2* | p | Hazard Ratio | χ2* | p | Hazard Ratio | χ2* | p | |
| Age | 1.134 | 5.00 | 0.03 | 1.155 | 4.96 | 0.03 | 1.134 | 2.92 | 0.09 |
| Change in MMSE | 1.036 | 0.94 | 0.33 | 1.039 | 0.89 | 0.35 | 1.087 | 2.75 | 0.09 |
| Education | 1.022 | 0.24 | 0.63 | 1.019 | 0.14 | 0.71 | 1.002 | 0.001 | 0.97 |
| Onset Age | 0.894 | 4.57 | 0.03 | 0.878 | 4.66 | 0.03 | 0.904 | 2.18 | 0.14 |
| Gender | 0.558 | 4.50 | 0.03 | 0.550 | 3.73 | 0.05 | 0.589 | 2.26 | 0.13 |
| CE Use | 1.467 | 1.77 | 0.18 | 1.413 | 1.09 | 0.30 | 1.552 | 1.28 | 0.26 |
Wald χ2 test with 1 df
MMSE, Mini Mental Status Examination; CE, Cognitive Enhancer
Effect of Diagnosis
Because some of the above results may have been influenced by diagnostic group, we reran the analyses including variables encoding MCI, Possible AD, and Probable AD diagnoses, and their interactions with MMSE. There were no main effects of diagnosis, and no interactions of diagnostic group by MMSE over time. In contrast, the findings presented in Tables 2, 3, and 4 remained essentially unchanged, with the exception that the two year lag MMSE analysis was now only significant in the MMSE ≥ 20 group.
Specific Cognitive Assessments and Time to onset of Psychosis
We conducted additional analyses in the 207 subjects with baseline MMSE ≥ 20 for whom complete neuropsychologic test scores were available to determine what neuropsychological domains contributed to the association of global cognitive impairment with subsequent onset of AD+P (Table 5). These analyses were confined to the early stage group because they demonstrated the strongest associations of MMSE with AD+P onset, and floor effects on the neuropsychologic measures utilized were least likely in this group. We found that time to onset of psychosis was associated with greater impairment in the Rey-Osterrieth Complex Figure Delayed Recall, Immediate Verbal Recall, and Phonological Fluency. In contrast, time to onset of AD+P was associated with better performance on Forward Digit Span. In order to examine whether reduced time to onset of psychosis was associated with performance in a specific cognitive domain that was disproportionate relative to global cognitive functioning, the above analysis was repeated with MMSE entered as a covariate. After accounting for MMSE, time to onset of psychosis continued to be associated with greater impairment on the Rey-Osterrieth Complex Figure Delayed Recall and better performance on Forward Digit Span.
Table 5. Cognitive assessments and time to onset of psychosis.
| Variable | AD+P | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | χ2* | p | Hazard Ratio | χ2* | p | |
| Age | 1.214 | 10.25 | 0.001 | 1.135 | 3.59 | 0.058 |
| Gender | 1.310 | 1.12 | 0.291 | 1.310 | 1.12 | 0.291 |
| Education | 0.954 | 1.14 | 0.286 | 0.954 | 1.14 | 0.286 |
| Race | 2.018 | 0.93 | 0.335 | 2.018 | 0.93 | 0.335 |
| Age of Onset | 0.828 | 10.48 | 0.001 | 0.886 | 3.54 | 0.060 |
| CE Use** | 0.986 | 0.003 | 0.957 | 0.986 | 0.003 | 0.957 |
| MMSE*** | ----- | ---- | ---- | 0.821 | 8.89 | 0.003 |
| Rey-Osterrieth Figure Delayed Memory | 0.939 | 5.73 | 0.017 | 0.945 | 4.63 | 0.031 |
| Phonologic Fluency | 0.968 | 7.21 | 0.007 | 0.980 | 2.79 | 0.095 |
| Verbal Immediate Recall | 0.924 | 4.55 | 0.033 | 0.967 | 0.62 | 0.429 |
| Verbal Delayed Recall | 0.868 | 1.26 | 0.262 | 0.940 | 0.23 | 0.630 |
| Forward Digit Span | 1.364 | 5.70 | 0.017 | 1.284 | 6.66 | 0.010 |
Wald χ2 test with 1 df
MMSE, Mini Mental Status Examination; CE, Cognitive Enhancer
Discussion
Subjects that developed AD+P exhibited greater global cognitive impairment prior to the onset of psychosis symptoms than subjects who did not develop psychosis. Results strengthened when we restricted the sample to subjects in early disease stages. Furthermore, we found that greater global cognitive impairment was present up to 2 years prior to onset of overt psychotic symptoms in subjects who ultimately develop AD+P, even in mild stages of AD. In contrast, we did not find evidence that more rapid decline in cognition preceded the onset of AD+P. While greater cognitive impairments associated with AD+P were most pronounced in measures of delayed recall, somewhat surprisingly we found relative preservation of performance on forward digit span to be associated with AD+P onset.
The relationship of cognition to AD+P onset might be characterized by one of several trajectories, with differing implications for the mechanism whereby genetic variation may result in AD+P and its associated excess cognitive burden. In Figure 1 Trajectory A, subjects with AD+P would only demonstrate excess cognitive impairment after onset of psychosis. In Trajectory B, subjects would have equal cognitive status at the onset of AD, but genetic variation leads to an accelerated rate of cognitive decline to produce AD+P. In contrast, in Trajectory C, individuals with genetic variation predisposing to AD+P would enter AD with worse cognition at the end of normal development and/or normal aging, then decline at the usual rate, with the additive effects resulting in AD+P. The current findings that greater cognitive impairment is already present 1 to 2 years prior to onset of psychosis in AD subjects, but cognition does not decline more rapidly during the same time period, would be most consistent with Trajectory C in our model.
The above interpretation should be tempered, however, by several additional considerations. In contrast to global and domain-specific evidence of greater cognitive impairment predicting subsequent AD+P, we found better performance to be present on forward digit span. If this is a true positive result, it would indicate that global cognitive trajectory is not a proxy for the relationship of all cognitive functions to AD+P onset. However, the possibility that this result is a false positive needs to be considered given that we did not find significant preservation of other attention and executive function measures in our cohort (Trails Test A and B, Backward Digit Span). Moreover, a prior study found decline in measures of attention to be predictive of AD+P (Paulsen et al., 2000b).
We only examined the course of individuals after onset of clinical symptoms of neurodegeneration, and until onset of psychosis. Others, however, have found that detectable differences in cognitive function may be present years (Saxton et al., 2004), or even decades (Snowdon et al., 1996;Whalley et al., 2000), prior to onset of AD. For example, Saxton et al (, 2004) examined cognitive performance in 693 normal elderly subjects. They found that differences in cognitive scores between individuals who developed AD and those who did not were evident up to 8 years prior to AD onset. Whether these differences are confined to, or greater in, subjects at risk for AD+P remains to be determined. Similarly, our data can not address whether there is an additional cognitive burden conferred subsequent to psychosis onset.
Although the degree of cognitive impairment predicted psychosis, we did not find the rate of cognitive decline was increased in individuals with subsequent onset of AD+P. The lack of more rapid decline corresponds to the observations by Paulsen et al (, 2000b) that the rate of change on the MMSE was not predictive of psychosis. However, Paulsen et al. examined neuropsychological tests in addition to the MMSE. They found more rapid decline in the Mattis Dementia Rating Scale Total Score, the Attention and Construction subscale scores, and a separate measure of verbal fluency to be predictive of AD+P. Thus it remains possible that AD+P is associated with more rapid cognitive decline during the early disease stages, at least in a subset of cognitive domains (Figure 1, Trajectory B). The conclusion that individuals destined to develop AD+P do not decline more rapidly in the early disease course is also subject to the limitation that only individuals already demonstrating symptomatic cognitive dysfunction were studied. However, the accumulation of neurodegenerative lesions likely precedes the onset of cognitive symptoms by years (Saxton et al., 2004). Thus it is possible that those of our subjects who developed AD+P underwent a phase of accelerated degeneration prior to entering our clinic.
If greater cognitive impairment is present in individuals destined to develop AD+P either prior to disease onset or early in disease course, how might this inform understanding of the pathophysiology of AD+P? Recent conceptualizations of AD have shifted the focus of early pathogenic events from formation of amyloid plaques and neurofibrillary tangles to the effects of β-amyloid and tau phosphorylation on synapse loss. This understanding has emerged in part from recognition that in transgenic mice overexpression of amyloid precursor protein results in cognitive deficits in advance of plaque formation (Guenette and Tanzi, 1999) and that early synapse loss can exceed neuronal loss (Hsia et al., 1999). There is similar evidence in humans that reduced synapse number is present early in disease (Masliah et al., 2001;Scheff and Price, 2003), is not accounted for by neuronal loss (Davies et al., 1987;Hsia et al., 1999), and is the strongest correlate of cognitive impairment (DeKosky and Scheff, 1990). Similarly, we have shown that markers of synaptic integrity in cortex are more disrupted in AD+P than in AD subjects without psychosis (Sweet et al., 2002b). Of interest, reduced synapse number is also a consistent finding in schizophrenic psychosis (Glantz and Lewis, 2000;Sweet et al., 2007). Reduced synaptic number could arise during development as an interaction of genetic and environmental factors, and be later additive with synapse loss during AD to yield greater cognitive impairment and subsequent onset of AD+P. Alternatively, genetic and environmental factors may influence the rate of neurodegenerative synapse loss during prodromal stages, and provide a similar outcome.
Some potential methodological limitations of this study should be noted. The study was conducted in an Alzheimer Disease Research Center referral clinic, and may not be representative of the general population. In a naturalistic follow-up of an elderly, infirm population, biases may emerge due to the effects of drop-outs and missed visits. Though we can not exclude this possibility, our approach to follow-up was designed to limit this potential bias, and overall resulted in relatively low missed visit rates (averaging <5%) and similarly low drop-out rates (averaging <4%) at each assessment time point. In contrast, one strength of our study was the inclusion of subjects in early clinical stages of disease. This includes subjects diagnosed with MCI, which in this setting consists of individuals with a high probability of subsequent conversion to AD.
In conclusion, we found that greater global cognitive impairment was associated with subsequent onset of AD+P. This effect was strongest in individuals who entered the study with the mildest degree of cognitive impairment, was detectable up to two years prior to AD+P onset, and did not appear to result from more rapid cognitive decline during the early course of symptomatic illness. Future prospective longitudinal studies assessing cognition and psychosis status should be conducted in subjects prior to the onset of AD to evaluate other cognitive predictors for AD+P and to confirm the results of this study. Studies to evaluate whether cognitive impairments mediate the effects of specific genetic variations, e.g. COMT (Sweet et al., 2005), on AD+P risk are also needed.
Acknowledgments
Supported in part by USPHS grant AG05133 and AG027224 from the National Institute of Aging and funding from the VISN 4 Mental Illness Research, Education, and Clinical Center (MIRECC, Director: D. Oslin; Co-Director: G. Haas), VA Pittsburgh Healthcare System. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Aging or the National Institutes of Health.
Footnotes
Previously presented at the 10th International Conference on Alzheimer's Disease and Related Disorders in Madrid, Spain, July 15-20
Conflict of Interest Declaration: The authors report no conflicts of interest. The sponsors had no part in formulation of the research questions, study design, data collection, data analysis, or decision to publish.
Description of Authors' Roles: All authors participated sufficiently in the manuscript to warrant authorship. Analyses were performed by PW and SM. The following authors participated in drafting and reviewing the text and interpreting the results: EW, JE, DV, SM, PW, OL, SD, and RS.
References
- Bacanu SA, Devlin B, Chowdari KV, DeKosky ST, Nimgaonkar VL, Sweet RA. Linkage analysis of Alzheimer disease with psychosis. Neurology. 2002;59:118–120. doi: 10.1212/wnl.59.1.118. [DOI] [PubMed] [Google Scholar]
- Ballard CG, O'Brien JT, Coope B, Wilcock G. Psychotic symptoms in dementia and the rate of cognitive decline. Journal of the American Geriatrics Society. 1997;45:1031–1032. doi: 10.1111/j.1532-5415.1997.tb02980.x. [DOI] [PubMed] [Google Scholar]
- Becker JT, Boller F, Lopez OL, Saxton J, McGonigle KL. The natural history of Alzheimer's disease: description of study cohort and accuracy of diagnosis. Archives of Neurology. 1994;51:585–594. doi: 10.1001/archneur.1994.00540180063015. [DOI] [PubMed] [Google Scholar]
- Becker JT, Boller F, Saxton J, McGonigle-Gibson KL. Normal rates of forgetting of verbal and non-verbal material in Alzheimer's disease. Cortex. 1987;23:59–72. doi: 10.1016/s0010-9452(87)80019-9. [DOI] [PubMed] [Google Scholar]
- Benton AL. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- Benton AL, Hamsher K, Varney NR, Spreen O. Contribution to Neuropsychological Assessment: A Clinical Manual. New York: Oxford University Press; 1983. [Google Scholar]
- Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–140. [Google Scholar]
- Davies CA, Mann DM, Sumpter PQ, Yates PO. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer's disease. Journal of the Neurological Sciences. 1987;78:151–164. doi: 10.1016/0022-510x(87)90057-8. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: Correlation with cognitive severity. Annals of Neurology. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Rubin EH. Psychotic symptoms and the longitudinal course of senile dementia of the Alzheimer type. Biological Psychiatry. 1989;25:39–48. doi: 10.1016/0006-3223(89)90145-5. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini mental state: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Archives of General Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Guenette SY, Tanzi RE. Progress toward valid transgenic mouse models for Alzheimer's disease. Neurobiology of Aging. 1999;20:201–211. doi: 10.1016/s0197-4580(99)00042-1. [DOI] [PubMed] [Google Scholar]
- Hollingworth P, Hamshere ML, Holmans PA, O'Donovan MC, Sims R, Powell J, Lovestone S, Myers A, Devrieze FW, Hardy J, Goate A, Owen M, Williams J. Increased familial risk and genomewide significant linkage for Alzheimer's disease with psychosis. Am J Med Genet B Neuropsychiatr Genet. 2007 doi: 10.1002/ajmg.b.30515. [DOI] [PubMed] [Google Scholar]
- Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc Natl Acad Sci U S A. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, Wragg RE, Salmon DP, Harris MJ, Thal LJ. Cognitive deficits of patients with Alzheimer's disease with and without delusions. American Journal of Psychiatry. 1992;149:184–189. doi: 10.1176/ajp.149.2.184. [DOI] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Levy ML, Cummings J, Fairbanks LA, Bravi D, Calvani M, Carta A. Longitudinal assessment of symptoms of depression, agitation, and psychosis in 181 patients with Alzheimer's disease. American Journal of Psychiatry. 1996;153:1438–1443. doi: 10.1176/ajp.153.11.1438. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Klunk WE, Saxton J, Hamilton RL, Kaufer D, Sweet RA, Cidis Meltzer C, Wisniewski SR, Kamboh MI, DeKosky ST. Research evaluation and diagnosis of possible Alzheimer's disease over the last two decades. II. Neurology. 2000a;55:1863–1869. doi: 10.1212/wnl.55.12.1863. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Klunk WE, Saxton J, Hamilton RL, Kaufer DI, Sweet RA, Cidis Meltzer C, Wisniewski SR, Kamboh MI, DeKosky ST. Research evaluation and diagnosis of probable Alzheimer's disease over the last two decades. I. Neurology. 2000b;55:1854–1862. doi: 10.1212/wnl.55.12.1854. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M, Kuller LH. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Archives of Neurology. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Kamboh MI, Becker JT, Kaufer DI, DeKosky ST. The apolipoprotein E e4 allele is not associated with psychiatric symptoms or extrapyramidal signs in probable Alzheimer's disease. Neurology. 1997;49:794–797. doi: 10.1212/wnl.49.3.794. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Wisniewski SR, Becker JT, Boller F, DeKosky ST. Psychiatric medication and abnormal behavior as predictors of progression in probable Alzheimer disease. Archives of Neurology. 1999;56:1266–1272. doi: 10.1001/archneur.56.10.1266. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Alford M, DeTeresa R, Hansen LA, McKeel DW, Jr, Morris JC. Altered expression of synaptic proteins occurs early during progression of Alzheimer's disease. Neurology. 2001;56:127–129. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Niendam TA, Bearden CE, Rosso IM, Sanchez LE, Hadley T, Nuechterlein KH, Cannon TD. A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. American Journal of Psychiatry. 2003;160:2060–2062. doi: 10.1176/appi.ajp.160.11.2060. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Ready RE, Stout JC, Salmon DP, Thal LJ, Grant I, Jeste DV. Neurobehaviors and psychotic symptoms in Alzheimer's disease. Journal of the International Neuropsychological Society. 2000a;6:815–820. doi: 10.1017/s1355617700677081. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Salmon DP, Thal L, Romero R, Weisstein-Jenkins C, Galasko D, Hofstetter CR, Thomas R, Grant I, Jeste DV. Incidence of and risk factors for hallucinations and delusions in patients with probable Alzheimer's disease. Neurology. 2000b;54:1965–1971. doi: 10.1212/wnl.54.10.1965. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery. Tuson: Neuropsychology Press; 1993. [Google Scholar]
- Rockwell E, Jackson E, Vilke G, Jeste DV. A study of delusions in a large cohort of Alzheimer's disease patients. American Journal of Geriatric Psychiatry. 1994;2:157–164. doi: 10.1097/00019442-199405000-00009. [DOI] [PubMed] [Google Scholar]
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. American Journal of Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- Saxton J, Lopez OL, Ratcliff G, Dulberg C, Fried LP, Carlson MC, Newman AB, Kuller L. Preclinical Alzheimer disease: neuropsychological test performance 1.5 to 8 years prior to onset. Neurology. 2004;63:2341–2347. doi: 10.1212/01.wnl.0000147470.58328.50. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Brandt J, Albert M, Hadjigeorgiou G, Papadimitriou A, Dubois B, Sarazin M, Devanand D, Honig L, Marder K, Bell K, Wegesin D, Blacker D, Stern Y. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Archives of Neurology. 2005;62:1601–1608. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA. Synaptic pathology in Alzheimer's disease: a review of ultrastructural studies. Neurobiology of Aging. 2003;24:1029–1046. doi: 10.1016/j.neurobiolaging.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Snitz BE, Macdonald AW, III, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophrenia Bulletin. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer's disease in late life - Findings from the Nun Study. Jama-Journal of the American Medical Association. 1996;275:528–532. [PubMed] [Google Scholar]
- Stern Y, Mayeux R, Sano M, Hauser WA, Bush T. Predictors of disease course in patients with probable Alzheimer's disease. Neurology. 1987;37:1649–1653. doi: 10.1212/wnl.37.10.1649. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Bergen SE, Sun Z, Marcsisin MJ, Sampson AR, Lewis DA. Anatomical evidence of impaired feedforward auditory processing in schizophrenia. Biological Psychiatry. 2007;61:854–864. doi: 10.1016/j.biopsych.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Devlin B, Pollock BG, Sukonick DL, Kastango KB, Bacanu SA, Chowdari KV, DeKosky ST, Ferrell RE. Catechol-O-methyltransferase haplotypes are associated with psychosis in Alzheimer disease. Molecular Psychiatry. 2005;10:1026–1036. doi: 10.1038/sj.mp.4001709. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Nimgaonkar VL, Devlin B, Lopez OL, DeKosky ST. Increased familial risk of the psychotic phenotype of Alzheimer disease. Neurology. 2002a;58:907–911. doi: 10.1212/wnl.58.6.907. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Nimgaonkar VL, Kamboh MI, Lopez OL, Zhang F, DeKosky ST. Dopamine receptor genetic variation, psychosis, and aggression in Alzheimer's disease. Archives of Neurology. 1998;55:1335–1340. doi: 10.1001/archneur.55.10.1335. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Panchalingam K, Pettegrew JW, McClure RJ, Hamilton RL, Lopez OL, Kaufer DI, DeKosky ST, Klunk WE. Psychosis in Alzheimer disease: postmortem magnetic resonance spectroscopy evidence of excess neuronal and membrane phospholipid pathology. Neurobiology of Aging. 2002b;23:547–553. doi: 10.1016/s0197-4580(02)00009-x. [DOI] [PubMed] [Google Scholar]
- Tariot PN, Mack JL, Patterson MB, Edland SD, Weiner MF, Fillenbaum G, Blazina L, Teri L, Rubin E, Mortimer JA, Stern Y, Behavioral Pathology Committee of the Consortium to Establish a Registry for Alzheimer's Disease The behavior rating scale for dementia of the Consortium to Establish a Registry for Alzheimer's Disease. American Journal of Psychiatry. 1995;152:1349–1357. doi: 10.1176/ajp.152.9.1349. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler Memory Scale Manual-Revised. New York: The Psychological Corporation; 1987. [Google Scholar]
- Whalley LJ, Starr JM, Athawes R, Hunter D, Pattie A, Deary IJ. Childhood mental ability and dementia. Neurology. 2000;55:1455–1459. doi: 10.1212/wnl.55.10.1455. [DOI] [PubMed] [Google Scholar]
- Wilkosz PA, Miyahara S, Lopez OL, DeKosky ST, Sweet RA. Prediction of Psychosis Onset in Alzheimer Disease: The Role of Cognitive Impairment, Depressive Symptoms, and Further Evidence for Psychosis Subtypes. American Journal of Geriatric Psychiatry. 2006;14:352–356. doi: 10.1097/01.JGP.0000192500.25940.1b. [DOI] [PubMed] [Google Scholar]