Abstract
The Study of the Effects of Diet on Metabolism and Nutrition (STEDMAN) Project uses comprehensive metabolic profiling to probe biochemical mechanisms of weight loss in humans. Measurements at baseline, 2 and 4 weeks, 6 and 12 months included diet, body composition, metabolic rate, hormones, and 80 intermediary metabolites measured by mass spectrometry. In 27 obese adults in a behavioral weight loss intervention, median weight decreased 13.9 lb over the first 6 months, then reverted towards baseline by 12 months. Insulin resistance (HOMA) was partially ameliorated in the first 6 months and showed sustained improvement at 12 months despite weight regain. Ghrelin increased with weight loss and reverted to baseline, whereas leptin and PYY fell at 6 months and remained persistently low. NPY levels did not change. Factors possibly contributing to sustained improvement in insulin sensitivity despite weight regain include adiponectin (increased by 12 months), IGF-1 (increased during weight loss and continued to increase during weight regain), and visceral fat (fell at 6 months but did not change thereafter). We observed a persistent reduction in free fatty acids, branched chain amino acids, and related metabolites that may contribute to improved insulin action. These findings provide evidence for sustained benefits of weight loss in obese humans and insights into mechanisms.
Introduction
The prevalence of overnutrition and sedentary behavior has led to a world-wide epidemic of obesity and attendant comorbidities (Flegal et al., 2002; Hedley et al., 2004; Kuczmarski et al., 1997, 2000; Willett et al., 1999). In the United States, current estimates are that 65% of adults are overweight or obese [defined as a body mass index (BMI) ≥ 25 kg/m2] and 30% are obese (BMI ≥ 30 kg/m2) (Flegal et al., 2002). Obesity is strongly linked to increased incidence of chronic diseases such as diabetes, cardiovascular disease, hypertension, and cancer (Burton and Foster, 1985; Calle and Than, 2004; Sowers, 2003). Weight loss reduces cardiovascular risk factors (Anonymous, 1998; Moore et al., 2005; Sowers, 2003) and prevents diabetes and hypertension (Anonymous, 1998; Knowler et al., 2002), but the precise biological factors that link weight loss to improved health are incompletely understood. Knowledge is limited in part because most research in this field involves measurement of one or a small number of analytes chosen to address a particular hypothesis. The current study has taken an alternative, comprehensive approach for studying weight loss in humans, comprising 130 separate measurements. Included were 18 hormones of energy balance, 4 pro- and anti-inflammatory cytokines, physiological variables such as body composition and resting metabolic rate, 10 metabolites assayed by conventional analytical assays, and, as a novel feature, 80 intermediary metabolites in 4 chemical classes measured by targeted mass spectrometry (MS) (Haqq et al., 2005). This approach has allowed us to provide a comprehensive metabolic profile of obese humans during a 6-month intensive dietary/behavioral weight loss intervention followed by 6 months of continued follow-up, during which there was significant weight regain.
Metabolic profiling in the Study of Effects of Diet on Metabolism and Nutrition (STEDMAN) Project included constellations of variables that may relate to comorbidities of obesity that resolve with weight loss. One such condition is insulin resistance, which occurs in response to a complex interplay of metabolic and inflammatory mediators of energy balance. For example, there is a strong association between levels of circulating free fatty acids and insulin resistance, correlated with accumulation of lipid-derived byproducts such as triglycerides, long-chain acyl CoAs, ceramides, diacylglycerides, and products of incomplete fatty acid oxidation in the mitochondria (An et al., 2004; Morino et al., 2006; Muoio and Newgard, 2006; Summers, 2006). Inflammatory cytokines are also involved in this obesity-related disorder, including TNF-α, IL-6, and C-reactive protein (CRP) (Hotamisligil et al., 1993; Monzilllo et al., 2003; O'Brien et al., 2005; Shoelson et al., 2006; Wellen and Hotamisligil, 2005). Finally, adipose-derived hormones, such as leptin, adiponectin, and resistin (Bjorbaek and Kahn, 2004; Lehrke et al., 2004; Trujillo and Scherer, 2006; Weyer et al., 2001), have been implicated in modulation of insulin sensitivity. The current investigation aimed to better understand the changes in these previously implicated factors during an intervention that causes partial amelioration of insulin resistance, and to supplement this knowledge by measuring a wider array of biochemical variables over the same time period.
Although modest (e.g. 5% of baseline) weight loss has metabolic and clinical benefits (Klein et al., 2004), it is often assumed that these positive effects last only as long as the reduction in weight persists. In the current study, we describe the metabolic changes that occur in response to a 6-month intensive behavioral intervention. We also describe the metabolic profile of subjects in the 6 months following the intensive intervention, during which a number of subjects experienced partial weight regain. Surprisingly, a number of metabolic variables associated with improved insulin sensitivity show sustained improvement that persisted beyond the 6-month period of intervention when weight loss occurred and into the subsequent 6-month period when weight regain was observed. Moreover, the broad-based approach and the multiple time points of sampling have allowed us to describe temporal changes in metabolic variables during weight loss and regain, thereby providing insight into potential cause-and-effect relationships between such variables and changes in insulin sensitivity.
Materials and Methods
Individuals joined the STEDMAN Project by virtue of their participation in the Weight Loss Maintenance Study (WLM) (Brantley et al., 2006) at Duke. All WLM participants undergo 6 months of an intensive behavioral intervention for achieving weight loss, consisting of 20 weekly group sessions with a trained interventionist. The intervention focuses on behavior change aimed at reducing energy intake, increasing physical activity, and adoption of a healthy dietary pattern [the Dietary Approaches to Stop Hypertension (DASH) dietary pattern] (Appel et al., 1997; Sacks et al., 1995). At enrollment into WLM, participants who met STEDMAN eligibility criteria were also recruited into the STEDMAN Project. STEDMAN inclusion criteria consisted of: age ≥ 18 years; BMI 30–50 kg/m2; eligible and enrolled in WLM. STEDMAN exclusion criteria consisted of: regular use of diabetes, weight loss, or oral corticosteroid medications; serious medical illness; evidence of secondary obesity; weight loss of more than 20 lbs in the previous 3 months; prior bariatric surgery; pregnancy/nursing (Haqq et al., 2005). The protocol was approved by the Duke institutional review board, and all participants provided written informed consent.
As part of the WLM design, those who lost at least 4 kg (8.8 lb) during the initial weight loss program were then randomized to one of three behavioral strategies for maintaining weight loss (personal counseling by phone, an interactive Web site, or a no-intervention control group). Since WLM is a blinded trial still in progress, randomization assignments are not known. Because our primary interest here is the biologic effect of weight change and the maintenance interventions are unlikely to differ biologically, the current paper pools the treatment arms.
Measurements
Major data collection visits occurred at baseline, 6, and 12 months; a subset measurements were obtained at 2 and 4 weeks (Table 1).The 6-month measurements corresponded to the end of the behavioral weight loss intervention, and the 12-month measurements to the end of a 6-month period of follow-up with or without a behavioral maintenance intervention.
Table 1.
Measurements and Data Collection Schedule
| Measurements | 0 weeks | 2 weeks | 4 weeks | 6 months | 12 months |
|---|---|---|---|---|---|
| Eligibility, demographics, medical conditions | X | ||||
| Medications | X | X | X | X | X |
| Dietary intake (Block Food Frequency Questionnaire) (Harlan and Block 1990) | X | X | |||
| Physical activity (International Physical Activity Questionnaire) (Craig et al., 2003) | X | X | X | X | |
| Biological samples | |||||
| Hormones | X | X | X | X | X |
| Insulin, adiponectin, ghrelin, leptin, PYY, NPY | |||||
| IGF-1, IGFBP-1, IGFBP-2, IGFBP-3, hGH | |||||
| Glucagon, C-Peptide, Amylin, Resistin, | |||||
| PP, GLP-2, GIP | |||||
| Cytokines | X | X | X | X | X |
| IL-6, IL-10, TNF-alpha, CRP | |||||
| Conventional metabolites | X | X | X | X | X |
| Glucose, free fatty acids, triglycerides, Total cholesterol, HDL, LDL, Totak Ketones, β-hydroybutyrate | |||||
| Plasma lactate and plasma pyruvate | |||||
| Metabolic profiling | X | X | X | X | X |
| Plasma acylcarnitines, amino acids, and free fatty acids | |||||
| Urinary organic acids | |||||
| Anthropometrics and physiology | |||||
| Height | X | ||||
| Weight | X | X | X | X | X |
| Waist circumference | X | X | X | X | |
| Body composition (DEXA and CT) | X | X | X | ||
| Resting metabolic rate (indirect calorimetry) | X | X | X | X | |
| Insulin sensitivity (HOMA) | X | X | X | X | X |
Measures of behavior
At major data collection visits, dietary intake was measured by Block Food Frequency Questionnaire (Harlan and Block, 1990), and physical activity by the International Physical Activity Questionnaire (IPAQ) (Craig et al., 2003).
Physiologic measurements
Weight was measured at each study visit. Body composition was measured by dual energy X-ray absorptiometry (DEXA) (Hologic QDR-1000/W, Bedford, MA), with calculation of total and regional fat and lean mass (Haqq et al., 2005).
Resting Energy Expenditure (REE) and Respiratory Exchange Rate (RER) were assessed by indirect calorimetry (ParvoMedics metabolic cart, Sandy, UT). Oxygen consumption was converted into kilocalories using the Weir equation (Weir, 1949).
Insulin resistance (IR) was estimated by the Homeostasis Model Assessment (HOMA) Index (fasting insulin microunits per milliliter* fasting glucose mmol/L) / 22.5) (Matthews et al., 1985).
Biochemical measurements
Blood and urine samples were obtained after an overnight fast and processed as previously described (Haqq et al., 2005).
Hormone analysis. Radioimmunoassay (RIA) kits were used for plasma leptin, insulin, C-peptide, glucagon, total ghrelin, total adiponectin, high molecular weight adiponectin, and total peptide YY (PYY) (Linco, St. Charles, MO); Neuropeptide Y (NPY), and serum pancreatic polypeptide (PP) (Alpco, Salem, NH), and human growth hormone (hGH), insulin-like growth factor-I (IGF-I) and three of its binding proteins (IGFBP-1, 2, and 3) (DSL, Webster, TX) (Haqq et al., 2005). ELISAs kits were used to measure plasma amylin, total gastric inhibitory polypeptide (GIP) (Linco), and resistin (Biovendor, Candler, NC) levels. Total plasma GLP-1 was measured by electrochemiluminescent assay (MesoScale Devices, Gaithersburg, MD).
Conventional metabolite analysis. Plasma glucose, lactate, total-, HDL- and LDL-cholesterol, triglycerides (Roche Diagnostics, Indianapolis, IN), free fatty acids (total) and ketones (total and 3-hydroxybutyrate) (Wako, Richmond, VA) were measured as previously described (Haqq et al., 2005). Plasma pyruvate was measured using a modified method (Hansen and Freier, 1978) based on utilization of NADH by endogenous lactate dehydrogenase.
Cytokine analysis. ELISA-based assays (Biosource, Camarillo, CA) were used to measure serum IL-6, IL-10, and TNF-α. Plasma CRP was measured by high-sensitivity immunoturbidimetry (Roche).
Mass-spectrometry-based metabolic profiling: Serum acylcarnitines and amino acids. Proteins were first removed by precipitation with methanol. Aliquoted supernatants were dried, and then esterified with hot, acidic methanol (acylcarnitines) or n-butanol (amino acids). Acylcarnitines and amino acids were analyzed by tandem mass spectrometry (MS/MS) with a Quattro Micro instrument (Waters Corporation, Milford, MA) (An et al., 2004; Hansen and Freier, 1978; Millington et al., 1990; Wu et al., 2004). Leu/Ile are reported as a single analyte because they are not resolved by our MS/MS method (Chace et al., 1995). In addition, the acidic conditions used to form butyl esters results in partial hydrolysis of glutamine to glutamic acid and asparagine to aspartate. Accordingly, values reported as Glu/Gln or Asp/Asn measure the amount of glutamate or aspartate plus the contribution of the partial hydrolysis reactions of glutamine and asparagine, respectively.
Plasma total fatty acids, serum free fatty acids, and urinary organic acids. (Patterson et al., 1999). For determination of total fatty acids (free + esterified) in plasma samples, fatty acid residues were aggressively transesterified to their methyl esters in a solution of 4 % v/v acetyl chloride in methanol, using a process adapted from Lepage and Roy (Trujillo and Scherer, 2006). To measure nonesterified free fatty acids, separate serum samples were gently methylated using iodomethane and purified by solid-phase extraction (Lehrke et al., 2004). For analysis of urinary organic acids, analytes were extracted in ethyl acetate, dried, and then converted to trimethyl silyl esters by N,O-bis (trimethylsilyl) trifluoroacetamide, with protection of alpha-keto groups by oximation with ethoxyamine hydrochloride. Derivatized fatty and organic acids were analyzed by capillary gas chromatography/mass spectrometry (GC/MS) using a Trace DSQ instrument (Thermo Electron Corporation, Austin, TX).
All mass-spectrometric analyses employed stable-isotope-dilution. Quantification of the foregoing “targeted” intermediary metabolites was facilitated by addition of mixtures of known quantities of stable-isotope internal standards from Isotec (St. Louis, MO), Cambridge Isotope Laboratories (Andover, MA), and CDN Isotopes (Pointe-Claire, Quebec, CN) to samples, as follows: Acylcarnitine assays—D3-acetyl, D3-propionyl, D3-butyryl, D9-isovaleryl, D3-octanoyl, and D3-palmitoyl carnitines; amino acid assays—15N1,13C1-glycine, D4-alanine, D8-valine, D7-proline, D3-serine, D3-leucine, D3-methionine, D5-phenylalanine, D4-tyrosine, D3-aspartate, D3-glutamate, D2-ornithine, D2-citrulline, and D5-arginine; organic acid assays—D3-lactate, D3-pyruvate, D3-methylmalonate, D3-ethylmalonate, 13C4-succinate, D6-methylsuccinate, D2-fumarate, D2-isobutyrylglycine, D4-glutarate, D3-butyrylglycine, D2-isovalerylglycine, 13C1-malate, D4-adipate, D6-alpha-ketoglutarate, D3-3-hydroxy-3-methylglutarate, D3-hexanoylglycine, D4-suberate, 15N2-orotate, D2-homovanillate, and D3-citrate; fatty acid assays—D3-octanoate, D3-decanoate, D3-laurate, D3-myristate, D3-palmitate, 13C1-oleate, and D3-stearate.
Statistical analysis
This study is exploratory, rather than hypothesis testing; statistical methods reflect this purpose. Because of the limited sample size and nonnormal distribution for many variables, all results are presented as medians and interquartile range (IQR), and comparisons between any two time points are made by Wilcoxon signed-rank tests on change scores. A nominal p-value of 0.01 was considered statistically significant, with no adjustment for multiple comparisons, and actual p-values are provided. In addition, data tables include all variables for which p < 0.05 for at least one comparison as well as other variables of interest. Complete data are presented in the Appendix.
Results
Of 50 WLM participants enrolled in the STEDMAN Project, 27 individuals completed the 12 month data collection visit and are included in this report. Of these 27 individuals, 16 (59%) were women; 17 (63%) were African-American. Median age and BMI at baseline were 51 years and 32.6 kg/m2, respectively. Baseline measurements were similar for the 27 participants in this analysis and the 23 that did not complete follow-up.
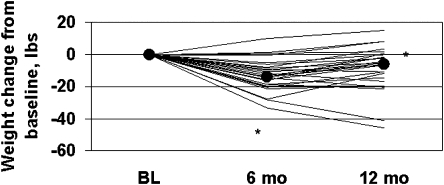
During the 6-month behavioral weight loss intervention, participants lost 13.9 pounds (p < 0.0001), with significant weight regain after the intensive intervention was completed (p = 0.0065) (Table 2).This pattern of weight change embodied some heterogeneity (Fig. 1): 23 of the 27 participants lost at least 5 pounds (decrease of 5.40 to 33.30 pounds) at 6 months. Between 6 and 12 months, 2 remained weight stable (less than 1-pound gain or loss), 6 continued to lose weight, and 19 regained at least 2 pounds (increase of 2.00 to 17.05 pounds).
Table 2.
Baseline Values and Changes in Weight, Diet, Physical Activity, Body Composition, Metabolic Rate, and Conventional Metbolites
| Measurement n = 27a | Baseline (BL) | Week 2–BL | Week 4–BL | Month 6–BL | Month 12–Month 6 | Month 12–BL |
|---|---|---|---|---|---|---|
| Weight (lbs) | 220.20 (190.10, 245.00) | −1.40 (−4.30, 0.30) | −3.80 (−6.90, −1.70) | −13.90 (−18.65, −8.00) | 5.10 (0.15, 8.75) | −6.15 (−16.90, −0.20) |
| p-value | 0.0065 | <.0001 | <.0001 | 0.0065 | 0.001 | |
| BMI (kg/m2) | 32.58 (30.76, 38.19) | −0.23 (−0.66, 0.05) | −0.69 (−1.15, −0.22) | −2.08 (−2.70, −1.07) | 0.78 (0.03, 1.42) | −0.99 (−2.57, −0.03) |
| p-value | 0.0096 | <.0001 | <.0001 | 0.006 | 0.001 | |
| Dietary intake % of calories from fat | 39.44 (34.83, 44.38) | −6.71 (−10.47, −2.81) | 3.04 (−0.38, 5.22) | −4.16 (−7.14, 0.11) | ||
| p-value | <.0001 | 0.0069 | 0.0132 | |||
| % of calories from carbohydrate | 45.82 (40.01, 49.98) | 6.53 (3.32, 11.64) | −1.87 (−6.26, 0.17) | 2.89 (−0.90, 8.47) | ||
| p-value | <.0001 | 0.0188 | 0.0231 | |||
| % of calories from protein | 15.65 (13.75, 18.23) | 0.98 (−2.08, 2.12) | 0.00 (−1.42, 1.30) | 1.16 (−2.30, 2.72) | ||
| p-value | 0.5787 | 0.8385 | 0.5036 | |||
| Physical activity: IPAQ (METs) | 1801.5 (410.00, 3084.00) | −564.00 (−1291.50, 205.00) | 422.25 (−1510.00, 741.75) | −274.50 (−2798.00, 478.25) | 99.00 (−972.00, 1177.50) | |
| p-value | 0.3652 | 0.9697 | 0.2024 | 0.4922 | ||
| Body composition Fat mass (kg) | 35.25 (29.54, 38.83) | −3.78 (−5.51, −1.38) | 2.14 (−0.64, 3.04) | −2.16 (−4.77, 0.84) | ||
| p-value | 0.0001 | 0.0305 | 0.0094 | |||
| Lean mass (kg) | 61.67 (49.74, 71.74) | −1.43 (−2.53, 0.27) | 0.51 (−0.56, 1.70) | −1.10 (−2.86, 0.27) | ||
| p-value | 0.0121 | 0.4586 | 0.0441 | |||
| Subcutaneous fat (mm2) | 36192 (31652, 46257) | −5483 (−6785, −1176) | 3675 (−342, 4525) | −1017 (−4042, 679) | ||
| p-value | 0.0008 | 0.0042 | 0.1674 | |||
| Visceral fat (mm2) | 14694 (12244, 18933) | −3298 (−4741, 60) | 678 (−893, 3931) | −1542 (−2206, 959) | ||
| p-value | 0.0139 | 0.2069 | 0.1815 | |||
| Resting metabolic rate REE/LBM (kcal/kg) | 2743 (24.97, 29.39) | −1.61 (−3.25, −0.11) | 2.77 (1.99, 4.58) | 1.24 (−0.28, 2.55) | ||
| p-value | 0.0083 | 0.0008 | 0.0897 | |||
| RER (RQ) | 0.87 (0.85, 0.90) | 0.02 (−0.06, 0.03) | 0.05 (−0.01, 0.07) | −0.06 (−0.14, −0.04) | −0.03 (−0.11, 0.04) | |
| p-value | 0.8461 | 0.0154 | 0.0001 | 0.04 | ||
| Conventional metabolites Ketones (μmol/L) | 55.00 (40.50, 92.50) | 12.00 (−7.00, 34.00) | 7.50 (−15.50, 34.00) | −7.50 (−28.00, 11.00) | 9.50 (−13.00, 22.50) | −1.50 (−35.00, 25.00) |
| p-value | 0.051 | 0.1753 | 0.0954 | 0.2088 | 0.6655 | |
| 3-hydroxybutyrate (μmol/L) | 31.00 (22.50, 55.00) | 6.50 (−4.50, 20.00) | 6.50 (−6.00, 22.00) | −18.50 (−28.00, −3.50) | 18.00 (2.00, 29.00) | −1.50 (−17.00, 10.50) |
| p-value | 0.0439 | 0.2043 | <.0001 | 0.8335 | ||
| Plasma lactic acid (mM) | 1.25 (1.08, 1.61) | −0.17 (−0.35, 0.10) | −0.27 (−0.53, −0.17) | −0.19 (−0.46, 0.10) | 0.04 (−0.22, 0.29) | −0.10 (−0.43, 0.30) |
| p-value | 0.0224 | <.0001 | 0.2667 | 0.6823 | 0.334 | |
| Plasma Pyruvic acid (mM) | 0.12 (0.09, 0.15) | −0.01 (−0.04, 0.02) | −0.02 (−0.04, 0.01) | −0.01 (−0.03, 0.02) | 0.01 (−0.05, 0.04) | −0.01 (−0.04, 0.02) |
| p-value | 0.2826 | 0.0092 | 0.2665 | 0.786 | 0.4668 | |
| Total Cholesterol (mg/dL) | 191.00 (174.00, 206.00) | −11.00 (−19.50, −2.00) | −12.50 (−19.50, 5.00) | −0.50 (−15.50, 18.50) | −5.00 (−21.50, 16.50) | −3.50 (−22.50, 10.00) |
| p-value | 0.0593 | 0.0216 | 0.9721 | 0.5442 | 0.2934 | |
| LDL (mg/dL) | 119.40 (100.85, 139.70) | −5.35 (−14.60, 2.90) | −3.85 (−19.10, 5.75) | −3.05 (−14.15, 13.65) | 20.10 (−20.10, 14.15) | 16.15 (−16.15, 6.70) |
| p-value | 0.0594 | 0.0667 | 0.5740 | 0.5191 | 0.0835 | |
| HDL (mg/dL) | 51.85 (3.900, 59.45) | −0.05 (−4.05, 2.00) | −0.35 (−4.45, 0.45) | −0.10 (−4.35, 3.45) | −0.65 (−3.20, 2.05) | −1.25 (−3.70, 4.05) |
| p-value | 0.2719 | 0.0706 | 0.9442 | 0.7435 | 0.9534 | |
| Triglycerides (mg/dL) | 89.00 (72.50, 110.50) | −2.50 (−14.50, 15.50) | −1.00 (−26.00, 14.50) | 0.00 (−24.50, 14.00) | 0.50 (−11.50, 23.50) | −2.50 (−18.50, 20.50) |
| p-value | 0.6230 | 0.7702 | 0.5645 | 0.7346 | 0.8978 | |
| Free fatty acids (mmol/L) | 0.51 (0.41, 0.71) | 0.02 (−0.02, 0.1) | −0.03 (−0.2, 0.1) | −0.11 (−0.3, 0.1) | −0.04 (−0.1, 0.1) | −0.05 (−0.3, 0.0) |
| p-value | 0.96 | 0.35 | 0.04 | 0.3 | 0.0065 |
All data presented as median (25th, 75th percentile).
N may vary due to completeness of data collection. Of note, N < 20 for the following measurements: International Physical Activity Questionnaire, Resting Metabolic Rate and Body Composition.
FIG. 1.
Patterns of weight change for individual participants during the 6-month behavioral weight loss intervention.
Dietary fat decreased and carbohydrate increased during the initial 6 months (p < 0.0001), consistent with the DASH dietary pattern (Table 2).Subsequently, fat intake increased and carbohydrate intake decreased (p = 0.0069 and p = 0.0188, respectively), consistent with dietary recidivism.
Total fat and lean mass decreased with weight loss (3.8 kg, p = 0.0001, and 1.43 kg, p = 0.0121, respectively), with some reversion with weight regain (Table 2).Both subcutaneous and visceral fat decreased dramatically in the first 6 months (p < 0.014), whereas only subcutaneous fat increased substantially in the second 6-month period (p = 0.004).
Weight loss was accompanied by decreased resting energy expenditure, which reverted with weight regain (Table 2).RER or Rq increased during weight loss (p = 0.015), consistent with the relatively high carbohydrate content of the DASH diet and presumed preferential oxidation of carbohydrates and amino acids relative to lipids. RER then declined (p = 0.0001) in the second 6-month period of the study, consistent with increased fat consumption.
HOMA decreased from a baseline of 4.73 to 3.28 at 6 months, with a median change of −0.91 (p = 0.007) (Table 3).Fasting insulin also decreased with weight loss. There was no significant reversion of these measures of insulin resistance during weight regain, such that values at 12 months remained significantly lower than baseline (p ≤ 0.0014).
Table 3.
Baseline Values and Changes in Insulin Resistance, Hormones, and Cytokines
| Measurement n = 27a | Baseline (BL) | Week 2–BL | Week 4–BL | Month 6–BL | Month 12–Month 6 | Month 12–BL |
|---|---|---|---|---|---|---|
| Insulin sensitivity | ||||||
| HOMA | 4.73 (3.32, 5.93) | 0.07 (−1.27, 1.02) | −0.10 (−1.76, 0.55) | −0.91 (−2.31, 0.04) | 0.20 (−0.98, 0.85) | −0.86 (−2.10, 0.13) |
| p-value | 0.8335 | 0.3461 | 0.0068 | 0.9256 | 0.0003 | |
| Fasting Insulin (μIU/mL) | 18.33 (14.45, 23.26) | 1.25 (−5.08, 3.69) | −0.28 (−5.87, 2.05) | −3.29 (−9.35, −1.02) | 1.31 (−2.47, 4.53) | −3.68 (−7.43, −0.28) |
| p-value | 0.9628 | 0.4243 | 0.0022 | 0.3972 | 0.0014 | |
| Hormones Adiponectin (μg/mL) | 5.28 (3.44, 8.25) | −0.32 (−0.62, 0.29) | −0.12 (−0.97, 0.42) | 0.03 (−0.23, 1.14) | 0.36 (−0.38, 1.93) | 0.88 (0.22, 1.86) |
| p-value | 0.0747 | 0.2772 | 0.3972 | 0.0317 | 0.0014 | |
| Ghrelin (pg/mL) | 809.59 (511.47, 962.67) | −28.23 (−55.85, 52.69) | −19.87 (−97.22, 74.17) | 127.26 (84.16, 250.17) | −111.92 (−179.14, −46.36) | −9.24 (−65.22, 112.15) |
| p-value | 0.6231 | 0.7613 | <0.001 | <0.001 | 0.7435 | |
| Leptin (ng/mL) | 23.17 (15.61, 43.89) | −1.84 (−6.28, −0.03) | −3.95 (−8.04, −0.61) | −5.46 (−11.89, −1.62) | 0.24 (−2.35, 5.23) | −4.74 (−6.52, −0.99) |
| p-value | 0.0276 | 0.0001 | <0.001 | 0.4107 | ||
| PYY (pg/mL) | 148.71 (125.52, 178.02) | 5.03 (−7.16, 26.20) | −0.31 (−12.63, 21.34) | −35.97 (−56.35, −20.53) | −18.31 (−30.08, −1.68) | −43.07 (−78.35, −38.67) |
| p-value | 0.0835 | 0.4243 | <0.001 | <0.001 | <0.001 | |
| NPY (pM) | 50.43 (44.54, 60.14) | −4.03 (−7.14, 4.76) | −0.08 (−6.87, 7.75) | −0.20 (−10.08, 2.92) | ||
| p-value | 0.2667 | 0.7389 | 0.2772 | |||
| IGF-1 (ng/mL) | 151.45 (90.48, 184.54) | −4.31 (−19.04, 29.31) | 3.30 (−13.87, 29.67) | 18.67 (−14.40, 57.03) | 61.61 (16.87, 85.48) | 73.00 (30.85, 128.46) |
| p-value | 0.8153 | 0.2772 | 0.024 | <0.001 | <0.001 | |
| IGFBP-1 (ng/mL) | 13.52 (7.75, 29.51) | 0.23 (−3.82, 16.29) | 0.48 (−2.25, 7.82) | −4.63 (−11.34, −0.50) | −1.22 (−4.24, 0.85) | −4.93 (−11.66, −2.95) |
| p-value | 0.4668 | 0.2178 | <0.001 | 0.0386 | <0.001 | |
| IGFBP-2 (ng/mL) | 111.99 (41.56, 394.56) | 28.70 (−18.71, 103.15) | 27.59 (−13.40, 104.99) | 17.58 (−88.53, 30.76) | 20.81 (−89.57, 75.09) | 0.00 (−107.15, 46.96) |
| p-value | 0.0276 | 0.0361 | 0.4383 | 0.8701 | 0.9116 | |
| IGFBP-3 (ng/mL) | 3257.32 (2830.47, 3655.17) | −80.54 (−349.02, 392.96) | 102.26 (−85.82, 268.95) | −164.69 (−363.89, 273.00) | −252.88 (−520.99, −103.55) | −417.10 (−556.82, −49.69) |
| p-value | 0.6231 | 0.2178 | 0.3585 | <0.001 | ||
| Growth hormone (ng/mL) | 0.09 (0.00, 0.63) | 0.00 (−0.12, 0.19) | 0.04 (0.00, 0.35) | 0.11 (−0.12, 0.13) | −0.02 (−0.10, 0.37) | 0.02 (−0.09, 0.84) |
| p-value | 0.5344 | 0.1572 | 0.3105 | 0.0929 | 0.3221 | |
| Cytokines IL-6 (pg/mL) | 5.35 (3.40, 9.15) | −1.17 (−2.86, 2.38) | 0.27 (−1.41, 2.14) | −0.06 (−1.93, 1.58) | ||
| p-value | 0.227 | 0.6909 | 0.9256 | |||
| IL-10 (pg/mL) | 0.02 (0.00, 1.39) | 0.00 (0.00, 0.31) | 0.00 (−0.40, 0.45) | 0.01 (−0.05, 0.09) | ||
| p-value | 0.5245 | 0.8911 | 0.6692 | |||
| TNF-alpha (pg/mL) | 9.42 (8.12, 11.86) | −0.15 (−1.09, 0.53) | 0.06 (−0.79, 1.25) | −0.25 (−1.62, 1.98) | ||
| p-value | 0.3221 | 0.8153 | 0.6567 | |||
| CRP (mg/L) | 2.55 (1.50, 6.10) | −0.25 (−0.90, 0.40) | −0.65 (−1.25, 0.70) | −0.45 (−1.00, 0.15) | −0.25 (−1.20, 0.70) | −0.45 (−1.30, 0.15) |
| p-value | 0.6312 | 0.2935 | 0.0373 | 0.2891 | 0.0148 | |
All data presented as median (25th, 75th percentile).
N may vary due to completeness of data collection. (N ranges from 22 to 27).
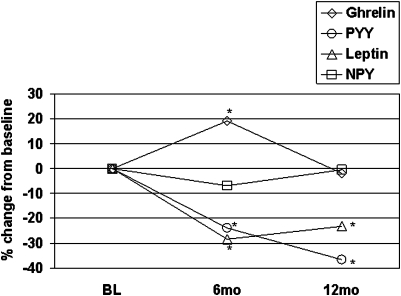
As expected, fasting plasma ghrelin levels increased with weight loss (p < 0.0001 compared to baseline), with reversion to baseline values by 12 months (Table 3).In contrast, leptin and PYY levels fell with overall weight loss but then remained persistently lower than baseline despite weight regain. Circulating levels of NPY remained unchanged at 6 and 12 months, despite the large decrease in leptin, a key inhibitor of NPY synthesis in the central nervous system, and the increase in ghrelin, a stimulator of NPY production (Kalra and Kalra, 2003; Palmiter et al., 1998).
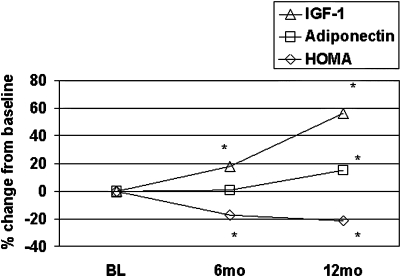
Table 3 shows changes in hormones that track with the persistent improvement in insulin sensitivity. Thus, levels of total adiponectin remained stable during weight loss but increased by 17% compared to baseline between 6 and 12 months (p = 0.001) despite weight regain. A similar pattern was observed for high molecular weight adiponectin, thought to be even more tightly correlated with insulin sensitivity than total adiponectin (data not shown) (Trujillo and Scherer, 2006). Total IGF-I increased during overall weight loss and continued to increase by approximately 48% over the 12 months of observation. In contrast, IGFBP-1 and IGFBP-3 persistently decreased.
Circulating levels of the cytokines IL-6, IL-10, and TNF-α were not affected by weight loss or regain. However, the inflammatory marker CRP declined with weight loss and remained persistently lower than baseline values (Table 3).
Total nonesterified fatty acids measured by biochemical assay trended down at 6 months (p = 0.04), and continued to decline to significantly lower levels by 12 months (p = 0.0065; Table 2).We also measured individual species of fatty acids in blood-derived samples in the nonesterified (free fatty acids) and total (free + esterified) pools by GC/MS, using distinct methods of extraction of separate blood product aliquots to isolate these pools (see Materials and Methods). Consistent with the fall in total nonesterified fatty acids measured with the biochemical assay, several individual nonesterifed fatty acids, including myristic (C14:0), palmitic (C16:0), palmitoleic (C16:1), oleic (C18:1), and stearic (C18:0) acids showed trends toward a decline at 6 months and sustained lowering at 12 months (Table 4)(p values ranging from 0.01 to 0.09 for these analytes, comparison of 12 month values to baseline). In contrast, the polyunsaturated fatty acids α-linolenic (α-C18:3), linoleic (C18:2), and arachidonic (C20:4) showed no trends for change at any time.
Table 4.
Baseline Values and Changes in Metabolites Measured by Mass Spectrometry
| Measurement n = 27a | Baseline (BL) | Week 2–BL | Week 4–BL | Month 6–BL | Month 12–Month 6 | Month 12–BL |
|---|---|---|---|---|---|---|
| Free fatty acids (μM) | ||||||
| Myristic (cl4) | 9.22 (6.33, 12.41) | −0.28 (−5.18, 2.51) | −1.17 (−5.57, 3.10) | −1.93 (−5.73, 1.48) | 0.04 (−2.48, 2.10) | −2.2 (−6.97, 1.25) |
| p-value | 0.5423 | 0.2772 | 0.0276 | 0.7435 | 0.0104 | |
| Palmitic (cl6) | 121.50 (92.08, 148.59) | 11.25 (−21.88, 24.50) | −5.54 (−58.03, 23.19) | −23.20 (−62.18, 13.67) | −3.43 (−36.17, 19.48) | −11.16 (−74.30, 17.52) |
| p-value | 0.7435 | 0.3461 | 0.0496 | 0.6567 | 0.0496 | |
| Palmitoleic (cl6:l) | 16.23 (12.73, 30.30) | 2.03 (−6.03, 7.17) | −0.42 (−7.62, 5.16) | −2.13 (−11.99, 1.88) | 0.35 (−4.37, 4.13) | −1.83 (−12.33, 1.43) |
| p-value | 0.7972 | 0.4383 | 0.0594 | 0.9814 | 0.0527 | |
| Stearic (cl8) | 39.27 (31.28, 47.71) | −2.88 (−10.07, 5.55) | −2.57 (−14.30, 7.77) | −5.80 (−12.23, 3.76) | −0.92 (−7.20, 4.76) | −2.06 (−11.82, 1.55) |
| p-value | 0.5114 | 0.1459 | 0.0747 | 0.7792 | 0.0929 | |
| Oleic (cl8:l) | 168.79 (127.16, 250.38) | 13.98 (−41.96, 55.45) | −2.74 (−59.15, 46.94) | −20.94 (−94.24, 34.43) | −10.11 (−47.05, 40.07) | −21.58 (−112.99, 27.17) |
| p-value | 0.3841 | 0.9814 | 0.1677 | 0.7972 | 0.056 | |
| Linolenic (cl8:3) | 6.52 (4.41, 9.92) | 0.51 (−2.26, 2.35) | 0.93 (−1.69, 3.16) | −0.45 (−2.79, 2.26) | 0.17 (−1.85, 2.06) | −1.35 (−4.42, 1.46) |
| p-value | 0.5581 | 0.6909 | 0.4524 | 0.9814 | 0.1602 | |
| Linoleic (cl8:2) | 74.03 (61.47, 101.76) | 8.76 (−10.86, 23.01) | 4.57 (−33.63, 32.08) | −8.45 (−39.45, 14.85) | 3.42 (−13.83, 14.00) | −4.37 (−40.65, 9.12) |
| p-value | 0.3972 | 0.7972 | 0.1143 | 0.9814 | 0.1326 | |
| Arachidonic (c20:4) | 6.73 (5.20, 9.39) | −1.46 (−5.21, 0.56) | −0.21 (−2.49, 1.56) | −0.17 (−2.26, 2.53) | −0.41 (−2.99, 1.26) | −0.39 (−3.70, 1.05) |
| p-value | 0.0747 | 0.4668 | 0.8335 | 0.4107 | 0.288 | |
| Total fatty acids (piM) Octanoic (c8) | 26.54 (23.40, 29.59) | 12.73 (−3.15, 20.45) | 10.06 (−4.30, 18.27) | 12.56 (−2.86, 23.43) | 1.07 (−13.13, 21.50) | 13.86 (2.64, 22.46) |
| p-value | 0.0104 | 0.0296 | 0.0132 | 0.7389 | 0.0043 | |
| Decanoic (c10) | 5.39 (4.42, 7.86) | 0.20 (−1.31, 2.15) | 0.19 (−1.44, 1.80) | 1.52 (0.09, 3.96) | 1.06 (−1.55, 4.20) | 2.71 (0.64, 5.29) |
| p-value | 0.7972 | 0.4963 | 0.0008 | 0.6909 | 0.0033 | |
| Laurie (cl2) | 10.02 (8.05, 14.44) | −0.23 (−6.03, 5.69) | 0.49 (−6.01, 5.97) | 7.75 (0.34, 17.62) | −6.10 (−16.71, 10.23) | 3.49 (−4.54, 14.13) |
| p-value | 0.9163 | 0.9070 | 0.0011 | 0.3221 | 0.1143 | |
| Palmitoleic (cl6:l) | 410.15 (295.45, 486.62) | 81.40 (−14.24, 280.72) | 93.70 (−46.70, 168.85) | 91.91 (42.28, 258.36) | −92.5 (−166.8, 58.5) | 30.15 (−69.31, 172.38) |
| p-value | 0.0040 | 0.1032 | 0.0016 | 0.0496 | 0.3585 | |
| Palmitic (cl6) | 1479.6 (1282.3, 2017.8) | 637 (368, 1031) | 708 (276, 985) | 591 (304, 863) | 206 (−262, 357) | 662 (330, 1010) |
| p-value | <.0001 | <.0001 | <.0001 | 0.3712 | <.0001 | |
| Stearic (cl8) | 883.91 (804.29, 991.98) | 6.3 (−75.1, 143.9) | 41.3 (−121.6, 131.7) | 75.3 (−63.9, 201.5) | 90.2 (−153.8, 245.6) | 107.2 (−96.4, 317.7) |
| p-value | 0.6398 | 0.3841 | 0.0438 | 0.2772 | 0.0386 | |
| Amino acids (μM) | ||||||
| Alanine | 435.55 (399.32, 482.60) | −25.26 (−88.62, 19.35) | −59.84 (−87.36, −32.05) | −24.02 (−57.33, 11.48) | −8.85 (−31.76, 36.61) | −16.94 (−77.74, 16.29) |
| p-value | 0.0386 | <.0001 | 0.1032 | 0.779 | 0.1202 | |
| Glycine | 292.63 (258.10, 330.34) | −8.89 (−66.06, 24.98) | −23.5 (−56.67, 3.12) | 10.81 (−24.69, 39.01) | −11.77 (−43.02, 29.84) | −2.7 (−41.28, 51.10) |
| p-value | 0.2001 | 0.0296 | 0.4243 | 0.334 | 0.9256 | |
| Leucine/isoleucine | 169.55 (152.13, 206.96) | −4.33 (−27.30, 14.98) | −6.4 (−21.36, 8.74) | −10.20 (−25.22, 1.31) | 0.20 (−10.31, 18.61) | −4.37 (−28.57, 6.38) |
| p-value | 0.4383 | 0.0835 | 0.004 | 0.6567 | 0.2001 | |
| Methionine | 30.03 (26.51, 33.78) | −0.30 (−3.46, 0.50) | −2.09 (−5.79, −0.74) | −0.32 (−4.32, 2.32) | −0.99 (−3.12, 0.67) | −0.96 (−5.80, 1.23) |
| p-value | 0.2001 | 0.0001 | 0.4524 | 0.0496 | 0.0835 | |
| Phenylalanine | 72.92 (66.17, 80.13) | −1.64 (−5.10, 4.31) | −2.05 (−8.25, 0.57) | −3.51 (−5.49, 4.51) | −0.45 (−9.54, 5.50) | −4.36 (−8.85, 3.82) |
| p-value | 0.9256 | 0.0258 | 0.4668 | 0.3972 | 0.0648 | |
| Acylcarnitines (μM) | ||||||
| Octenoyl (c8:l) | 0.20 (0.15, 0.29) | 0.01 (−0.03, 0.11) | 0.03 (0.00, 0.06) | 0.03 (−0.05, 0.08) | −0.03 (−0.07, 0.06) | 0.02 (−0.04, 0.11) |
| p-value | 0.0957 | 0.02 | 0.2662 | 0.7389 | 0.2888 | |
| Decatrienoyl (cl0:3) | 0.11 (0.07, 0.13) | 0.02 (−0.01, 0.08) | 0.02 (−0.01, 0.06) | 0.02 (−0.03, 0.05) | −0.01 (−0.03, 0.05) | 0.02 (0.00, 0.07) |
| p-value | 0.205 | 0.0102 | 0.1834 | 0.8241 | 0.0242 | |
| Urinary organic acids (mmol/mol creatinine) | ||||||
| Methylmalonate | 0.94 (0.64, 1.09) | 0.07 (−0.05, 0.30) | 0.17 (−0.02, 0.41) | 0.42 (0.10, 0.54) | 0.14 (−0.15, 0.42) | 0.44 (0.28, 0.79) |
| p-value | 0.0812 | 0.004 | <0.0001 | 0.1391 | <0.0001 | |
| Ethylmalonate | 2.51 (2.26, 2.98) | 0.66 (0.44, 0.90) | 0.87 (0.64, 1.02) | 0.81 (0.46, 1.01) | −0.42 (−0.73, 0.03) | 0.45 (−0.07, 0.66) |
| p-value | 0.0001 | <0.0001 | <0.0001 | 0.0009 | 0.01 | |
| Fumarate | 0.63 (0.42, 0.69) | −0.02 (−0.10, 0.03) | −0.02 (−0.15, 0.08) | 0.00 (−0.12, 0.10) | −0.13 (−0.27, 0.08) | −0.11 (−0.30, −0.06) |
| p-value | 0.201 | 0.4668 | 0.8426 | <0.0001 | 0.0001 | |
| Isobutyrylglycine | 0.41 (0.26, 0.58) | 0.04 (0.00, 0.18) | 0.06 (−0.03, 0.25) | 0.03 (−0.02, 0.14) | −0.12 (−0.24, 0.07) | −0.07 (−0.24, 0.01) |
| p-value | 0.0038 | 0.0224 | 0.066 | 0.0576 | 0.1022 | |
| Glutarate | 0.85 (0.69, 1.21) | 0.05 (−0.18, 0.04) | −0.05 (−0.18, 0.15) | 0.03 (−0.09, 0.22) | 0.04 (−0.26, 0.24) | 0.02 (−0.10, 0.28) |
| p-value | 0.0316 | 0.6566 | 0.4524 | 0.9256 | 0.2826 | |
| Butyrylglycine | 0.31 (0.19, 0.39) | 0.01 (−0.07, 0.11) | 0.09 (−0.10, 0.16) | 0.12 (−0.04, 0.32) | −0.09 (−0.18, 0.02) | 0.00 (−0.08, 0.21) |
| p-value | 0.7258 | 0.2667 | 0.018 | 0.0194 | 0.6398 | |
| 3-hydroxy-3methylglutarate | 2.25 (1.88, 2.55) | 0.60 (0.24, 0.83) | 0.59 (0.39, 0.84) | 0.42 (0.17, 1.00) | −0.30 (−0.77, 0.27) | 0.44 (−0.41, 0.68) |
| p-value | <0.0001 | <0.0001 | <0.0001 | 0.1358 | 0.1754 | |
| Adipate | 0.93 (0.80, 1.32) | 0.13 (−0.07, 0.39) | 0.13 (−0.13, 0.62) | 0.21 (−0.13, 0.55) | −0.17 (−0.83, 0.19) | −0.02 (−0.24, 0.23) |
| p-value | 0.1791 | 0.1143 | 0.1754 | 0.0496 | 0.8701 | |
| Alpha-ketoglutarate | 4.71 | 0.12 (−1.09, 0.82) | −0.10 (−1.75, 0.67) | 2.16 (0.35, 4.31) | −1.35 (−3.21, 1.45) | 1.63 (−0.15, 2.71) |
| p-value | 0.6652 | 0.3105 | 0.0018 | 0.1459 | 0.0022 | |
| Suberate | 0.57 (0.46, 0.84) | 0.03 (−0.08, 0.19) | 0.03 (−0.14, 0.23) | 0.14 (−0.12, 0.26) | −0.13 (−0.51, 0.07) | 0.07 (−0.16, 0.18) |
| p-value | 0.334 | 0.5423 | 0.0438 | 0.056 | 0.6737 | |
| Orotate | 0.49 (0.33, 0.60) | −0.04 (−0.14, 0.04) | −0.05 (−0.10, 0.08) | −0.07 (−0.16, −0.01) | −0.02 (−0.16, 0.13) | −0.10 (−0.27, 0.08 |
| p-value | 0.0576 | 0.2667 | 0.0026 | 0.8532 | 0.0954 | |
| Citrate | 310.38 (207.20, 372.02) | −38.61 (−94.41, 1.18) | −23.78 (−108.81, 23.90) | −26.33 (−61.65, 12.43) | −34.56 (−102.17, 60.92) | −76.90 (−114.83, 0.06) |
| p-value | 0.0074 | 0.1754 | 0.056 | 0.2088 | 0.0033 | |
All data presented as median (25th, 75th percentile).
N may vary due to completeness of data collection. (N ranges from 26 to 27).
In contrast, several individual saturated fatty acids were markedly increased during weight loss in the total fatty acid pool, which predominantly represents esterified species (Table 4).Two patterns of increase were observed. First, octanoic (C8:0), and palmitic (C16:0) acids increased sharply in the first 2 weeks of the intervention, and then remained elevated at all other time points of the study. Second, decanoic (C10:0), lauric (C12:0), and stearic (C18:0) acids trended to increase or increased significantly at the 6-month time point (p values ranging from 0.0008 to 0.0438), and then remained elevated at 12 months. Among unsaturated species, only palmitoleic (C16:1) acid showed a sporadic rise at 2 weeks and 6 months, but these changes were not sustained in the 12 month samples.
Other metabolites also changed in concert with improved insulin sensitivity. Serum leucine/isoleucine declined significantly (p = 0.004) at 6 months, and remained low at 12 months. The tricarboxylic acid cycle intermediate α-ketoglutarate (α-KG) was significantly increased at 6 months (p = 0.0018), and citrate decreased at 12 months (p = 0.0033).
Early in the intervention (2 weeks and 4 weeks), the ketone β-hydroxybutyrate (3-HB) increased, with a similar trend in total ketone levels (Table 2).This was accompanied by an increase in organic acid metabolites, such as methylmalonate, ethylmalonate, and 3-hydroxy-3-methylglutarate (p ≤ 0.08 at 2 weeks, and ≤0.004 at 4 weeks for all analytes) (Table 4), presumably a result of increased fatty acid and amino acid catabolism. Plasma alanine, methionine, pyruvate, and lactate fell within the first 4 weeks of intervention (p ≤ 0.009), and then reverted towards baseline levels (Tables 3 and 4). As the intervention proceeded and consumption of the DASH diet continued, 3-HB fell at 6 months (p < 0.001). As subjects began to consume diets with higher fat content in the second 6 months, 3-HB increased dramatically (p < 0.001), reverting completely to baseline levels; similar trends were noted for total ketone levels at 6 and 12 months (Table 2).These results are consistent with a pattern of enhanced oxidation of endogenous fat and protein depots in response to caloric restriction early in the intervention, followed by transition to preferential oxidation of carbohydrate at 6 months during the intensive intervention with the DASH diet, and a switch to preferential lipid oxidation at 12 months as subjects reverted to a higher rate of fat consumption.
Discussion
Comprehensive metabolic profiling provides a unique opportunity to gain new insights into biochemical, endocrine, inflammatory marker, and physiologic changes occurring during weight loss and regain in humans. In this analysis, we report 12-month follow-up data on 27 obese adults who lost weight by means of a 6-month behavioral intervention incorporating the DASH dietary pattern and moderate caloric restriction. Several changes, including RER, REE, and circulating ketones, were consistent with adherence to the DASH dietary pattern during the first 6-months followed by a return to a diet higher in fat and partial weight regain during the subsequent 6 months.
In the initial 6 months of intervention, ghrelin levels increased, and PYY and leptin levels decreased. Leptin is produced in adipose tissue and inhibits food intake, whereas ghrelin and PYY3–36 are gut hormones thought to stimulate and inhibit food intake, respectively (Bjorbaek and Kahn, 2004; Ellacott et al., 2006; Hosoda et al., 2006). All three peptides are thought to exert their effects on food intake via binding to receptors in the arcuate nucleus of the hypothalamus. Thus, the changes in these hormones in the first 6 months can be viewed as a concerted response to maintain body weight set-point, and as an impediment for successful long-term weight loss. In the 6- to 12-month period of partial weight regain in the current study, levels of the orexigenic hormone ghrelin decreased as expected as resting energy expenditure and body weight increased, whereas levels of the anorexigenic hormones leptin and PYY remained persistently lower than baseline. These findings suggest that one entire arm of the concerted mechanism for control of food intake is rendered unresponsive during partial weight regain.
Leptin, ghrelin, and PYY are thought to regulate body weight through effects on production of hypothalamic NPY (Bjorbaek and Kahn, 2004; Ellacott et al., 2006; Hosoda et al., 2006). In this context, a noteworthy finding of the current study is that serum levels of NPY remained unchanged at all time points, seemingly impervious to the rise and fall of ghrelin concentrations or the sustained lowering of circulating leptin and PYY levels (Fig. 2). In a separate arm of the STEDMAN project, we have found that circulating NPY levels are significantly elevated in 74 obese compared to 67 lean subjects, suggesting that obese individuals have a constitutive elevation in NPY levels (Newgard et al., 2008). Since NPY readily crosses the blood–brain barrier (Kastin and Akerstrom, 1999), these increases in circulating NPY may be relevant to control of feeding behaviors via signaling in the hypothalamus. If so, the fixed level of NPY, in concert with the failure of the anorexigenic peptides leptin and PYY to respond to weight regain, may contribute to weight “rebound.”
FIG. 2.
Changes in satiety hormones over time.
During the 6-month intensive intervention period, study participants lost weight and demonstrated an improvement in several measures of insulin resistance, including HOMA and circulating insulin levels. During the 6–12-month period, one might have expected to see a reversion of these effects, but the improvements persisted throughout the period of weight regain. Similarly, we as well as others (Brinkworth et al., 2004) note a sustained decrease over 12 months of an inflammatory marker associated with cardiovascular disease, CRP, providing another indication of persistent improvement in “metabolic health” in response to a transient period of weight loss. Two potential hormonal mediators of this response are adiponectin and IGF-1. Figure 3 shows the changes in these hormones in relation to changes in HOMA. Circulating adiponectin levels have been reported to rise with weight loss (Hotta et al., 2000; Trujillo and Scherer, 2006; Yamamoto et al., 2004). However, in our study, total adiponectin levels did not rise significantly in the first 6 months of weight loss (consistent with other studies) (Ryan et al., 2003), but rather exhibited a rise during weight regain in concert with the persistent improvement in insulin sensitivity. IGF-1 levels increased during weight loss and then continued to rise to a significant level, whereas IGF-binding proteins-1 and −3, were decreased throughout the 12 months, and hGH was unchanged, suggesting a persistent increase in free IGF-I that may contribute to the improvement in insulin sensitivity (Clemmons, 2004). Overall, our study suggests long-term metabolic benefits of modest weight loss, and identifies IGF-1 and adiponectin as possible mediators of this response.
FIG. 3.
Changes in insulin resistance, adiponectin, and IGF-1 over time.
As noted above, not every individual experienced the same degree of weight loss and regain. However, we confirmed our findings in a subset of study participants who lost at least 5 pounds during the first 6 months and regained at least 2 pounds in the second 6 months (n = 16) (data not shown). In addition, although our observations of persistent metabolic improvement despite weight regain may seem counterintuitive, they are not completely unprecedented. In a comparison of low-carbohydrate versus low-fat weight loss diets, insulin secretion during a glucose tolerance test was significantly lower than baseline in both groups at 12 months, after a period of partial weight regain (Foster et al., 2003). Also, in a trial of 3 months of hypocaloric meal replacements, there were significant improvements in blood glucose and insulin levels 4 years later associated with persistent weight loss (Flechtner-Mors et al., 2000). However, these studies did not provide a comprehensive metabolic profile that identifies factors that may contribute to the sustained improvement in insulin sensitivity.
Several saturated and monounsaturated nonesterifed long chain fatty acids as well as total non-esterifed free fatty acid levels decreased in response to weight loss and remained low during the period of sustained improvement in insulin sensitivity despite weight regain. A large number of studies in experimental animals and humans have linked free fatty acid levels and tissue lipid accumulation to insulin resistance (An et al., 2004; Morino et al., 2006; Muoio and Newgard, 2006; Summers, 2006). However, a novel finding of this study is that the decrease in several nonesterified free fatty acids was accompanied by an increase in several species of saturated fatty acids in the total serum fatty acid pool (nonesterified + esterified). Since the total fatty acid levels are primarily reflective of esterified species, these results suggest a repartitioning of saturated fatty acids from the nonesterified to the esterified pool in response to weight loss, possibly accompanied by changes in expression or activity of steroyl CoA desaturase. Our findings could indicate either that this change in lipid partitioning and/or saturation index contributed to sustained improvements in insulin sensitivity, or conversely, that resolution of insulin resistance by other mechanisms led to more effective insulin-mediated lipid storage and inhibition of lipolysis, leading to the observed changes in fatty acid levels. Further studies will be required to identify the specific esterified lipid moieties (triglycerides, phospholipids, cholesterol esters) that contain the increase pool of saturated fatty acids. We also noted a significant increase in subcutaneous, but not visceral fat during the 6–12-month time period, suggesting that the subcutaneous depot may serve a protective role by being replenished before the visceral depot; this is consistent with findings that insulin resistance correlates strongly with visceral, but not subcutaneous fat mass (Bjorntorp, 1991; Carey et al., 1996; Coon et al., 1992; Gastaldelli et al., 2002).
Changes in other classes of metabolic analytes are intriguing, and reflect the potential for hypothesis generation from this exploratory research. For example, changes in the amino acids leucine/isoleucine and alanine suggest that weight loss and regain are associated with changes in catabolism of branched-chain amino acids (BCAA). Several recent studies have linked BCAA to impaired insulin action (Nobukuni et al., 2005; Um et al., 2006) and to central control of satiety and food intake (Cota et al., 2006). These findings warrant further investigation.
As an initial approach to metabolic profiling, we examined variables individually. However, a deeper understanding of the interrelationships among the metabolic variables will require data reduction techniques, novel integrative approaches, and the development of predictive models, which are techniques ideally applied in larger populations. In addition, results of integrated analyses will require validation in other populations, ideally including populations that lose weight by different strategies. These integrated statistical techniques are beyond the scope of this paper but will follow in future analyses.
Finally, while it is important to consider the effect of sex and ethnicity on the relationships identified above, in this preliminary study, the numbers are too small (12 month n = 27) to allow for meaningful subgroup comparisons, but these are important future considerations in a larger population.
In summary, our findings suggest that persistent reductions in PYY and leptin, and unchanged levels of NPY may promote weight regain. Our data further suggest that persistent elevation of adiponectin and IGF-1, preferential restoration of subcutaneous relative to visceral fat depots, lowering of circulating free fatty acid and BCAA levels, and changes in the content of saturated fatty acids in the total fatty acid pool may promote sustained improvement in insulin sensitivity despite weight regain. If confirmed, these findings may help identify methods to promote long-term weight loss maintenance and maximize the persistent metabolic benefits of weight loss, thereby reducing obesity-related morbidity and mortality.
Acknowledgments
This study was supported by a sponsored research agreement from GlaxoSmithKline, and we would like to thank Drs. Derek Nunez, Jeff Cobb, William Wilkison, Nandu Gattu, Kenneth Batchelor, Nick Livingston, and Terry Walker from GSK for their support and good advice. The work was also supported by NIH Grant 1K23-RR-021979 (to A.M.H.). Participants in this study joined the STEDMAN Project by virtue of their participation in the Weight Loss Maintenance Study (WLM) at Duke, an NHLBI-sponsored multicenter clinical trial, supported by U01 HL068734-02 (to L.P.S.). The authors gratefully acknowledge Laveina Dash, Tonya Milligan, Lori Aiken, Chelle Yin, Janice Lee, Lorraine Elliott, Lauren C. Naliboff, Erin L. Chu, Dr. William Kraus, and Dr. Jarol Boan for their advice and assistance during this study. We thank Drs. Larry Gene Moss and Thomas Coffman for critical review of this manuscript. We also are grateful for the generous time and effort devoted by study participants.
Author Disclosure Statement
No competing financial interests exist.
References
- An J. Muoio D.M. Shiota M. Fujimoto Y. Cline G.W. Shulman G.I., et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med. 2004;10:268–274. doi: 10.1038/nm995. [DOI] [PubMed] [Google Scholar]
- Anonymous. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- Appel L.J. Moore T.J. Obarzanek E. Vollmer W.M. Svetkey L.P. Sacks F.M., et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C. Kahn B.B. Leptin signaling in the central nervous system and periphery. Recent Prog Horm Res. 2004;59:305–331. doi: 10.1210/rp.59.1.305. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P. Metabolic implications of body fat distribution. Diabetes Care. 1991;14:1132–1143. doi: 10.2337/diacare.14.12.1132. [DOI] [PubMed] [Google Scholar]
- Brantley P.J. Appel L.J. Hollis J.F. Stevens V.J. Ard J. Champagne C.M., et al. Weight loss maintenance (WLM): design and rationale of a multi-center trial to sustain weight loss (abstract) Presented at the Society of Behavioral Medicine Scientific Meeting. 2006 doi: 10.1177/1740774508096315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkworth G.D. Noakes M. Parker B. Foster P. Clifton P.M. Long-term effects of advice to consume a high-protein, low-fat diet, rather than a conventional weight-loss diet, in obese adults with type 2 diabetes: one-year follow-up of a randomised trial. Diabetologia. 2004;47:1677–1686. doi: 10.1007/s00125-004-1511-7. [DOI] [PubMed] [Google Scholar]
- Burton B.T. Foster W.R. Health implications of obesity: an NIH Consensus Development Conference. J Am Diet Assoc. 1985;85:1117–1121. [PubMed] [Google Scholar]
- Calle E.E. Thun M.J. Obesity and cancer. Oncogene. 2004;23:6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- Carey D.G. Jenkins A.B. Campbell L.V. Freund J. Chisholm D.J. Abdominal fat and insulin resistance in normal and overweight women: direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes. 1996;45:633–638. doi: 10.2337/diab.45.5.633. [DOI] [PubMed] [Google Scholar]
- Chace D.H. Hillman S.L. Millington D.S. Kahler S.G. Roe C.R. Naylor E.W. Rapid diagnosis of maple syrup urine disease in blood spots from newborns by tandem mass spectrometry. Clin Chem. 1995;41:62–68. [PubMed] [Google Scholar]
- Clemmons D.R. Role of Insulin-like growth factor in maintaining normal glucose homeostasis. Hormone Res. 2004;62(Supp 1):77–82. doi: 10.1159/000080763. [DOI] [PubMed] [Google Scholar]
- Coon P.J. Rogus E.M. Drinkwater D. Muller D.C. Goldberg A.P. Role of body fat distribution in the decline in insulin sensitivity and glucose tolerance with age. J Clin Endocrinol Metab. 1992;75:1125–1132. doi: 10.1210/jcem.75.4.1400882. [DOI] [PubMed] [Google Scholar]
- Cota D. Proulx K. Smith K.A. Kozma S.C. Thomas G. Woods S.C., et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- Craig C.L. Marshall A.L. Sjostrom M. Bauman A.E. Booth M.L. Ainsworth B.E., et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Ellacott K.L. Haltchev I.G. Cone R.D. Interactions between gut peptides and the central melanocortin system. Peptides. 2006;27:340–349. doi: 10.1016/j.peptides.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Flechtner-Mors M. Ditschuneit H.H. Johnson T.D. Suchard M.A. Adler G. Metabolic and weight loss effects of long-term dietary intervention in obese patients: four-year results. Obes Res. 2000;8:399–402. doi: 10.1038/oby.2000.48. [DOI] [PubMed] [Google Scholar]
- Flegal K.M. Carroll M.D. Ogden C.L. Johnson C.L. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- Foster G.D. Wyatt H.R. Hill J.O. McGuckin B.G. Brill C. Mohammed B.S., et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348:2082–2090. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- Gastaldelli A. Miyazaki Y. Pettiti M. Matsuda M. Mahankali S. Santini E., et al. Metabolic effects of visceral fat accumulation in type 2 diabetes. J Clin Endocrinol Metab. 2002;87:5098–5103. doi: 10.1210/jc.2002-020696. [DOI] [PubMed] [Google Scholar]
- Hansen J.L. Freier E.F. Direct assays of lactate, pyruvate, beta-hydroxybutryrate, and acetoacetate with a centrifugal analyzer. Clin Chem. 1978;24:475–479. [PubMed] [Google Scholar]
- Haqq A.M. Lien L.F. Boan J. Arlotto M. Slentz C.A. Muehlbauer M.J., et al. The Study of the Effects of Diet on Metabolism and Nutrition (STEDMAN) weight loss project: rationale and design. Contemp Clin Trials. 2005;26:616–625. doi: 10.1016/j.cct.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Harlan L.C. Block G. Use of adjustment factors with a brief food frequency questionnaire to obtain nutrient values. Epidemiology. 1990;1:224–231. doi: 10.1097/00001648-199005000-00008. [DOI] [PubMed] [Google Scholar]
- Hedley A.A. Ogden C.L. Johnson C.L. Carroll M.D. Curtin L.R. Flegal K.M. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- Hosoda H. Kojima M. Kangawa K. Biological, physiological, and pharmacological aspects of ghrelin. J Pharmacol Sci. 2006;100:398–410. doi: 10.1254/jphs.crj06002x. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S. Shargill N.S. Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Hotta K. Funahashi T. Arita Y. Takahashi M. Matsuda M. Okamoto Y., et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- Kalra S.P. amd Kalra P.S. Neuropeptide Y: a physiological orexigen modulated by the feedback action of ghrelin and leptin. Endocrine. 2003;22:49–56. doi: 10.1385/ENDO:22:1:49. [DOI] [PubMed] [Google Scholar]
- Kastin A.J. Akerstrom V. Nonsaturable entry of neuropeptide Y into brain. Am J Physiol. 1999;276:E479–E482. doi: 10.1152/ajpendo.1999.276.3.E479. [DOI] [PubMed] [Google Scholar]
- Klein S. Burke L.E. Bray G.A. Blair S. Allison D.B. Pi-Sunyer X., et al. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–2967. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- Knowler W.C. Barrett-Connor E. Fowler S.E. Hamman R.F. Lachin J.M. Walker E.A., et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski R.J. Flegal K.M. Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr. 2000;72:1074–1081. doi: 10.1093/ajcn/72.5.1074. [DOI] [PubMed] [Google Scholar]
- Kuczmarski R.J. Carroll M.D. Flegal K.M. Troiano R.P. Varying body mass index cutoff points to describe overweight prevalence among U.S. adults: NHANES III (1988 to 1994) Obes Res. 1997;5:542–548. doi: 10.1002/j.1550-8528.1997.tb00575.x. [DOI] [PubMed] [Google Scholar]
- Lehrke M. Reilly M.P. Millington S.C. Izbal N. Rader D.J. Lazar M.A. An inflammatory cascade leading to hyperresistinemia in humans. PLoS Med. 2004;1:e45. doi: 10.1371/journal.pmed.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D.R. Hosker J.P. Rudenski A.S. Naylor B.A. Treacher D.F. Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Millington D.S. Kodo N. Norwood D.L. Roe C.R. Tandem mass spectrometry: a new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inherit Metab Dis. 1990;13:321–324. doi: 10.1007/BF01799385. [DOI] [PubMed] [Google Scholar]
- Monzillo L.U. Hamdy O. Horton E.S. Ledbury S. Mullooly C. Jarema C., et al. Effect of lifestyle modification on adipokine levels in obese subjects with insulin resistance. Obes Res. 2003;11:1048–1054. doi: 10.1038/oby.2003.144. [DOI] [PubMed] [Google Scholar]
- Moore L.L. Visioni A.J. Qureshi M.M. Bradlee M.L. Ellison R.C. D'Agostino R. Weight loss in overweight adults and the long-term risk of hypertension: the Framingham study. Arch Intern Med. 2005;165:1298–1303. doi: 10.1001/archinte.165.11.1298. [DOI] [PubMed] [Google Scholar]
- Morino K. Petersen K.F. Shulman G.I. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;(Suppl 2):S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoio D.M. Newgard C.B. Obesity-related derangements in metabolic regulation. Ann Rev Biochem. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- Newgard C.B. An J. Bain J.R. Muehlbauer M. Stevens R.D. Lien L.F., et al. Study of the Effect of Diet on Metabolism and Nutrition (The STEDMAN Project. (2008) Metabolic Signatures that Differentiate Obese and Lean Subjects at Baseline. 2008 manuscript submitted for publication. [Google Scholar]
- Nobukuni T. Joaquin M. Roccio M. Dann S.G. Kim S.Y. Gulati P, et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci USA. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien K.D. Brehm B.J. Seeley R.J. Bean J. Wener M.H. Daniels S., et al. Diet-induced weight loss is associated with decreases in plasma serum amyloid a and C-reactive protein independent of dietary macronutrient composition in obese subjects. J Clin Endocrinol Metab. 2005;90:2244–2249. doi: 10.1210/jc.2004-1011. [DOI] [PubMed] [Google Scholar]
- Palmiter R.D. Erickson J.C. Hollopeter G. Baraban S.C. Schwartz M.W. Life without neuropeptide Y. Recent Prog Horm Res. 1998;53:163–199. [PubMed] [Google Scholar]
- Patterson B.W. Zhao G. Elias N. Hachey D.L. Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res. 1999;40:2118–2124. [PubMed] [Google Scholar]
- Ryan A.S. Nicklas B.J. Berman D.M. Elahi D. Adiponectin levels do not change with moderate dietary induced weight loss and exercise in obese postmenopausal women. Int J Obes Relat Metab Disord. 2003;27:1066–1071. doi: 10.1038/sj.ijo.0802387. [DOI] [PubMed] [Google Scholar]
- Sacks F.M. Obarzanek E. Windhauser M.M. Svetkey L.P. Vollmer W.M. McCullough M., et al. Rationale and design of the Dietary Approaches to Stop Hypertension trial (DASH). A multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol. 1995;5:108–118. doi: 10.1016/1047-2797(94)00055-x. [DOI] [PubMed] [Google Scholar]
- Shoelson S.E. Lee J. Goldfine A.B. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers J.R. Obesity as a cardiovascular risk factor. Am J Med. 2003;115(Suppl 8A):37S–41S. doi: 10.1016/j.amjmed.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Summers S.A. Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Trujillo M.E. Scherer P.E. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. 2006;27:762–778. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- Um S.H. D'Alessio D. Thomas G. Nutrient overload, insulin resistance and ribosomal protein S6 kinase 1, D6K1. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Weir J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen K.E. Hotamisligil G.S. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer C. Funahashi T. Tanaka S. Hotta K. Matsuzawa Y. Pratley R.E., et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- Willett W.C. Dietz W.H. Colditz G.A. Guidelines for healthy weight. N Engl J Med. 1999;341:427–434. doi: 10.1056/NEJM199908053410607. [DOI] [PubMed] [Google Scholar]
- Wu J.Y. Kao H.J. Li S.C. Stevens R. Hillman S. Millington D., et al. ENU mutagenesis identifies mice with mitochondrial branched-chain aminotransferase deficiency resembling human maple syrup urine disease. J Clin Invest. 2004;113:434–440. doi: 10.1172/JCI19574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y. Hirose H. Saito I. Nishikai K. Saruta T. Adiponectin, an adipocyte-derived protein, predicts future insulin resistance: two-year follow-up study in Japanese population. J Clin Endocrinol Metab. 2004;89:87–90. doi: 10.1210/jc.2003-031163. [DOI] [PubMed] [Google Scholar]