Abstract
Cancer cells, relative to normal cells, demonstrate increased sensitivity to glucose deprivation-induced cytotoxicity. To determine if oxidative stress mediated by O2•− and hydroperoxides contributed to the differential susceptibility of human epithelial cancer cells to glucose deprivation, oxidation of dihydroethidine (DHE; for O2•−) and 5-(and-6)-carboxy-2', 7'-dichlorodihydrofluorescein diacetate (CDCFH2; for hydroperoxides) were measured in human colon and breast cancer cells (HT29, HCT116, SW480, MB231) and compared to normal human cells (FHC, 33Co, HMEC). Cancer cells showed significant increases in DHE (2–20 fold) and CDCFH2 (1.8–10 fold) oxidation, relative to normal cells that were more pronounced in the presence of the mitochondrial electron transport chain blocker, antimycin A. Furthermore, HCT116 and MB231 cells were more susceptible to glucose deprivation-induced cytotoxicity and oxidative stress, relative to 33Co and HMEC. HT-29 cells were also more susceptible to 2-deoxyglucose-(2DG)-induced cytotoxicity, relative to FHC. Over expression of manganese superoxide dismutase and mitochondrially targeted catalase significantly protected HCT116 and MB231 cells from glucose deprivation-induced cytotoxicity and oxidative stress, as well as protecting HT-29 cells from 2DG-induced cytotoxicity. These results show cancer cells (relative to normal cells) demonstrate increased steady-state levels of reactive oxygen species (ROS, i.e. O2•− and H2O2) that contribute to differential susceptibility to glucose deprivation-induced cytotoxicity and oxidative stress. These studies support the hypotheses that cancer cells increase glucose metabolism to compensate for excess metabolic production of ROS as well as that inhibition of glucose and hydroperoxide metabolism may provide a biochemical target for selectively enhancing cytotoxicity and oxidative stress in human cancer cells.
Keywords: Cancer, superoxide, hydrogen peroxide, glucose deprivation, oxidative stress, manganese superoxide dismutase
Introduction
For more than eight decades, it has been known that cancer cells exhibit altered metabolism when compared to their normal counterparts [1–4]. These alterations include increased rates of glycolysis and pentose phosphate cycle activity along with slightly reduced mitochondrial respiration [1–4]. Originally these metabolic abnormalities were thought to result from some impairment or damage to the cancer cell’s ability to undergo respiration [2] but the underlying mechanisms responsible for these metabolic abnormalities are still not well understood.
In normal mammalian cells, mitochondria represent the major cellular organelle responsible for respiration [1]. Electron transport chains (ETCs) in the inner mitochondrial membrane are believed to be responsible for the majority of cellular O2 consumption as well as being hypothesized to be a source of reactive oxygen species (ROS) during metabolism [5–9]. In normal cells, as much as 1 % of the electrons flowing through ETCs are thought to undergo one-electron reductions of O2 to form superoxide (O2•−), which can then react to form other ROS such as hydrogen peroxide (H2O2) and organic hydroperoxides [5, 10–13]. However, studies directly comparing steady-state levels of O2•− in cancer vs. normal epithelial cells are lacking.
Studies of cancer cell mitochondria have noted many structural abnormalities [14, 15] and epithelial cancers from colon and breast have also demonstrated higher rates of mutations in mitochondrial DNA [16]. These data have lead to the speculation that cancer cells may have mitochondrial ETC defects that could contribute to increased steady-state levels of O2•− and H2O2 in human tumor cells when compared to normal cells but direct evidence supporting this speculation is lacking [10–13, 16, 17]. In addition, previous studies using glucose deprivation have suggested that increases in glucose metabolism in cancer cells, relative to normal cells, could be necessary to provide reducing equivalents in the form of NADPH and pyruvate for the detoxification of ROS [10–13]. However, there is no direct evidence linking increased steady-state levels of O2•− and H2O2 to the differential susceptibility of epithelial cancer vs. normal cells to glucose deprivation-induced cytotoxicity and oxidative stress.
In the current study carcinoma cells from human colon and breast epithelial tissue are shown to have increased steady-state levels of endogenous O2•− and ROS compared to normal cells derived from the same tissues as well as consuming more glucose, having higher activities of Pentose Phosphate Pathway enzymes associated with NADPH regeneration, and being more sensitive to oxidative stress and cell killing induced by glucose deprivation or treatment with an inhibitor of glucose metabolism (2-DG). Furthermore, glucose deprivation or 2DG-induced cytotoxicity as well as parameters indicative of oxidative stress could be inhibited by co-over expression of mitochondrially targeted superoxide dismutase (MnSOD) and mitochondrially targeted catalase (mitoCAT), that scavenge O2•− and H2O2, respectively. The results demonstrate that metabolic oxidative stress mediated by O2•− and H2O2 significantly contributes to the differential susceptibility of cancer vs. normal epithelial cells to glucose deprivation or 2DG-induced cytotoxicity. These results support the hypothesis that cancer cells exhibit increased glucose metabolism to compensate for excess metabolic production of ROS as well as the hypothesis that inhibition of glucose and hydroperoxide metabolism may provide a biochemical target for selectively enhancing cytotoxicity and oxidative stress in human cancer cells.
Materials and Methods
Cell and culture conditions
HT29, HCT116, and SW480 human colon carcinoma cells were obtained from American Type Culture Collection (ATCC; Manassas, VA); MDA-MB231 breast cancer cells were a gift from Dr. Mary Hendrix, Northwestern University. Cells were cultured in RPMI media supplemented with 10% fetal bovine serum (FBS, HyClone, Logan, UT). Normal non-immortalized human mammary epithelial cells (HMEC) were purchased from Clonetics (East Rutherford, NJ) and the cells were maintained in MEBM media (Clonetics). Normal non-immortalized human colon cells, FHC (epithelial) and CCD-33Co (fibroblasts) were obtained from ATCC. 33Co cells were maintained in EMEM with 2 mM L-glutamine and Earle's balanced salt solution with 1.5 g/L sodium bicarbonate, 0.1 mM non-essential amino acids, 1.0 mM sodium pyruvate, and 10% FBS. FHC cells were grown in DMEM medium and F-12 medium (1:1; vol/vol) supplemented with 10% FBS, insulin (5 mg/ml), hydrocortisone (0.5 mg/ml), transferrin (50 mg/ml), cholera toxin (500 ng/µl) and HEPES (20 mM). Normal (non-immortalized) human cells (passage 2–5) were maintained in a humidified 4% O2 chamber with 5% CO2 and N2, while other cultures were maintained in 5% CO2 and air in a humidified 37°C incubator. All experimental treatments comparing normal vs. tumor cells were performed in the same RPMI media preparations in the 4% O2 chamber until the collection of cells for each assay. For clonogenic survival assays, the complete media and incubator normally used to maintain each cell line was used for the cloning interval. We have also tested the effects of growing HCT116 cells in different pyruvate concentrations (0–1 mM) for 2 weeks or HCT116 and MB231 cells in different O2 tensions (4% O2 vs. 21% O2) for 4 weeks to determine if these variables could affect the conclusions of our studies, when comparing these tumor cells to normal cells. The results of these studies (data not shown) indicate that as long as all experimental comparisons are made 24 hours following switching the cells being compared to the identical culture conditions; no statistical differences between different maintenance conditions can be detected that would affect the overall conclusions.
Measurement of intracellular superoxide levels
Steady-state levels of superoxide were estimated using oxidation of the fluorescent dye, dihydroethidium (DHE) obtained from Molecular Probes, Eugene, Oregon. Cells were washed once with phosphate buffered saline (PBS) and labeled on culture plates at 37°C for 40 min in PBS (containing 5 mM pyruvate) with DHE (10 µM; in 0.1% DMSO). Culture plates were placed on ice to stop the labeling, trypsinized, and re-suspended in ice cold PBS. Samples were analyzed using a FACScan flowcytometer (Becton Dickinson Immunocytometry System, INC., Mountain View, CA) (excitation 488 nm, emission 585 nm band-pass filter). The mean fluorescence intensity (MFI) of 10,000 cells was analyzed in each sample and corrected for autofluorescence from unlabeled cells. The MFI data was normalized to corresponding normal cell levels for each cell type [18, 19].
Measurement of intracellular prooxidant levels
Steady–state levels of prooxidants (presumably hydroperoxides) were determined using the oxidation-sensitive {5-(and-6)-carboxy-2’,7’-dichlorodihydrofluorescein diacetate, (CDCFH2), 10 µg/mL} and oxidation-insensitive {5-(and-6)-carboxy-2’,7’-dichlorofluorescein diacetate, (CDCF), 10 µg/mL} fluorescent dyes (dissolved in 0.1% DMSO) that were obtained from Molecular Probes. The cells were washed once with PBS and labeled on the culture plates with the fluorescent dyes for 15 min at 37°C in PBS. At the end of the incubation time culture plates were placed on ice, trypsinized, re-suspended in ice cold PBS, and analyzed using a FACScan flow cytometer (excitation 488 nm, emission 530 nm band-pass filter). In each replicate experiment the numbers obtained for mean florescence intensity (MFI) of 10,000 cells/sample are arbitrary, based on the gain setting of the flow cytometer adjusted to the normal unlabeled cells in that particular experiment. In order to be able to combine the results of replicate experiments that were performed on different days, normalization to the MFI exhibited by the labeled normal cell type in each experiment was done. The MFI from the normal cell type on a given day was used as the denominator and the MFI obtained from each cancer cell type done on that same day was used as the numerator. Using this procedure data from each experiment were normalized to the corresponding normal cell type and combined for analysis [19, 20].
Glucose consumption
150,000–300,000 cells being compared were plated and allowed to grow 48 h in 4% O2. Cells were given fresh media at time zero. Cells were counted and media samples were obtained at 24 h and analyzed for glucose content using a YSI glucometer. Glucose consumption was determined by subtracting glucose content at 24 h point from the 0 h sample. Results were normalized to the number of the cells in each culture [19].
Measurement of glucose-6-phosphate dehydrogenase (G6PD) and 6-phosphogluconate dehydrogenase (6PGD) activities
Exponentially growing HCT116 cancer cells and 33Co normal colon fibroblasts in complete media at 4%O2 were washed with PBS and scrape harvested in PBS at 4°C. Combined G6PD+6PGD and 6PGD enzyme activities were measured in cell homogenates as previously described [21]. The first reaction used glucose-6-phosphate and 6-phosphogluconate as substrates and in the second reaction the substrate consisted of only 6-phosphogluconate without glucose-6-phosphate. The change in absorbance associated with the regeneration of NADPH was monitored at 340 nm. G6PD activity was calculated from the differences observed between the first reaction and the second reaction and errors were estimated using propagation of error theory. Results were normalized to protein content.
NADPH/ NADP+ Measurement
Following 24 h treatment with (+ Glu) or without (− Glu) glucose, HCT116 and MB231 cells were washed with PBS and scrape harvested in PBS at 4°C. After centrifugation at 1200 rpm for 5 min, cell pellets were re-suspended in extraction buffer containing 0.1 M Tris-HCl, pH 8.0, 0.01 M EDTA, and 0.05% (v/v) Triton X-100. The cell suspension was sonicated at a duty cycle of 34% (Sonics Vibracell, VC750) in ice water. The solution was centrifuged at 5000 rpm for 5 min. The supernatants were collected and analyzed immediately for NADPH and NADP+ as previously described [20]. Results were obtained by comparison with a standard curve using genuine NADPH and normalized per mg cellular protein.
Thiol analysis
Cells were grown to 70–80% confluency on 100 mm dishes. Following treatment, cells were washed with ice cold PBS, scraped into cold PBS, and centrifuged at 4 °C for 5 min at 400 × g to obtain cell pellets which were then frozen at −80 °C. Pellets were then thawed and homogenized in 50 mM potassium phosphate buffer, pH 7.8, containing 1.34 mM diethylenetriaminepentaacetic acid. All biochemical determinations were normalized to the protein content using the method of Lowry et al [22]. Glutathione content was determined by the GSH/GSSG recycling method [20, 23] and the yellow color change was detected spectrophotometrically at 412 nm. To determine the amount of glutathione disulfide, 2 µL of 2-vinylpyridine (1:1 volume/volume in 100% ethanol) was added to 30 uL of sample and incubated for 1 h and assayed as described [20, 23].
Measurement of glucose deprivation-induced cytotoxicity
Cytotoxicity due to glucose deprivation was measured by plating 200,000 cells on a 60 mm culture dish and incubated in 4% O2 at 37°C. The cells were allowed 24 h to recover from trypsin. At time zero cells were given fresh glucose free RPMI media containing 10% dialyzed FBS and gentamicin. Control cultures were treated identically except 11 mM glucose was added back at time zero. Clonogenic survival was determined as previously described [24] at 24, 48, and 72 h and normalized to the control cultures for each time point [20, 24].
Transduction of Antioxidant Enzymes
Replication incompetent adenoviral vectors, AdCMV Bgl II (AdBglII), and AdCMV MnSOD (AdMnSOD) were purchased from Viraquest (North Liberty, Iowa). They were prepared by inserting the gene of interest into the E1 region of an Ad5 E1/particle E3 deleted replication deficient adenoviral vector. The cDNAs were all under the control of a CMV promotor. Except for AdmitoCAT, the adenovirus constructs were originally prepared by Dr. John Engelhardt, University of Iowa [25]. The full-length catalase cDNA with the MnSOD mitochondrial leader sequence added to the construct was originally prepared by Dr. Andre Melendez [26]. Cells were plated the day before virus administration. The desired amount of viral particles was added with 1.8 mL of media per 60 mm dish for 24 h, and then the media was changed to complete media and left for another 24 h prior to each experiment.
Measurement of Antioxidant Enzyme Activities
Catalase activity was measured on whole cell homogenates in 50 mM potassium phosphate, pH 7.0. This method measures the exponential disappearance of H2O2 (10 mM) at 240 nm in the presence of cellular homogenates. The equation which is used to fit the exponential decay of H2O2 for 60 seconds is A60 = A0e−kt, where k = the rate constant which is dependent on the catalase activity. The activities were expressed in milli k (mk) units/mg protein added to each assay cuvette as described [27]. MnSOD activity of cell homogenates was determined as previously described [28] using an indirect competitive inhibition of nitroblue tetrazolium (NBT) reduction in the presence of cyanide.
Viability Assay
Cells were trypsinized and resuspended in medium. Cell suspension (0.1 mL) was then mixed with 0.1 mL of 0.04% trypan blue dye solution in PBS and incubated at 37°C for 2 min. The number of cells excluding the dye (viable) and cell stained by the dye (non-viable) were counted. Viability was expressed as the number of cells that excluded the dye divided by the total number (at least 100 cells) counted.
Statistical analysis
Data was expressed as mean ± 1 S.D unless otherwise specified. One-way ANOVA analysis with Tukey’s post hoc test was used to study the differences among three or more means. Two-way ANOVA analysis with Tukey’s post test was done to determine the differences over various time points. For analysis limited to two groups, Student’s t test was used. Significance was determined at p<0.05 and the 95% confidence interval.
Results
In order to be able to confirm that colon cancer cells used in this study (HT29, HCT116 and SW480) have higher Pentose Phosphate Pathway activity and higher glucose consumption compared to their normal counterparts (FHC and 33Co), the cell-mediated rate of glucose disappearance from media as well as glucose-6-phosphate dehydrogenase (G6PD) and 6-phosphogluconate dehydrogenase (6PGD) activities were measured (Table 1 and Table 2). All 3 colon carcinoma cell lines demonstrated increased levels of glucose consumption (3- to 7-fold) compared to FHC cells (Table 1). In addition G6PD and 6PGD activities were significantly elevated (3-fold and 20-fold, respectively) in HCT116 colon cancer cells, relative to 33Co normal colon fibroblasts (Table 2). These results clearly showed that colon cancer cells demonstrated increased glucose consumption and increased Pentose Phosphate Pathway activity, relative to normal cells derived from colon tissue.
Table 1.
Rates of glucose consumption in normal and cancerous colon epithelial cells (n=3)
| Cell Line | Glucose Consumption Rate (µmoles glucose/1 million cells/24hr) |
|---|---|
| FHC | 3 ± 0 |
| HT29 | 9 ± 1* |
| SW480 | 17 ± 3* |
| HCT116 | 21 ± 3* |
Significantly different from FHC, p<0.05
Table 2.
Glucose-6-phosphate dehydrogenase (G6PD) and 6-phosphogluconate dehydrogenase (6PGD) activity in normal and cancerous colon cells (n=3).
| Cell Line | G6PD+6PGD Activity (mU/mg protein) |
6PGD Activity (mU/mg protein) |
G6PD Activity (mU/mg protein) |
|---|---|---|---|
| 33Co | 20 ± 5 | 1 ± 1 | 19 ± 3 |
| HCT116 | 84 ± 3* | 29 ± 1* | 55 ± 4* |
Significantly different from 33Co, p<0.05
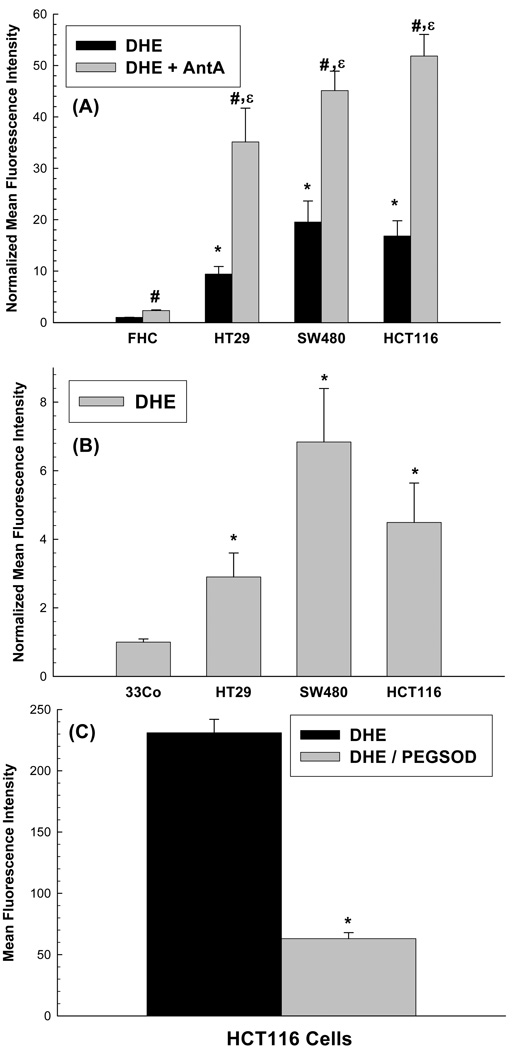
Steady state levels of O2•− were found to be significantly (p<0.05) elevated (10–20 fold) in colon carcinoma cells (HT29. HCT116 and SW480) compared to normal non-transformed colon epithelial cells (FHC) as determined by increased oxidation of DHE (Figure 1A). When the same cells were treated with the electron transport chain blocker antimycin A (known to increase mitochondrial O2•− production), DHE oxidation was significantly increased in all 4 cell lines (Figure 1A). The fact that the magnitude of increase in DHE oxidation caused by antimycin A in the cancer cells, (relative to FHC) was at least as great (if not greater) than that seen in the absence of antimycin A, strongly suggests that the cancer cell mitochondria are producing much greater amounts of O2•− then are the normal cell mitochondria. Independent comparisons using another normal cell type from colon tissue (33Co; colon fibroblasts) also demonstrated (Figure 1B) significantly greater DHE oxidation (3- to 7-fold) in HT29, HCT116, and SW480 colon carcinoma cells (p<0.05). HCT116 cells treated with 100 U/mL polyethylene-glycol conjugated CuZn superoxide dismutase (PEG-SOD) for 2 hours prior and during DHE labeling demonstrated that 75% of the fluorescent signal was inhibitable by SOD supporting the conclusion that DHE oxidation was truly reflective of steady-state levels of intracellular O2•− (Figure 1C). Overall, these results strongly support the hypothesis that human colon cancer cells demonstrate greater steady-state levels of intracellular O2•− , relative to normal colon epithelial cells and fibroblasts. The results with antimycin A also support the hypothesis that tumor cell mitochondria have a greater capacity for producing superoxide, relative to normal epithelial cell mitochondria.
Figure 1.
(A) Increased steady-state levels of superoxide demonstrated by increased DHE oxidation in human colon cancer cells (HT29, HCT116, SW480) compared to normal human colon epithelial cells (FHC). Cells were plated in 60 mm dishes, grown for 48 h and then incubated with 10 µM DHE in 2 ml PBS containing 5 mM pyruvate at 37°C for 40 min in the presence or absence of 10 µM AntA. Cells were trypsinized on ice and analyzed by flow cytometry. Mean fluorescence intensity (MFI) of 10,000 cells was measured. Values are expressed as the ratio of MFI, relative to FHC cells. Error bars represent ± 1SD of 3– 9 treatment dishes done in 3 separate experiments. [* Significantly different from FHC, DHE only, p<0.05, N=3; # significantly different from FHC, DHE+AntA, p<0.05, N=3–9 ; ε significantly different from each respective DHE only group, p<0.05, N=3–9]
(B) Increased steady-state levels of superoxide demonstrated by increased DHE oxidation in human colon cancer cells (HT29, HCT116, SW480) compared to normal human colon fibroblasts (33Co). Cells were grown and labeled with 10 µM DHE as described above and analyzed by flow cytometry. Mean fluorescence intensity (MFI) of 10,000 cells was measured. Values are expressed as the ratio of MFI, relative to 33Co cells. Error bars represent ± 1SD of 9 treatment dishes done in 3 separate experiments. [*Significantly different from 33Co, DHE only, p<0.05, N=9 ]
(C) HCT116 cells demonstrated PEG SOD inhibitable DHE fluorescence. Cells were plated in 60 mm dishes, grown for 48 h and treated with 100 U/ml PEG SOD for 2 h prior and during DHE labeling. Cells were trypsinized on ice and analyzed by flow cytometry. Each sampling measured the MFI of 10,000 cells and corrected for autofluorescence. Error bars represent ± 1SD of 3 treatment dishes. [*Significantly different from DHE only group, p<0.05, N=3]
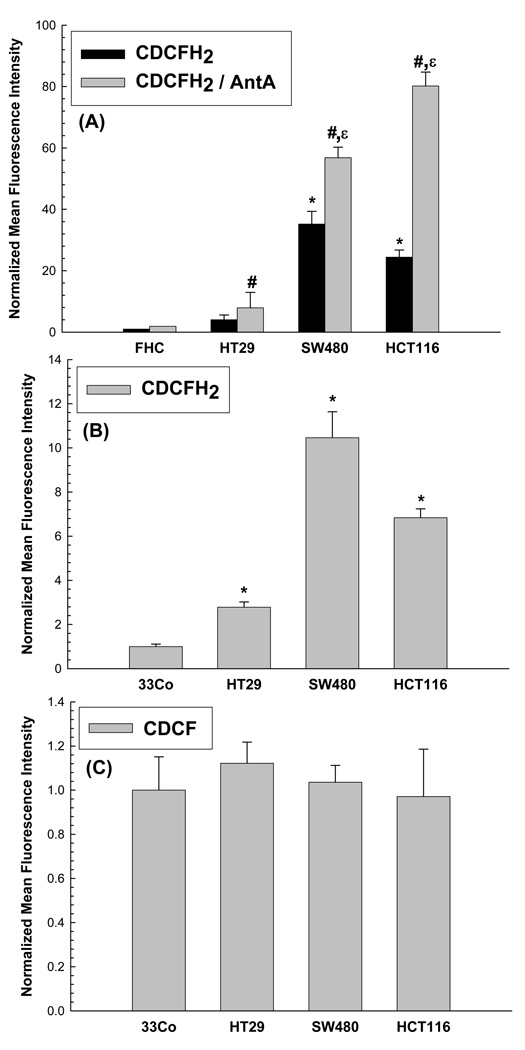
Intracellular pro-oxidant production (presumably hydroperoxides) was also determined in colon carcinoma versus normal colon cells using the oxidation sensitive 5-(and-6)-carboxy-2’,7’-dichlorodihydrofluorescein diacetate, (CDCFH2) in the presence and absence of the ETC blocker antimycin A. Using this assay in the absence of antimycin A, 20- to 30-fold increases in CDCFH2 oxidation were noted in HCT116 and SW480 cells, relative to FHC, and these results were similar to the increases in DHE oxidation seen in the same cell lines (compare Figure 1A to Figure 2A). In addition when treated with the antimycin A, HCT116 and SW480 cells demonstrated 30- to 40- fold increases in CDCFH2 oxidation, relative to FHC (Figure 2A). Interestingly, relative to FHC, Figure 2A shows that HT29 cells demonstrated 2- and 4-fold increases in CDCFH2 oxidation in the absence and presence of antimycin A, in contrast to the 10-fold increases in DHE oxidation noted in Figure 1A. However, the magnitude of increase in CDCFH2 oxidation caused by antimycin A in all the cancer cells, (relative to FHC) was at least as great (if not greater) than that seen in the absence of antimycin A, again strongly suggesting that cancer cell mitochondria are capable of producing greater amounts of hydroperoxides then are normal cell mitochondria. As can be seen in Figure 2B all 3 colon carcinoma cell lines also demonstrated significantly higher CDCFH2 oxidation when compared to 33Co normal colon fibroblasts. The differences seen in mean fluorescence intensity (MFI) values for the cancer cell lines between Figure 1A and 1B (as well as Figures 2A and 2B) are due to the normalization procedure used to express the data comparing the cancer cell lines to the two different normal cell types originally isolated from colon tissue (FHC colon epithelial cells in Figure 1A–Figure 2A and 33Co fibroblasts in Figure 1B–Figure 2B). Since the normalization procedure was done using two different normal cell types (with different MFIs) the fold changes in normalized MFIs for carcinoma cells were different, depending upon which normal cell type was being used for comparison (FHC epithelial cells or 33Co fibroblasts). These results show that regardless of which normal cell type from the colon was used for comparison, the cancer cells clearly demonstrated increased oxidation of DHE or CDCFH2, relative to normal cells. When the cells were labeled with oxidation insensitive analog of CDCFH2 [5-(and-6)-carboxy-2’,7’-dichlorofluorescein diacetate (CDCF)], there was no significant difference in any of the cell types (Figure 2C) confirming that the differences seen in Figure 2B were indeed due to changes in steady-state levels of probe oxidation and not changes in probe influx, efflux, or ester cleavage. Overall, these results strongly support the hypothesis that human colon cancer cells demonstrate greater steady-state levels of intracellular hydroperoxides, relative to normal colon epithelial cells and fibroblasts.
Figure 2.
(A) Human cancer cells (HT29, HCT116, SW480) demonstrated significantly increases oxidation of CDCFH2 relative to normal human colon epithelial cells (FHC). Cells were plated in 60 mm dishes, grown for 48 h and then incubated with 10 µg/ml CDCFH2 in 2 ml PBS at 37°C for 15 min in the presence or absence of 10 µM AntA. Cells were trypsinized on ice and analyzed by flow cytometry. Mean fluorescence intensity (MFI) of 10,000 cells were measured. Values are expressed as the ratio of MFI relative to FHC cells. Error bars represent ± 1SD of 3– 9 treatment dishes done in 3 separate experiments. [* Significantly different from FHC, CDCFH2 only, p<0.05, N=3; significantly different from FHC, CDCFH2+AntA, p<0.05, N=3–9 ; ε significantly different from each respective CDCFH2 only group, p<0.05, N=3–9]
(B) Human colon cancer cells (HT29, HCT116, SW480) demonstrated significantly increased oxidation of CDCFH2 relative to normal human colon fibroblasts (33Co). Cells were grown and labeled with 10 µg/ml CDCFH2 and analyzed by flow cytometry. Mean fluorescence intensity (MFI) of 10,000 cells was measured. Values are expressed as the ratio of MFI, relative to 33Co cells. Error bars represent ± 1SD of 9 treatment dishes done in 3 separate experiments. [*Significantly different from 33Co, CDCFH2 only, p<0.05, N=9]
(C) The oxidation insensitive probe demonstrated no differences in fluorescence among normal versus cancer cells from colon tissue. Cells were plated in 60 mm dishes, grown for 48 h and then incubated with 10 µg/ml CDCF in 2 ml PBS at 37°C for 15 min. Cells were trypsinized on ice and analyzed by flow cytometry. Mean fluorescence intensity (MFI) of 10,000 cells was measured. Values are expressed as the ratio of MFI, relative to 33Co cells. Error bars represent ± 1SD of 3 treatment dishes/group done on three separate days.
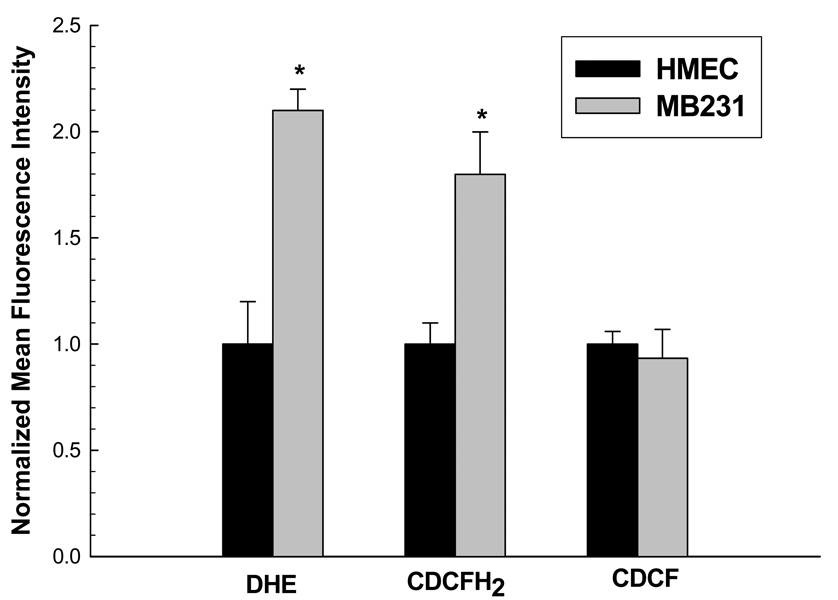
In order to extend these observations to another epithelial cell model system, steady-state levels of DHE and CDCFH2 oxidation were determined in MDA-MB231 breast carcinoma cells and normal non-transformed human mammary epithelial cells (HMECs). Figure 3 shows that MB231 cells had approximately 2-fold increases in DHE and CDCFH2 oxidation (p<0.05), compared to normal HMECs. Again, no differences were noted when the cells were labeled with the oxidation insensitive probe fluorescence (CDCF, Figure 3). As was seen with colon epithelial cells, these results support the hypothesis that human breast cancer cells also demonstrate increased steady-state levels of intracellular ROS, relative to normal breast epithelial cells.
Figure 3. Human breast cancer cells (MB231) demonstrated significantly increased oxidation of DHE and CDCFH2, relative to normal human mammary epithelial cells (HMEC).
Cells were grown and labeled with 10 µM DHE, 10 µg/ml CDCFH2, or 10 µg/ml CDCF as described in Fig 1 and Fig 2 and analyzed by flow cytometry. Mean fluorescence intensity (MFI) of 10,000 cells was measured. Values are expressed as the ratio of MFI, relative to HMEC cells. Error bars represent ± 1SD of 3 treatment dishes done in 3 separate experiments. [*Significantly different from HMEC, p<0.05, N=3].
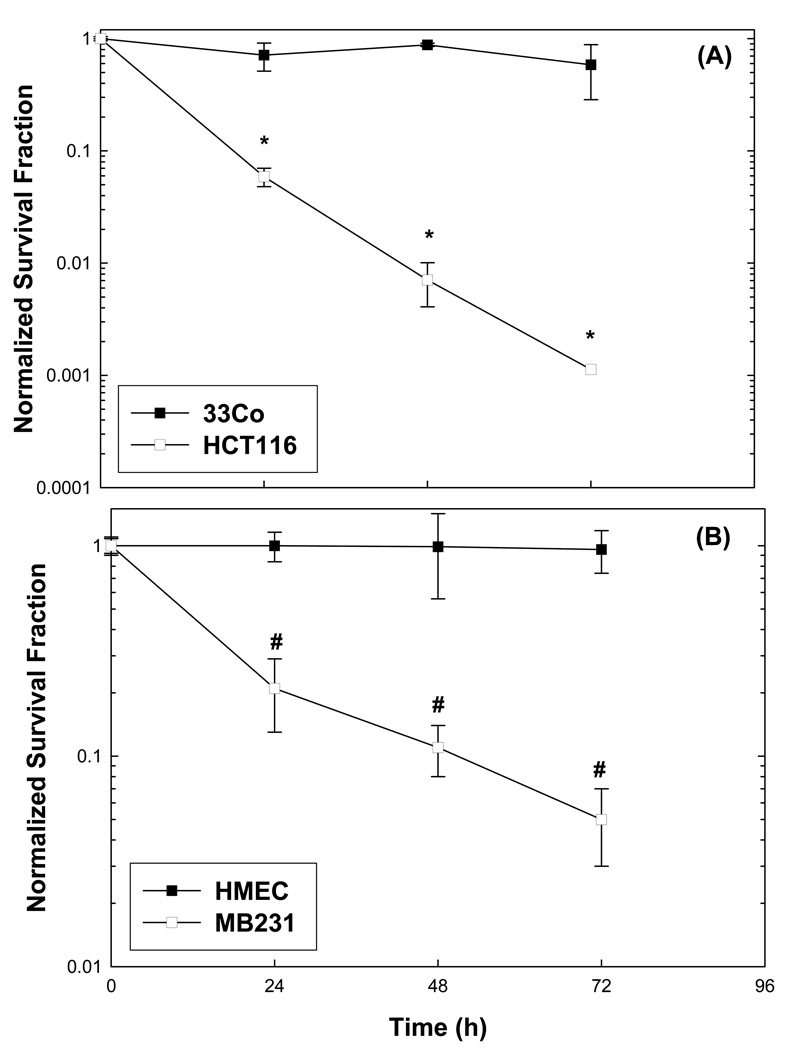
Since we hypothesized that cancer cells utilize glucose more extensively than their normal counterparts to provide reducing equivalents for the detoxification of endogenous ROS, it was logical to investigate the effects of glucose deprivation on cytotoxicity in normal vs. cancer cells. When colon (HCT116) and breast (MB231) carcinoma cells were exposed to glucose free media, a significant differential susceptibility to time-dependent clonogenic cell killing (Figures 4A and 4B) was clearly demonstrated in both cancer cell types, relative to normal cells (33Co, HMEC). Furthermore, NADPH levels were significantly decreased (and virtually undetectable) after 24 hours of glucose deprivation in both cancer cell lines (Table 3). In addition, NADP+ levels increased in both cancer cell lines during 24 hrs of glucose deprivation, but this increase only reached statistical significance in HCT116 colon carcinoma cells (Table 3). These results strongly suggest that depletion of NADPH levels in colon and breast carcinoma cells could contribute to glucose deprivation-induced cytotoxicity and oxidative stress in cancer cells.
Figure 4. Clonogenic survival of normal versus cancer cells from colon (A) and breast tissues (B) exposed to glucose deprivation.
Cells were plated in complete media and 24 hours later they were given fresh glucose free media containing 10% dialyzed FBS, non-essential amino acids, and gentamicin. Clonogenic survival was determined at 24, 48, and 72 h and normalized to the respective control group at time zero. Errors represent ±1 SEM of 4–6 cloning dishes counted from each treatment dish done in three separate experiments [*Significantly different from 33Co for each time point, p<0.05, N=3; # Significantly different from HMEC, for each time point, p<0.05, N=3].
Table 3.
NADPH and NADP+ levels in MB231 and HCT116 cells treated with +/− Glu for 24 h (n=3).
| Cell Line | NADPH (nmol/mg protein) |
NADP+ (nmol/mg protein) |
|---|---|---|
| MB231/ Glu + | 12 ± 6* | 58 ± 20 |
| MB231/ Glu − | N.D. | 77 ± 19 |
| HCT116/ Glu + | 25 ± 3* | 22 ± 13* |
| HCT116/ Glu − | N.D. | 81 ± 18 |
Significantly different from corresponding “Glu −” group, p<0.05.
N.D. Not detectable (considered as zero for statistical analysis)
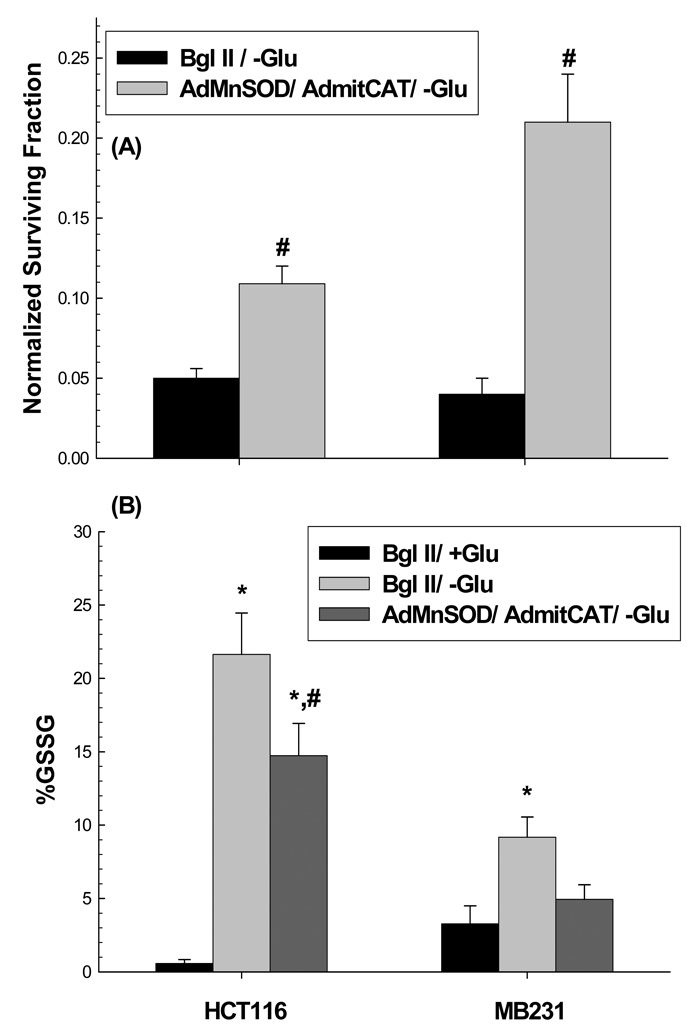
To further establish the causal role of ROS in the cytotoxic mechanisms responsible for differential susceptibility to glucose-deprivation induced cytotoxicity in colon and breast cancer cells, the effects of co-over expression of MnSOD and mitoCAT were evaluated. When HCT116 and MB231 cells were transduced with both AdMnSOD and AdmitoCAT (50 MOI each) and treated for 24 hours in the presence or absence of glucose, there was a partial but statistically significant (p<0.05) protection of both cell lines from glucose deprivation induced cytotoxicity (Figure 5A). Furthermore, Figure 5B demonstrates that during glucose deprivation % GSSG (an indicator of oxidative stress) was significantly elevated and this was partially suppressed by co-over expression of MnSOD and mitoCAT activity (Figure 5BC) in HCT116 and MB231 cells, relative to vector controls. GSH and GSSG levels in 33Co, normal colon fibroblasts and HMEC, normal breast epithelial cells were also measured after glucose deprivation treatment under the same conditions as HCT116 and MB231 cells. In 33Co colon fibroblasts %GSSG values in the presence and absence of glucose were found to be 5.5±1.8 (mean±1SD) and 5.7±0.6 (mean±1SD) respectively (p>0.05). GSSG levels of HMEC cells were undetectable in the presence or absence of glucose. Overall, the results presented in Figure 4 and Figure 5 strongly support a causal link between increased steady-state levels of mitochondrial ROS (i.e. of O2•− and H2O2) and differential cancer cell susceptibility to glucose deprivation induced cytotoxicity as well as oxidative stress.
Figure 5. Over expression of MnSOD and mitoCAT in HCT116 and MB231 cells suppressed the cytotoxicity (A) as well as %GSSG (B) seen at 24 h of glucose deprivation.
HCT116 and MB231 cells were transiently transduced with 50 MOI of AdMnSOD and 50 MOI of AdmitoCAT 24 h after plating. The media was changed 24 h after infection and cells were allowed to recover 24 h hours in fresh media. Cells then were treated with glucose free media for an additional 24 h and then plated for clonogenic survival. Survival data were normalized to sham treated cultures. In panel A, errors represent ±1 SEM of at least six cloning dishes counted from each treatment dish taken from two separate experiments. In panel B, errors represent ±1 SEM of three treatment dishes from each group assayed on three different days. [* Significantly different from Bgl II/+Glu, p<0.05, N=3; # significantly different from Bgl II/-Glu, p<0.05, N=3]. In panel C, MnSOD and catalase activities measured in HCT116 and MB231 cells given the indicated treatments with adenoviral vectors.
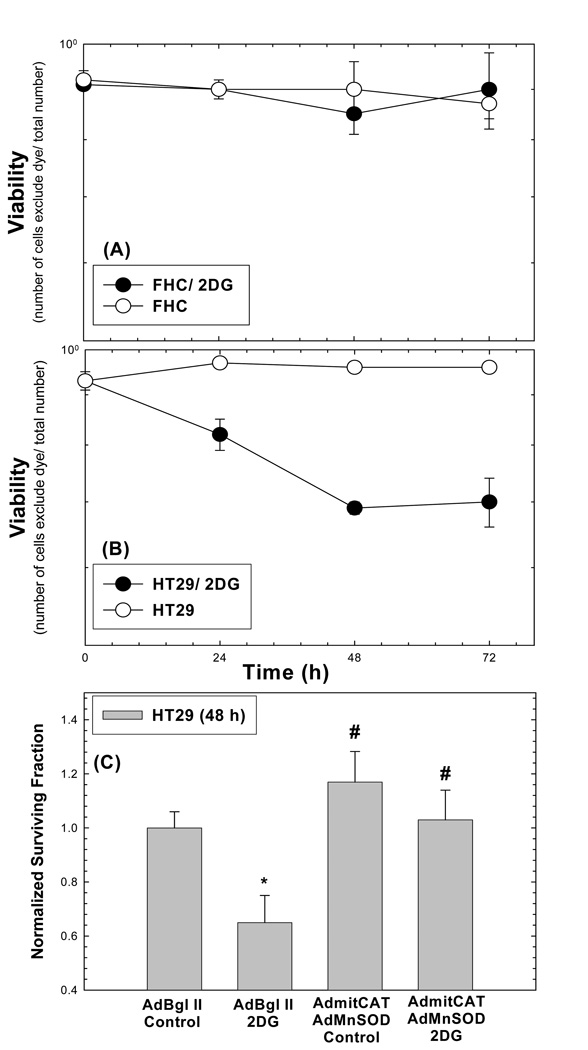
In order to extend these observations to a clinically relevant inhibitor of glucose metabolism (2-deoxyglucose, 2DG) capable of mimicking glucose deprivation, FHC and HT29 were treated with 20 mM 2DG for 0–72 hrs. The results in Figure 6AB show that HT29 cancer cells were significantly more susceptible to 2DG-induced cell killing as assayed by trypan blue dye exclusion, relative to FHC. Since mimicking glucose deprivation by using 2DG is a less severe stress on the cells than the complete glucose deprivation, a 48 h treatment with 2DG was used in an experiment to determine if over expression of cellular antioxidants could protect cancer cells from 2DG-induced cytotoxicity and oxidative stress. Figure 6C demonstrates that co-expression of MnSOD and mitoCAT activities using adenoviral vectors (Figure 6D) significantly protected the HT29 from 2DG-induced toxicity at 48 hours of exposure. Overall, the results presented in Figure 6 strongly support a causal link between increased steady-state levels of mitochondrial ROS (i.e. of O2•− and H2O2) and differential cancer cell susceptibility to the clinically relevant inhibitor of glucose metabolism.
Figure 6. Toxicity of 20 mM 2DG in normal (FHC) cells (A) vs cancer (HT29) cells (B). Over expression of MnSOD and mitoCAT in HT29 cells suppressed cytotoxicity at 48 h of 2DG treatment (C).
In panels A and B viability was assessed using tyrpan blue dye exclusion assay of at least 100 cells from each group. Errors represent +1 SD of three measurements from each of two dishes harvested at each time point (A and B). HT29 cells were transiently transduced with 50 MOI of AdMnSOD and 50 MOI of AdmitoCAT 24 h after plating. The media was changed 24 h after infection and cells were allowed to recover 24 h hours in fresh media. Cells then were treated with 20 mM 2DG for an additional 48 h. Survival data were normalized to empty vector treated cultures (C). Errors represent ±1 SEM of at least six cloning dishes counted from each treatment dish taken from two experiments [*Significantly different from Bgl II/Control, p<0.05; # significantly different from Bgl II/2DG, p<0.05]. In panel D, MnSOD and catalase activities were measured in HT29 cells given the indicated treatments with adenoviral vectors.
Discussion
The results presented in this study allow for a reformulation of the hypothesis regarding the mechanisms underlying the observed abnormalities in cancer cell glucose metabolism collectively known as the “Warburg Effect” [2]. According to Warburg [2] cancer cells demonstrate increased aerobic glycolysis due to damage or impairment of their respiratory mechanism leading to an inability to maintain [ATP] during energy metabolism. However several lines of evidence suggest that increased aerobic glycolysis in cancer cells may not be the result of an inability to maintain [ATP] during mitochondrial energy metabolism [29–31]. First, in many cases tumor cells appear to respire at levels comparable to their non-transformed counterparts [29]. Secondly recent data suggests that tumor cells do not lack the capacity to utilize oxidative phosphorylation to produce ATP when glycolysis is suppressed [30].
While the ability of mitochondria to consume O2 and produce ATP does not appear to be compromised in cancer cells, mitochondrial structure and mitochondrial DNA integrity have been reported to be abnormal in cancer cells [14–16, 32]. The mitochondria of malignant cells have been shown to exhibit significant histological abnormalities characterized by unusual arrangements of mitochondrial cristae, mitochondrial hypertrophy, and fragmentation when compared to normal cells [14, 15]. Furthermore, many tumors, including epithelial cancers (i.e., colon, breast, as well as head and neck), have been shown to have high rates of mtDNA mutations (relative to normal human tissues) and this has been suggested to lead to increased O2•− and H2O2 production [16, 32]. Therefore, it appears that while mitochondrial ATP production may not be compromised in cancer cells, mitochondrial O2•− and H2O2 production could be altered in cancer vs. normal cells. Finally extensive studies using several different cancer cell lines revealed that inhibition of ATP production did not correlate with 2DG-induced radiosensitization, but the extent that glucose uptake and metabolism was increased in tumor cells was an important factor predicting the extent of 2DG-induced radio- and chemo-sensitization [33, 34]. While these results seem counter intuitive to the hypothesis that deficits in energy metabolism are the basis for the “Warburg Effect”, they are consistent with the hypothesis that increased glucose metabolism in cancer cells could be compensating for elevated steady-state levels of intracellular ROS resulting from defects in mitochondrial respiration that lead to O2•− and H2O2 production.
To the best of our knowledge this is the first study clearly showing cancerous human colon and breast epithelial cells have elevated steady state levels of O2•− and ROS [relative to normal epithelial cells; Figure 1, Figure 2, and Figure 3] that are accentuated by treatment with an electron transport chain blocker (antimycin A, Figure 1 and Figure 2) known to cause increases in mitochondrial O2•− and H2O2 [6, 8]. The fact that cancer cells demonstrated profound increases in O2•− and pro-oxidant production (presumably hydroperoxides) when treated with antimycin A, strongly suggests that one electron reductions of O2 from ETC complexes I, II, and III are more favorable in colon cancer cells, relative to normal cells (Figure 1 and Figure 2). This phenomenon could be explained by: 1) defects in mitochondrial respiratory chain assembly and/ or 2) mutations in genes coding for ETC proteins in cancer vs. normal cells that increase residence time and/or accessibility of electrons to sites favorable for one electron reductions of O2 to form O2•− and the resulting dismutation product, H2O2. In this scenario tumor cells, relative to normal cells, would be expected to increase glucose metabolism in order to obtain reducing equivalents such as NADPH via pentose cycle as well as pyruvate from glycolysis [10–13] to detoxify H2O2 and other hydroperoxides produced by this defect in respiration.
In strong support of this hypothesis, the data in Figure 4–Figure 6 clearly show that cancer cells are more susceptible to killing induced by glucose deprivation or 2DG via a mechanism that can be inhibited by specific scavengers of O2•− and H2O2 targeted to the mitochondria that also inhibited parameters indicative of oxidative stress. These results are consistent with the hypothesis that, relative to normal cells, cancer cells have higher steady-state levels of ROS that are compensated for by increased metabolism of glucose and that when glucose metabolism is restricted, O2•− and H2O2 significantly contribute to the differential susceptibility of cancer cells to oxidative stress and cell killing. This hypothesis is also consistent with previous reports describing biochemical changes consistent with oxidative stress associated with differential susceptibility of transformed vs. normal cells to glucose deprivation-induced cytotoxicity [10, 12, 13, 35].
A mechanistic understanding of the fundamental relationship between glucose metabolism and ROS production between normal vs. cancer cells, could provide a clear biochemical rationale for the development of combined modality cancer therapies based on inhibiting glucose and hydroperoxide metabolism. Using this rationale it would be predicted that compromising glucose and hydroperoxide metabolism would lead to much greater endogenous metabolic oxidative stress in cancer cells relative to normal cells, which could be exploited to selectively sensitize cancer cells to conventional therapies (i.e., radiation and chemotherapy) that induce oxidative stress while causing minimal normal tissue damage. In support of this idea, previous studies have shown that 2DG selectively radiosensitizes fully transformed rodent cells to a greater extent than non-transformed cells by a mechanism that can be inhibited using the thiol antioxidant, N-acetylcysteine [13]. Furthermore, in MDA-MB231 breast and FaDu human head and neck cancer cells, 2DG-induced cytotoxicity can be significantly enhanced when combined with L-Buthionine-[S,R]-sulfoximine (BSO), an inhibitor of GSH synthesis and hydroperoxide metabolism [36, 37]. In addition 2DG sensitizes cancer cells to cisplatin and adriamycin [34, 36, 38], which are thought to induce oxidative stress. Finally, it has recently been shown that 2DG + cisplatin + radiation is a very effective combination in a FaDu human cancer xenograft model and 2DG + radiation is an effective combination in a human xenograft model of pancreatic cancer [34, 39]. Overall, these results support the hypothesis that a clear mechanistic understanding of the relationship between glucose metabolism and oxidative metabolic defects in cancer vs. normal cells can provide a new paradigm useful to the design of combined modality therapies that are selectively cytotoxic to cancer cells.
Acknowledgements
We would like to dedicate this publication to Dr. Larry W. Oberley, who passed away on April 21st, 2008. He was a great mentor and friend as well as an originator of the Free Radical Theory of Cancer. He also inspired the current work with his 1980 Cancer Research publication studying mitochondrial abnormalities in cancer cells. The authors thank Dr. Mary Hendrix for providing MB231 human breast carcinoma cells and Dr. Andre Melendez for the mitoCAT cDNA construct as well as Justin Fishbaugh and Gene Hess for technical assistance with flow cytometry. This work was supported by NIH R01-CA100045, NIH P01-CA66081, NIH P30-CA086862, NIH F32-CA110611.
Abbreviations
- 2DG
2-deoxyglucose
- 6PGD
6-phosphogluconate dehydrogenase
- Ant A
antimycin A
- ATP
adenosine triphosphate
- BSO
L-Buthionine-[S,R]-sulfoximine
- CDCF
5-(and-6)-carboxy-2’,7’-dichlorofluorescein diacetate
- CDCFH2
5-(and-6)-carboxy-2', 7'-dichlorodihydrofluorescein diacetate
- DHE
dihydroethidine
- ETC
electron transport chain
- FBS
fetal bovine serum
- G6PD
glucose-6-phosphate dehydrogenase
- GSH
glutathione
- GSSG
glutathione disulfide
- H2O2
hydrogen peroxide
- MFI
mean fluorescence intensity
- mitoCAT
mitochondrially targeted catalase
- MnSOD
Mn containing superoxide dismutase
- MOI
multiplicity of infection
- NBT
nitroblue tetrazolium
- O2•−
superoxide
- PBS
phosphate buffered saline
- PEG-SOD
polyethylene conjugated CuZn superoxide dismutase
- ROS
reactive oxygen species
REFERENCES
- 1.Lehninger AL. Biochemistry. New York: Worth Publishers Inc.; 1976. [Google Scholar]
- 2.Warburg O. On the origin of cancer cells. Science. 1956;132:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 3.Weber G. Enzymology of cancer cells (first of two parts) New Engl. J. Med. 1977;296:486–492. doi: 10.1056/NEJM197703032960905. [DOI] [PubMed] [Google Scholar]
- 4.Weber G. Enzymology of cancer cells (second of two parts) New Engl. J. Med. 1977;296:541–551. doi: 10.1056/NEJM197703102961005. [DOI] [PubMed] [Google Scholar]
- 5.Boveris A, Cadenas E. Production of superoxide radicals and hydrogen peroxide in mitochondria (Chapter 2) In: Oberley LW, editor. Superoxide Dimsutase. Boca Raton: CRC Press; 1982. pp. 15–30. [Google Scholar]
- 6.Boveris A. Advances in Experimental Medicine and Biology. Buenos Aires; 1977. Mitochondrial production of superoxide radical and hydrogen peroxide; pp. 67–82. [DOI] [PubMed] [Google Scholar]
- 7.Voet D, Voet JG, Pratt CW. Fundementals of Biochemistry. John Wiley and Sons, Inc.; 1999. Electron transport and oxidative phosphorylationn (Chapter 17) pp. 492–525. [Google Scholar]
- 8.Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch. Biochem. Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 9.Nohl H, Jordan W. The mitochondrial site of superoxide formation. Biochem. Biophys. Res. Commun. 1986;138:533–539. doi: 10.1016/s0006-291x(86)80529-0. [DOI] [PubMed] [Google Scholar]
- 10.Spitz DR, Sim JE, Ridnour LA, Galoforo SS, Lee YJ. Glucose deprivation-induced oxidative stress in human tumor cells: a fundamental defect in metabolism? Ann. N.Y. Acad. Sci. 2000;899:349–362. doi: 10.1111/j.1749-6632.2000.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee YJ, Galoforo SS, Berns CM, Chen JC, Davis BH, Sim JE, Corry PM, Spitz DR. Glucose deprivation-induced cytotoxicity and alterations in mitogen-activated protein kinase activation are mediated by oxidative stress in multidrug-resistant human breast carcinoma cells. J. Biol. Chem. 1998;273:5294–5299. doi: 10.1074/jbc.273.9.5294. [DOI] [PubMed] [Google Scholar]
- 12.Blackburn RV, Spitz DR, Liu X, Galoforo SS, Sim JE, Ridnour LA, Chen JC, Davis BH, Corry PM, Lee YJ. Metabolic Oxidative Stress Activates Signal Transduction and Gene Expression During Glucose Deprivation in Human Tumor Cells. Free Radic. Biol. Med. 1999;26:419–430. doi: 10.1016/s0891-5849(98)00217-2. [DOI] [PubMed] [Google Scholar]
- 13.Lin X, Zhang F, Bradbury CW, Kaushal A, Li L, Spitz DR, Aft R, Gius D. 2-Deoxy-d-Glucose-induced cytotoxicity and radiosensitization in tumor cells is mediated via disruptions in thiol metabolism. Cancer Res. 2003;63:3413–3417. [PubMed] [Google Scholar]
- 14.Springer EL. Comparative study of the cytoplasmic organelles of the epithelial cell lines derived from human carcinomas and nonmalignant tissues. Cancer Res. 1980;40:803–817. [PubMed] [Google Scholar]
- 15.Bize IB, Oberley LW, Morris HP. Superoxide dismutase and superoxide radical in Morris hepatomas. Cancer Res. 1980;40:3686–3693. [PubMed] [Google Scholar]
- 16.Penta JS, Johnson FM, Wachsman JT, Copeland WC. Mitochondrial DNA in Human Malignancy. Mutat. Res. 2001;488:119–133. doi: 10.1016/s1383-5742(01)00053-9. [DOI] [PubMed] [Google Scholar]
- 17.Szatrowski TP, Nathan CF. Production of Large Amounts of Hydrogen Peroxide by Human Tumor Cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 18.Li WG, Miller FJ, Zhang HJ, Spitz DR, Oberley LW, Weintraub NL. H2O2-induced O2.− production by a non-phagocytic NAD(P)H oxidase causes oxidant injury. J. Biol. Chem. 2001;276:29251–29256. doi: 10.1074/jbc.M102124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slane BG, Aykin-Burns N, Smith BJ, Kalen AL, Goswami PC, Domann FE, Spitz DR. Mutation of succinate dehydrogenase subunit C results in increased O2.−, oxidative stress, and genomic instability. Cancer Res. 2006;66:7615–7620. doi: 10.1158/0008-5472.CAN-06-0833. [DOI] [PubMed] [Google Scholar]
- 20.Ahmad IM, Aykin-Burns N, Sim JE, Walsh SA, Higashikubo R, Buettner GR, Venkataraman S, Mackey MA, Flanagan SW, Oberley LW, Spitz DR. Mitochondrial superoxide and hydrogen peroxide mediate glucose deprivation-induced cytotoxicity and oxidative stress in human cancer cells. J. Biol. Chem. 2005;280:4254–4263. doi: 10.1074/jbc.M411662200. [DOI] [PubMed] [Google Scholar]
- 21.Glock GE, McLean P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem. J. 1953;55:400–408. doi: 10.1042/bj0550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 23.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 24.Spitz DR, Malcolm RR, Roberts RJ. Cytotoxicity and metabolism of 4-hydroxy-2-nonenal and 2-nonenal in H2O2-resistant cell lines. Do aldehydic by-products of lipid peroxidation contribute to oxidative stress? Biochem. J. 1990;267:453–459. doi: 10.1042/bj2670453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zwacka RM, Dudus L, Epperly MW, Greenberger JS, Engelhardt JF. Redox gene therapy protects human IB-3 lung epithelial cells against ionizing radiation-induced apoptosis. Hum. Gene. Ther. 1998;9:1381–1386. doi: 10.1089/hum.1998.9.9-1381. [DOI] [PubMed] [Google Scholar]
- 26.Bai J, Rodriguez AM, Melendez JA, Cederbaum AI. Overexpression of Catalase in Cytosolic or Mitochondrial Compartment Protects HepG2 Cells against Oxidative Injury. J. Biol. Chem. 1999;274:26217–26224. doi: 10.1074/jbc.274.37.26217. [DOI] [PubMed] [Google Scholar]
- 27.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 28.Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- 29.Weinhouse S. Isozyme alterations, gene regulation and the neoplastic transformation. Adv. Enzyme. Regul. 1983;21:369–386. doi: 10.1016/0065-2571(83)90024-9. [DOI] [PubMed] [Google Scholar]
- 30.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 31.Maity A, Tuttle SW. 2-Deoxyglucose and radiosensitization: teaching an old DOG new tricks? Cancer Biol. Ther. 2006;5:824–826. doi: 10.4161/cbt.5.7.3024. [DOI] [PubMed] [Google Scholar]
- 32.Fliss MS, Usadel H, Caballero OL, Wu L, Buta MR, Eleff SM, Jen J, Sidransky D. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science. 2000;287:2017–2019. doi: 10.1126/science.287.5460.2017. [DOI] [PubMed] [Google Scholar]
- 33.Dwarkanath BS, Zolzer F, Chandana S, Bauch T, Adhikari JS, Muller WU, Streffer C, Jain V. Heterogeneity in 2-deoxy-D-glucose-induced modifications in energetics and radiation responses of human tumor cell lines. Int. J. Radiat. Oncol. Biol. Phys. 2001;50:1051–1061. doi: 10.1016/s0360-3016(01)01534-6. [DOI] [PubMed] [Google Scholar]
- 34.Simons AL, Fath MA, Mattson DM, Smith BJ, Walsh SA, Graham MM, Hichwa RD, Buatti JM, Dornfeld K, Spitz DR. Enhanced response of human head and neck cancer xenograft tumors to cisplatin combined with 2-deoxy-D-glucose correlates with increased 18F-FDG uptake as determined by PET imaging. Int. J. Radiat. Oncol. Biol. Phys. 2007;69:1222–1230. doi: 10.1016/j.ijrobp.2007.07.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jelluma N, Yang X, Stokoe D, Evan GI, Dansen TB, Haas-Kogan DA. Glucose withdrawal induces oxidative stress followed by apoptosis in glioblastoma cells but not in normal human astrocytes. Mol. Cancer Res. 2006;4:319–330. doi: 10.1158/1541-7786.MCR-05-0061. [DOI] [PubMed] [Google Scholar]
- 36.Simons AL, Ahmad IM, Mattson DM, Dornfeld KJ, Spitz DR. 2-Deoxy-D-glucose combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res. 2007;67:3364–3370. doi: 10.1158/0008-5472.CAN-06-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andringa KK, Coleman MC, Aykin-Burns N, Hitchler MJ, Walsh SA, Domann FE, Spitz DR. Inhibition of glutamate cysteine ligase activity sensitizes human breast cancer cells to the toxicity of 2-deoxy-D-glucose. Cancer Res. 2006;66:1605–1610. doi: 10.1158/0008-5472.CAN-05-3462. [DOI] [PubMed] [Google Scholar]
- 38.Maschek G, Savaraj N, Priebe W, Braunschweiger P, Hamilton K, Tidmarsh GF, De Young LR, Lampidis TJ. 2-deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res. 2004;64:31–34. doi: 10.1158/0008-5472.can-03-3294. [DOI] [PubMed] [Google Scholar]
- 39.Coleman MC, Asbury CR, Daniels D, Du J, Aykin-Burns N, Smith BJ, Li L, Spitz DR, Cullen JJ. 2-deoxy-D-glucose causes cytotoxicity, oxidative stress, and radiosensitization in pancreatic cancer. Free Radic. Biol. Med. 2008;44:322–331. doi: 10.1016/j.freeradbiomed.2007.08.032. [DOI] [PubMed] [Google Scholar]