Abstract
The overall objective of this study was to examine the effects of in vitro expansion on neocartilage formation by auricular chondrocytes photoencapsulated in a hyaluronic acid (HA) hydrogel as a next step towards the clinical application of tissue engineering therapies for treatment of damaged cartilage. Swine auricular chondrocytes were encapsulated either directly after isolation (p = 0), or after further in vitro expansion (p = 1 and p = 2) in a 2 wt%, 50 kDa HA hydrogel and implanted subcutaneously in the dorsum of nude mice. After 12 weeks, constructs were explanted for mechanical testing and biochemical and immunohistochemical analysis and compared to controls of HA gels alone and native cartilage. The compressive equilibrium moduli of the p = 0 and p = 1 constructs (51.2 ± 8.0 and 72.5 ± 35.2 kPa, respectively) were greater than the p = 2 constructs (26.8 ± 14.9 kPa) and the control HA gel alone (12.3 ± 1.3 kPa) and comparable to auricular cartilage (35.1 ± 12.2 kPa). Biochemical analysis showed a general decrease in glycosaminoglycan (GAG), collagen, and elastin content with chondrocyte passage, though no significant differences were found between the p = 0 and p = 1 constructs for any of the analyses. Histological staining showed intense and uniform staining for aggrecan, as well as greater type II collagen versus type I collagen staining in all constructs. Overall, this study illustrates that constructs with the p = 0 and p = 1 auricular chondrocytes produced neocartilage tissue that resembled native auricular cartilage after 12 weeks in vivo. However, these results indicate that further expansion of the chondrocytes (p = 2) can lead to compromised tissue properties.
Keywords: cartilage, auricular chondrocyte, hyaluronic acid, hydrogel, passage number
Introduction
As the need for cartilage repair becomes a growing problem in today’s society, a wide range of materials and techniques for cartilage repair and regeneration are being developed1,2. Tissue engineering, where cells are combined with a biocompatible, biodegradable scaffold to provide a suitable environment for tissue regeneration, may prove to be an ideal technique to repair damaged cartilage. One obstacle that cartilage tissue engineering faces is the development of a suitable environment for cell encapsulation that promotes the retention of the chondrogenic phenotype, the production of ECM, and the integration of scaffold and native tissue.
In previous studies, Elisseeff et al3 established photopolymerization of a hydrogel system as an efficient method to encapsulate chondrocytes, demonstrating chondrocyte survival, uniform cell distribution, and ECM protein production within poly(ethylene oxide)-based hydrogels. Recently, Nettles et al4 demonstrated that HA hydrogels provided a suitable environment for articular chondrocytes, where retention of a chondrogenic phenotype and the production of ECM were observed. Photopolymerization may be advantageous for clinical applications because defects can be filled directly with the liquid prepolymer solution containing cells and polymerized in vivo, providing filling of irregular defects and good contact between the hydrogel and surrounding cartilage tissue.
However, before reaching clinical feasibility, other challenges such as finding an efficient cell source and developing techniques to expand the cells while maintaining a chondrogenic phenotype need to be addressed5. To this effect, auricular cartilage proves to be a promising cell source for cartilage regeneration for applications in plastic surgery and potentially for articular surface repair. Auricular chondrocytes are easily harvested with little donor-site morbidity and can be obtained at yields twice as high as articular chondrocytes6 and proliferate approximately four times faster than articular chondrocytes when grown in monolayer culture7. Additionally, when implanted in vivo on three-dimensional scaffolds, primary auricular chondrocytes have been shown to express high levels of type II collagen and glycosaminoglycans6. Furthermore, auricular chondrocytes have been successfully encapsulated in a variety of materials such as poly(glycolic acid)8,9, alginate10, chitosan11, and Pluronic F12712, and have been shown to produce extracellular matrix and form neocartilage. In a study by Xu et al13, auricular chondrocytes encapsulated in fibrin polymer exhibited the highest equilibrium modulus compared to those encapsulated with articular and costal chondrocytes. In addition, we showed, in a previous study, that auricular chondrocytes that were photoencapsulated in hyaluronic acid (HA) hydrogels could produce neocartilage in vivo after the optimization of hydrogel properties14.
However, due to the large number of chondrocytes that would be needed to repair a clinically relevant cartilage defect, expansion of isolated chondrocytes may be necessary. Unfortunately, for rapid expansion in monolayer culture, chondrocytes isolated from both articular and auricular cartilage have been shown to dedifferentiate, losing their chondrogenic phenotype15,16. Originally rounded in shape, chondrocytes flatten and take on a more fibroblastic phenotype with in vitro expansion16,17. Additionally, when chondrocytes are removed from their extracellular matrix (ECM) environment, a decrease in type II collagen and an increase in type I collagen are seen18, leading to a mechanically inferior fibrocartilage tissue. Although dedifferentiation seems inevitable in monolayer culture, some studies have shown slower dedifferentiation, stabilization of the differentiated phenotype, or even redifferentiation (i.e., return to a chondrocytic phenotype after dedifferentiation) when chondrocytes are cultured under conditions such as in liquid suspension19, agarose15, alginate20, or methacrylated HA hydrogels4. In our previous work, we also showed retention of the chondrogenic phenotype by auricular chondrocytes when photoencapsulated in 2 wt%, 50 kDa hyaluronic acid (HA) hydrogels, which exhibited continued glycosaminoglycan and type II collagen production14.
The overall objective of this study was to examine the effects of in vitro expansion of auricular chondrocytes on neocartilage formation in a previously optimized HA hydrogel. This work will also allow more insight into the potential use of auricular chondrocytes as a cell source for cartilage regeneration. To accomplish this, initially isolated (p = 0) and expanded (p = 1 and p = 2) swine auricular chondrocytes were photoencapsulated in a HA hydrogel, implanted subcutaneously in nude mice for 12 weeks, and explanted for mechanical, biochemical, and immunohistological analysis with comparisons to controls of the HA gel alone and native cartilage tissue.
Materials and Methods
Macromer Synthesis and Polymerization
Methacrylated HA (MeHA) was synthesized by the addition of methacrylic anhydride (Sigma) to a solution of 1 wt% HA (Lifecore, MW = 50 kDa) in deionized water, adjusted to a pH of 8 with 5 N NaOH, and reacted on ice for 24 hours, as previously reported21,22. The macromer solution was purified via dialysis (MW cutoff 5–8k) against deionized water for a minimum of 48 hours with repeated changes of water. The final product was obtained by lyophilization and stored at −20°C in powder form prior to use. The macromer was sterilized using a germicidal lamp in a laminar flow hood for 30 minutes and dissolved in a sterile solution of phosphate buffered saline (PBS) containing 0.05 wt% 2-methyl-1-[4-(hydroxyethoxy)phenyl]-2-methyl-1-propanone (Irgacure 2959, I2959) for cell encapsulation.
Chondrocyte Isolation, Expansion, and Photoencapsulation
Cartilage tissue was harvested in a sterile fashion from the ears (auricular) and the knees (articular) of 3 to 6 months old swine that were euthanized with an overdose of Pentobarbital (100 mg/kg IV). The harvested auricular cartilage was cut into ~1mm3 pieces, washed in PBS, and digested overnight at 37°C in Ham’s F-12 medium containing 0.1% collagenase (Worthington). Digested tissue was passed through a 100 μm filter and centrifuged to obtain a chondrocyte pellet. Chondrocytes were washed twice with PBS, counted using a hemacytometer, and determined viable using the trypan blue exclusion dye test prior to encapsulation and plating. Chondrocytes (40 × 106 cells/ml) were photoencapsulated in hydrogel networks by suspension in a 2 wt% macromer (MeHA) solution containing 0.05 wt% I2959. The solution was pipetted into sterile molds (50 μl volume) and polymerized with ~4 mW/cm2 ultraviolet light for 10 minutes using a long-wave ultraviolet lamp (Model 100AP, Blak-Ray). Remaining chondrocytes were plated in T-150 flasks at a seeding density of 1×106 cells/150 cm2 (~6700 chondrocytes/cm2) for expansion in Ham’s F-12 culture medium containing 10% FBS, 1% penicillin-streptomycin, and 1% non-essential amino acids. After reaching ~90% confluency, chondrocytes were trypsinized and photoencapsulated as stated above (p=1) or replated at 1×106 cells/150 cm2, trypsinized, and photoencapsulated after reaching ~90% confluency again (p=2). Constructs were placed in culture media and implanted within 2 hours of gelation.
Implantation in Nude Mice
Nude mice were anesthetized with ketamine (80 mg/kg) and xylazine (12 mg/kg). A 2 cm midline incision was made on the dorsum of each mouse and 5 subcutaneous pockets were made using blunt dissection. One chondrocyte/hydrogel construct was placed in each of these pockets and the wound was closed with sterile stainless steel skin clips. After 12 weeks, mice were euthanized and constructs were harvested for analysis. NIH guidelines for the care and use of laboratory animals (NIH Publication #85-23 Rev. 1985) were observed.
Mechanical Testing
Samples (n=5) were cored with a 3/16 inch diameter punch and weighed (wet weight). Cored samples were mechanically tested in confined compression in a PBS bath. For complete confinement, samples were initially loaded in creep to a tare load of 5 grams until reaching equilibrium (defined as less than 10μm of change in 10 min) before undergoing stress relaxation. Stress relaxation was carried out by applying a ramped displacement to 10% strain, and then the sample was allowed to relax to equilibrium (defined as less than 0.5 g of change in 10 min). The equilibrium confined compression modulus (HA) for each sample was calculated by dividing the equilibrium load by the area loaded.
Biochemical Analysis
For biochemical analysis (n = 5), mechanically tested samples were lyophilized, weighed (dry weight), and digested in a proteinase K solution (200μg/ml proteinase K (Roche), 100 mM ammonium acetate, pH 7.0) overnight at 60°C. Proteinase K was then inactivated at 100°C for 5 min. Total DNA, GAG, and collagen contents were determined using the PicoGreen dsDNA Assay23, the dimethylmethylene blue dye method24 with chondroitin sulfate as a standard, and the hydroxyproline assay25 using a collagen to hydroxyproline ratio of 7.2526,27, respectively. Values reported for DNA, GAG, and collagen content were normalized to the sample wet weight. Elastin content was measured using the Fastin Elastin Assay (Accurate Chemical & Scientific Corp)28 with an α–elastin solution as a standard. Briefly, 100μl of the sample digest solution was combined with 200 μl of 90% ammonium sulfate and 1 ml of Fastin dye reagent to form the elastin-dye complex. Contents were reacted for 1 hr and centrifuged to pellet the complex. The pellet was solubilized with the Fastin dissociation reagent and the absorbance was measured at a wavelength of 513 nm. The proteinase K digestion solution was used as a negative control for the hydroxyproline and elastin assays.
Histological Analysis
For histological analysis, constructs were fixed in 10% formalin for 24 hours, embedded in paraffin, and processed using standard histological procedures. The histological sections (7 μm thick) were stained for chondroitin sulfate and collagen distributions using the Vectastain ABC kit (Vector Labs) and the DAB Substrate kit for peroxidase (Vector Labs). Sections were predigested in 0.5 mg/ml hyaluronidase for 30 min at 37°C and incubated in 0.5 N acetic acid for 4 hours at 4°C to swell the samples prior to overnight incubation with primary antibodies at dilutions of 1:100, 1:200, and 1:3 for chondroitin sulfate (mouse monoclonal anti-chondroitin sulfate, Sigma), and type I (mouse monoclonal anti-collagen type 1, Sigma) and type II collagen antibodies (mouse monoclonal anti-collagen type II, Developmental Studies Hybridoma Bank), respectively. Non-immune controls underwent the same procedure without primary antibody incubation.
Statistical Analysis
Anova with Tukey’s post-hoc test was used to determine significant difference among groups, with p < 0.05. All values are reported as the mean ± the standard deviation.
Results
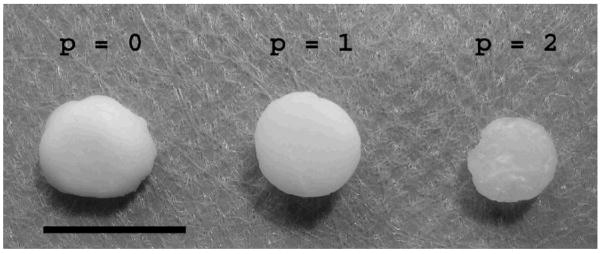
To determine the effects of auricular chondrocyte expansion on neocartilage formation in photopolymerizable HA hydrogels, swine auricular chondrocytes isolated directly from cartilage or expanded in vitro were encapsulated and implanted in the dorsum of nude mice for 12 weeks. The specific hydrogel composition used (2 wt%, 50 kDa MeHA) was previously optimized for chondrocyte encapsulation and neocartilage formation14. Constructs were explanted, mechanically tested, analyzed, and compared to controls of the HA gel alone and native auricular and articular cartilage. A schematic of this process is shown in Figure 1. Macroscopically, the explants were white and opaque and resembled native cartilage tissue. The p = 0 and p = 1 constructs are noticeably larger (0.5 cm diameter when implanted) and more opaque than the p = 2 constructs (Figure 2) that are slightly translucent.
Figure 1.
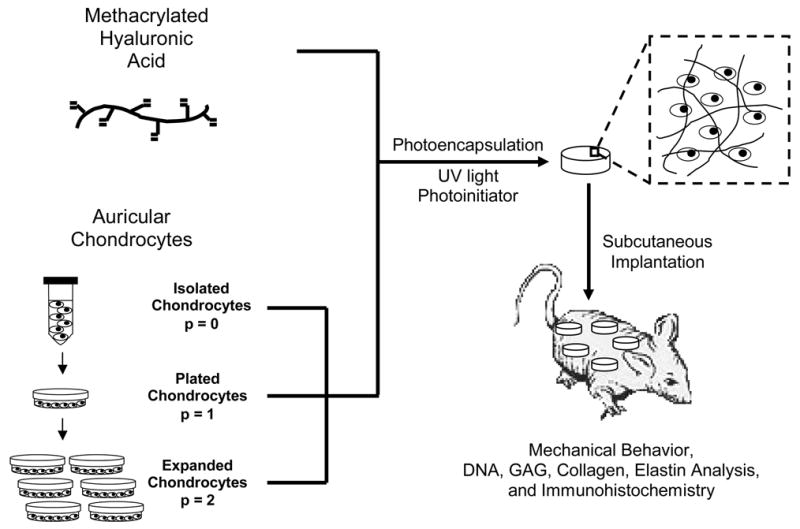
General schematic of chondrocyte expansion, photoencapsulation, and subsequent analysis.
Figure 2.
Explanted HA constructs 12 weeks after subcutaneous implantation in nude mice. Scale bar = 1cm.
Mechanical Behavior
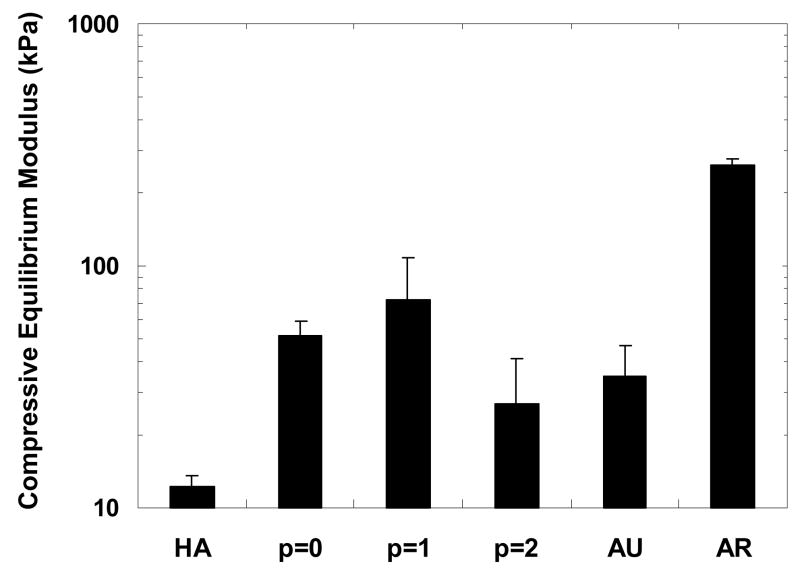
Samples were tested in confined compression in a PBS bath to simulate a cartilage defect environment. The compressive equilibrium moduli were calculated by dividing the equilibrium load by the area loaded and are reported in Figure 3. The p = 0 and p = 1 engineered constructs (51.2 ± 8.0 kPa and 72.5 ± 35.2 kPa, respectively) exhibited a significant increase in compressive equilibrium moduli from the HA gel (12.3 ± 1.3 kPa), while no significant difference was detected for the p = 2 constructs (26.8 ± 14.9 kPa). Though the moduli of the engineered constructs were all significantly lower than the articular cartilage (259.2 ± 20.0 kPa), they were all either higher than or not statistically different than that of native auricular cartilage (35.1 ± 12.2 kPa).
Figure 3.
Compressive equilibrium modulus of constructs after 12 weeks of subcutaneous implantation in nude mice compared to controls of the HA gel alone and native auricular and articular cartilage. The moduli of the p = 0 and p = 1 constructs are significantly greater than the HA gel and the average modulus of the p = 1 constructs is significantly greater than both the p = 2 constructs and auricular cartilage. Additionally, the average modulus of the articular cartilage is significantly greater than all other groups.
Biochemical Analysis
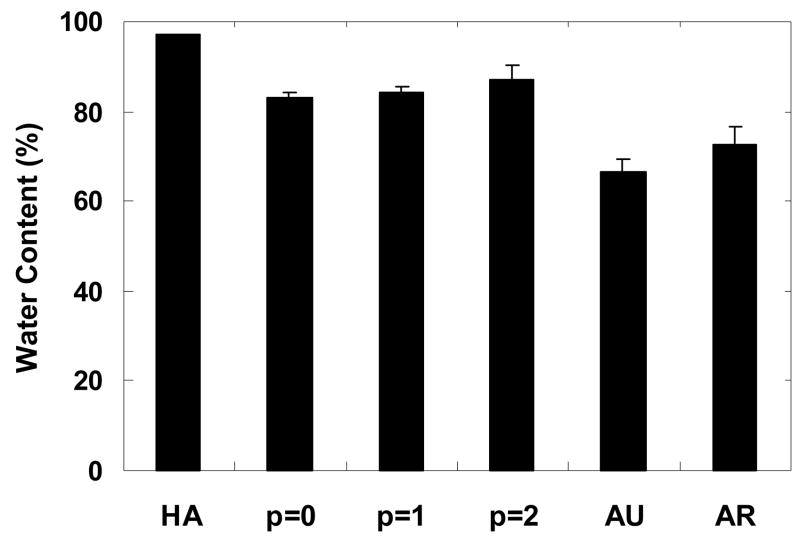
The water content of the tissue engineered constructs and control samples were determined from wet and dry weights (Figure 4). In general, constructs exhibited an increase in water content with passage number. All constructs exhibited a significantly lower water content when compared to the HA gel (97.0 ± 0.3% water) and showed a significantly higher water content when compared to native auricular (66.5 ± 2.7% water) and articular (72.7 ± 4.0% water) cartilage. The p = 0 constructs (83.1 ± 1.3% water) were most comparable to native articular cartilage.
Figure 4.
Water content of constructs after 12 weeks of subcutaneous implantation in nude mice compared to controls of the HA gel alone and auricular and articular cartilage. The HA gel exhibits a significantly greater water content than all other groups. The water content also showed slight increases with auricular chondrocyte passage number, although no significant differences were measured. The water content of auricular and articular cartilage is significantly lower than both the HA gel and the tissue engineered constructs.
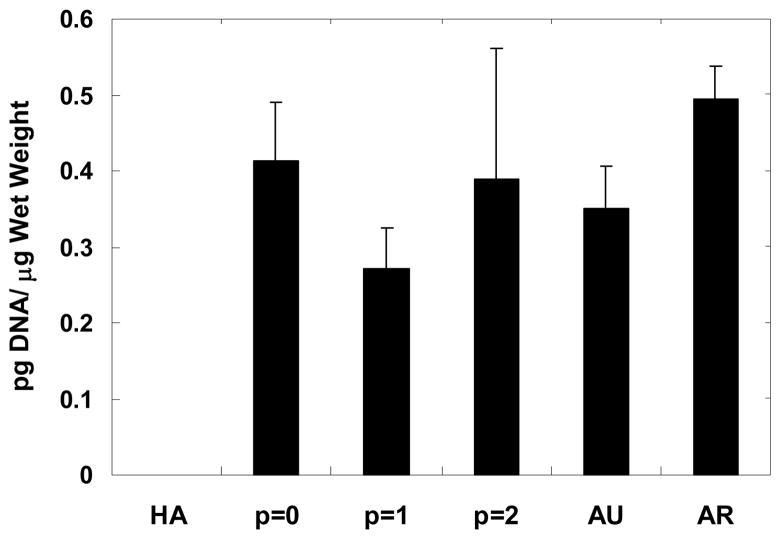
Total DNA content was determined using the dsPicoGreen assay and normalized to sample wet weight in Figure 5. Minimal background fluorescence was detected for the HA gel and was determined to be insignificant when normalized to wet weight. In general, no significant differences were detected among sample groups, with DNA content ranging from ~0.3 to 0.5 pg DNA/μg wet weight. However, the p = 1 constructs exhibited the lowest amount of DNA/wet weight among the engineered constructs.
Figure 5.
DNA content normalized to wet weight for constructs after 12 weeks of subcutaneous implantation in nude mice compared to controls of the HA gel alone and native auricular and articular cartilage. No significant differences were detected among groups with the exception of p = 1 constructs versus articular cartilage. The HA gel showed insignificant DNA measurements and thus, exhibited no interference with the fluorescent assay.
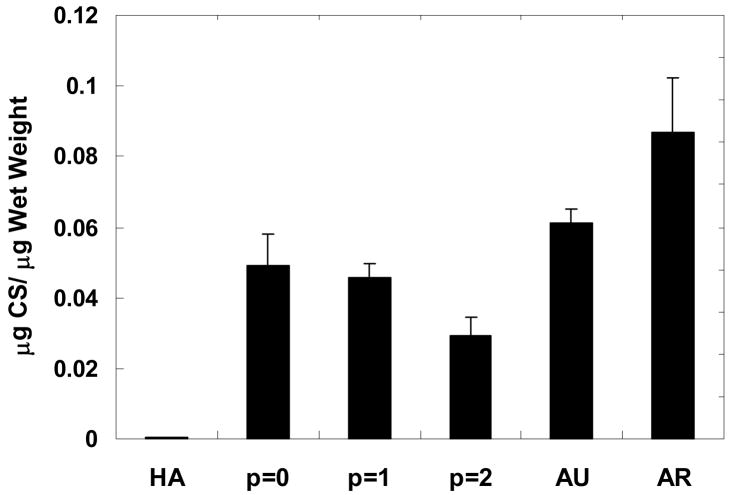
In Figure 6, GAG content is reported as the quantity of chondroitin sulfate normalized to sample wet weight. In general, constructs exhibited a decrease in GAG content with passage number, where p = 0 and p = 1 constructs (0.049 ± 0.009 and 0.046 ± 0.004 μg CS/μg wet weight, respectively) are significantly greater than the p = 2 constructs (0.029 ± 0.005 μg CS/μg wet weight). When compared to native cartilage, the p = 0 and p = 1 constructs are ~75–80% and ~53–57% of the GAG content measured for auricular and articular cartilage, respectively. Articular cartilage was significantly greater than all engineered constructs, but there was no significant difference detected in GAG content between auricular cartilage and the p = 0 constructs. Minimal GAG content was detected for the HA gels.
Figure 6.
Glycosaminoglycan content of samples normalized to construct wet weight after 12 weeks of subcutaneous implantation in nude mice compared to controls of the HA gel alone and native auricular and articular cartilage. Articular cartilage has significantly greater GAG content than all other groups while no significant difference was detected between auricular cartilage and the p = 0 constructs. In the engineered constructs, the GAG content generally decreased with chondrocyte passage, with the GAG content of the p = 0 and p = 1 constructs significantly greater than that of the p = 2 constructs. The GAG content detected in the HA gels was minimal and statistically lower than all constructs and native cartilage.
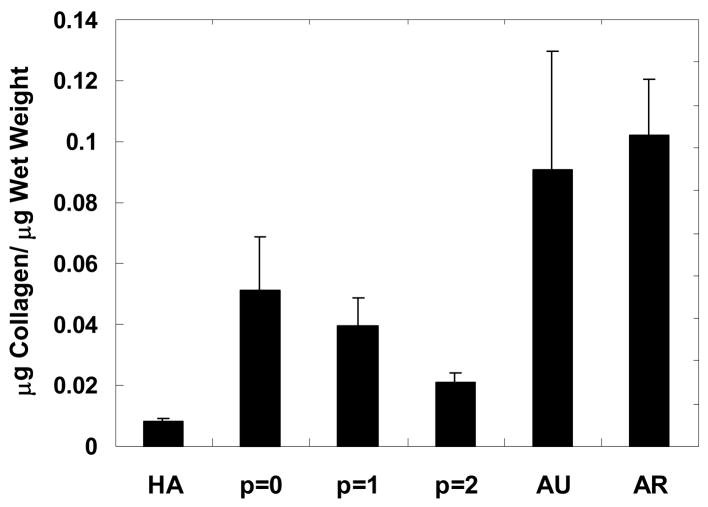
Total collagen content, normalized to wet weight and reported in Figure 7, exhibited similar trends to those observed for GAG content, with a general decrease in collagen observed with passage number. The collagen content of the p = 0 constructs (0.051 ± 0.017 μg collagen/μg wet weight) was most comparable to native cartilage (i.e., ~57% and ~50% of measured collagen content for auricular and articular cartilage, respectively). Again, the control HA gels showed minimal levels with this assay.
Figure 7.
Collagen content of constructs normalized to construct wet weight after 12 weeks of subcutaneous implantation in nude mice compared to controls of the HA gel alone and native auricular and articular cartilage. Collagen content in articular cartilage is significantly greater than the HA gel and all engineered constructs. No significant difference was found between auricular cartilage and the p = 0 constructs, but there is significantly more collagen in auricular cartilage than the HA gel and the p = 1 and p = 2 constructs. In general, the collagen content decreased with chondrocyte passage.
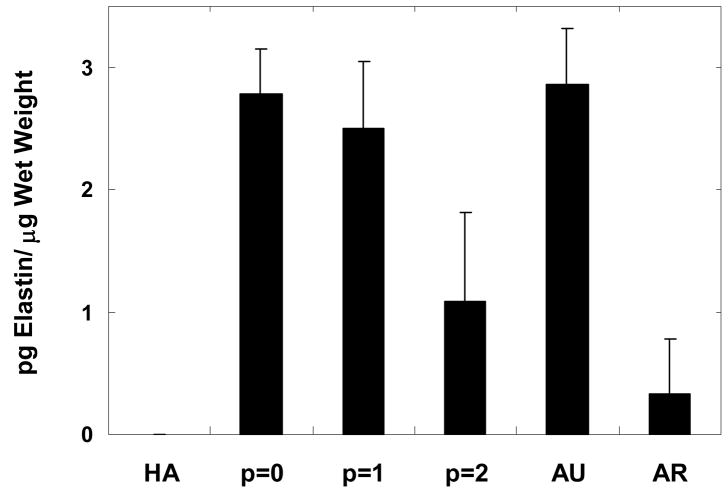
As a final measure of construct biochemical levels, elastin was quantified to determine if the implanted auricular chondrocytes still produced elastin after isolation and expansion. In general, elastin content decreased with passage number, with a significant decrease from p = 1 to p = 2. However, no significant difference for p = 0 (2.7 ± 0. 4 pg elastin/μg wet weight) or p = 1 constructs (2.5 ± 0.5 pg elastin/μg wet weight) was observed when compared to auricular cartilage (2.9 ± 0.5 pg elastin/μg wet weight). Lower levels of elastin were detected in articular cartilage and p = 2 constructs (0.3 ± 0.4 and 1.1 ± 0.7 pg elastin/μg wet weight, respectively), which were significantly lower than elastin found in the p = 0 and p = 1 constructs and auricular cartilage. No elastin was detected in the HA gels.
Immunohistochemical Analysis
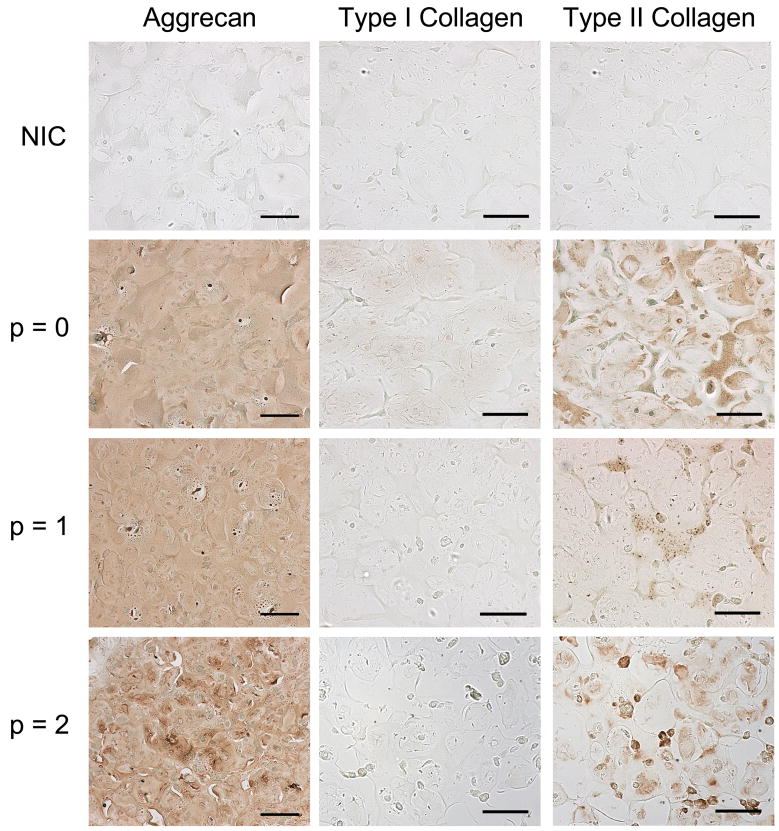
Representative stains for chondroitin sulfate, type I collagen, and type II collagen are shown in Figure 9 with non-immune controls for comparison. In general, histological sections illustrate the morphology and distribution of the auricular chondrocytes and the distribution of extracellular components. Chondroitin sulfate is evenly distributed throughout all constructs with similar intensity. Though little to no type I collagen staining was detected, type II collagen staining was detected in all constructs, where it was most intense and most widely distributed for the p = 0 constructs. Overall, aggrecan is more evenly distributed throughout the constructs than collagen. The non-immune controls exhibited no background staining of the constructs.
Figure 9.
Histological sections of constructs stained for chondroitin sulfate and type I and type II collagen compared to non-immune controls (NIC), with no primary antibody, after 12 weeks of subcutaneous implantation in nude mice. Chondroitin sulfate is evenly distributed throughout all constructs with similar intensity, regardless of passage number. All constructs exhibited greater type II collagen staining versus type I collagen, where the p = 0 constructs exhibit the greatest intensity and distribution of type II collagen. Scale bar =100 μm.
Discussion
One of the major obstacles for cartilage tissue engineering is finding a cell source and adequate cell numbers for delivery to a defect. For this study, auricular chondrocytes were chosen for their ease of harvest, high yield, and rapid proliferation6. Our previous work screened various HA macromer concentrations and molecular weights for their ability to support neocartilage formation and we found that hydrogels fabricated from 2 wt% of a 50 kDa HA macromer most resembled the properties of native cartilage and showed the greatest promise for cartilage regeneration. In this study we examined the effects of auricular chondrocyte expansion on the final properties of engineered cartilage in photopolymerizable HA hydrogels. Directly isolated (p = 0) and expanded (p = 1 and p = 2) auricular chondrocytes were photoencapsulated in HA gels, cultured subcutaneously in the dorsum of nude mice for 12 weeks, and explanted for testing.
Macroscopic observations of the explants showed an increase in size and opaqueness of all constructs since initial implantation, regardless of expansion. The p = 0 and p = 1 constructs were larger and more opaque than the p = 2 constructs, potentially indicating more ECM production and neocartilage formation. Our mechanical testing results show an increase in the moduli of the p = 0 and p = 1 constructs over the HA hydrogel alone. The testing protocol used in this analysis is similar to previously used protocols for measuring the equilibrium modulus of hydrogel systems29 and native cartilage30. This expected increase in compressive equilibrium modulus is indicative of neocartilage formation (i.e., chondrocyte proliferation and ECM deposition). However, the significant difference between all engineered constructs and articular cartilage may limit the application of these hydrogels to load bearing joints. It is important to note that the equilibrium moduli reported here for native cartilage is lower than the aggregate moduli reported previously for auricular10 and articular30 cartilage. These differences may be attributed to the specific conditions of the testing system (e.g. percent total strain) or testing method (e.g. indentation). Even though comparison among literature may be difficult, within our paradigm, relative comparisons can be made between our hydrogel constructs and native cartilage. Furthermore, the equilibrium moduli we obtained for articular cartilage is comparable to that determined by Strauss et al31, using a similar protocol.
As expected, the water content of all constructs was significantly lower than the control HA gel. This is due to the production of ECM molecules, which replaced water within the construct during the 12 week culture. Biochemical analysis of GAG and collagen content exhibited similar trends, with a general decrease observed with chondrocyte expansion. All engineered constructs exhibited a significantly higher GAG content than the control HA gels, indicating neocartilage formation, while GAG content significantly decreased from p = 1 to p = 2, indicating potential dedifferentiation. Furthermore, no significant difference was found between p=0 constructs and auricular cartilage, but constructs with expanded chondrocytes exhibited significantly lower collagen content than that of native cartilage, again showing a potential loss of phenotype. The trend for elastin content was similar to results from the GAG content analysis. This observed trend is similar to findings by van Osch et al6, which showed that as the cells are expanded 2-dimensionally in vitro, their phenotype changes and a decrease in elastin, a component of auricular cartilage, is seen. No significant differences were found between the p = 0 and p = 1 constructs when compared to each other and to auricular cartilage, indicating a retention of the auricular chondrocyte phenotype.
Immunohistochemical analysis was used to detect the distribution of aggrecan and type I and type II collagen within the constructs. The histological sections of all constructs showed intense, uniform staining for chondroitin sulfate, which reflected the even distribution of chondrocytes within the hydrogel. Compared to type II collagen staining, the more even distribution of chondroitin sulfate, a major component of aggrecan, may have resulted from a difference in molecule size, where smaller chondroitin sulfate molecules were able to fill the voids of the hydrogel with greater ease. Histological observations indicate ECM production by encapsulated chondrocytes and are consistent with the results from the biochemical analysis.
Overall, this analysis shows that p = 0 and p = 1 auricular chondrocytes retain a more chondrogenic phenotype when photoencapsulated in a HA hydrogel for 12 weeks while possible changes in phenotype may occur after p = 1 and may compromise neocartilage formation in vivo. Although constructs were all mechanically inferior to articular cartilage, the p = 0 and p = 1 constructs showed comparable if not greater compressive moduli than auricular cartilage and show an increase in modulus over the HA gel. Even though biochemical content generally decreased with passage, significant decreases were only found after p = 1. Histologically, all constructs exhibited aggrecan and type II collagen staining, characteristic of native cartilage. These results show that p = 0 and p = 1 auricular chondrocytes produce cartilaginous tissue in a 2 wt%, 50 kDa hyaluronic acid hydrogel that is comparable to auricular cartilage, but are lower than values for articular cartilage. However, it is possible that mechanical stimuli or the introduction of growth factors could lend to the production of more hyaline-like cartilage (i.e., articular cartilage). Auricular chondrocytes may be a viable source of cells for cartilage regeneration, but extensive expansion of the cells 2-dimensionally in vitro must be limited to prevent compromised properties.
Figure 8.
Elastin content of constructs normalized to construct wet weight after 12 weeks of subcutaneous implantation in nude mice compared to controls of the HA gel alone and native auricular and articular cartilage. There was no significant difference between auricular cartilage when compared to either the p = 0 or p = 1 constructs. Minimal elastin was found in articular cartilage and the elastin content in the p = 2 constructs was significantly lower than that in the p = 0 and p = 1 constructs and auricular cartilage. No elastin was detected in the HA gels.
Acknowledgments
Support for this research was provided through an NIH grant (K22 DE-015761). The authors would like to thank Dr. Steven Nicoll for helpful discussions and use of his laboratory for sample analysis.
Footnotes
Portions of this work were performed at the University of Pennsylvania (polymer synthesis, sample analysis) and portions were performed at the Massachusetts General Hospital (implantation in mice)
References
- 1.Galois L, Freyria AM, Grossin L, Hubert P, Mainard D, Herbage D, Stoltz JF, Netter P, Dellacherie E, Payan E. Cartilage repair: surgical techniques and tissue engineering using polysaccharide- and collagen-based biomaterials. Biorheology. 2004;41:433. [PubMed] [Google Scholar]
- 2.Sharma B, Elisseeff JH. Engineering structurally organized cartilage and bone tissues. Ann Biomed Eng. 2004;32:148. doi: 10.1023/b:abme.0000007799.60142.78. [DOI] [PubMed] [Google Scholar]
- 3.Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51:164. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 4.Nettles DL, Vail TP, Morgan MT, Grinstaff MW, Setton LA. Photocrosslinkable hyaluronan as a scaffold for articular cartilage repair. Ann Biomed Eng. 2004;32:391. doi: 10.1023/b:abme.0000017552.65260.94. [DOI] [PubMed] [Google Scholar]
- 5.Saadeh PB, Brent B, Mehrara BJ, Steinbrech DS, Ting V, Gittes GK, Longaker MT. Human cartilage engineering: chondrocyte extraction, proliferation, and characterization for construct development. Ann Plast Surg. 1999;42:509. [PubMed] [Google Scholar]
- 6.Van Osch GJ, Mandl EW, Jahr H, Koevoet W, Nolst-Trenite G, Verhaar JA. Considerations on the use of ear chondrocytes as donor chondrocytes for cartilage tissue engineering. Biorheology. 2004;41:411. [PubMed] [Google Scholar]
- 7.Kafienah W, Jakob M, Demarteau O, Frazer A, Barker MD, Martin I, Hollander AP. Three-dimensional tissue engineering of hyaline cartilage: comparison of adult nasal and articular chondrocytes. Tissue Eng. 2002;8:817. doi: 10.1089/10763270260424178. [DOI] [PubMed] [Google Scholar]
- 8.Rotter N, Ung F, Roy AK, Vacanti M, Eavey RD, Vacanti CA, Bonassar LJ. Role for interleukin 1alpha in the inhibition of chondrogenesis in autologous implants using polyglycolic acid-polylactic acid scaffolds. Tissue Eng. 2005;11:192. doi: 10.1089/ten.2005.11.192. [DOI] [PubMed] [Google Scholar]
- 9.Cao Y, Rodriguez A, Vacanti M, Ibarra C, Arevalo C, Vacanti CA. Comparative study of the use of poly(glycolic acid), calcium alginate and pluronics in the engineering of autologous porcine cartilage. J Biomater Sci Polym Ed. 1998;9:475. doi: 10.1163/156856298x00578. [DOI] [PubMed] [Google Scholar]
- 10.Chang SC, Tobias G, Roy AK, Vacanti CA, Bonassar LJ. Tissue engineering of autologous cartilage for craniofacial reconstruction by injection molding. Plast Reconstr Surg. 2003;112:793. doi: 10.1097/01.PRS.0000069711.31021.94. [DOI] [PubMed] [Google Scholar]
- 11.Xia W, Liu W, Cui L, Liu Y, Zhong W, Liu D, Wu J, Chua K, Cao Y. Tissue engineering of cartilage with the use of chitosan-gelatin complex scaffolds. J Biomed Mater Res B Appl Biomater. 2004;71:373. doi: 10.1002/jbm.b.30087. [DOI] [PubMed] [Google Scholar]
- 12.Kamil SH, Kojima K, Vacanti MP, Bonassar LJ, Vacanti CA, Eavey RD. In vitro tissue engineering to generate a human-sized auricle and nasal tip. Laryngoscope. 2003;113:90. doi: 10.1097/00005537-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Xu JW, Zaporojan V, Peretti GM, Roses RE, Morse KB, Roy AK, Mesa JM, Randolph MA, Bonassar LJ, Yaremchuk MJ. Injectable tissue-engineered cartilage with different chondrocyte sources. Plast Reconstr Surg. 2004;113:1361. doi: 10.1097/01.prs.0000111594.52661.29. [DOI] [PubMed] [Google Scholar]
- 14.Chung C, Mesa J, Randolph MA, Yaremchuk M, Burdick JA. Influence of Gel Properties on Neocartilage Formation by Auricular Chondrocytes Photoencapsulated in Hyaluronic Acid Networks. J Biomed Mater Res. doi: 10.1002/jbm.a.30660. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 16.von der Mark K, Gauss V, von der Mark H, Muller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977;267:531. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- 17.Takigawa M, Shirai E, Fukuo K, Tajima K, Mori Y, Suzuki F. Chondrocytes dedifferentiated by serial monolayer culture form cartilage nodules in nude mice. Bone Miner. 1987;2:449. [PubMed] [Google Scholar]
- 18.Mayne R, Vail MS, Mayne PM, Miller EJ. Changes in type of collagen synthesized as clones of chick chondrocytes grow and eventually lose division capacity. Proc Natl Acad Sci U S A. 1976;73:1674. doi: 10.1073/pnas.73.5.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villar-Suarez V, Calles-Venal I, Bravo IG, Fernandez-Alvarez JG, Fernandez-Caso M, Villar-Lacilla JM. Differential Behavior Between Isolated and Aggregated Rabbit Auricular Chondrocytes on Plastic Surfaces. J Biomed Biotechnol. 2004;2004:86. doi: 10.1155/S1110724304312039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chia SH, Homicz MR, Schumacher BL, Thonar EJ, Masuda K, Sah RL, Watson D. Characterization of human nasal septal chondrocytes cultured in alginate. J Am Coll Surg. 2005;200:691. doi: 10.1016/j.jamcollsurg.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6:386. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smeds KA, Pfister-Serres A, Miki D, Dastgheib K, Inoue M, Hatchell DL, Grinstaff MW. Photocrosslinkable polysaccharides for in situ hydrogel formation. J Biomed Mater Res. 2001;54:115. doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 23.Singer VL, Jones LJ, Yue ST, Haugland RP. Characterization of PicoGreen reagent and development of a fluorescence-based solution assay for double-stranded DNA quantitation. Anal Biochem. 1997;249:228. doi: 10.1006/abio.1997.2177. [DOI] [PubMed] [Google Scholar]
- 24.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 25.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 26.Herbage D, Bouillet J, Bernengo JC. Biochemical and physiochemical characterization of pepsin-solubilized type-II collagen from bovine articular cartilage. Biochem J. 1977;161:303. doi: 10.1042/bj1610303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williamson AK, Chen AC, Sah RL. Compressive properties and function-composition relationships of developing bovine articular cartilage. J Orthop Res. 2001;19:1113. doi: 10.1016/S0736-0266(01)00052-3. [DOI] [PubMed] [Google Scholar]
- 28.Brown AN, Kim BS, Alsberg E, Mooney DJ. Combining chondrocytes and smooth muscle cells to engineer hybrid soft tissue constructs. Tissue Eng. 2000;6:297. doi: 10.1089/107632700418029. [DOI] [PubMed] [Google Scholar]
- 29.Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, Hung CT, Ateshian GA. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 30.Mow VC, Gibbs MC, Lai WM, Zhu WB, Athanasiou KA. Biphasic indentation of articular-cartilage.2. A numerical algorithm and experimental study. J Biomech. 1989;22:853. doi: 10.1016/0021-9290(89)90069-9. [DOI] [PubMed] [Google Scholar]
- 31.Strauss EJ, Goodrich LR, Chen CT, Hidaka C, Nixon AJ. Biochemical and biomechanical properties of lesion and adjacent articular cartilage after chondral defect repair in an equine model. Am J Sports Med. 2005;33:1647. doi: 10.1177/0363546505275487. [DOI] [PubMed] [Google Scholar]