Abstract
Mesenchymal stem cells (MSCs) are multipotent progenitor cells whose plasticity and self-renewal capacity have generated significant interest for applications in tissue engineering. The objective of this study was to investigate MSC chondrogenesis in photo-cross-linked hyaluronic acid (HA) hydrogels. Because HA is a native component of cartilage, and MSCs may interact with HA via cell surface receptors, these hydrogels could influence stem cell differentiation. In vitro and in vivo cultures of MSC-laden HA hydrogels permitted chondrogenesis, measured by the early gene expression and production of cartilage-specific matrix proteins. For in vivo culture, MSCs were encapsulated with and without transforming growth factor beta-3 (TGF-β3) or pre-cultured for 2 weeks in chondrogenic medium before implantation. Up-regulation of type II collagen, aggrecan, and sox 9 was observed for all groups over MSCs at the time of encapsulation, and the addition of TGF-β3 further enhanced the expression of these genes. To assess the influence of scaffold chemistry on chondrogenesis, HA hydrogels were compared with relatively inert poly(ethylene glycol) (PEG) hydrogels and showed enhanced expression of cartilage-specific markers. Differences between HA and PEG hydrogels in vivo were most noticeable for MSCs and polymer alone, indicating that hydrogel chemistry influences the commitment of MSCs to undergo chondrogenesis (e.g., ∼43-fold up-regulation of type II collagen of MSCs in HA over PEG hydrogels). Although this study investigated only early markers of tissue regeneration, these results emphasize the importance of material cues in MSC differentiation microenvironments, potentially through interactions between scaffold materials and cell surface receptors.
Introduction
Mesenchymal stem cells (MSCs) are multipotent progenitor cells that have the ability to self-replicate and differentiate down multiple cell lineages when given the appropriate environmental cues.1 Although Friedenstein and colleagues2 first identified them in bone marrow in the 1970s, MSCs have since been isolated from various adult tissues and differentiated into several cell types, including osteoblasts, chondrocytes, and adipocytes.1,3–5 With their plasticity and self-renewal capacity, these cells have generated significant interest for applications in cell-replacement therapies and tissue regeneration.
MSCs have garnered interest as an alternative cell source for cartilage tissue engineering, because they can be isolated from adults via a bone marrow biopsy. To induce chondrogenic differentiation, MSCs are typically grown in pellet culture in the presence of transforming growth factor betas (TGF-βs),6–10 and differentiation is monitored according to the production of cartilaginous matrix proteins such as sulfated glycosaminoglycans (GAGs) and type II collagen. In recent years, natural and synthetic biomaterials have been used to create niches or microenvironments to control stem cell behavior and differentiation toward cartilage formation.11,12 These biomaterials serve as three-dimensional (3D) scaffolds capable of enhancing and templating tissue formation through cell morphology and organization, intercellular interactions, mechanical forces, and the delivery of bioactive molecules.11,13
One molecule of particular interest is hyaluronic acid (HA), which is found natively in cartilage tissue. HA, a linear polysaccharide of alternating D-glucuronic acid and N-acetyl-D-glucosamine, functions as a core molecule for the binding of keratin sulfate and chondroitin sulfate in forming aggrecan.14 Hyaluronidases found in the body degrade it enzymatically, as do oxidative mechanisms to yield oligosaccharides and glucuronic acid. This natural polymer plays a role in cartilage homeostasis; is involved in cellular processes such as cell proliferation, morphogenesis, inflammation, and wound repair;15–17 and can interact with cell surface receptors for HA18 (e.g., CD44, CD54, and CD168). HA can be modified through its carboxyl and hydroxyl groups and subsequently cross-linked into hydrogels or made hydrophobic and processed into macroporous scaffolds. These modification strategies include esterfication,19,20 methacrylation,21,22 and cross-linking with divinyl sulfone23,24 or dialdehyde.25
Researchers have developed HA-based scaffolds in the form of hydrogels,26–30 sponges,31 and meshes.32 These scaffolds are biocompatible and can serve as delivery vehicles for cells and bioactive molecules. Chondrocytes and MSCs have been successfully encapsulated in HA and HA composite hydrogels.22,26,33 Liu et al. showed that MSC-laden HA–gelatin hydrogels were able to produce elastic, firm, translucent cartilage with zonal architecture in rabbit osteochondral defects.26 Sponges and non-woven meshes made of hydrophobic HA ester derivatives (Hyaff-7 and -11) seeded with MSCs and chondrocytes have been shown to support a chondrocyte phenotype and the production of cartilaginous extracellular matrix (ECM) proteins.31,32,34,35 In the clinical setting, Hyalograft-C, a tissue-engineered graft composed of Hyaff-11 scaffolds seeded with autologous chondrocytes, has been used to treat cartilage lesions in patients. Results from 2- to 5-year follow-ups showed better repair of cartilage lesions in 91.5% of patients than predicted by pre-operative assessments, and the repaired cartilage was hyaline-like in appearance.20 Recently, a thiolated HA derivative was successfully electrospun into a nano-fibrous mesh with the potential to more closely mimic the size-scale of native ECM.36
For this study, we used photo-cross-linked HA hydrogels to investigate the chondrogenesis of MSCs in HA microenvironments. Previously, we optimized a methacrylated HA (MeHA) hydrogel system for the encapsulation of chondrocytes and characterized cytocompatibility, phenotype retention, and neocartilage formation within these hydrogels using auricular and articular chondrocytes.30,33,37,38 However, inherent limitations to the use of chondrocytes (e.g., low cell yields and a tendency to dedifferentiate when expanded in vitro) have motivated the use of MSCs as an alternative cell source. MSCs are easily expanded in vitro without loss of differentiation potential and express CD44,39 one of the primary receptors for HA. Thus, we hypothesized that photo-cross-linked MeHA hydrogels could provide a favorable niche for MSC chondrogenesis.
Materials and Methods
CD44 staining and flow cytometry
To determine the presence of CD44 receptors, human MSCs (Lonza Walkersville, Inc., Walkersville, MD; donor: 33-year-old man, original passage number 2) were cultured in two dimensions on glass coverslips and fixed in Accustain (Sigma) for immunofluorescent staining. Briefly, the cells were blocked with 5% fetal bovine serum (FBS), stained with primary antibody anti-CD44 clone A3D8 (4 μg/mL, Sigma) for 2 h, incubated with secondary antibody anti-mouse immunoglobulin G (IgG; whole molecule) F(ab’)2 fragment-fluorescein isothiocyanate (1:50 dilution) for 15 min, and counterstained with 4',6-diamidino-2-phenylindole (2 μg/mL) for nuclei visualization. In addition, MSCs were labeled with phycoerythrin (PE)-conjugated CD44 (0.25 μg/mL, eBioscience, San Diego, CA) monoclonal antibody for 1 h on ice and analyzed using flow cytometry (Guava EasyCyte 3.10, Guava Technologies, Hayward, CA).
Macromer syntheses
MeHA was synthesized as previously reported.21 Briefly, methacrylic anhydride (Sigma, St. Louis, MO) was added to a solution of 1 wt% HA (MW = 64 kDa, Lifecore, Chaska, MN) in deionized water, adjusted to a pH of 8 with 5 N NaOH, and reacted on ice for 24 h. The macromer solution was purified via dialysis (MW cutoff 6–8 k) against deionized water for a minimum of 48 h with repeated changes of water. The final product was obtained using lyophilization and stored at −20°C in powder form before use. Poly(ethylene glycol)-diacrylate (PEGDA) was synthesized as previously reported.40 Briefly, triethylamine (1.5 molar excess) was added to PEG-4600 (Sigma) dissolved in methylene chloride. Acryloyl chloride (1.5 molar excess) was added drop-wise under nitrogen and reacted on ice for 6 h, followed by reaction at room temperature for 30 h. The product was precipitated in ethyl ether, filtered, dried in a vacuum oven, re-dissolved in deionized water, dialyzed for 3 days, lyophilized, and stored at −20°C in powder form before use. The macromers were characterized using 1H nuclear magnetic resonance (Bruker Advance 360 MHz, Bruker, Billerica, MA). Macromers were sterilized using a germicidal lamp in a laminar flow hood for 30 min and dissolved in a sterile solution of phosphate buffered saline (PBS) containing 0.05 wt% 2-methyl-1-[4-(hydroxyethoxy)phenyl]-2-methyl-1-propanone (Irgacure 2959, I2959, Ciba, Basel, Switzerland) for polymerization. Hydrogels were polymerized by injecting the macromer solution into a mold or between glass slides and exposing to ultraviolet light (Eiko, ∼1.9 mW/cm2, Topbulb, East Chicago, IN) for 10 min.
Mechanical characterization
Acellular hydrogels (n = 5) approximately 7 mm in diameter and 1 mm thick were tested in unconfined compression using a Dynamic Mechanical Analyzer Q800 (DMAQ800, TA Instruments, New Castle, DE) in a PBS bath. Hydrogels were compressed at a rate of 10%/min until failure or until they reached 70% of their initial thickness. The modulus was determined as the slope of the stress-versus-strain culture at low strains (<20%). The elastic modulus of PEG hydrogels was matched to 2wt% MeHA hydrogels by varying the macromer concentration (5–10 wt%).
MSC photo-encapsulation and culture
Human MSCs were expanded for four additional passages in growth medium consisting of alpha minimum essential medium with 16.7% FBS and 1% penicillin/streptomycin. MSCs (20 × 106 cells/mL) were photo-encapsulated in hydrogels by suspension in 2 wt% MeHA or 5.5 wt% PEG solutions with or without 200 ng/mL TGF-β3 (R&D Systems, Minneapolis, MN). The cell–macromer solutions were pipetted into sterile molds (50 μL volume) and polymerized using ultraviolet light (Eiko, ∼1.9 mW/cm2, Topbulb, East Chicago, IN) for 10 min. Because of the polymerization technique, all growth factor is encapsulated within the hydrogel, resulting in a concentration of 10 ng TGF-β3/50 μL gel.
To evaluate chondrogenesis, MSC-laden MeHA hydrogels were cultured in vitro in growth medium or Dulbecco's modified Eagle medium supplemented with 1% penicillin/streptomycin, 1% insulin-transferrin-selenium + universal culture supplement, 1 mM sodium pyruvate, 40 mg/mL L-proline, 100 nM dexamethasone, 50 μg/mL ascorbic acid 2-phosphate, and 10 ng/mL TGF-β3 (chondrogenic medium). For in vivo culture, MSCs were encapsulated in MeHA hydrogels with (HA + T3) and without (HA-MSCs) TGF-β3 and implanted in nude mice or cultured in vitro in chondrogenic media for 2 weeks before implantation (HA-C). Nude mice were anesthetized with isoflurane, a 2-cm midline incision was made on the dorsum of each mouse, and five subcutaneous pockets were made using blunt dissection. One construct was placed in each pocket, and the wound was closed using sterile stainless steel skin clips. Constructs were cultured in vivo for 2 weeks. National Institutes of Health guidelines for the care and use of laboratory animals were observed.41 For scaffold comparison, MSCs were encapsulated in PEG hydrogels and cultured in vitro and in vivo in the same manner as the MeHA hydrogels.
Viability
The viability of MSCs in the MeHA and PEG hydrogels was assessed using a live/dead cytotoxicity kit (Molecular Probes, Eugene, OR) and a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (ATCC, Manassas, VA). Live/dead images were taken 1 and 24 h after encapsulation. Mitochondrial activity (n = 3) was assessed after 7 and 14 days of in vitro culture. Briefly, 100 μL of MTT reagent was added to 1 mL of medium and incubated for 4 h. Samples were then removed from the medium, homogenized in the detergent solution using a tissue grinder, incubated for 4 h, and read at an absorbance of 570 nm in a Synergy HT (Bio-Tek Instruments, Winooski, VT) spectrophotometer.
Gene expression analysis
Samples (n = 4) were homogenized in Trizol Reagent (Invitrogen, Carlsbad, CA) using a tissue grinder, and RNA was extracted according to the manufacturer's instructions. RNA concentration was determined using an ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE). One microgram of RNA from each sample was reverse transcribed into cDNA using reverse transcriptase (Superscript II, Invitrogen, Carlsbad, CA) and oligoDT (Invitrogen, Carlsbad, CA). Polymerase chain reaction (PCR) was performed on an Applied Biosystems 7300 real-time PCR system using a 25-μL reaction volume for TaqMan (5’-nuclease) and sybr green reactions. Primers and probes specific for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), type I and type II collagen, aggrecan, sox 9, and hyaluronidases (Hyal) 1, 2, and 3 are listed in Table 1. GAPDH was used as the housekeeping gene. Relative gene expression was calculated using the ΔΔCT method, where fold difference was calculated using the expression 2-ΔΔCt.
Table 1.
Human Quantitative Polymerase Chain Reaction Primers and Probes
| Gene | Forward Primer | Reverse Primer | Probe |
|---|---|---|---|
| Glyceraldehyde 3-phosphate dehydrogenase | AGGGCTGCTTTTAACTCTGGTAAA | GAATTTGCCATGGGTGGAAT | CCTCAACTACATGGTTTAC |
| Type I Collagen | AGGACAAGAGGCATGTCTGGTT | GGACATCAGGCGCAGGAA | TTCCAGTTCGAGTATGGC |
| Type II Collagen | GGCAATAGCAGGTTCACGTACA | CGATAACAGTCTTGCCCCACTT | CTGCACGAAACATAC |
| Aggrecan | TCGAGGACAGCGAGGCC | TCGAGGGTGTAGCGTGTAGAGA | ATGGAACACGATGCCTTTCACCACGA |
| Sox 9 | AAGCTCTGGAGACTTCTGAACGA | GCCCGTTCTTCACCGACTT | |
| HYAL1 | AAAATACAAGAACCAAGGAATCATGTC | CGGAGCACAGGGCTTGACT | |
| HYAL2 | GGCGCAGCTGGTGTCATC | CCGTGTCAGGTAATCTTTGAGGTA | |
| HYAL3 | GCCTCACACACCGGAGATCT | GCTGCACTCACACCAATGGA |
HYAL, hyaluronidase.
Histological analysis
For histological analysis, constructs were fixed in 10% formalin for 24 h, embedded in paraffin, and processed using standard histological procedures. The histological sections (7 μm thick) were stained for aggrecan and collagen distributions using the Vectastain ABC kit (Vector Labs, Burlingame, CA) and the DAB Substrate kit for peroxidase (Vector Labs). Sections were predigested in 0.5 mg/mL hyaluronidase for 30 min at 37°C and incubated in 0.5 N acetic acid for 4 h at 4°C to swell the samples before overnight incubation with primary antibodies at dilutions of 1:100, 1:200, and 1:3 for chondroitin sulfate (mouse monoclonal anti-chondroitin sulfate, Sigma) and type I (mouse monoclonal anti-collagen type 1, Sigma) and type II collagen antibodies (mouse monoclonal anti-collagen type II, Developmental Studies Hybridoma Bank, Iowa City, IA), respectively. Non-immune controls underwent the same procedure without primary antibody incubation.
TGF-β3 release
TGF-β3 (10 ng/50 μL gel) was encapsulated in acellular MeHA hydrogels, and release of the growth factor was monitored via diffusion in PBS alone or in the presence of hyaluronidase (500 U/mL) at 37°C on an orbital shaker. PBS and hyaluronidase solutions were changed every other day, and aliquots were stored at −20°C until analysis with a TGF-β3 enzyme-linked immuno-adsorbent assay (R&D Systems, Minneapolis, MN).
Statistical analysis
All values are reported as means ± standard errors of the mean. The Student t-test and Wilcoxon sum-rank test were used to determine significant differences between groups, with p < 0.05.
Results
For this study, we investigated the chondrogenesis of MSCs in photo-cross-linked HA hydrogels. The chondrogenic differentiation of MSCs in HA hydrogels was monitored in vitro and in vivo; increases in the gene expression and production of cartilaginous matrix proteins were used as markers for chondrogenesis, as well as limited expression and production of type I collagen. In addition, benefits of potential cell–HA scaffold interactions were explored using short-term culture comparisons with a relatively inert PEG hydrogel system that would provide minimal direct interactions with encapsulated cells.
MSC interactions with HA
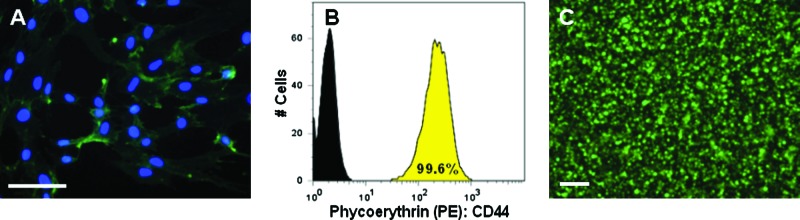
The potential for interactions between MSCs and HA was first assessed using immunofluorescent staining and flow cytometry (Fig. 1A, B) for CD44. This HA receptor was present on 99.6% of the cell population and stained uniformly on MSCs cultured in two dimensions. Additionally, MSCs cultured in two dimensions expressed multiple isoforms of hyaluronidases (e.g., Hyal 1, Hyal 2, Hyal 3) (results not shown). When photo-encapsulated in 3D HA hydrogels, nearly all of the MSCs remained viable (>98%) as indicated by live/dead staining 6 h after encapsulation (Fig. 1C).
FIG. 1.
CD44 expression by mesenchymal stem cells (MSCs) and MSC viability in methacrylated hyaluronic acid (MeHA) hydrogels. Immunofluorescence staining of CD44 (green) with nuclear counterstain (blue) of MSCs cultured in two dimensions on glass coverslips (scale bar = 100 μm) (A), flow cytometry staining for CD44 (yellow) compared with unstained (black) population of MSCs before encapsulation (B), and live (green)/dead (red) image of MSCs encapsulated in MeHA hydrogel 6 h after photopolymerization (scale bar = 200 μm) (C). Color images available online at www.liebertonline.com/ten.
MSC chondrogenesis
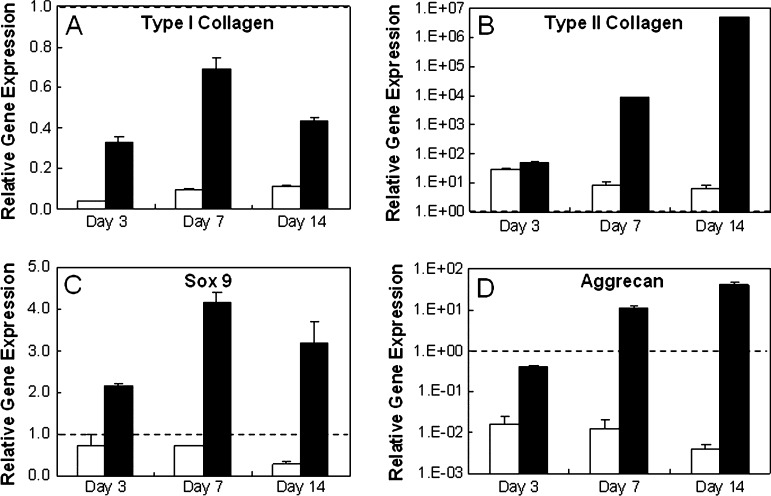
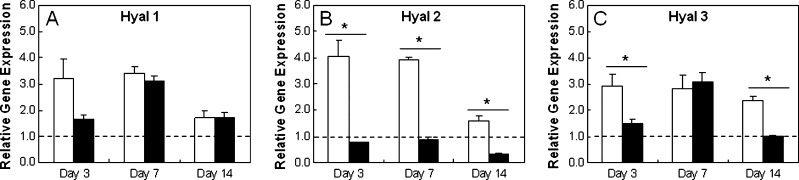
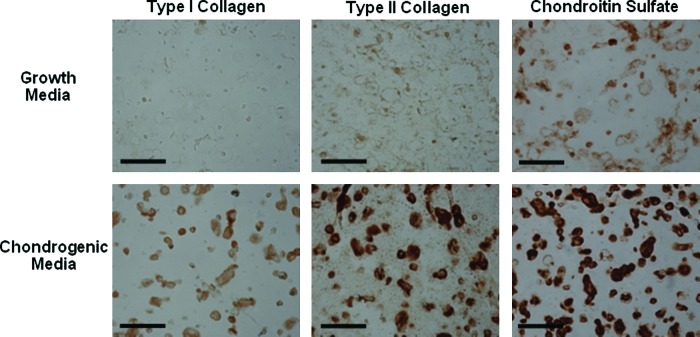
Chondrogenic differentiation was induced in vitro with the addition of TGF-β3 to cultures of MSCs in HA hydrogels. Comparisons between MSC-laden HA hydrogels cultured in growth and chondrogenic media (+TGF-β3) showed significant differences in gene expression at 3, 7, and 14 days of culture (Fig. 2). Specifically, greater up-regulation of sox 9, type II collagen, and aggrecan was observed for constructs cultured in chondrogenic medium than cultures in growth medium at all time points. Except for aggrecan at day 3, greater up-regulation of the chondrogenic genes (type II collagen, aggrecan, sox 9) was observed in hydrogels cultured in chondrogenic media over the gene expression of MSCs at the time of encapsulation. Significant differences in hyaluronidase expression were also observed based on culture medium for hyal 2 and 3 (Fig. 3) at several time points. In addition, type I collagen was more down-regulated in growth and chondrogenic media than expression at the time of encapsulation. Histologically, greater deposition of type II collagen and chondroitin sulfate (CS) was observed for MSC-laden HA hydrogels cultured in chondrogenic medium (Fig. 4), where intense pericellular staining was observed after 14 days of culture. The cells remained rounded in all gels, and no obvious spatial variations were observed between the perimeter and the center of the gels. Light staining for CS in hydrogels cultured in growth medium and light staining for type I collagen in hydrogels cultured in chondrogenic medium was also observed.
FIG. 2.
Mesenchymal stem cell (MSC) chondrogenesis in methacrylated hyaluronic acid hydrogels in vitro. Relative gene expression of type I (A) and type II (B) collagen, sox 9 (C), and aggrecan (D) for MSCs encapsulated in hydrogels cultured in growth (white) and chondrogenic (black) media. Glyceraldehyde 3-phosphate dehydrogenase was used as the housekeeping gene, and expression was normalized to cells at the time of encapsulation (indicated by the dashed line). Gene expression of MSCs cultured in chondrogenic media was significantly different than that of MSCs cultured in growth medium (p < 0.05) for all genes at all time points.
FIG. 3.
Hyaluronidase (Hyal) expression of mesenchymal stem cell (MSC)-laden methacrylated hyaluronic acid hydrogels in vitro. Relative gene expression of Hyals for MSCs encapsulated in hydrogels cultured in growth (white) and chondrogenic (black) media. Glyceraldehyde 3-phosphate dehydrogenase was used as the housekeeping gene, and expression was normalized to cells at the time of encapsulation (indicated by the dashed line). *Significant differences (p < 0.05) between hydrogels cultured in growth and chondrogenic medium.
FIG. 4.
Immunohistochemistry of mesenchymal stem cell (MSC)-laden methacrylated hyaluronic acid hydrogels in vitro. Representative stains for type I and II collagen and chondroitin sulfate for MSC-laden hydrogels cultured in growth and chondrogenic media for 14 days in vitro. Scale bar = 100 μm. Color images available online at www.liebertonline.com/ten.
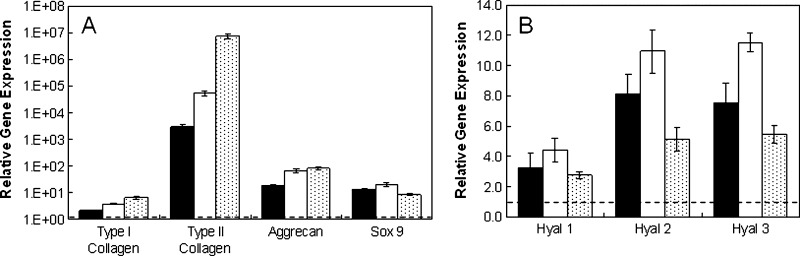
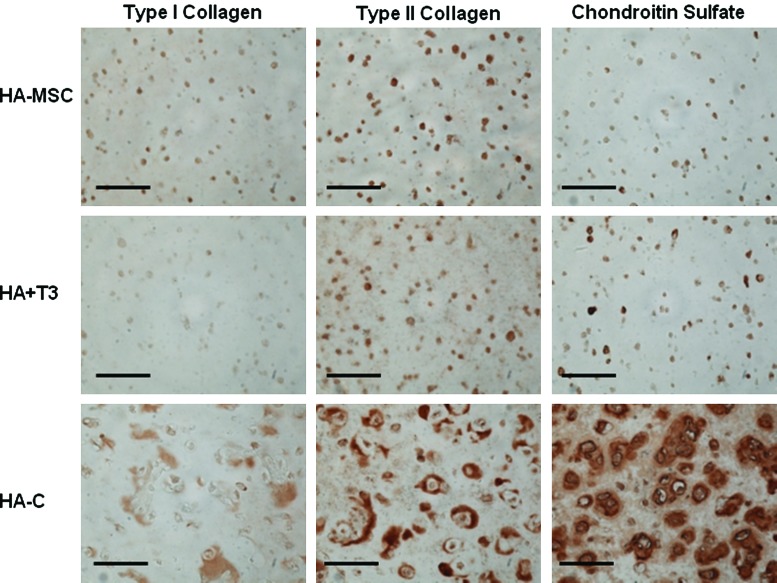
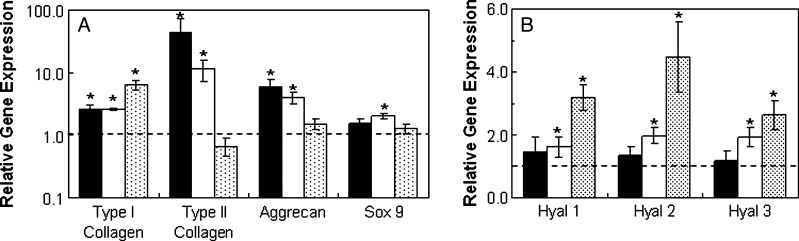
When cultured in vivo, MSCs in all groups (HA-MSC, HA + T3, HA-C) exhibited greater expression of all genes of interest (Fig. 5) than cells at the time of encapsulation after 14 days of implantation. Without growth factors present, there were approximately 3000, 18, and 13 times more type II collagen, aggrecan, and sox 9 gene expression, respectively. The HA + T3 group (with TGF-β3) and the HA-C group (with 2 weeks of pre-culture in chondrogenic media) exhibited approximately 17.5 and 2370 times more type II collagen and 3.7 and 4.6 times more aggrecan, respectively, than the HA-MSC group. Also, positive staining for type II collagen and CS, indicating chondrogenesis, was observed for all groups, with the greatest amount of staining observed for the HA-C group, which had an additional 2 weeks of pre-culture in vitro (Fig. 6). In addition, a separate in vitro assessment of TGF-β3 release for the HA + T3 group indicated that more than 75% of loaded TGF-β3 remained in the hydrogels after 2 weeks and that the addition of exogenous hyaluronidase could trigger release (data not shown). Bioactivity of TGF-β3 was not specifically assessed in this study beyond evidence of chondrogenesis within the hydrogel with the incorporation of TGF-β3.
FIG. 5.
Mesenchymal stem cell (MSC)-laden methacrylated hyaluronic acid (HA) hydrogels in vivo. Relative gene expression for type I and type II collagen, aggrecan, sox 9 (A), and hyaluronidases (B) for MSCs encapsulated in hydrogels cultured 2 weeks in vivo. Glyceraldehyde 3-phosphate dehydrogenase was used as the housekeeping gene, and expression was normalized to cells at the time of encapsulation (indicated by the dashed line). The groups included the hydrogel alone (HA-MSC, black), hydrogels with transforming growth factor beta-3 co-encapsulated with cells (HA + T3, white), and hydrogels pre-cultured in chondrogenic medium for 2 weeks (HA-C, shaded). All groups were significantly different (p < 0.05) for type I and II collagen, whereas HA-MSC was significantly different from HA + T3 and HA-C for aggrecan. In addition, HA + T3 was significantly different from HA-C for hyaluronidase 2 and 3 and sox 9.
FIG. 6.
Immunohistochemistry of mesenchymal stem cell (MSC)-laden methacrylated hyaluronic acid (HA) hydrogels in vivo. Representative stains for type I and II collagen and chondroitin sulfate for hydrogel alone (HA-MSC), hydrogels with transforming growth factor beta-3 co-encapsulated with cells (HA + T3), and hydrogels pre-cultured in chondrogenic medium for 2 weeks (HA-C) groups after 3 week culture in vivo. Scale bar = 100 μm. Color images available online at www.liebertonline.com/ten.
Comparison between HA and PEG hydrogels
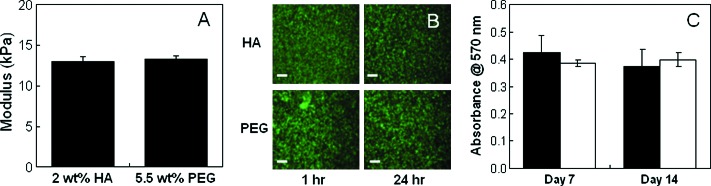
To explore potential cell–HA scaffold interactions, HA hydrogel cultures were compared with a relatively inert PEG hydrogel in short-term in vitro and in vivo cultures. First, the elastic modulus of PEG hydrogels was matched to 2 wt% MeHA hydrogels by altering the PEGDA macromer concentration. A 5.5 wt% PEG formulation with a modulus of 13.3 ± 1.0 kPa was found to be comparable (i.e., no statistical differences between moduli) with the 2 wt% MeHA hydrogels (13.0 ± 1.4 kPa) and was used for all comparison studies to minimize mechanical influences on cellular differentiation (Fig. 7A). In addition, live/dead staining and an MTT assay (Fig. 7B, C) demonstrated that viable MSCs were successfully encapsulated in both hydrogel systems and that there were no statistical differences in cell viability between hydrogel types.
FIG. 7.
Methacrylated hyaluronic acid (HA) compared with polyethylene glycol (PEG). Modulus of acellular HA and PEG hydrogels (A), live (green)/dead (red) images of mesenchymal stem cell–laden hydrogels at 1 and 24 h after polymerization; scale bar = 200 μm (B), relative mitochondrial activity for HA (black) and PEG (white) hydrogels after 7 and 14 days of in vitro culture (C). There were no statistical differences in hydrogel moduli and viability between the HA and PEG groups. Color images available online at www.liebertonline.com/ten.
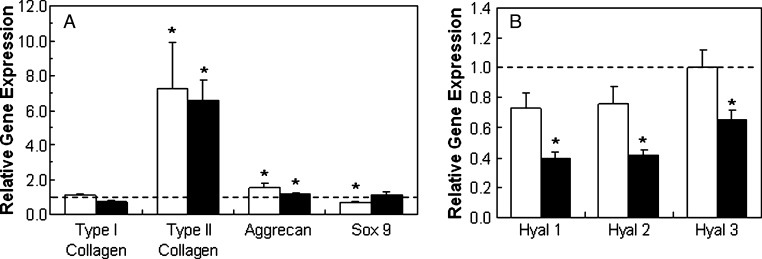
With in vitro and in vivo cultures, the gene expression of encapsulated MSCs differed depending on the hydrogel chemistry. With in vitro culture, type II collagen expression by MSCs in MeHA hydrogels was up-regulated approximately 7.3 and 6.6 times more than PEG counterparts after 7 and 14 days, respectively (Fig. 8). Aggrecan was also up-regulated in MeHA hydrogels (∼1.5 and ∼1.2 times after 7 and 14 days, respectively), although to a lesser extent. These differences were also observed in immunohistochemical staining, with more-intense type II collagen and CS staining in MeHA than PEG hydrogels (Fig. 9).
FIG. 8.
Methacrylated hyaluronic acid (HA) compared with polyethylene glycol (PEG) in vitro. Relative gene expression of type I and type II collagen, sox 9, and aggrecan (A) and hyaluronidases (B) for mesenchymal stem cells encapsulated in HA hydrogels cultured in vitro in chondrogenic media for 7 (white) and 14 days (black). Glyceraldehyde 3-phosphate dehydrogenase was used as the housekeeping gene, and expression was normalized to PEG counterparts (indicated by the dashed line). *Significant differences (p < 0.05) between HA and PEG hydrogels.
FIG. 9.
Immunohistochemistry of mesenchymal stem cell (MSC)-laden methacrylated hyaluronic acid (HA) and polyethylene glycol (PEG) hydrogels in vitro. Representative stains for type I and II collagen and chondroitin sulfate for MSC-laden HA and PEG hydrogels cultured in chondrogenic medium for 14 days in vitro. Scale bar = 200 μm. Color images available online at www.liebertonline.com/ten.
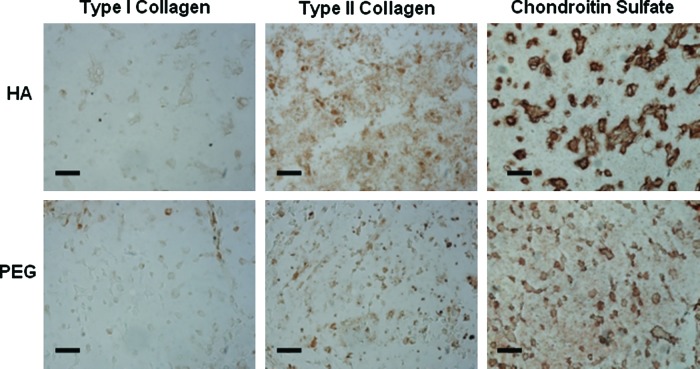
For in vivo culture, differences between MeHA and PEG hydrogels were most noticeable for MSCs plus scaffold alone, with type II collagen and aggrecan up-regulated approximately 43 and 6 times more, respectively, for MSCs in MeHA hydrogels than PEG hydrogels (Fig. 10). These differences between MeHA and PEG decreased to approximately 11.5 and 4.1 times with the addition of TGF-β3 directly encapsulated within the hydrogel and became negligible (∼0.7 and ∼1.5 times) with two weeks of pre-culture in chondrogenic media in vitro. Hyaluronidase expression also differed, with the expression of enzymes more down-regulated in vitro but more up-regulated in vivo for HA + T3 and HA-C groups than for their PEG counterparts.
FIG. 10.
Methacrylated hyaluronic acid (HA) compared with polyethylene glycol (PEG) in vivo. Relative gene expression for type I and II collagen, aggrecan, sox 9 (A), and hyaluronidases (B) of mesenchymal stem cells (MSCs) in hydrogel alone (HA-MSC, black), hydrogels with transforming growth factor beta-3 co-encapsulated with cells (HA + T3, white), and hydrogels pre-cultured in chondrogenic medium for 2 weeks (HA-C, shaded) groups cultured in vivo for 2 weeks. Glyceraldehyde 3-phosphate dehydrogenase was used as the housekeeping gene, and expression was normalized to PEG counterparts (indicated by the dashed line). *Significant differences (p < 0.05) between HA and PEG hydrogels.
Discussion
Recently, MSCs have been explored as an alternative cell source for cartilage regeneration and repair because of their chondrogenic potential and their ease of isolation from sources such as bone marrow without damage to native cartilage tissue. To this end, 3D scaffolds have been developed to create microenvironments for stem cells in which numerous factors, including material chemistry, functionalization with biological cues, interactions with surrounding cells, and mechanical properties,11 play a role in directing stem cell differentiation, in addition to soluble cues. In our laboratory, we investigated the use of a photo-cross-linked HA hydrogel to provide a favorable niche for MSC chondrogenesis in vitro and in vivo by providing cell interactive cues with a naturally found polysaccharide.
One of the advantages of using a HA-based scaffold is the potential for cell–scaffold interactions via cell surface receptors, which could direct cell behaviors and assist in stem cell differentiation. Previous studies have shown that exogenous HA added to culture medium elicits a chondrogenic effect on equine MSCs grown in pellet culture,42 which may be explained by the interaction between HA and HS CD44 receptor. CD44 is a cell-surface receptor that binds to HA, providing a means to retain and anchor proteoglycan aggregates to the plasma membrane of a cell. In addition, intimate association with the underlying cytoskeleton permits CD44 to initiate intracellular signaling,42,43 allowing it to sense changes in the ECM environment and signal a cellular response. This receptor is of particular interest because it is essential for the maintenance of cartilage homeostasis44 and plays a role in the catabolism of HA via phagocytosis.45 Positive expression of CD44 on MSCs was verified using immunofluorescent staining and flow cytometry. In addition, the expression of hyaluronidases was observed in MSCs, indicating the potential to remodel the MeHA hydrogel. Hyaluronidases are enzymes that cleave the β-1,4-glycosidic bonds between glucuronic acid and N-acetylglucosamine,46 which can affect cell differentiation and matrix catabolism. Each enzyme isoform plays a specific role in cleaving HA into discrete fragment sizes that regulate different cellular processes.47–49
The high viability of MSCs after photo-encapsulation in MeHA hydrogels demonstrated that these hydrogels could be successfully used as cell delivery vehicles. In addition, photopolymerization, with its numerous advantages for a clinical setting, served as an easy means to encapsulate cells uniformly in a 3D hydrogel matrix. MSC chondrogenesis in MeHA hydrogels was induced in vitro by culture in chondrogenic medium containing TGF-β3, which has been shown to induce chondrogenesis in a variety of scaffolds, including alginate beads, agarose, and poly(DL-lactic-co-glycolic acid)-collagen hybrid meshes.50–52 Accordingly, the culture of MSC-laden MeHA hydrogels in chondrogenic medium resulted in the up-regulation of type II collagen and aggrecan, which are typical markers for chondrogenic differentiation, and sox 9, a transcription factor that is required for successive steps in chondrogenesis. This up-regulation of cartilaginous protein expression was also reflected in immunohistochemical staining, which showed intense pericellular staining of type II collagen and CS after only 2 weeks of culture. Culture in growth medium also resulted in more down-regulation of type I collagen and slightly greater up-regulation of type II collagen than the cells at the time of encapsulation, suggesting that the scaffold alone could promote chondrogenesis. Likewise, this was also observed through the immunohistochemical staining of CS. This could be due to the morphology of the cells in the hydrogels (rounded in 3D vs spread in 2D culture) and interactions with the hydrogel. There were no spatial variations in ECM elaboration, indicating that growth factor transport through the hydrogels was not inhibited. Differences in hyaluronidase expression were observed and may be due in part to medium components, where serum can also contain HA. Similar gene expression observed on day 7 for hyal 3 does not follow the observed trends, but the cause of this anomaly is unclear.
In vivo, MSC chondrogenesis was explored with and without TGF-β3, which was delivered without a carrier via direct encapsulation within the hydrogel. Because each hydrogel is exposed to 10 ng of TGF-β3 during in vitro culture, a concentration of 10 ng of TGF-β3 per hydrogel was chosen for in vivo encapsulation. The assessment of TGF-β3 release from MeHA hydrogels in vitro indicated that there was growth factor remaining in the hydrogels after 14 days, unless the addition of exogenous hyaluronidase triggered release. Thus, we hypothesized that TGF-β3 could be retained within the hydrogel to induce chondrogenic differentiation when implanted. Gene expression analysis after 2 weeks of in vivo culture reflected increases in type II collagen, aggrecan, and sox 9, as was found in vitro, for all groups. These results indicate that the MeHA hydrogel as a cell delivery vehicle alone supports some MSC chondrogenesis, which the addition of TGF-β3 then further enhances. The single dose of encapsulated TGF-β3 was capable of altering gene expression during short-term in vivo culture. In addition, data showed that the pre-programming of MSCs toward chondrogenesis with 2 weeks of pre-culture in vitro was also sufficient to maintain chondrogenesis in short-term in vivo culture. This was also reflected in the immunohistochemical staining of type II collagen and CS, as seen in Figure 6. Furthermore, increases in hyaluronidase expression in vivo may reflect potential cell-dictated remodeling of the MeHA hydrogel. Differences between HA-C and the other in vivo groups (HA-MSC and HA + T3) seen in the gene expression and immunohistochemistry data can also be attributed, in part, to the 2 weeks of additional culture time.
To better evaluate the effect of scaffold material on MSC chondrogenesis, MeHA hydrogels were compared with a relatively inert PEG hydrogel system. PEG hydrogels were used as comparative controls because of their resistance to protein adsorption and minimal interaction with cells. To eliminate the influence of other parameters on MSC differentiation, the mechanical properties of the hydrogels were correlated. Although the macromers have different structures (diacrylate for PEG and an acrylated chain for HA) and molecular weights, the modulus is proportional to the hydrogel cross-linking density and should reflect the mechanical stresses sensed by the cells and also reflect the diffusion rate of growth factors to the cells. In addition, the 2 wt% MeHA hydrogel has not been optimized for MSC differentiation and was chosen based on our previous work with chondrocytes. Thus, there is ample opportunity for further optimization of the hydrogel through tunable properties (e.g., mechanics and degradation), which can influence chondrogenesis.
In vitro cultures reflected significant differences in gene expression for type II collagen and hyaluronidases between the hydrogels (i.e., greater expression in HA than in PEG hydrogels). Accordingly, the up-regulation in gene expression translated to greater type II collagen and CS deposition within the HA hydrogels. For in vivo culture, differences between HA and PEG hydrogels were most noticeable for MSCs and polymer alone, indicating that hydrogel chemistry alone can greatly influence the commitment of MSCs to undergo chondrogenesis. However, these differences decreased with the addition of TGF-β3, suggesting that the hydrogel chemistry may play a less prominent role when chondrogenic growth factors are present. Furthermore, once chondro-induced, MSC gene expression for chondrogenic markers between polymers was comparable in vivo. With pericellular deposition after 2 weeks of in vitro culture, MSCs may begin interacting more with newly deposited matrix than with the surrounding scaffold material; thus, differences as a result of polymer choice may be minimized when compared in longer in vivo cultures.
Others have successfully used PEG hydrogels for cartilage tissue engineering with chondrocytes53 and stem cells,54,55 and the inertness of the hydrogels may be advantageous for many applications because they can be modified specifically to control interactions. Additionally, PEG hydrogels have been further designed to incorporate degradable moieties, bioactive molecules, and adhesive functionality to control overall matrix distribution and cell interactions.12,56,59 Also, HA has been used to direct embryonic stem cell differentiation in hydrogels as the major matrix component60 or intermixed with PEG hydrogels.54,61 HA, as a natural polymer, is able to provide specific biological interactions and physiological degradation by enzymes, whereas PEG, as a synthetic polymer, has the benefits of being reproducible and having easily controllable physical properties. Blends of cross-linked HA and PEG have resulted in concentration-dependent physical properties (e.g., swelling, degradation, and mechanics),27,62 whereas the addition of soluble HA encapsulated in PEGDA hydrogels has been shown to independently induce chondrogenesis in goat MSCs.54 This work indicates that the cell type is important in the cellular response and that the method of exposure (e.g., bound versus soluble) is also important.
In conclusion, we have shown that MSCs undergo chondrogenesis in photo-cross-linked HA hydrogels in vitro and in vivo in short-term culture. Gene expression also showed that scaffold choice affects the expression of cartilaginous matrix proteins, where favorable cell–scaffold interactions can assist in chondrogenic differentiation. Additionally, TGF-β3 can be delivered within HA hydrogels and can alter gene expression of encapsulated MSCs. The next step in assessing the use of MSCs as a cell source for cartilage regeneration in HA hydrogels is the completion of long-term studies to assess the quality and function of the matrix formed by MSCs with a range of methods for the delivery of TGF-β3.
Acknowledgments
Support for this research was provided through NIH Grant K22 DE-015761 and a National Science Foundation Graduate Research Fellowship (CC). The authors would also like to acknowledge Jamie L. Ifkovits for her help in synthesizing the PEGDA macromer and 1H NMR characterization and Cheng-Hung Chou for his help in performing the TGF-β3 release experiment.
References
- 1.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Friedenstein A.J. Gorskaja J.F. Kulagina N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267. [PubMed] [Google Scholar]
- 3.Alhadlaq A. Mao J.J. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. 2004;13:436. doi: 10.1089/scd.2004.13.436. [DOI] [PubMed] [Google Scholar]
- 4.Zuk P.A. Zhu M. Ashjian P. De Ugarte D.A. Huang J.I. Mizuno H. Alfonso Z.C. Fraser J.K. Benhaim P. Hedrick M.H. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashton B.A. Allen T.D. Howlett C.R. Eaglesom C.C. Hattori A. Owen M. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop Relat Res. 1980:294. [PubMed] [Google Scholar]
- 6.Barry F.P. Biology and clinical applications of mesenchymal stem cells. Birth Defects Res C Embryo Today. 2003;69:250. doi: 10.1002/bdrc.10021. [DOI] [PubMed] [Google Scholar]
- 7.Johnstone B. Hering T.M. Caplan A.I. Goldberg V.M. Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 8.Mackay A.M. Beck S.C. Murphy J.M. Barry F.P. Chichester C.O. Pittenger M.F. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 9.Sekiya I. Vuoristo J.T. Larson B.L. Prockop D.J. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci U S A. 2002;99:4397. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoo J.U. Barthel T.S. Nishimura K. Solchaga L. Caplan A.I. Goldberg V.M. Johnstone B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Dawson E. Mapili G. Erickson K. Taqvi S. Roy K. Biomaterials for stem cell differentiation. Adv Drug Deliv Rev. 2008;60:215. doi: 10.1016/j.addr.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 12.Varghese S. Hwang N.S. Canver A.C. Theprungsirikul P. Lin D.W. Elisseeff J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008;27:12. doi: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Chung C. Burdick J.A. Engineering cartilage tissue. Adv Drug Deliv Rev. 2008;60:243. doi: 10.1016/j.addr.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgelin M. Heinegard D. Engel J. Paulsson M. The cartilage proteoglycan aggregate: assembly through combined protein-carbohydrate and protein-protein interactions. Biophys Chem. 1994;50:113. doi: 10.1016/0301-4622(94)85024-0. [DOI] [PubMed] [Google Scholar]
- 15.Chen W.Y. Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7:79. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 16.Knudson C.B. Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J. 1993;7:1233. [PubMed] [Google Scholar]
- 17.Laurent T.C. Fraser J.R. Hyaluronan. FASEB J. 1992;6:2397. [PubMed] [Google Scholar]
- 18.Menzel E.J. Farr C. Hyaluronidase and its substrate hyaluronan: biochemistry, biological activities and therapeutic uses. Cancer Lett. 1998;131:3. doi: 10.1016/s0304-3835(98)00195-5. [DOI] [PubMed] [Google Scholar]
- 19.Grigolo B. Roseti L. Fiorini M. Fini M. Giavaresi G. Aldini N.N. Giardino R. Facchini A. Transplantation of chondrocytes seeded on a hyaluronan derivative (hyaff-11) into cartilage defects in rabbits. Biomaterials. 2001;22:2417. doi: 10.1016/s0142-9612(00)00429-4. [DOI] [PubMed] [Google Scholar]
- 20.Marcacci M. Berruto M. Brocchetta D. Delcogliano A. Ghinelli D. Gobbi A. Kon E. Pederzini L. Rosa D. Sacchetti G.L. Stefani G. Zanasi S. Articular cartilage engineering with Hyalograft (R) C - 3-year clinical results. Clin Orthop Relat Res. 2005:96. doi: 10.1097/01.blo.0000165737.87628.5b. [DOI] [PubMed] [Google Scholar]
- 21.Smeds K.A. Pfister-Serres A. Miki D. Dastgheib K. Inoue M. Hatchell D.L. Grinstaff M.W. Photocrosslinkable polysaccharides for in situ hydrogel formation. J Biomed Mater Res. 2001;54:115. doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 22.Nettles D.L. Vail T.P. Morgan M.T. Grinstaff M.W. Setton L.A. Photocrosslinkable hyaluronan as a scaffold for articular cartilage repair. Ann Biomed Eng. 2004;32:391. doi: 10.1023/b:abme.0000017552.65260.94. [DOI] [PubMed] [Google Scholar]
- 23.Ramamurthi A. Vesely I. Ultraviolet light-induced modification of crosslinked hyaluronan gels. J Biomed Mater Res A. 2003;66:317. doi: 10.1002/jbm.a.10588. [DOI] [PubMed] [Google Scholar]
- 24.Hahn S.K. Jelacic S. Maier R.V. Stayton P.S. Hoffman A.S. Anti-inflammatory drug delivery from hyaluronic acid hydrogels. J Biomater Sci Polym Ed. 2004;15:1111. doi: 10.1163/1568562041753115. [DOI] [PubMed] [Google Scholar]
- 25.Luo Y. Kirker K.R. Prestwich G.D. Cross-linked hyaluronic acid hydrogel films: new biomaterials for drug delivery. J Control Release. 2000;69:169. doi: 10.1016/s0168-3659(00)00300-x. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y.C. Shu X.Z. Prestwich G.D. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng. 2006;12:3405. doi: 10.1089/ten.2006.12.3405. [DOI] [PubMed] [Google Scholar]
- 27.Park Y.D. Tirelli N. Hubbell J.A. Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials. 2003;24:893. doi: 10.1016/s0142-9612(02)00420-9. [DOI] [PubMed] [Google Scholar]
- 28.Shu X.Z. Liu Y. Luo Y. Roberts M.C. Prestwich G.D. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules. 2002;3:1304. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- 29.Baier Leach J. Bivens K.A. Patrick C.W., Jr. Schmidt C.E. Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds. Biotechnol Bioeng. 2003;82:578. doi: 10.1002/bit.10605. [DOI] [PubMed] [Google Scholar]
- 30.Burdick J.A. Chung C. Jia X.Q. Randolph M.A. Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6:386. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solchaga L.A. Dennis J.E. Goldberg V.M. Caplan A.I. Hyaluronic acid-based polymers as cell carriers for tissue-engineered repair of bone and cartilage. J Orthop Res. 1999;17:205. doi: 10.1002/jor.1100170209. [DOI] [PubMed] [Google Scholar]
- 32.Brun P. Abatangelo G. Radice M. Zacchi V. Guidolin D. Gordini D.D. Cortivo R. Chondrocyte aggregation and reorganization into three-dimensional scaffolds. J Biomed Mater Res. 1999;46:337. doi: 10.1002/(sici)1097-4636(19990905)46:3<337::aid-jbm5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 33.Chung C. Mesa J. Randolph M.A. Yaremchuk M. Burdick J.A. Influence of gel properties on neocartilage formation by auricular chondrocytes photoencapsulated in hyaluronic acid networks. J Biomed Mater Res A. 2006;77:518. doi: 10.1002/jbm.a.30660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aigner J. Tegeler J. Hutzler P. Campoccia D. Pavesio A. Hammer C. Kastenbauer E. Naumann A. Cartilage tissue engineering with novel nonwoven structured biomaterial based on hyaluronic acid benzyl ester. J Biomed Mater Res. 1998;42:172. doi: 10.1002/(sici)1097-4636(199811)42:2<172::aid-jbm2>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 35.Fraser S.A. Crawford A. Frazer A. Dickinson S. Hollander A.P. Brook I.M. Hatton P.V. Localization of type VI collagen in tissue-engineered cartilage on polymer scaffolds. Tissue Eng. 2006;12:569. doi: 10.1089/ten.2006.12.569. [DOI] [PubMed] [Google Scholar]
- 36.Ji Y. Ghosh K. Shu X.Z. Li B. Sokolov J.C. Prestwich G.D. Clark R.A. Rafailovich M.H. Electrospun three-dimensional hyaluronic acid nanofibrous scaffolds. Biomaterials. 2006;27:3782. doi: 10.1016/j.biomaterials.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 37.Chung C. Mesa J. Miller G.J. Randolph M.A. Gill T.J. Burdick J.A. Effects of auricular chondrocyte expansion on neocartilage formation in photocrosslinked hyaluronic acid networks. Tissue Eng. 2006;12:2665. doi: 10.1089/ten.2006.12.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung C. Erickson I.E. Mauck R.L. Burdick J.A. Differential behavior of auricular and articular chondrocytes in hyaluronic acid hydrogels. Tissue Eng. 2008 Apr 12; doi: 10.1089/ten.tea.2007.0291. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu H. Mitsuhashi N. Klein A. Barsky L.W. Weinberg K. Barr M.L. Demetriou A. Wu G.D. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24:928. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 40.Burdick J.A. Anseth K.S. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 41.National Institutes of Health. Guidelines for the care and use of laboratory animals. NIH Publication; 1985. 85-23 rev. [Google Scholar]
- 42.Hegewald A.A. Ringe J. Bartel J. Kruger I. Notter M. Barnewitz D. Kaps C. Sittinger M. Hyaluronic acid and autologous synovial fluid induce chondrogenic differentiation of equine mesenchymal stem cells: a preliminary study. Tissue Cell. 2004;36:431. doi: 10.1016/j.tice.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Jiang H. Peterson R.S. Wang W. Bartnik E. Knudson C.B. Knudson W. A requirement for the CD44 cytoplasmic domain for hyaluronan binding, pericellular matrix assembly, and receptor-mediated endocytosis in COS-7 cells. J Biol Chem. 2002;277:10531. doi: 10.1074/jbc.M108654200. [DOI] [PubMed] [Google Scholar]
- 44.Knudson W. Loeser R.F. CD44 and integrin matrix receptors participate in cartilage homeostasis. Cell Mol Life Sci. 2002;59:36. doi: 10.1007/s00018-002-8403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vachon E. Martin R. Plumb J. Kwok V. Vandivier R.W. Glogauer M. Kapus A. Wang X. Chow C.W. Grinstein S. Downey G.P. CD44 is a phagocytic receptor. Blood. 2006;107:4149. doi: 10.1182/blood-2005-09-3808. [DOI] [PubMed] [Google Scholar]
- 46.Chow G. Knudson C.B. Knudson W. Expression and cellular localization of human hyaluronidase-2 in articular chondrocytes and cultured cell lines. Osteoarthritis Cartilage. 2006;14:849. doi: 10.1016/j.joca.2006.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frost G.I. Csoka A.B. Wong T. Stern R. Purification, cloning, and expression of human plasma hyaluronidase. Biochem Biophys Res Commun. 1997;236:10. doi: 10.1006/bbrc.1997.6773. [DOI] [PubMed] [Google Scholar]
- 48.Lepperdinger G. Strobl B. Kreil G. HYAL2, a human gene expressed in many cells, encodes a lysosomal hyaluronidase with a novel type of specificity. J Biol Chem. 1998;273:22466. doi: 10.1074/jbc.273.35.22466. [DOI] [PubMed] [Google Scholar]
- 49.Flannery C.R. Little C.B. Hughes C.E. Caterson B. Expression and activity of articular cartilage hyaluronidases. Biochem Biophys Res Commun. 1998;251:824. doi: 10.1006/bbrc.1998.9561. [DOI] [PubMed] [Google Scholar]
- 50.Chen G. Liu D. Tadokoro M. Hirochika R. Ohgushi H. Tanaka J. Tateishi T. Chondrogenic differentiation of human mesenchymal stem cells cultured in a cobweb-like biodegradable scaffold. Biochem Biophys Res Commun. 2004;322:50. doi: 10.1016/j.bbrc.2004.07.071. [DOI] [PubMed] [Google Scholar]
- 51.Huang C.Y. Reuben P.M. D'Ippolito G. Schiller P.C. Cheung H.S. Chondrogenesis of human bone marrow-derived mesenchymal stem cells in agarose culture. Anat Rec A Discov Mol Cell Evol Biol. 2004;278:428. doi: 10.1002/ar.a.20010. [DOI] [PubMed] [Google Scholar]
- 52.Mehlhorn A.T. Schmal H. Kaiser S. Lepski G. Finkenzeller G. Stark G.B. Sudkamp N.P. Mesenchymal stem cells maintain TGF-beta-mediated chondrogenic phenotype in alginate bead culture. Tissue Eng. 2006;12:1393. doi: 10.1089/ten.2006.12.1393. [DOI] [PubMed] [Google Scholar]
- 53.Elisseeff J. McIntosh W. Anseth K. Riley S. Ragan P. Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51:164. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 54.Sharma B. Williams C.G. Khan M. Manson P. Elisseeff J.H. In vivo chondrogenesis of mesenchymal stem cells in a photopolymerized hydrogel. Plast Reconstr Surg. 2007;119:112. doi: 10.1097/01.prs.0000236896.22479.52. [DOI] [PubMed] [Google Scholar]
- 55.Williams C.G. Kim T.K. Taboas A. Malik A. Manson P. Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003;9:679. doi: 10.1089/107632703768247377. [DOI] [PubMed] [Google Scholar]
- 56.Bryant S.J. Anseth K.S. Controlling the spatial distribution of ECM components in degradable PEG hydrogels for tissue engineering cartilage. J Biomed Mater Res A. 2003;64:70. doi: 10.1002/jbm.a.10319. [DOI] [PubMed] [Google Scholar]
- 57.Bryant S.J. Arthur J.A. Anseth K.S. Incorporation of tissue-specific molecules alters chondrocyte metabolism and gene expression in photocrosslinked hydrogels. Acta Biomater. 2005;1:243. doi: 10.1016/j.actbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 58.Rice M.A. Anseth K.S. Controlling cartilaginous matrix evolution in hydrogels with degradation triggered by exogenous addition of an enzyme. Tissue Eng. 2007;13:683. doi: 10.1089/ten.2006.0142. [DOI] [PubMed] [Google Scholar]
- 59.Salinas C.N. Cole B.B. Kasko A.M. Anseth K.S. Chondrogenic differentiation potential of human mesenchymal stem cells photoencapsulated within poly(ethylene glycol)-arginine-glycine-aspartic acid-serine thiol-methacrylate mixed-mode networks. Tissue Eng. 2007;13:1025. doi: 10.1089/ten.2006.0126. [DOI] [PubMed] [Google Scholar]
- 60.Gerecht S. Burdick J.A. Ferreira L.S. Townsend S.A. Langer R. Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:11298. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hwang N.S. Varghese S. Elisseeff J. Controlled differentiation of stem cells. Adv Drug Deliv Rev. 2008;60:199. doi: 10.1016/j.addr.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kirker K.R. Prestwich G.D. Physical properties of glycosaminoglycan hydrogels. J Polym Sci B Polym Phys. 2004;42:4344. [Google Scholar]