Abstract
AIM: To investigate the relationship between 90-kuD ribosomal S6 kinase (p90RSK) and collagen type I expression during the development of hepatic fibrosis in vivo and in vitro.
METHODS: Rat hepatic fibrosis was induced by intraperitoneal injection of dimethylnitrosamine. The protein expression and cell location of p90RSK and their relationship with collagen type I were determined by co-immunofluoresence and confocal microscopy. Subsequently, RNAi strategy was employed to silence p90RSK mRNA expression in HSC-T6, an activated hepatic stellate cell (HSC) line. The expression of collagen type I in HSC-T6 cells was assessed by Western blotting and real-time polymerase chain reaction. Furthermore, HSCs were transfected with expression vectors or RNAi constructs of p90RSK to increase or decrease the p90RSK expression, then collagen type I promoter activity in the transfected HSCs was examined by reporter assay. Lastly HSC-T6 cells transfected with p90RSK siRNA was treated with or without platelet-derived growth factor (PDGF)-BB at a final concentration of 20 μg/L and the cell growth was determined by MTS conversion.
RESULTS: In fibrotic liver tissues, p90RSK was over-expressed in activated HSCs and had a significant positive correlation with collagen type I levels. In HSC-T6 cells transfected with RNAi targeted to p90RSK, the expression of collagen type I was down-regulated (61.8% in mRNA, P < 0.01, 89.1% in protein, P < 0.01). However, collagen type I promoter activity was not increased with over-expression of p90RSK and not decreased with low expression either, compared with controls in the same cell line (P = 0.076). Furthermore, p90RSK siRNA exerted the inhibition of HSC proliferation, and also abolished the effect of PDGF on the HSC proliferation.
CONCLUSION: p90RSK is over-expressed in activated HSCs and involved in regulating the abnormal expression of collagen type I through initiating the proliferation of HSCs.
Keywords: 90-kuD ribosomal S6 kinase, Collagen type I, Hepatic fibrosis, Hepatic stellate cell, RNAi
INTRODUCTION
Hepatic fibrogenesis is a response to injury in the liver. It is characterized by both a quantitative and qualitative change in the extracellular matrix (ECM), within which collagen type I predominates[1,2]. The activated hepatic stellate cell (HSC) is primarily responsible for excessive collagen deposition during liver fibrosis[3–6]. Recently, multiple cellular signals, especially extracellular signal-regulated kinase 1 and 2 (ERK1/2), have been reported to be involved in the process of activation of HSCs by increasing protein phosphorylation and up-regulation of gene transcription[7–9]. 90-kuD ribosomal S6 kinase (p90RSK) is an important downstream substrate of ERK1/2. p90RSK itself interacts with numerous substrates in the cytoplasm and nucleus, and is involved in gene expression, protein synthesis, cell survival, cell cycle proliferation and progression[10–13]. p90RSK has been implicated in the pathogenesis of some tumors and some other chronic diseases[14]. However, the role of p90RSK in hepatic fibrosis has not yet to be fully elucidated. It is known that in rat hepatic fibrosis, p90RSK is significantly up-regulated in association with elevated collagen type I levels[15]. However, the relationship between p90RSK and collagen type I, including any regulatory effects of p90RSK on the expression of collagen type I, is elusive.
Hence the present study was undertaken to explore the relationship between p90RSK and collagen type I expression in the fibrotic liver.
MATERIALS AND METHODS
Animal model
Male adult Sprague-Dawley rats weighing 250 ± 12.3 g were purchased from the Centre of Experimental Animals in Jinling Hospital. The rats received intraperitoneal injections of dimethylnitrosamine (DMN) (Sigma, Saint-Quentin Fallavier, France) at 10 mg/kg body weight (n = 30) or 0.9% sodium chloride (n = 10) thrice a week as previously described[16]. The rats were injected for 1 wk (n = 10), 2 wk (n = 10), and 3 wk (n = 10), and were sacrificed 3 d after the last administration. At the time of sacrifice, a hepatectomy was performed and liver tissue samples were fixed in 10% buffered formalin and embedded in paraffin. The experimental protocol was approved by the Institutional Animal Care committee of Jinling Hospital.
Immunofluorescent staining
Liver sections were blocked with 5% normal goat serum after fixing and then simultaneously incubated with both monoclonal anti-p90RSK (1:200 dilution, BD Biosciences, San Jose, CA, USA) and polyclone anti-collagen type I (1:50 dilution, Rockland, Gilbertsville, PA, USA), or polyclonal antibody of α-smooth muscle actin (α-SMA) (1:100 dilution, Rockland, Gilbertsville, PA, USA) prepared in phosphate-buffered saline (PBS). The sections were incubated overnight at 4°C or 1 h at room temperature and then washed with PBS. Sections were then simultaneously incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1:100 dilution, Jackson Immunoresearch Laboratories, West Grove, PA, USA) and rhodamine-conjugated secondary antibody (1:200 dilution, Jackson Immunoresearch Laboratories) for 30 min at 37°C in the dark. After extensive washing with PBS, the slides were mounted in a drop of Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA) to reduce photobleaching. Control experiments were performed in parallel with the omission of one of the primary antibodies. For double-staining experiments, both primary antibodies were produced in the different species.
Confocal microscopy and image analysis
Antibody labeling was examined under a Zeiss LSM-510 laser scanning confocal microscope. Optical slices (1.8 μm) were taken perpendicular to the liver section. A 488-nm argon laser was used in combination with a 499/505-530-nm excitation/emission filter set for fluorescence examination. For rhodamine, the 543-nm helium neon laser was used with a 543-nm excitation filter and 560-nm emission filter. Simultaneous images of FITC or rhodamine were captured from the same optical section. The captured images were then pseudocolored: red for rhodamine and green for FITC. Regions of co-localization appeared in yellow, reflecting the additive effect of superimposing green and red pixels. Image analysis was performed using the standard system operating software provided with the Zeiss LSM-510 series microscope.
Design of p90RSK siRNA and cell transfection
The RNAi targeting the p90RSK mRNA was designed by the software on the www.ambion.com. Forward oligo: TCGACAAAAGAGATCCCTCCGAAGTTCGCTTCGGAGGGATCTCTTTTTTTT. Reverse oligo: CTAGAAAAAAAAGTAGATCCCTCCGAAGCGAACTTCGGAGGGATCTCTTTTG. The vector of p90RSK siRNA was constructed using standard techniques[17]. p90RSK siRNA fragments and the vector pAVU6+27 were ligated, and the constructed plasmid with p90RSK siRNA was referred as pAVU6-siRSK. The activated cell line HSC-T6, a kind gift from Dr. Friedman (Mount Sinai School of Medicine), was cultured as previously described[18], and transfected with pAVU6-siRSK or empty plasmid pAVU6+27 by lipofectamine reagents (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions.
RNA extraction and real-time polymerase chain reaction (RT-PCR)
Total RNA was isolated from HSC-T6 cells transfected with pAVU6-siRSK or pAVU6+27 respectively, using Trizol in accordance with the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). One microgram of total RNA was then treated with DNAse for 30 min at 37°C. Reverse transcription was performed using the Omniscript RT kit (Qiagen, Valencia, CA, USA) and random primers (Promega, Madison, WI, USA). RT-PCR for rat p90RSK1 and collagen type I were performed using the ABI Prism 7700 Sequence Detection System, the Taqman universal PCR Master Mix, and assay-on-demand probes and primers (Shanghai Shengong Ltd., Shanghai, China), according to standard protocols. The primers in RT-PCR are presented in Table 1. Parameters for baseline and threshold-cycle (Ct) settings were kept constant for each gene. To calculate ΔCt, the Ct value for each target gene was standardized against that for the internal rRNA (18S) control probe.
Table 1.
RT- PCR primers
| Forward | Reverse | |
| P90RSK | 5′-TCTCTGTCCAGCGGCGGGTGAGGA-3′ | 5′-GCATTCACAGCGCCCATGCGCAG-3′ |
| Collagen I | 5′-CCAGCCGCAAAGAGTCTACATGTC-3′ | 5′-TCACCTTCTCATCCCTCCTAA-3′ |
| 18S RNA | 5′-GTCTGTGATGCCCTTAGATG-3′ | 5′-AGCTTATGACCCGCACTTAC-3′ |
Western blotting
Rat HSCs were lysed in 1 × sample buffer (62.5 mmol/L Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 5% 2-mercaptoethanol, 1 mmol/L Na3VO4). Ten micrograms of each sample were subjected to SDS-PAGE (7.5%-15%) and then transferred onto an Immobilon P membrane (Millipore Corp., Bedford, MA, USA). After blocking non-specific binding sites, the filters were incubated in Tween PBS at 4°C for 16 h with one of the following antibodies: (1) mouse monoclonal antibodies directed against rat p90RSK (1:200) (BD); or (2) rabbit polyclonal antibody against rat collagen I (1:1000). Revelation was performed by a chemiluminescence-based method (ECL; Amersham Pharmacia Biotech, San Francisco, CA, USA).
Reporter assays
COL1A1 promoter (-378 to -340 bp)-luciferase reporter constructs were kindly provided by Dr. Huang (Nanjing Medical University). HSC-T6 cells (4 × 106), were electroporated (270 V, 950 μF) with 10 μg of the COL1A1-luciferase reporter and 2 μg of a Renilla luciferase expression construct (Promega), alone or in combination with pAVU6-siRSK, pAVU6+27 (empty vector), pMT2·RSK1 or pMT2 (empty vector) expression construct, respectively. HSC-T6 transfection efficiency was monitored by electroporation of a green fluorescent protein expression construct (10 mg). The relative luciferase value (RLV) was defined as the ratio of the luciferase activity divided by the activity of Renilla luciferase in transfected cell lysates. The RLV of unstimulated cultures was given the arbitrary value of 1. Each experiment was repeated a minimum of three times.
Analysis of HSC proliferation
Cell growth curves of HSC-T6 cells transfected with pAVU6-siRSK or control plasmid pAVU6+27 were analyzed by MTS conversion. Furthermore, to examine the effect of p90RSK siRNA on HSC proliferation induced by platelet-derived growth factor (PDGF), rhPDGF-BB (Boehringer Mannheim Co., Mannheim, Germany) was added to the medium at a final concentration of 20 μg/L in HSC-T6 cells transfected with pAVU6-siRSK or control plasmid pAVU6+27; cell growth was determined by MTS conversion as mentioned. The absolute number of HSCs in different groups by counting cells under microscopy after staining was also measured at the same time.
Statistics analysis
Statistical Package for the Social Sciences (version 10.0 for Windows; SPSS, Chicago, IL, USA) was used for statistical analysis. The calculation of Spearman’s rank correlation coefficient was used to assess the relationship between quantitative parameters. Student’s t test and the Mann-Whitney U test were used to compare data from different treatment groups. Data are expressed as mean ± SE. Differences were considered significant when P was less than 0.05.
RESULTS
Expression and relation of p90RSK with collagen type I in DMN-treated rats
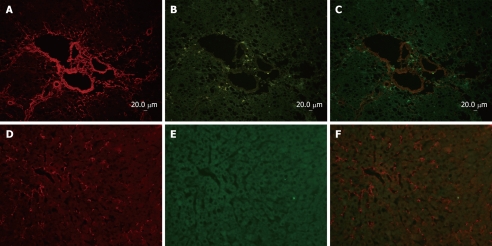
Immunofluorescent double-staining showed abundant expression of collagen type I and p90RSK in the fibrotic liver (Figure 1A and B). However, in normal liver, only a little collagen type I could be observed and no p90RSK was detected (Figure 1D and E). Image analysis showed that both of p90RSK and collagen type I were up-regulated simultaneously, but these two signals did not co-localize (Figure 1C and F).
Figure 1.
Co-immunofluoresence of p90RSK and collagen type I in fibrotic liver and normal liver. Sections (A-C) are from rat liver with intraperitoneal injection of DMN for 3 wk, and sections (D-F) are from normal livers as control. Sections of fibrotic liver mostly demonstrate that collagen type I (rhodamine) and p90RSK (FITC) immunoreactivity were both present around central veins as well as in the interstitium, and up-regulated in fibrotic liver. Sections of normal liver mostly demonstrate that collagen type I (rhodamine) immunoreactivity was less in normal liver than in fibrotic liver and p90RSK (FITC) was hardly observed in nomal liver simultaneously.
Cellular localization of p90RSK in DMN-treated rats
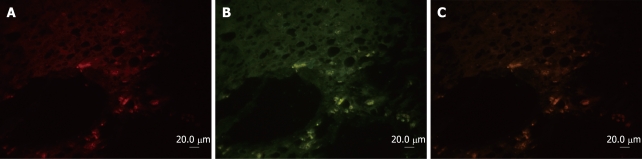
α-SMA, a typical marker of activated HSCs, was selected for this study to determine the cellular localization of p90RSK in fibrotic liver. The localization of p90RSK and α-SMA was visualized by immunofluorescent double labeling and laser scanning confocal microscopy. Image analysis showed a diffused distribution of p90RSK throughout the fibrotic liver (Figure 2A), and a similar distribution was observed with α-SMA staining (Figure 2B). When the two images merged, p90RSK showed a very high degree of co-localization with α-SMA throughout the fibrotic liver (Figure 2C).
Figure 2.
Cellular localization of p90RSK in fibrotic liver and co-immunofluoresence and confocal microscopy of p90RSK and αSMA in fibrotic liver. A: α-SMA (rhodamine)-positive cell represent the activated HSC, which deposited in interstitium; B: P90RSK (FITC)-positive cell were also present in interstitium; C: The yellow areas on the merged image show co-localization of α-SMA and p90RSK.
Identification of p90RSK siRNA
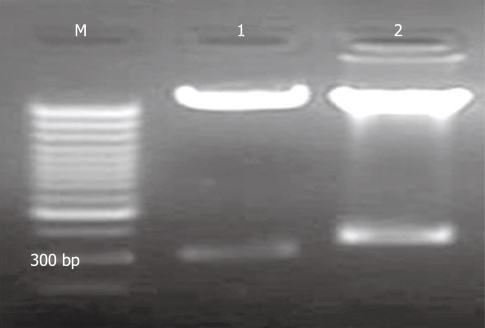
The recombinant pAV-siRSK was identified by enzyme digestion (Figure 3) and sequencing, which showed that digestion product of pAV-siRSK was about 350 bp, compared with 300 bp production of pAVU6+27. The sequencing result showed siRSK was 52 bp.
Figure 3.
Agarose gel electrophoresis of restriction enzyme digestion of pAV-siRSK. M: 1 kb marker; 1: Restriction enzyme digestion product of pAVU6+27 was about 300 bp; 2: Restriction enzyme digestion product of pAV-siRSK. The siRNA of p90RSK was designed of 52 bp.
Regulation of p90RSK siRNA on collagen type I
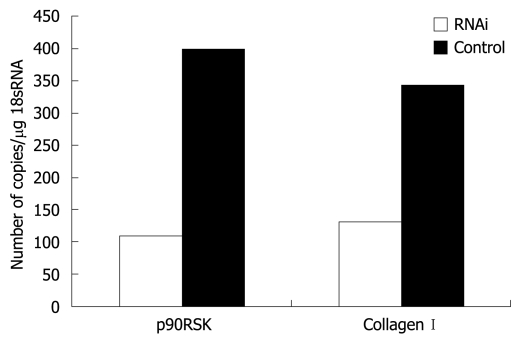
The RT-PCR experimental conditions were optimized to obtain an efficacy up to 90% of standard curves. When p90RSK mRNA in HSC-T6 cells was silenced using RNAi and the mRNA of p90RSK and collagen type I examined in HSC-T6 cells transfected with pAVU6-siRSK or empty pAVU6+27, there was an obvious reduction of 72.6% in p90RSK mRNA levels within HSC-T6 cells transfected with pAVU6-siRSK. There was also a reduction of 61.8% in collagen type I (P < 0.01, Figure 4).
Figure 4.
RT-PCR assessment of p90RSK and collagen type I in HSC-T6 transfected with or without pAVU6-siRSK. Quantification of p90RSK and collagen type I normalized to 18S RNA in HSC-T6 cells transfected with pAVU6-siRSK decreased 72.6% and 61.8%, respectively, compared with control (P < 0.01). Results are expressed as mean ± SE of three separate experiments.
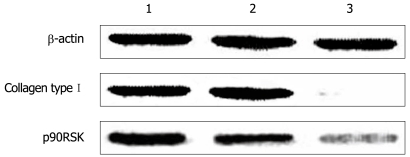
The protein level of p90RSK and collagen type I were examined by Western blotting in HSC-T6 cells transfected with pAVU6-siRSK or empty pAVU6+27. The protein level of p90RSK and collagen type I was reduced to 75.6% and 89.1%, respectively, after RNAi (P < 0.01, Figure 5).
Figure 5.
Western blotting analysis of p90RSK, collagen type I in HSC-T6 cells transfected with or without pAVU6-siRSK. β-actin provided as an inner control. 1: Normal HSC-T6 cells; 2: HSC-T6 transfected with empty plasmid; 3: HSC-T6 transfected with pAVU6-siRSK. With p90RSK siRNA, the expression of p90RSK decreased 75.6% compared with controls, and the expression of collagen type I decreased 89.1% accordingly (P < 0.01). Results are expressed as mean ± SE of three separate experiments.
Effect of p90RSK siRNA on collagen type I promoter activity
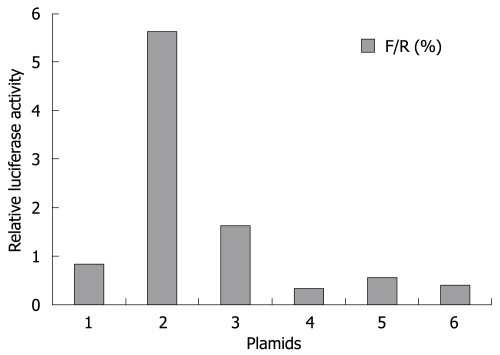
Collagen type I is a heterotrimer composed of two coordinately expressed α1 chains and one α2 chain. They are encoded by distinct genes, COL1A1 and COL1A2, respectively[19]. The -378 to -340 region of the COL1A1 promoter exploited in this study is the site of convergence of different stimuli to modulate the gene transcription[20]. In this study, we observed that over-expression of p90RSK had little effect on activity of this region. Similarly, silencing of p90RSK expression did not decrease its activity either (Figure 6). The results showed that p90RSK did not work on COL1A1 promoter. Herein, we identified p90RSK could not alter transcriptional activity of collagen type I in HSCs.
Figure 6.
Effect of p90RSK on collagen type I promoter activity. HSCs transfected with the pAVU6-siRSK (bar 3, to decrease p90RSK expression), pMT2·RSK1 (bar 5, to increase p90RSK expression) showed no alteration of collagen type I promoter activity compared to control cells sham-transfected with pAVU6+27 (bar 4), pMT2 (bar 6), respectively. HSCs transfected with collagen type I luciferase reporter construct (bar 1) as normal control and Renilla luciferase expression construct (bar 2) as positive control. After 24 h incubation, the luciferase activity was determined. Data represent luciferase activity relative to the control (bar 1) and are expressed as mean ± SD of three separate experiments.
Effect of p90RSK siRNA on HSC proliferation
PDGF is a well known ligand able to elicit proliferation as well as to operate through ERK1/2 pathway and the most potent mitogen for HSCs in vitro. To further investigate the role of the p90RSK in the mitogenic effects on HSCs, we used the RNAi strategy, to produce the post-transcriptional gene expression silencing of p90RSK in HSCs. In accordance with previous studies, our data showed that p90RSK siRNA significantly inhibited the proliferation of HSC-T6 (Figure 7A) and also abolished the effect of PDGF-BB on HSC proliferation (Figure 7B).
Figure 7.
Effect of p90RSK siRNA on the proliferation of HSCs. A: p90RSK siRNA inhibited the proliferation of HSC-T6 cells. Cell growth curves of the recombinant cells with or without p90RSK siRNA were analyzed by MTS conversion; B: p90RSK siRNA abolished the effect of PDGF-BB on the proliferation of HSCs. rhPDGF-BB was added to the medium at a final concentration of 20 μg/L in HSC-T6 cells transfected with pAVU6-siRSK (RNAi), which showed decreased proliferative activity compared to sham transfected control cells (control). Each sample was tested in triplicate and error bars were included.
DISCUSSION
Northern blot and immunohistochemical analysis has previously demonstrated that the expression of p90RSK has a significant correlation with that of collagen type I during the development of hepatic fibrosis[15]. In that study, the measurements were performed separately, preventing the determination of any spatial relationship between p90RSK and collagen type I. Therefore, to ascertain whether there was any association between them, the expression of p90RSK and collagen type I was measured simultaneously by immunofluorescent double-staining and confocal microscopy. The results indicate that both p90RSK and collagen type I increase simultaneously in the same section of the fibrotic liver.
The activated HSC is the primary cell type in the liver responsible for the excess synthesis and deposition of ECM, within which collagen type I predominates[5,9]. It resides in the perisinusoidal space of Disse in the liver[21]. In our previous studies, p90RSK was observed in interstitial cells, which include activated HSCs and some other interstitial cells. To determine whether the over-expression of p90RSK was located in activated HSCs, α-SMA was employed as an HSC activation marker[21–23]. The result of confocal microscopy showed that p90RSK and α-SMA are co-localized within the interstitium. Hence, up-regulated p90RSK is located within activated HSC.
HSC-T6 is the immortalized rat HSC line, which retains all features of activated stellate cells, including expression of desmin, α-SMA, and glial acidic fibrillary protein, as well as collagen[24]. Because primary stellate cell cultures and isolation is extremely time-consuming, yields are modest, and there is considerable preparation-to-preparation variability, we used HSC-T6 cells to study the role of p90RSK in vitro. We observed that down-regulation of the post-transcriptional gene expression of p90RSK in the HSC-T6 cells, was achieved through the administration of p90RSK siRNA. Subsequently, the expression of collagen type I mRNA was significantly reduced, leading to a reduction of collagen type I in cell culture supernatant. This is in agreement with previous reports[15], and strengthens the evidence for p90RSK production having an influence on collagen type I expression in activated HSCs.
It is known that HSCs are directly involved in mediating the fibrogenic response in hepatic fibrosis. They become fibrogenic by synthesizing ECM proteins and activated HSCs proliferate, thereby amplifying the fibrogenic response[25]. It is becoming clear that both proliferative (i.e. PDGF)[17] and fibrogenic (i.e. transforming growth factor-β)[26] cytokines activate ERK1/2 signaling cascades in the development of hepatic fibrosis. p90RSK could be activated by the above stimuli and has widely distributed substrates[17,27–29]. The diversity of these stimuli and substrates suggests that p90RSK may be involved in the regulation of a wide range of cellular functions[11–13]. ERK1/2 has an important role in the signaling pathway that leads to the proliferation of HSCs. From this, it might be speculated that p90RSK, as a potent downstream substrate of the ERK1/2 signaling pathway, is involved in the fibrogenic activation of HSCs, or proliferation of HSCs, or both. Reporter assays designed to address the ability of p90RSK to regulate the activity of the collagen type I promoter were used to explore the role of p90RSK in the transcriptional induction of collagen type I gene expression in HSCs. The results showed that neither an increase nor decrease of p90RSK has any effect on the collagen type I promoter activity. Otherwise, the analysis of HSC proliferation showed that p90RSK siRNA significantly inhibited the proliferation of activated HSCs and also abolished the effect of PDGF-BB on that of HSCs. This suggests that p90RSK has no effect on the fibrogenic activation of HSCs, rather that p90RSK increases the collagen type I expression via the initiation of HSC proliferation. This observation is in line with the recent report that p90RSK phosphorylates C/EBPβ to inhibit activated HSC apoptosis in liver fibrosis[30].
Therefore, we conclude that p90RSK is over-expressed in activated HSCs and involved in the regulation of collagen type I expression through the initiation of HSC proliferation.
COMMENTS
Background
Hepatic fibrogenesis is a response to injury in the liver. It is characterized by both a quantitative and qualitative change in the extracellular matrix, within which collagen type I predominates. The activated hepatic stellate cell (HSC) is primarily responsible for excessive collagen deposition during liver fibrosis. Recently, multiple cellular signals, especially extracellular signal-regulated kinase 1 and 2 (ERK1/2), have been reported to be involved in the process of activation of HSCs by increasing protein phosphorylation and up-regulation of gene transcription. However, the molecular mechanism is not fully elucidated.
Research frontiers
90-ku ribosomal S6 kinase (p90RSK) is an important downstream substrate of ERK1/2. p90RSK itself interacts with numerous substrates in the cytoplasm and nucleus, and is involved in gene expression, protein synthesis, cell survival, cell cycle proliferation and progression. p90RSK has been implicated in the pathogenesis of some tumors and some other chronic diseases. The authors’ previous research has demonstrated that p90RSK is significantly up-regulated in rat hepatic fibrosis. However, the role of p90RSK in hepatic fibrosis has yet to be fully elucidated. In this study, the authors demonstrate that the overexpression of p90RSK could be a potential mechanism for mediating collagen type I expression.
Innovations and breakthroughs
Recent reports have highlighted the importance of p90RSK in cell proliferation and differentiation. In particular, p90RSK is required for cytostatic factor arrest in Xenopus laevis eggs. This is the first study to investigate the regulatory mechanism of p90RSK on collagen type I expression in rat HSCs.
Applications
This study may represent a future strategy for therapeutic intervention in the treatment of hepatic fibrosis.
Terminology
p90RSK is a serine/threonine kinase, which is the key substrate of the ERK1/2 signal pathway and involved in the phosphorylation of transcription factors, including nuclear factor-κB, c-Fos, Nur77, and cAMP response element-binding protein.
Peer review
The study by Yang et al investigated the relationship between p90RSK and collagen type I expression during the development of experimental hepatic fibrosis induced by dimethylnitrosamine. By also employing a number of experimental procedures and the T6 rat-model of immortalized HSCs, the authors conclude that p90RSK is over-expressed in activated HSCs and involved in the abnormal expression of collagen type I, although collagen type I promoter activity is not affected by either p90RSK over-expression or silencing. The study, of appreciable technical quality, is of potential interest for a reader interested in liver fibrosis. Data are mostly straightforward.
Acknowledgments
We gratefully acknowledge Dr. Scott L Friedman (Professor in Mount Sinai School of Medicine) for kindly providing the cell line HSC-T6. We are grateful to Dr. Joseph Avruch (Professor of the Harvard Medical School) for the plasmid pMT2·RSK1 and Dr. David R Engelke (Professor of biological chemistry in University of Michigan) for the plasmid pAVU6+27.
Supported by Jinling Hospital Medical Research Fund, No. 2005029
Peer reviewer: Maurizio Parola, Professor, Department Medicina e Oncologia Sperimentale, University of Torino Corso Raffaello 30, 10125 Torino, Italy
S- Editor Li LF L- Editor Ma JY E- Editor Zheng XM
References
- 1.Brenner DA, Rippe RA, Rhodes K, Trotter JF, Breindl M. Fibrogenesis and type I collagen gene regulation. J Lab Clin Med. 1994;124:755–760. [PubMed] [Google Scholar]
- 2.Friedman SL. Cytokines and fibrogenesis. Semin Liver Dis. 1999;19:129–140. doi: 10.1055/s-2007-1007105. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 4.Geerts A, Lazou JM, De Bleser P, Wisse E. Tissue distribution, quantitation and proliferation kinetics of fat-storing cells in carbon tetrachloride-injured rat liver. Hepatology. 1991;13:1193–1202. [PubMed] [Google Scholar]
- 5.Takahara T, Kojima T, Miyabayashi C, Inoue K, Sasaki H, Muragaki Y, Ooshima A. Collagen production in fat-storing cells after carbon tetrachloride intoxication in the rat. Immunoelectron microscopic observation of type I, type III collagens, and prolyl hydroxylase. Lab Invest. 1988;59:509–521. [PubMed] [Google Scholar]
- 6.Yin MF, Lian LH, Piao DM, Nan JX. Tetrandrine stimulates the apoptosis of hepatic stellate cells and ameliorates development of fibrosis in a thioacetamide rat model. World J Gastroenterol. 2007;13:1214–1220. doi: 10.3748/wjg.v13.i8.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 8.Marra F, Arrighi MC, Fazi M, Caligiuri A, Pinzani M, Romanelli RG, Efsen E, Laffi G, Gentilini P. Extracellular signal-regulated kinase activation differentially regulates platelet-derived growth factor's actions in hepatic stellate cells, and is induced by in vivo liver injury in the rat. Hepatology. 1999;30:951–958. doi: 10.1002/hep.510300406. [DOI] [PubMed] [Google Scholar]
- 9.Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis. 2001;21:397–416. doi: 10.1055/s-2001-17554. [DOI] [PubMed] [Google Scholar]
- 10.Anjum R, Roux PP, Ballif BA, Gygi SP, Blenis J. The tumor suppressor DAP kinase is a target of RSK-mediated survival signaling. Curr Biol. 2005;15:1762–1767. doi: 10.1016/j.cub.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 11.Butcher GQ, Lee B, Hsieh F, Obrietan K. Light- and clock-dependent regulation of ribosomal S6 kinase activity in the suprachiasmatic nucleus. Eur J Neurosci. 2004;19:907–915. doi: 10.1111/j.0953-816x.2004.03155.x. [DOI] [PubMed] [Google Scholar]
- 12.Mori M, Hara M, Tachibana K, Kishimoto T. p90Rsk is required for G1 phase arrest in unfertilized starfish eggs. Development. 2006;133:1823–1830. doi: 10.1242/dev.02348. [DOI] [PubMed] [Google Scholar]
- 13.Richards SA, Dreisbach VC, Murphy LO, Blenis J. Characterization of regulatory events associated with membrane targeting of p90 ribosomal S6 kinase 1. Mol Cell Biol. 2001;21:7470–7480. doi: 10.1128/MCB.21.21.7470-7480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frödin M, Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol. 1999;151:65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 15.Qiang H, Lin Y, Zhang X, Zeng X, Shi J, Chen YX, Yang MF, Han ZG, Xie WF. Differential expression genes analyzed by cDNA array in the regulation of rat hepatic fibrogenesis. Liver Int. 2006;26:1126–1137. doi: 10.1111/j.1478-3231.2006.01353.x. [DOI] [PubMed] [Google Scholar]
- 16.Haratake J, Hisaoka M, Yamamoto O, Horie A. Morphological changes of hepatic microcirculation in experimental rat cirrhosis: a scanning electron microscopic study. Hepatology. 1991;13:952–956. [PubMed] [Google Scholar]
- 17.Mérienne K, Jacquot S, Zeniou M, Pannetier S, Sassone-Corsi P, Hanauer A. Activation of RSK by UV-light: phosphorylation dynamics and involvement of the MAPK pathway. Oncogene. 2000;19:4221–4229. doi: 10.1038/sj.onc.1203712. [DOI] [PubMed] [Google Scholar]
- 18.Vogel S, Piantedosi R, Frank J, Lalazar A, Rockey DC, Friedman SL, Blaner WS. An immortalized rat liver stellate cell line (HSC-T6): a new cell model for the study of retinoid metabolism in vitro. J Lipid Res. 2000;41:882–893. [PubMed] [Google Scholar]
- 19.Slack JL, Parker MI, Robinson VR, Bornstein P. Regulation of collagen I gene expression by ras. Mol Cell Biol. 1992;12:4714–4723. doi: 10.1128/mcb.12.10.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iraburu MJ, Domínguez-Rosales JA, Fontana L, Auster A, García-Trevijano ER, Covarrubias-Pinedo A, Rivas-Estilla AM, Greenwel P, Rojkind M. Tumor necrosis factor alpha down-regulates expression of the alpha1(I) collagen gene in rat hepatic stellate cells through a p20C/EBPbeta- and C/EBPdelta-dependent mechanism. Hepatology. 2000;31:1086–1093. doi: 10.1053/he.2000.5981. [DOI] [PubMed] [Google Scholar]
- 21.Inagaki Y, Truter S, Greenwel P, Rojkind M, Unoura M, Kobayashi K, Ramirez F. Regulation of the alpha 2(I) collagen gene transcription in fat-storing cells derived from a cirrhotic liver. Hepatology. 1995;22:573–579. [PubMed] [Google Scholar]
- 22.Mak KM, Leo MA, Lieber CS. Alcoholic liver injury in baboons: transformation of lipocytes to transitional cells. Gastroenterology. 1984;87:188–200. [PubMed] [Google Scholar]
- 23.Ramadori G, Veit T, Schwögler S, Dienes HP, Knittel T, Rieder H, Meyer zum Büschenfelde KH. Expression of the gene of the alpha-smooth muscle-actin isoform in rat liver and in rat fat-storing (ITO) cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59:349–357. doi: 10.1007/BF02899424. [DOI] [PubMed] [Google Scholar]
- 24.Kim Y, Ratziu V, Choi SG, Lalazar A, Theiss G, Dang Q, Kim SJ, Friedman SL. Transcriptional activation of transforming growth factor beta1 and its receptors by the Kruppel-like factor Zf9/core promoter-binding protein and Sp1. Potential mechanisms for autocrine fibrogenesis in response to injury. J Biol Chem. 1998;273:33750–33758. doi: 10.1074/jbc.273.50.33750. [DOI] [PubMed] [Google Scholar]
- 25.Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22 Suppl 1:S79–S84. doi: 10.1111/j.1440-1746.2006.04659.x. [DOI] [PubMed] [Google Scholar]
- 26.Pinzani M, Gesualdo L, Sabbah GM, Abboud HE. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest. 1989;84:1786–1793. doi: 10.1172/JCI114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panta GR, Kaur S, Cavin LG, Cortés ML, Mercurio F, Lothstein L, Sweatman TW, Israel M, Arsura M. ATM and the catalytic subunit of DNA-dependent protein kinase activate NF-kappaB through a common MEK/extracellular signal-regulated kinase/p90(rsk) signaling pathway in response to distinct forms of DNA damage. Mol Cell Biol. 2004;24:1823–1835. doi: 10.1128/MCB.24.5.1823-1835.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah BH, Farshori MP, Jambusaria A, Catt KJ. Roles of Src and epidermal growth factor receptor transactivation in transient and sustained ERK1/2 responses to gonadotropin-releasing hormone receptor activation. J Biol Chem. 2003;278:19118–19126. doi: 10.1074/jbc.M212932200. [DOI] [PubMed] [Google Scholar]
- 29.Toledo-Pereyra LH, Lopez-Neblina F, Reuben JS, Toledo AH, Ward PA. Selectin inhibition modulates Akt/MAPK signaling and chemokine expression after liver ischemia-reperfusion. J Invest Surg. 2004;17:303–313. doi: 10.1080/08941930490884706. [DOI] [PubMed] [Google Scholar]
- 30.Buck M, Chojkier M. A ribosomal S-6 kinase-mediated signal to C/EBP-beta is critical for the development of liver fibrosis. PLoS ONE. 2007;2:e1372. doi: 10.1371/journal.pone.0001372. [DOI] [PMC free article] [PubMed] [Google Scholar]