Abstract
We eliminated type β transforming growth factor (TGF-β) signaling by adenovirus-mediated local expression of a dominant-negative type II TGF-β receptor (AdCATβ-TR) in the liver of rats treated with dimethylnitrosamine, a model of persistent liver fibrosis. In rats that received a single application of AdCATβ-TR via the portal vein, liver fibrosis as assessed by histology and hydroxyproline content was markedly attenuated. All AdCATβ-TR-treated rats remained alive, and their serum levels of hyaluronic acid and transaminases remained at low levels, whereas all the AdCATβ-TR-untreated rats died of liver dysfunction. The results demonstrate that TGF-β does play a central role in liver fibrogenesis and indicate clearly in a persistent fibrosis model that prevention of fibrosis by anti-TGF-β intervention could be therapeutically useful.
Many people suffer from fibrosis of their critical organs. Clarification of the molecular mechanisms underlying fibrotic disorders and the development of effective therapy are both of clinical importance. It has been considered that type β transforming growth factor (TGF-β) is an important cytokine in the regulation of the production, degradation, and accumulation of extracellular matrix (ECM) proteins and that, as a consequence, it may play a pivotal role in the fibroproliferative changes that follow tissue damage in many vital organs, including liver, lung, kidney, skin, heart, and arterial wall (1–4). However, whether TGF-β indeed is of crucial importance in fibrogenesis in vivo and whether inhibition of TGF-β actually would be effective in preventing fibrosis have not yet been elucidated using persistent fibrosis models. Importantly, work on fibrosis models has yet to elucidate whether prevention of fibrosis through anti-TGF-β intervention would be therapeutic or whether it might hinder the physiological repair process after tissue injury.
As a model of irreversible fibrosis in vital organs, we focused on liver fibrosis and investigated these questions by inducing a specific blockade of TGF-β signaling in vivo. For this purpose, we used dimethylnitrosamine (DMN)-treated rats (5, 6), an established animal model of persistent liver fibrosis with a pathophysiology closely resembling that of human liver cirrhosis (3, 5, 6). To achieve a specific block of the action of TGF-β, we used an adenoviral vector to locally express a truncated type II TGF-β receptor (AdCATβ-TR); this specifically can abolish all the diverse signaling by TGF-β by acting as a dominant-negative receptor (7).
In AdCATβ-TR-treated rats liver fibrosis was reduced markedly by comparison with the controls, demonstrating that TGF-β does indeed play a critical role in fibrogenesis in vivo. Strikingly, all the AdCATβ-TR-treated rats survived to the end of the study period, whereas all the control rats died of liver dysfunction. This evidence indicates that prevention of fibrosis through anti-TGF-β treatment could preserve organ function and be therapeutically useful. Our data may suggest that AdCATβ-TR could be effective in future gene therapy for fibrosis in vital organs.
MATERIALS AND METHODS
Replication-Defective Recombinant Adenoviruses.
Replication-defective E1− and E3− adenoviral vectors expressing either a truncated human TGF-β type II receptor (AdCATβ-TR) (7) or β-galactosidase (AdCALacZ) (8) under a CA promoter comprising a cytomegalovirus enhancer and chicken β-actin promoter (9) were prepared as described previously (7, 8, 10).
Animal Models.
Male Sprague–Dawley rats, 10 weeks old and weighing around 380 g, were given a single infusion of 1 ml of either AdCATβ-TR (1.0 × 109 pfu/ml), AdCALacZ, or saline via the portal vein. Immunohistostaining of the liver of the AdCALacZ-infused rats using an antibody against β-galactosidase showed that virtually all liver cells expressed β-galactosidase, as reported previously (11, 12), although the expression level varied between cells (data not shown).
Each rat subsequently received an i.p. injection of DMN [10 μg/g body weight (BW) or, for the survival assay, 12 μg/g BW] (Wako) three times a week for 3 weeks. The amount of DMN administered was adjusted for body weight each week. At the end of the 3-week study period, venous blood was collected and the rats were sacrificed. Biochemical parameters were measured by using standard methods. The liver either was fixed with 4% buffered paraformaldehyde for histological examination or frozen immediately in liquid nitrogen for the extraction of hydroxyproline, the content of which was measured as described previously (13). Not every rat was subjected to all the assays described.
Northern Blotting.
Via the portal vein, rats were infused with 1 ml either of saline, AdCALacZ, or AdCATβ-TR (1.0 × 109 pfu/ml). Three days later, RNA was extracted. Polyadenylated mRNA (from 150 μg of total RNA) was subjected to Northern blotting analysis, as described previously (7). A nylon membrane (Hybond-N, Amersham) was probed for 16 h at 42°C with 32P-labeled cDNAs corresponding to the ectodomain of the human TGF-β type II receptor. Hybridization signals were detected either by autoradiography (Kodak XA-R film) or by a quantitative imaging analyzer (BAS 2000; Fuji). Some rats were infected with AdCATβ-TR and treated with DMN for 3 days before RNA was extracted.
Histological Examination.
Tissue sections either were stained with Masson-trichrome or subjected to immunohistostaining by using antibodies against collagen type I (14), fibronectin (Chemicon), α-smooth muscle actin (Boehringer Mannheim), TGF-β1 (Promega), or monocytes/macrophages (ED-1; Serotec). Immunoreactive materials were visualized by use of a biotinylated anti-mouse (or -rabbit) IgG antibody, peroxidase-labeled streptavidin, and diaminobenzidine. For the semiquantitative analysis of fibrosis, the blue-stained area in the Masson-trichrome-stained sections was measured on a video-screen display (×400 magnification) by a technician blinded to the treatment regimen using a digital image analyzer (Digitizer KD4600; Graphtec, Tokyo, Japan). Three fields were selected randomly from each of 3 sections, and 3 rats from each group were examined; thus, a total of 27 fields were analyzed for each group.
Statistical Analysis.
Statistical analysis of values was performed by means of an unpaired Student‘s t test, with a P value <0.01 considered significant.
RESULTS
A Large Excess of mRNA for the Truncated TGF-β Type II Receptor Over That for the Rat Full-Length Receptor Is Expressed in the Liver of AdCATβ-TR-Infected Rats.
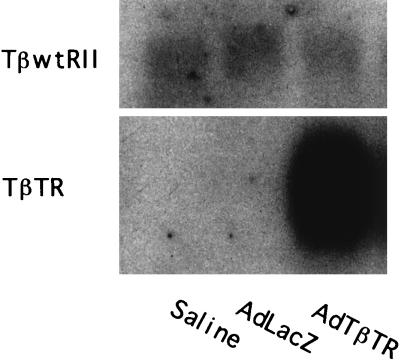
To inhibit signaling mediated by the wild-type receptor, the truncated receptor would need to be expressed in a considerable excess over the full-length receptor (7, 15). Because of the lack of an appropriate antibody for the detection of the extracellular domain of both the rat and the human type II TGF-β receptor at a similar level, we were unable to quantify the protein levels of those two receptors. For that reason, we compared the levels of the mRNAs for the two receptors. Via the portal vein, we infused either saline, a control adenovirus expressing bacterial β-galactosidase (AdCALacZ), or an adenovirus expressing a dominant-negative TGF-β receptor (a truncated human type II receptor) (AdCATβ-TR) (7). We extracted mRNA from the livers of rats 3 days after gene transfer and analyzed them by Northern blotting using a human receptor as a probe. Two mRNAs were detectable (Fig. 1), and these mRNAs, of 5.5 and 0.9 kb, would correspond to the rat full-length receptor and the truncated human receptor, respectively (7). The truncated receptor mRNA was much more abundant (around 20-fold more) than the rat full-length receptor (Fig. 1). Similar results were obtained in AdCATβ-TR-infected rats treated with DMN for 3 days (data not shown). It is true that we may have underestimated the level of the mRNA for the rat receptor because of our use of a human probe and, furthermore, that we did not investigate how great an excess of the truncated receptor may be required to eliminate TGF-β signaling in the liver in vivo. Nevertheless, these results seem to suggest that a large excess of the truncated receptor over the full-length receptor would be expressed in the liver; this then could lead to an elimination of signaling by TGF-β.
Figure 1.
Full-length rat type II and truncated human type II TGF-β receptor mRNA in liver of rat infected with AdCATβ-TR. Rats were infused via the portal vein with either saline, AdCALacZ, or AdCATβ-TR. Three days later, polyadenylated mRNA was extracted from the livers and subjected to Northern blotting analysis using the ectodomain of the human type II TGF-β receptor as a probe. Two signals (5.5 and 0.9 kb) were detectable. Two other independent experiments gave similar results.
DMN Treatment Induces Irreversible Liver Fibrosis.
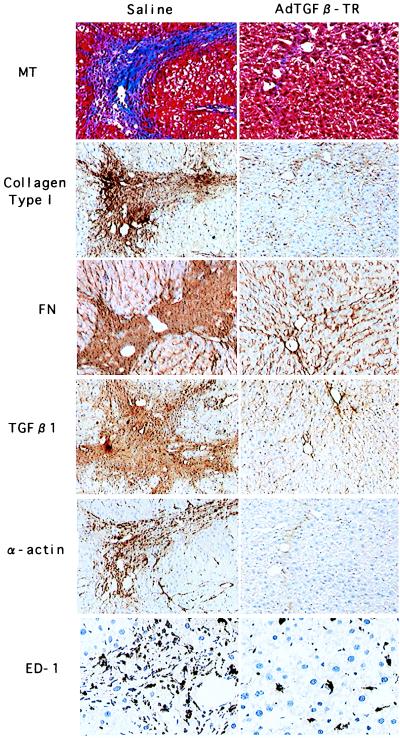
As an experimental liver fibrosis model, we used DMN-treated rats (5, 6). I.p. injection of DMN induced fatty degeneration of hepatocytes, activation and proliferation of lipocytes (also called Ito cells), infiltration by macrophages (possibly Kupffer cells), and secretion of TGF-β. Within 3 weeks, this resulted in tissue remodeling with matrix deposition mainly in the centrilobular area and portal tract, as assessed histologically (Fig. 2A) or by immunohistostaining using specific antibodies against either collagen type I or fibronectin (Fig. 3 Left). The fibrotic area also was stained intensely by an anti-TGF-β1 antibody, and the cells in the fibrotic area showed positive immunostaining either for anti-α-smooth muscle actin or for anti-monocytes/anti-macrophages (ED-1 antibody) (Fig. 3 Left). These findings suggest that the transformed lipocytes (and the infiltrated macrophages) may secrete TGF-β. This is consistent with the current idea that lipocytes are transformed into myofibroblasts (expressing α-smooth muscle actin) and secrete TGF-β, thus stimulating the production of ECM, and probably their proliferation, through a paracrine/autocrine loop (3).
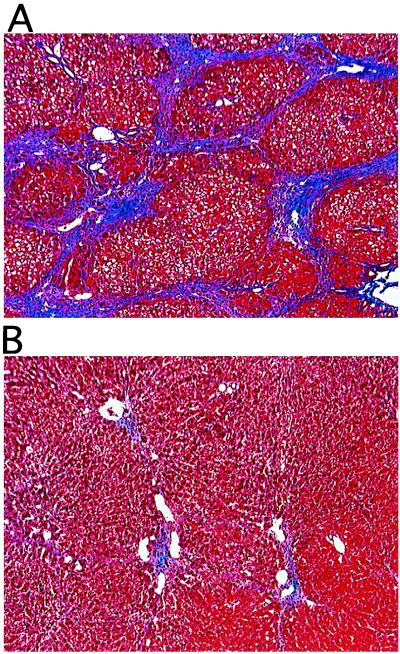
Figure 2.
Histology of liver from rats treated with DMN. Rats were infused once via the portal vein with saline (A), AdCATβ-TR (B), or AdCALacZ (not shown, because the histology was not significantly different from that of rats infused with saline), then treated with DMN for 3 weeks as described in Materials and Methods. Liver sections were examined histologically by Masson-trichrome staining. (×200.)
Figure 3.
Immunohistological analysis of liver from rats treated with DMN. Rats were treated as described in the legend to Fig. 1. Liver sections were examined by immunohistostaining by using antibodies against collagen type I, fibronectin (FN), TGF-β1, α-smooth muscle actin (α-actin), or monocyte/macrophage (ED-1). Masson-trichrome staining (MT) also is shown to indicate the fibrotic area. (Left) DMN-treated rats infused with saline. (Right) DMN-treated rats infused with AdCATβ-TR. The upper three sections are serial. [×200 (×400 for ED-1 immunostaining).] Sections from AdCALacZ-infused rats are not shown, because the results were similar to those seen in rats infused with saline.
Gene Transfer of a Dominant-Negative TGF-β Receptor Prevents Liver Fibrosis in DMN-Treated Rats.
By contrast, in rats in which we gave a single injection, via the portal vein, of AdCATβ-TR, liver fibrosis, as assessed by histological examination, was markedly reduced. Masson-trichrome staining is shown in Fig. 2B, and immunostaining against collagen type I, fibronectin, α-smooth muscle actin, TGF-β1, and monocytes/macrophages (ED-1) is shown in Fig. 3 Right. This inhibitory effect was observed in all areas of the liver, with no significant difference noticed between the different lobes. Semiquantitative analysis of the area of fibrosis in tissue sections showed that in AdCATβ-TR-treated rats the fibrotic area was reduced to 22 ± 4% (mean ± SD, 27 fields from 3 rats) of that seen in rats infused with saline. No reduction in fibrosis was observed in DMN-treated rats infused with a control adenovirus (AdCALacZ), and no significant histological difference was seen between AdCALacZ-treated rats and DMN-treated rats infused with saline, indicating that marked prevention of fibrosis was not the result of adenovirus infection per se.
For the purposes of quantitative evaluation, we measured the hydroxyproline content of the liver (13). In DMN-treated rats infused with either AdCALacZ or saline, the amount of hydroxyproline was 3.4 times higher than that found in intact rats (i.e., rats not treated with DMN) (Fig. 4). However, in DMN-treated rats infused once with AdCATβ-TR, the hydroxyproline content showed only a slight excess over the value seen in intact rats.
Figure 4.
Hydroxyproline content of the liver. Rats were treated as described in the legend to Fig. 1. Hydroxyproline content is shown as mean ± SD (n = 12). Three samples from each of four rats were analyzed for each group.
Serum levels of hyaluronate, which is used as a serum marker of the progression of liver fibrosis in humans (16, 17), were low in DMN-treated rats infused with AdCATβ-TR, but high in DMN-treated rats infused with either saline or AdCALacZ (Table 1). Surprisingly, the serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), which may reflect hepatocyte impairment, were within the low range in DMN-treated rats infused with AdCATβ-TR (Table 1).
Table 1.
Serum hyaluronate and hepatocyte enzymes
| Hyaluronate, ng/ml | AST, units/ml | ALT, units/ml | |
|---|---|---|---|
| Intact | <10 | 13 ± 5 | 8 ± 3 |
| DMN-saline | 430 ± 61 | 7,652 ± 1,695 | 4,211 ± 1,010 |
| DMN-AdLacZ | 411 ± 46 | 8,078 ± 1,515 | 4,041 ± 768 |
| DMN AdTβ-TR | 22 ± 4 | 78 ± 6 | 67 ± 7 |
Serum hyaluronate and hepatocyte enzymes in intact and DMN-treated rats. Rats were treated as described in the legend to Fig. 1. Blood was collected 3 weeks after gene transfer and DMN treatment. Hyaluronate, AST, and ALT are shown as mean ± SE (n = 7). Intact rats were also analyzed as controls.
When we measured AST and ALT in the serum of rats 3 days after both gene transfer and the initial treatment with DMN, the increases in the levels of these hepatic enzymes were the same regardless of whether the DMN-treated rats were infused with saline, AdCALacZ, or AdCATβ-TR (Table 2). This indicates that in the initial stage of DMN treatment, a comparable degree of hepatic injury was induced in the livers of all rats.
Table 2.
Serum hepatocyte enzymes
| AST, international units/ml | ALT, international units/ml | |
|---|---|---|
| Intact | 58 ± 2 | 36 ± 2 |
| DMN-saline | 1,301 ± 202 | 1,038 ± 217 |
| DMN-AdLacZ | 1,350 ± 302 | 1,325 ± 310 |
| DMN-AdTbTR | 1,395 ± 138 | 1,450 ± 304 |
Serum hepatocyte enzymes in intact and DMN-treated rats. Rats were treated as described in the legend to Fig. 1. Three days after the initial treatment with DMN, blood was collected and serum enzymes were measured. AST and ALT both are shown as mean ± SE (n = 6). Intact rats also were analyzed as controls. There is no statistical difference between the values from AdCALacZ-infused rats and those from AdCATβ-TR-infused rats.
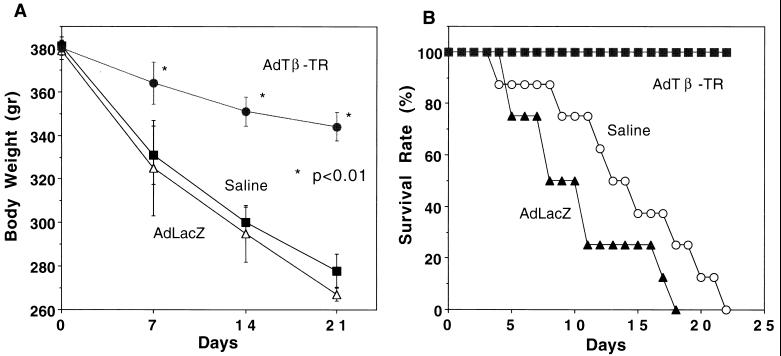
Protection Against Fibrosis by AdCATβ-TR Reduced Loss of Body Weight and Led to Greater Survival Among Rats.
Fibrosis may be part of the physiological response to tissue damage. Thus, whether prevention of fibrosis indeed is beneficial is an important question. This question cannot be examined in a temporary model of ECM deposition. There was a gradual loss of body weight in DMN-treated rats infused with either saline or AdCALacZ, but this loss was attenuated significantly in DMN-treated rats infused with AdCATβ-TR (Fig. 5A). Furthermore, the DMN-treated rats infused with AdCATβ-TR all remained alive throughout the study, whereas all the DMN-treated rats infused with either saline or AdCALacZ died within 3 weeks (Fig. 5B). It should be noted that for the body weight and survival studies, DMN was given at 12 μg/g BW, instead of 10 μg/g BW, to accelerate liver fibrosis and dysfunction.
Figure 5.
Temporal changes in body weight (A) and survival rate (B) in DMN-treated rats. Rats were treated as described in the legend to Fig. 1 (DMN was given at 12 μg/g BW). Rats that died within the 24 h after the gene-transfer procedure were excluded from the evaluation. For rats infused with either AdCALacZ or saline who died during the 3rd week, body weight at death is shown at day 21 (n = 5 in A and n = 15 in B for each group.)
DISCUSSION
It is now widely accepted that TGF-β is a major cytokine in the regulation of the production, degradation, and accumulation of ECM, and it has been suggested that overexpression (or activation) of TGF-β for a prolonged period of time after tissue damage may induce a fibroproliferative response and deposition of ECM, resulting in fibrosis in vital organs (2–4). Many studies have detected the presence of TGF-β, in the form of either protein or message, in the fibrotic tissues of animal models or human samples. Partial inhibition of the accumulation of ECM using either anti-TGF-β serum or a TGF-β-binding protein has been reported in a temporary fibrosis model, such as the antithymocyte antibody-induced glomerulonephritis model (18, 19), in which ECM deposition will, even without treatment, persist for only a week or so. However, it has not been demonstrated clearly in a persistent fibrosis model whether TGF-β does play a central role in fibrogenesis; in other words, whether prevention of fibrosis can be achieved by anti-TGF-β intervention. Moreover, the important question of whether prevention of fibrosis is therapeutic or hinders the physiological response leading to wound repair has not been answered clearly yet. The present study of rat liver fibrosis in an irreversible fibrosis model has answered all these questions by adenovirus-mediated local expression of a dominant-negative TGF-β receptor (7) that can abolish all the known signaling by TGF-β. Our results indicate that TGF-β does indeed play a central role in liver fibrogenesis. Our study also provides clear evidence that blockade of fibrosis via anti-TGF-β intervention preserves liver function and will be therapeutically useful. The study also suggests that the use of the adenovirus-mediated transfer of a molecule that blocks TGF-β signaling may have potential as an effective means of therapy.
Overexpression of kinase-truncated or kinase-defective receptors for particular growth factors (15, 20–22) would be a useful way of clarifying the in vivo role of these factors (23–28), because signaling by a given growth factor is blocked at the receptor level without affecting signaling mediated by other receptors (7, 15, 20, 21). This is especially true in studies of disorders developed in mature adults; because most growth factors are indispensable for normal embryogenesis, gene targeting would be lethal. In this study a dominant-negative TGF-β receptor has been expressed in adult animals as a way of elucidating the role of TGF-β in fibrogenesis and evaluating the therapeutic effects of its inhibition in a persistent fibrosis model.
The immunohistostaining analyses of the liver of DMN-treated rats in our study produced results that are consistent with the current concept that activated lipocytes, which are transformed to myofibroblasts expressing α-smooth muscle actin, may be the primary cellular source of ECM proteins in response to TGF-β. This idea has been proposed in studies of both rodent models and humans (3, 29, 30). The factor(s) responsible for the activation of lipocytes in the initial phase has yet to be identified, although it is thought that Kupffer cells (liver macrophages) may secrete the activating factor(s) and that at least one of those factors may be TGF-β (31, 32). Although TGF-β has an antiproliferation effect on cells of epithelial origin, it also exerts a proliferative action, depending on its concentration, particularly on mesenchymal cells (2, 33). Thus, the notion that TGF-β may stimulate proliferation of lipocytes is not inconsistent with its known actions. It has also been reported that lipocyte proliferation was augmented when the cells were cultured on type I collagen (34), which is the predominant matrix component increased in liver fibrosis. Thus, a suppression of the accumulation of ECM by elimination of TGF-β signaling may also reduce lipocyte proliferation, which, in turn, should further reduce the accumulation of ECM. Alternatively, TGF-β may stimulate the secretion of other cytokines such as platelet-derived growth factor (PDGF), which induces proliferation of lipocytes, as reported previously in fibroblasts and arterial smooth muscle cells (35, 36). Up-regulation of the PDGF receptor in cultured lipocytes also has been reported (37–39), and this would tend to enhance the above effect of TGF-β. These studies suggest that an elimination of PDGF signaling might suppress the activation and/or proliferation of lipocytes and thus contribute to a more effective prevention of fibrosis if it occurs in addition to an inhibition of TGF-β signaling. This possibility is now under investigation in our laboratory using an adenovirus expressing a truncated PDGF receptor that works as a dominant-negative PDGF receptor (15, 40).
An important finding in this study was that prevention of fibrosis through anti-TGF-β intervention was accompanied by a reduction of liver dysfunction (as assessed by the level of hepatocyte enzymes in the serum), a smaller loss of body weight, and enhanced survival (Table 1 and Fig. 5). At present, we do not know the identity of the underlying mechanism responsible for this maintenance (or enhancement) of liver function. Because ECM (through an interaction with integrins) affects cell structure and function, induces gene expression, and stimulates cellular proliferation (41), it is theoretically possible that the reduced fibrosis may have led to an attenuation of the hepatic “injury” at a later stage in the disease process and thus to a lessening of the liver dysfunction. Indeed, the more than 60-fold reduction in ALT levels seen in AdCATβ-TR-treated rats at 3 weeks may raise this possibility. An accumulation of fibril-forming collagen and fibronectin in the subendothelial Disse space is associated with clinical liver disease (42). Fibrosis in the Disse space may block the exchange of molecules between the sinusoidal space and the hepatocytes, thereby impairing the functioning of hepatocytes (3). Thus, a reduction in fibrosis in this space might improve liver function more than might otherwise be expected. It has been reported that TGF-β is an inhibitor of the proliferation of hepatocytes (43, 44) and that, at higher concentrations, TGF-β induces oxidative stress leading to hepatocyte apoptosis (44). Thus, attenuation of the action of TGF-β by expression of the truncated TGF-β receptor might facilitate the process of hepatic regeneration or prevent hepatocyte damage from occurring. Further studies including electronmicroscopic analysis are needed to test these ideas.
The present data may suggest that AdCATβ-TR is a possible candidate for use in future gene therapy. Whether AdCATβ-TR will be effective in halting fibrosis and allowing its reversal in individuals in which fibrosis is already established is a question to be examined in future studies. Conceivably, the elimination of the effects of TGF-β for a prolonged period of time might have unfavorable consequences, such as the inflammation and tissue necrosis observed in TGF-β1 gene-disrupted mice (45). This also will need to be investigated carefully in future studies.
Acknowledgments
We thank Ms. S. Masuda for her technical assistance in histological analysis and Drs. I. Saito and J. Miyazaki for a cosmid vector. This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan, and by a grant from Takeda Medical Research Foundation (Osaka, Japan) (to H.U.).
ABBREVIATIONS
- TGF-β
type β transforming growth factor
- DMN
dimethylnitrosamine
- ECM
extracellular matrix
- AdCATβ-TR
adenovirus expressing a dominant-negative type II TGF-β receptor
- AdCALacZ
adenovirus expressing bacterial β-galactosidase
- BW
body weight
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
References
- 1.Massague J. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- 2.Sporn M B, Roberts A B. J Cell Biol. 1992;119:1017–1021. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman S L. N Engl J Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 4.Border W A, Noble N A. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins S A, Grandison A, Bazter J N, Day D W, Taylor I, Shields R. J Hepatol. 1985;1:489–499. doi: 10.1016/s0168-8278(85)80747-9. [DOI] [PubMed] [Google Scholar]
- 6.Jezequel A M, Mancini R, Rinaldesi M L, Macarri G, Venturini C, Orlandi F. J Hepatol. 1987;5:174–181. doi: 10.1016/s0168-8278(87)80570-6. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto H, Ueno H, Ooshima A, Takeshita A. J Biol Chem. 1996;271:16253–16259. doi: 10.1074/jbc.271.27.16253. [DOI] [PubMed] [Google Scholar]
- 8.Ueno H, Li J-J, Tomita H, Yamamoto H, Pan Y, Kanegae Y, Saito I, Takeshita A. Arterioscl Thromb Vasc Biol. 1995;15:2246–2253. doi: 10.1161/01.atv.15.12.2246. [DOI] [PubMed] [Google Scholar]
- 9.Niwa H, Yamamura K, Miyazaki J. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 10.Miyake S, Makimura M, Kanegae Y, Harada S, Sato Y, Takamori K, Tokuda C, Saito I. Proc Natl Acad Sci USA. 1996;93:1320–1324. doi: 10.1073/pnas.93.3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Kay M A, Finegold M, Stradford-Perricaudet L D, Woo S L C. Hum Gene Ther. 1993;4:403–409. doi: 10.1089/hum.1993.4.4-403. [DOI] [PubMed] [Google Scholar]
- 12.Kozarsky K F, McKinley D R, Austin L L, Raper S E, Stratford-Perricaudet L D, Wilson J M. J Biol Chem. 1994;269:13695–13702. [PubMed] [Google Scholar]
- 13.Woessner J F J. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 14.Kumagai J, Sarkar K, Uhthoff H K, Okawara Y, Ooshima A. J Anat. 1994;185:279–284. [PMC free article] [PubMed] [Google Scholar]
- 15.Ueno H, Colbert H, Escobedo J A, Williams L T. Science. 1991;252:844–848. doi: 10.1126/science.1851331. [DOI] [PubMed] [Google Scholar]
- 16.Guechot J, Poupon R E, Poupon R. J Hepatol. 1995;22:103–106. [PubMed] [Google Scholar]
- 17.Korner T, Kropf J, Gressner A M. J Hepatol. 1996;25:684–688. doi: 10.1016/s0168-8278(96)80239-x. [DOI] [PubMed] [Google Scholar]
- 18.Border W A, Okuda S, Languino L R, Sporn M B, Ruoslahti E. Nature (London) 1990;346:371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- 19.Border W A, Noble N A, Yamamoto T. Nature (London) 1992;346:371–374. [Google Scholar]
- 20.Ueno H, Gunn M, Dell K, Tseng A, Williams L. J Biol Chem. 1992;267:1470–1476. [PubMed] [Google Scholar]
- 21.Kashles O, Yarden Y, Fischer R, Ullrich A, Schlessinger J. Mol Cell Biol. 1991;11:1454–1463. doi: 10.1128/mcb.11.3.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wieser R, Attisano L, Wrana J L, Massague J. Mol Cell Biol. 1993;13:7329–7247. doi: 10.1128/mcb.13.12.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amaya E, Musci T J, Kirschner M W. Cell. 1991;66:257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- 24.Hemmati-Brivanlou A, Melton D A. Nature (London) 1992;359:609–614. doi: 10.1038/359609a0. [DOI] [PubMed] [Google Scholar]
- 25.Mima T, Ueno H, Fischman D A, Williams L T, Mikawa T. Proc Natl Acad Sci USA. 1995;92:467–471. doi: 10.1073/pnas.92.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millauer B, Shawver L K, Plate K H, Risau W, Ullrich A. Nature (London) 1994;367:576–579. doi: 10.1038/367576a0. [DOI] [PubMed] [Google Scholar]
- 27.Werner S, Smola H, Liao X, Longaker M T, Krieg T, Hofschneider P H, Williams L T. Science. 1994;266:819–822. doi: 10.1126/science.7973639. [DOI] [PubMed] [Google Scholar]
- 28.Peters K, Werner S, Liao X, Wert S, Whitsett J, Williams L. EMBO J. 1994;13:3296–3301. doi: 10.1002/j.1460-2075.1994.tb06631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maher J J, McGuire R F. J Clin Invest. 1990;86:1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman S L, Rockey D C, MuGuire R F, Maher J J, Boyles J K, Yamasaki G. Hepatology. 1992;15:234–243. doi: 10.1002/hep.1840150211. [DOI] [PubMed] [Google Scholar]
- 31.Shiratori Y, Geerts A, Ichida T, Kawase T, Wisse E. J Hepatol. 1986;3:294–303. doi: 10.1016/s0168-8278(86)80481-0. [DOI] [PubMed] [Google Scholar]
- 32.Matsuoka M, Tsukamoto H. Hepatology. 1990;11:599–605. doi: 10.1002/hep.1840110412. [DOI] [PubMed] [Google Scholar]
- 33.Battegay E J, Raines E W, Seifert R A, Bowen-Pope D F, Ross R. Cell. 1990;63:515–524. doi: 10.1016/0092-8674(90)90448-n. [DOI] [PubMed] [Google Scholar]
- 34.Davis B H, Pratt B M, Madri J A. J Biol Chem. 1987;262:10280–10286. [PubMed] [Google Scholar]
- 35.Paulsson Y, Beckmann M P, Westmark B, Heldin C-H. Growth Factors. 1988;1:19–27. doi: 10.3109/08977198809000243. [DOI] [PubMed] [Google Scholar]
- 36.Majack R A, Majesky M W, Goodman L V. J Cell Biol. 1990;111:239–247. doi: 10.1083/jcb.111.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinzani M, Gesualdo L, Sabbah G M, Abboud H E. J Clin Invest. 1989;84:1786–1793. doi: 10.1172/JCI114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedman S L, Arthur M J P. J Clin Invest. 1989;84:1780–1785. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinzani M, Gentilini A, Caligiuri A, De Franco R, Pellegrini G, Milani S, Marra F, Gentilini P. Hepatology. 1995;21:232–239. [PubMed] [Google Scholar]
- 40.Ueno H, Escobedo J A, Williams L T. J Biol Chem. 1993;268:22814–22819. [PubMed] [Google Scholar]
- 41.Schwartz M A, Schaller M D, Ginsberg M H. Annu Rev Cell Dev Biol. 1998;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 42.Schaffner F, Popper H. Gastroenterology. 1963;44:239–242. [PubMed] [Google Scholar]
- 43.Carr B I, Hayashi I, Branum E L, Moses H L. Cancer Res. 1986;46:2330–2334. [PubMed] [Google Scholar]
- 44.Sanchez A, Alvarez A M, Benito M, Fabregat I. J Biol Chem. 1996;271:7416–7422. doi: 10.1074/jbc.271.13.7416. [DOI] [PubMed] [Google Scholar]
- 45.Shull M M, Ormsby I, Kier A B, Pawlowski S, Diebold R J, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Nature (London) 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]