Abstract
Chemical analyses of ancient organics absorbed into pottery jars from the beginning of advanced ancient Egyptian culture, ca. 3150 B.C., and continuing for millennia have revealed that a range of natural products—specifically, herbs and tree resins—were dispensed by grape wine. These findings provide chemical evidence for ancient Egyptian organic medicinal remedies, previously only ambiguously documented in medical papyri dating back to ca. 1850 B.C. They illustrate how humans around the world, probably for millions of years, have exploited their natural environments for effective plant remedies, whose active compounds have recently begun to be isolated by modern analytical techniques.
Keywords: ancient medicine, biomolecular archaeology, herbs, Middle East, wine
Before the rise of modern medicine and likely extending back into the Paleolithic period, humans treated disease and physical ailments by experimenting with natural products derived from plants, other animals, and minerals (1). Fruit-bearing trees, which appeared around 100 million years ago (Mya), offered unparalleled access to sugar and ethanol. The latter had already established themselves as prime energy sources in the animal kingdom. The sweet liquid that oozes out of ruptured ripened fruit provides the ideal conditions of water and nutrients for yeast on their surfaces to multiply and convert the sugar into alcohol (2).
A close symbiosis developed between plants and animals over time, in which the plants provided nourishment and other benefits to the animals, which, in turn, pollinated the plants' flowers, dispersed their seeds, and carried out other functions. The smaller molars and canines of Proconsul and other early hominid fossils as early as 24 Mya (3) were well adapted to consuming soft fleshy foods like fruit. These dentitions are broadly comparable to those of modern apes, including gibbons, orangutans, and lowland gorillas, which obtain most of their calories from fruit. Modern chimpanzees, whose genome is the closest to Homo sapiens, consume over 90% plants, of which more than 75% is fruit. It can be concluded that early hominids and their descendants favored ripe, often fermented, fruit for millions of years.
In the warm tropical climate of sub-Saharan Africa, where the human species emerged, sweet fruit slurries can achieve an alcoholic content of 5% or more (2). If early hominids were primarily fruit eaters, at least up until about 1–2 Mya, when they began focusing more on tubers and animal fat and protein, they can be expected to have adapted biologically. One result, among many, is that about 10% of the enzymes in the human liver, including alcohol dehydrogenase, function to generate energy from alcohol (4). The genetic underpinnings of the presumed early human penchant for alcoholic fruit compotes, according to the so-called “drunken monkey hypothesis” (5), has been partly borne out by the diet of Malaysian tree shrews (6). These nocturnal animals, which belong to a family believed to be ancestral to all living primates from more than 55 Mya ago, spend their nights feasting year-round on a frothy strongly scented palm “wine,” with an alcoholic content as high as 3.8%.
Plant fruits, exudates (including resins and nectar), and other structures, (such as flowers, roots, and leaves), also contain many additional compounds with anesthetic, antimicrobial, and psychotropic properties that early humans likely exploited (1, 7). Although some of these compounds might have been intended to protect the plants from predation, they could also have benefited host animals. By trial and error, humans might well have made use of some of these properties by preparing “medicinal wines” and external salves in which the plant products were dissolved or decocted in alcoholic media.
At present, the earliest biomolecular archaeological evidence for plant additives in fermented beverages dates from the early Neolithic period in China (8) and the Middle East (9), when the first plants and animals were taken into domestication and provided the basis for complex society and permanent settlement. Possibilities for extending the evidential base back into the Paleolithic period are limited by the lack of containers, which were probably made from perishable materials, such as wood, leather, or woven textiles. Under the right environmental conditions, however, we can expect future discoveries. For example, at Monte Verde in Chile, around 13,000 B.P., the bog-like conditions of one of the first human settlements in the Americas resulted in the preservation of an incredible diversity of marsh, dune, mountain, and sea plants, which were likely exploited for their alcoholic, medicinal, and nutritional benefits (10).
The dry climate of Egypt has similarly contributed to excellent preservation of ancient organic materials, in addition to providing very detailed literary and botanical evidence for medicinal wines from one of the most long-standing ancient traditions (11, 12). We deliberately chose samples from 2 ancient Egyptian jars that illuminate the earliest and latest stages of Egyptian winemaking history and applied highly sensitive chemical techniques to obtain biomolecular information about what the vessels originally contained. Because we had already analyzed both samples by less precise methods, the latest results provide a means to test our previous results and conclusions; at the same time, they shed additional light on the vessels' contents.
Archaeological Samples Analyzed.
The earlier sample comes from a multichambered tomb (U-j) at Abydos on the middle Nile River in Upper Egypt (2, 13). Dated to the Naqada IIIa2 period (ca. 3150 B.C.) by radiocarbon determinations, the tomb was built in the desert and belongs to one of the first rulers of the country, Scorpion I of Dynasty 0, at the beginning of Egyptian dynastic history. Its contents were exceptionally rich and included some 700 jars of foreign type that were stacked high in 3 chambers.
In 1994, a sample of yellowish flaky residue from jar no. 156 (Fig. 1) was analyzed by FTIR spectrometry, HPLC, and a Feigl spot test for tartaric acid/tartrate (14). The residue represented the agglomerated remains of materials on the surface of a liquid that had gradually evaporated and left a ring or tide-line on the interior of the jar (see Fig. S1). The 3 independent methods gave results that pointed strongly to the presence of tartrate, a principal biomarker for wine and other grape products in the Middle East. The HPLC data supported the interpretation that a tree resin—very likely terebinth—had been added to the vessel's contents.
Fig. 1.
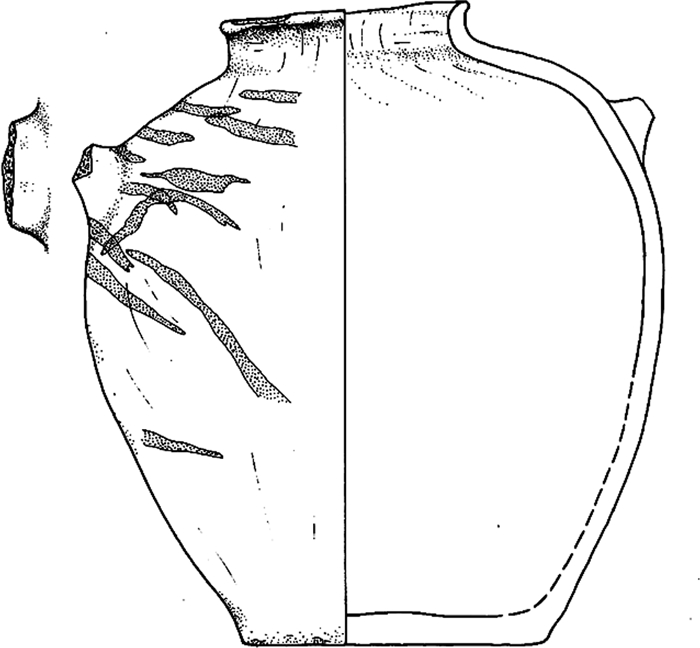
Jar no. 115 [Hartung U, ed (2001) Umm el-Qaab II: Importkeramik aus dem Friedhof U in Abydos (Umm el-Qaab) und die Beziehungen ägyptens zu Vorderasien im 4. Jahrtausend v. Chr. (P. von Zabern, Mainz, Germany): cat. no. 156, pls. 25 and 79:156] from chamber 10 of the Scorpion I tomb. Swirling “tiger-stripes” in red paint and the form of this narrow-mouthed jar are paralleled by southern Levantine vessels of the same period (Early Bronze I, ca. 3300–3000 B.C.); the manufacture of the vessel in this region was borne out by instrumental neutron activation analysis (INAA). The jar contained a yellowish flaky residue, which was analyzed by multiple chemical techniques, and several grape pips. Height: 31.7 cm. Drawing courtesy of German Archaeological Institute in Cairo.
The identification of tartrate, as the calcium salt, was based on the 3 methods independently agreeing with one another. If a single test result had proven negative, that would have invalidated the identification of calcium tartrate by the other methods. Although mixtures of compounds can be equivocal for IR and HPLC UV spectra, these were deconvoluted by statistical methods and scrutinized for the presence or absence of key absorptions. If a known absorption for a compound was lacking, that compound was excluded as a possibility. The IR and UV spectra of the Abydos sample were also searched for “matches” against large databases of relevant natural products and processed organic materials, synthetic compounds, modern wine samples, and “ancient wine reference samples.” The latter were residues from ancient vessels that likely originally contained wine, based on strong archaeological criteria or exterior inscriptions that recorded their contents.
Once calcium tartrate was identified to a high level of probability in the residue, archaeological and enological considerations come into play in determining whether the intended grape product was wine and not grape juice, syrup, or vinegar. A syrup, produced by heating grape juice and concentrating it down, was unlikely, because its viscosity would have left a uniform coating of residue on the inside of the vessel. Instead, the residue was confined to the tide-line and the bottom of the vessel, where precipitates from a liquid accumulate. Minimally, the jar had contained grape juice.
Any grape juice, however, will naturally ferment to wine in several days in the warm climate of the Middle East. The identification of a 256-bp DNA segment of a larger 840-bp fragment belonging to a ribosomal sequence of the principal wine yeast, Saccharomyces cerevisiae, bore out this interpretation (15). Intentional fermentation to vinegar was unlikely because of the precautions that were taken to protect the liquid in this jar from oxygen by sealing its mouth and adding a tree resin with antioxidant properties.
Grape seeds from jar no. 156 provided further corroboration that the jars originally held a grape wine. Additionally, and bearing on the issue of additives to the wine, some jars (not including jar no. 156) yielded a single whole fig, which had been preserved by desiccation. It had been sliced, perhaps to assure greater surface contact for enhancing the wine's sweetness, taste, and other properties and to provide sufficient yeast for starting and sustaining the fermentation.
The second sample, a residue deposited on or intentionally applied to the interior of an amphora (Fig. 2) from Lower Nubia in southern Egypt, dates more than 3,500 years later than the Abydos jar and represents a terminal phase of Egyptian winemaking before the Islamic conquest. Belonging to the fourth to early sixth century A.D. (Ballana period), the amphora was recovered from a tomb at Gebel Adda (16). Based on its form and an inscription on its shoulder, it has been identified as a wine jar (N.B. Millet, personal communication, 1989). Numerous such vessels littered the ground around taverns in Nubian villages of the period, demonstrating how wine had gone from being an elite beverage in Pharaonic times to becoming a beverage of commoners, who were also buried with it.
Fig. 2.
Wine amphora from tomb 217 of cemetery 4 at Gebel Adda (Egypt), dated to early Byzantine times (the Ballana period of Lower Nubia, fourth to early sixth century A.D.). Height: 67.3 cm. With permission of the Royal Ontario Museum; photograph courtesy of W. Pratt (museum no. 973.24.1217).
In our 1990 pioneering study of ancient Near Eastern wine (16), the Gebel Adda residue provided an ancient wine reference sample for detecting tartaric acid/tartrate and other wine constituents. As a known wine residue from antiquity, which had undergone aging processes, its composition served to assess whether other less definitive vessels, such as Abydos jar no. 156, once held grape wine. Modern reference samples of tartaric acid/tartrate and other wine constituents provided additional controls.
As expected, the Gebel Adda residue tested positive for tartaric acid/tartrate according to the 3 methods. Its FTIR spectometry spectrum also showed the characteristic and very specific absorptions for tartaric acid and a tartrate salt by location, shape, relative intensity, and multiplicity. These results were directly comparable to the residue in Abydos jar no. 156, except that the latter's FTIR spectrometry spectrum lacked the sharp intense carbonyl peak of the acid, with a shoulder, at 1,720–1,740 cm−1. In other words, although the tartaric acid in the Abydos jar had been converted wholly to the calcium salt, the younger Gebel Adda residue still retained the acid.
Another important inference could be drawn from the FTIR spectrometry spectra: other unidentified hydrocarbon-rich compounds must be present in the 2 ancient samples because of intense peaks around 2,900 cm−1 and many additional subsidiary absorptions in the “fingerprint region” from 1,550–800 cm−1 that were not attributable to tartaric acid/tartrate. Some of these components were later identified as probable tree resin compounds in the Abydos sample by HPLC, using a UV detector. This method, not yet available to us when the Gebel Adda sample was analyzed, has been very useful in our ongoing analytical program because it enables chromatographic separation and more precise chemical identifications. Together with GC/MS, these techniques have enabled us to substantiate our earlier finding that tree resins, both pine and terebinth, were often added to ancient wines.
Between 1990 and the present, many more samples from putative ancient wine jars have been analyzed, ranging in date from ca. 5400 B.C. through the Byzantine period and from sites throughout the Middle East and Greece (2, 17). A group of 9 samples from Pharaoh Amenhotep III's palace of Malkata (18), dated midway (ca. 1390–1350 B.C.) between the Abydos and Gebel Adda samples, is especially important in providing another set of ancient wine reference samples and additional evidence for resinated wine from this country. The analyzed amphorae bore black-inked inscriptions on their shoulders, which read like a modern wine label, providing the year of the pharaoh when the wine had been made, the name and location of the estate in the Nile Delta, the name of the winemaker, and even quality notes. Our chemical results from these samples, attesting to a resinated wine, were closely comparable to those for the Abydos and Gebel Adda jars.
Even when the archaeological, inscriptional, and chemical evidence are as congruent as they are for the Abydos, Malkata, and Gebel Adda samples, one can remain skeptical. As is generally true of historical sciences, the identification of any ancient natural product is limited by the database and the impossibility of replicating past events. An archaeologist might ask for samples from better archaeological contexts, question whether a particular vessel type was intended for wine, and propose alternative interpretations of the available evidence. A chemist will want ever-more definitive identifications of tartaric acid/tartrate and the tree resin compounds as chemical instrumentation is improved.
Liquid Chromatography Tandem Mass Spectrometry Analyses.
A major breakthrough in the detection of tartaric acid/tartrate was recently achieved by University of Barcelona researchers (19). Using liquid chromatography tandem mass spectrometry (LC/MS/MS), they analyzed ancient wine reference samples from Old and New Kingdom Egyptian wine vessels, viz., (i) a jar from the tomb of Pharaoh Semerkhet of Dynasty 1, who reigned about 150 years after Scorpion I, near the establishment of the royal winemaking industry in the Nile Delta; (ii) an amphora from the tomb of Pharaoh Tutankhamun, who reigned shortly after Amenhotep III; and (iii) an amphora said to contain wine from the Nile Delta of approximately the same date as the Tutankhamun amphora or somewhat later in early Dynasty 19.
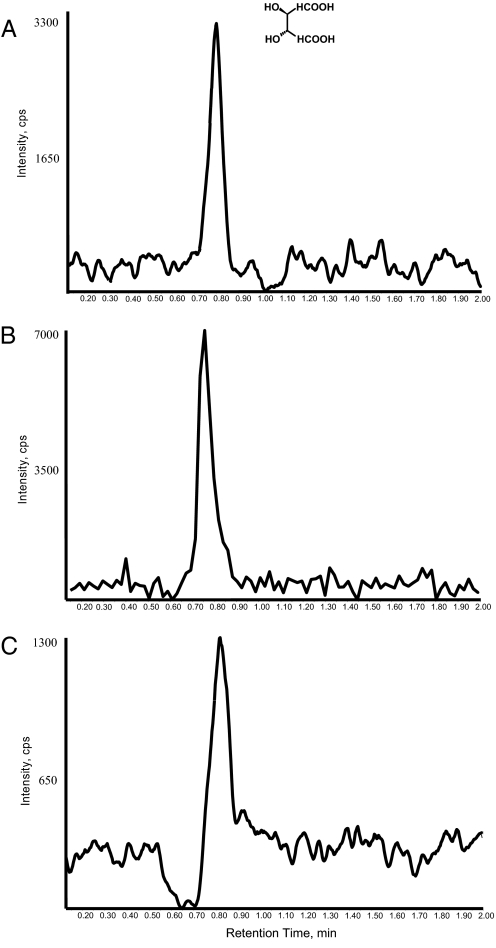
The Barcelona study left little doubt that tartaric acid/tartrate was preserved intact in ancient Egyptian jars, whether as an adherent residue or absorbed into the pottery fabric and held by polar clay constituents. It is unequivocally identified by using the multiple reaction monitoring (MRM) mode of the LC/MS/MS (Fig. 3). In brief, tartaric acid (Mr = 150.1) is ionized when it comes off an LC column at a specific time in the first cell of the quadrupole mass spectrometer, where it is mass-filtered. The deprotonated molecular ion is then fragmented in a collision cell, and the daughter ions are again filtered by a second quadrupole. Tartaric acid is identified based on discrete fragmentation products that are produced and detected.
Fig. 3.
MRM LC/MS/MS traces of L-tartaric acid. (A) Standard solution corresponding to m/z 149→87 molecular ion fragmentation. (B and C) Chromatograms for the aqueous extracts of the ancient Gebel Adda (Fig. 2) and Abydos (Fig. 1) samples, respectively.
In our ongoing collaboration to develop biomolecular archaeological techniques with the Scientific Services Division of the Tax and Trade Bureau, the primary government laboratory for the analysis of alcoholic beverages in the United States, we decided first to reanalyze the Abydos and Gebel Adda samples by LC/MS/MS. The methodological issue was clearcut: was tartaric acid/tartrate, in fact, present in these samples—one from a relatively late wine amphora representing an ancient wine reference sample and the other from a much more ancient jar with few clues of its original contents? If so, our earlier conclusions, based on 3 less precise methods and general archaeological considerations, would be greatly strengthened. The samples had been stored under climate-controlled conditions in the dark in the interim.
Our LC/MS/MS analyses of the 2 samples followed the general methodology of Guasch-Jané et al. (19), with some modifications. The monitored MRM m/z 149→87 transition corresponds to the loss of COOH and OH fragments of the [M-H]¯ molecule of tartaric acid. As can be seen in Fig. 3, both the Abydos and Gebel Adda samples gave peaks for tartaric acid at a retention time of 0.75 min, which matched a 0.1-μg/mL (0.1 ppm) standard of tartaric acid. The results of analyses on similar extracts of an ancient pottery blank and aqueous controls, run before and after each analysis, were negative (data not shown). An ancient bowl rim from a site in Jordan (Tell el-Fukhar, Late Bronze II, ca. 1400–1200 B.C., no. 763) served as the pottery blank. It had been made of local clay according to instrumental neutron activation analysis (INAA) (20) and had a very low probability of having originally contained wine or another grape product.
Headspace Solid Phase Microextraction and Thermal Desorption GC/MS Analyses
Having confirmed tartaric acid/tartrate by LC/MS/MS in the Abydos and Gebel Adda samples, we then analyzed them by headspace solid phase microextraction (SPME) and thermal desorption (TD) GC/MS. The goal was to discover whether the Egyptian wines contained any plant additives, which had been implied by our earlier analyses.
Headspace SPME and TD GC/MS have great utility in biomolecular archaeology because of their high sensitivity and selectivity for volatile compounds of interest (8, 21). SPME extraction efficiency and sensitivity are considerably increased by dissolving or suspending the solid sample in an aqueous saline solution. Moreover, the methods require only milligram quantities of valuable archaeological samples, and analyses can be performed rapidly without extraction in an organic solvent.
Compounds were identified in the Abydos and Gebel Adda samples (Table S1) by retention time and/or matches to a mass spectral library of more than 160,000 samples (NIST05). Illustrative SPME experimental data are provided in Figs. S2 and S3.
Compounds detected in the ancient pottery blank are assumed to derive either from ancient and/or modern “background contaminants,” attributable to groundwater percolation or sample handling (e.g., plasticizers and antioxidants from plastic, including compounds in the phthalate family and 3,5-di-tert-butyl-4-hydroxybenzaldehyde). Possibly, some of the low-boiling compounds up to hexenal were also contaminants; however, it is more likely that they were preserved within the ionic clay structure. Any ancient ethanol would have been metabolized by microorganisms.
A thorough search of the chemical literature [using SciFinder Scholar of the Chemical Abstract Services, American Chemical Society; Dr. Duke's Phytochemical and Ethnobotanical Databases (http://www.ars-grin.gov/duke/) of the U.S. Department of Agriculture, Agricultural Research Service; the chemical database of the Amber Research Laboratory of Vassar College (http://cima.ng-london.org.uk/arl/); and other bioinformatics tools (22)] enabled several groups of probable ancient compounds to be distinguished in the Abydos and Gebel Adda samples.
As might be anticipated for samples that tested positive for tartaric acid/tartrate, constituents of modern grape wine (23) are very well represented in one or both of the ancient samples, including alcohols, acids, esters, aldehydes, fatty acid derivatives, and terpenoids. Although benzaldehyde, 2-ethyl-1-hexanol, nonanal, and ethyl palmitate occur in wine, they might also be contaminants. Excellent preservation of the more ancient Abydos sample, in particular, is implied by these findings.
For the Abydos sample, 3 herbs—savory (Satureja), Artemisia seibeni (a member of the wormwood family), and blue tansy (Tanacetum annuum)—could account for the combined presence of 8 terpenoid compounds (labeled 2 in Table S1): linalool, camphor, borneol, L-menthol, alpha-terpineol, carvone, thymol, and geranyl acetone. The same compounds, except for geranyl acetone, occur in an additional 7 herbal genera, including balm (Melissa), senna (Cassia), coriander (Coriandrum), germander (Teucrium), mint (Mentha), sage (Salvia), and thyme (Thymus/Thymbra).
It may be significant that only Satureja, Cassia, and Salvia are possibly native to Egypt. Because A. seibeni and T. annuum are probably native to Iran and Morocco, respectively, they are unlikely to have been traded or transplanted to Egypt at this early date. Of the remaining 7 plants, all are arguably native to the southern Levant (24) (for coriander, see ref. 25). Today, they grow in the vicinity of the areas where the Abydos wine was made according to the INAA results (26), viz., the Jordan Valley, the uplands to its east and west, and the Mediterranean littoral near Gaza.
For the Gebel Adda sample, only rosemary (Rosmarinus officinialis), a member of the mint family (Lamiaceae or Labiatae), could explain fenchone, camphor, borneol, cuminaldehyde, and vanillin (labeled 3 in Table S1). Rosemary, which can tolerate relatively dry conditions, grows both in the southern Levant and Egypt (27).
As yet, unique biomarkers for the herbs that might have been added to the Abydos and Gebel Adda wines have not been delimited. Moreover, many of the compounds that we identified are found in other plants and herbs of the eastern Mediterranean region. For example, camphor, borneol, carvone, and thymol are reported in yarrow (Achillea); the same compounds, with the exception of borneol, in wild fennel (Foeniculum vulgare); linalool and thymol in marjoram; and only borneol in oregano. Two herbs of Egyptian origin should also be noted: Ambrosia maritima contains camphor and carvone and Conyza dioscorides has camphor and linalool. Linalool, alpha-terpineol, and geranyl acetone also occur in grape wine, and camphor and borneol are found in pine resin, in addition to rosemary and many other herbs. Thus, although many different permutations of the detected compounds deriving from various natural products might be proposed, the most straightforward and simplest explanation is that one or more of the herbs, which can account for the greatest number of relatively rare compounds in the Abydos and Gebel Adda samples, were the likely additives to the wines.
Both ancient samples also contained compounds found in pine resin (labeled 4 in Table S1). Our previous HPLC results for the Abydos sample had already pointed to a tree resin additive, which we had tentatively identified as terebinth. However, a diterpenoid compound specific to pine—retene, an oxidative product of abietic acid (17)—was observed by TD GC/MS. Conventional GC/MS analysis of the Abydos sample also revealed the presence of additional oxidative products of the acid, viz., dehydroabietatic acid and 7-oxodehydroabietic acid. Triterpenoids characteristic of terebinth resin (28) were absent.
The Gebel Adda sample had not been analyzed for tree resins in our initial study. In addition to retene, methyl dehydroabietate was detected by SPME and TD GC/MS. Methyl dehydroabietate (Fig. S4) points to the pine resin having been processed by heat to the tar, which is consistent with the entire interior of the amphora having been lined with a dark coating as a sealant.
Discussion and Conclusions
By employing LC/MS/MS to analyze the Abydos and Gebel Adda samples, 2 Egyptian samples separated in time by over 3,500 years, we have confirmed our earlier methodology for identifying tartaric acid/tartrate, the key biomarker for wine and grape products. By demonstrating that 3 independent techniques—FTIR spectrometry, HPLC, and a Feigl spot test—attested to the presence of tartaric acid/tartrate in the ancient samples and by making statistical comparisons with large IR and UV databases of ancient wine reference samples for wine and modern standards, we were reasonably confident that our results would hold up. Moreover, archaeological considerations—admittedly at a lower level of probability—supported a “wine hypothesis” as best accounting for the available evidence.
Certainly, a wine hypothesis stands or falls on whether tartaric acid/tartrate can be detected in the ancient residues. As analytical instrumentation improves, chemists and archaeologists alike should continually test previous results, cull out any “false-positives,” and generate data as they are made available by excavation or analytical techniques. Our analyses of the Abydos and Gebel Adda samples provide an illustrative example of how this can be accomplished in the constantly evolving field of biomolecular archaeology.
The reanalysis of the Abydos and Gebel Adda samples by SPME and TD GC/MS had another welcome unintended consequence in addition to confirming the presence of tartaric acid/tartrate. These sensitive versatile techniques enabled us to make a start on the chemical detection of ancient Egyptian herbal medicines. Much work remains to be done in refining and substantiating such findings, but ancient wine and other alcoholic beverages are known to be an excellent means to dissolve and administer herbal concoctions externally and internally (29). Indeed, before modern synthetic medicines became available, alcoholic beverages were the universal palliative.
Chemical analysis opens a door onto early Egyptian pharmacology by providing contemporaneous data of plant additives in ancient alcoholic beverages. In particular, adding a tree resin to wine, principally to protect against wine disease as already noted, was one of the most popular and widespread practices throughout the ancient world. Resinated wines were still being made in the Middle Ages according to the extensive agricultural and medical compilations based on classical writings collectively known as the Geoponica (e.g., ref. 30). An appreciation for the medicinal value of tree resins was not restricted to the Middle East and Mediterranean. In the Yellow River valley of China, probable resins of China fir, in the Elemi family of fragrant trees, and other native species were added to fermented beverages made from rice, millet, and fruits as early as 7000 B.C., according to multiple analyses by FTIR spectrometry, GC/MS, LC/MS, isotope analysis, and spot tests (8).
Until now, textual sources, in particular a series of medical papyri, have provided the primary evidence for the ancient Egyptian materia medica, which was renowned in the ancient world (11, 12, 31). The majority of the papyri belong to the New Kingdom, including the longest one, the 108-page Ebers papyrus, dated to ca. 1550 B.C. Several papyri have been dated as early as the mid-12th Dynasty, ca. 1850 B.C., and the Egyptian word for “physician” (swnw), which involved diagnosing disease and often included treatment with herbal remedies, occurs as early as Dynasty 3, ca. 2650 B.C. (12). A later tradition, which is not independently supported, states that Djer, the second pharaoh of Dynasty 1, was also a swnw (12); this is an intriguing possibility that may relate to the chemical findings from tomb U-j, because Djer ruled shortly after Scorpion I, ca. 3100 B.C., and his tomb at Abydos is close to U-j.
The prescriptions in the papyri, numbering more than 1,000, present a detailed picture of the ancient Egyptian pharmacopeia, even if more than 80% of some 160 hieroglyphic plant names defy translation. Many vegetables and fruits, including garlic, onion, celery, Cyperus grass tubers, watermelon, fig, moringa, persea, and zizyphus, for example, figure prominently as ingredients in the formulations; however, by far the most numerous are alcoholic beverages (wine and beer), tree resins (e.g., terebinth, pine, frankincense, myrrh, fir), and herbs of all kinds (e.g., bryony, coriander, cumin, mandrake, dill, aloe, wormwood). These plants and their exudates are described as being macerated; mixed together; steeped as a decoction or infusion in wine or vinegar, beer, honey, milk, oil, and/or water; strained; and administered for specific ailments (e.g., laxatives, emollients, expectorants, anthelmintics, analgesics, diuretics, aphrodisiacs). Many of the ingredients are still part of the herbal medical tradition of modern Egypt.
Among the most probable herbal additives to the Abydos wine, only coriander is known by its ancient Egyptian name (š3w). Eight baskets (half a liter) of coriander mericarp in the tomb of Tutankhamun underline its importance in ancient Egyptian culture and medicine (32). Coriander is explicitly listed in several medical prescriptions (11). Thus, stomach problems called for drinking a beer mixed with this herb, bryony, flax, and dates. For the treatment of blood in the stool, it was to be grated and mixed with chaste-tree and another unidentified fruit, infused into beer, strained, and drunk. For treating herpes, an external salve was prepared from coriander seeds, myrrh, and fermented honey.
The other herbal additive possibilities for the Abydos wine—balm, senna, germander, mint, sage, savory, and thyme—are yet to be certainly identified by their Egyptian names, although more intensive linguistic study promises their elucidation. For example, quite possibly, ‘k3y in a recipe for kyphi (11), a well-known temple fumigant and beverage additive, is to be translated as “mint.” According to inscriptions in the late first millennium B.C. temples at Edfu and Philae, kyphi was prepared by grinding and sieving equal amounts of sweet flag (Acorus calamus), aromatic rush (Andropogon schoenanthus), terebinth resin, cassia, mint, and possibly aspalathus. This powder was then combined with separately prepared concoctions of wine with juniper berries, Cyperus and other plants, raisins and wine, and frankincense and honey. The addition of finely ground myrrh completed the recipe.
This literary attestation of an ancient Egyptian resinated herbal wine has only been partly borne out by our analysis of the Abydos sample, because, apart from the wine itself and possibly mint, the other plant components of kyphi were not chemically attested. We are not arguing that kyphi, per se, had been developed and used as early as 3150 B.C. Rather, our contention is that plant additives, including various herbs and tree resins, were already being dispensed via alcoholic beverages millennia earlier than the Edfu and Philae temple inscriptions.
The most probable herbal additives to the Abydos wine, on current evidence, share another important feature in common: nearly all were domesticated or cultivated in the southern Levant in advance of their introduction into Egypt (33). This circumstance is in accord with the wine in the Abydos jars having most likely been made in this region. Beginning ca. 3000 B.C., as the domesticated grapevine was transplanted to the Nile Delta, one may reasonably hypothesize that some southern Levantine herbs accompanied or soon followed it into the gardens and fields of the country. These developments considerably expanded the Egyptian pharmacopeia.
Other researchers have begun to report botanical and chemical evidence for herbal concoctions in alcoholic beverages. From about the same time as the Abydos wine, native rosemary and mint, together with thyme, were added to a fermented emmer wheat-barley beverage at Genó in Spain (34). Wild rosemary was also an ingredient, along with bog myrtle, yarrow, and other herbs, in gruit, the principal bittering agent in early medieval European beer (35).
Other archaeochemical and archaeobotanical evidence for rosemary is of special interest, because only this herb had a high probability of having been added to the Gebel Adda resinated wine. Unfortunately, the ancient Egyptian word for the herb is unknown; thus, its place in Egyptian medicine cannot be tracked. It is known that rosemary was a popular food flavorant in Roman and Byzantine times (36) when the Gebel Adda resinated wine was produced. Moreover, it contains numerous antioxidant compounds (e.g., rosmarinic acid, carnosol) that have potentially wide-ranging medical benefits (37–38).
Although much remains to be discovered about ancient Egyptian herbal wines, our chemical findings from Abydos and Gebel Adda, together with the results from Malkata, attest to their great antiquity and importance from the country's initial unification under the pharaohs and continuing for millennia.
Further refinements in analytical techniques will undoubtedly reveal other important compounds in ancient wines. For example, the Gebel Adda sample was recently analyzed by Fourier-transform ion cyclotron resonance/MS. Signals consistent with the structure of resveratrol, the well-known antioxidant that has anticancer effects and has been shown to extend life in many organisms, were detected. Follow-up confirmatory studies by ultraperformance liquid chromatography/MS are now being carried out (P. Schmitt-Kopplin, R. Gougeon, personal communication, 2009).
Supplementary Material
Acknowledgments.
We thank Abdul Mabud, Jeffrey Ammann, and Vanessa Kinton of the Tax and Trade Bureau's Scientific Services Division for contributing technical expertise and ongoing support. Günter Dreyer of the German Archaeological Institute in Cairo advised on the archaeology of the Abydos tomb. W. Christian Petersen assisted in the GC/MS analyses at the Scientific Research and Analysis Laboratory of Winterthur Museum and Country Estate and consulted on chemical matters, together with Olga Jáuregui of the University of Barcelona and Theodore Davidson of the Museum Applied Science Center for Archaeology. Naomi F. Miller of the Museum Applied Science Center for Archaeology and Mark Nesbitt of the Royal Botanic Gardens, Kew, aided in the interpretation of the archaeobotanical data. Recent work using Fourier-transform ion cyclotron resonance/MS and ultraperformance liquid chromatography/MS is part of a collaboration with Philippe Schmitt-Kopplin of the Helmholtz Zentrum München and Régis Gougeon of the Institut Universitaire de la Vigne et du Vin-Jules Guyot, Dijon. Detailed information on the extraction methods for the LC/MS/MS analyses and on the experimental conditions for the SPME, TD, and liquid-injection GC/MS analyses is provided in SI Text. A grant from the Abramson Cancer Center of the University of Pennsylvania provided partial financial assistance.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811578106/DCSupplemental.
References
- 1.Johns T. With Bitter Herbs They Shall Eat It: Chemical Ecology and the Origins of Human Diet and Medicine. Tucson, AZ: Univ of Arizona Press; 1990. [Google Scholar]
- 2.McGovern PE. Ancient Wine: The Search for the Origins of Viniculture. Princeton: Princeton Univ Press; 2007. [Google Scholar]
- 3.Dudley R. Ethanol, fruit ripening, and the historical origins of human alcoholism in primate frugivory. Integr Comp Biol. 2004;44:315–323. doi: 10.1093/icb/44.4.315. [DOI] [PubMed] [Google Scholar]
- 4.Dudley R. Evolutionary origins of human alcoholism in primate frugivory. Q Rev Biol. 2000;75:3–15. doi: 10.1086/393255. [DOI] [PubMed] [Google Scholar]
- 5.Stephens D, Dudley R. The drunken monkey hypothesis. Nat Hist. 2004;113:40–44. [Google Scholar]
- 6.Wiens F, et al. Chronic intake of fermented floral nectar by wild treeshrews. Proc Natl Acad Sci USA. 2008;105:10426–10431. doi: 10.1073/pnas.0801628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nesse RM, Berridge KC. Psychoactive drug use in evolutionary perspective. Science. 1997;278:63–67. doi: 10.1126/science.278.5335.63. [DOI] [PubMed] [Google Scholar]
- 8.McGovern PE, et al. Fermented beverages of pre- and proto-historic China. Proc Natl Acad Sci USA. 2004;101:17593–17598. doi: 10.1073/pnas.0407921102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGovern PE, Voigt MM, Glusker DL, Exner LJ. Neolithic resinated wine. Nature. 1996;381:480–481. [Google Scholar]
- 10.Dillehay TD, et al. Monte Verde: seaweed, food, medicine, and the peopling of South America. Science. 2008;320:784–786. doi: 10.1126/science.1156533. [DOI] [PubMed] [Google Scholar]
- 11.Manniche L. An Ancient Egyptian Herbal. Austin, TX: Univ of Texas Press; 1989. pp. 57–58.pp. 94pp. 120pp. 144pp. 159–162. [Google Scholar]
- 12.Nunn JF. Ancient Egyptian Medicine. Norman, OK: Univ. of Oklahoma Press; 1996. pp. 24–42.pp. 121–122.pp. 124pp. 131–132.pp. 136–162.pp. 211–212. (Appendix B) [Google Scholar]
- 13.Dreyer G. Umm el-Qaab I: Das prädynastische Königsgrab U-j und seine frühen Schriftzeugnisse, Archäologische Veröffentlichungen. Vol. 86. Mainz, Germany: P von Zabern; 1998. [Google Scholar]
- 14.McGovern PE, Glusker DL, Exner LJ. The organic contents of the Tomb U-j Syro-Palestinian type jars: resinated wine flavored with fig. In: Hartung U, editor. Umm el-Qaab II: Importkeramik aus dem Friedhof U in Abydos (Umm el-Qaab) und die Beziehungen ägyptens zu Vorderasien im 4. Jahrtausend v. Chr. Mainz, Germany: P von Zabern; 2001. pp. 399–403. [Google Scholar]
- 15.Cavalieri D, McGovern PE, Hartl DL, Mortimer R, Polsinelli M. Evidence for S. cerevisiae fermentation in ancient wine. J Mol Evol. 2003;57:S226–S232. doi: 10.1007/s00239-003-0031-2. [DOI] [PubMed] [Google Scholar]
- 16.McGovern PE, Badler V, Michel RH. MASCA Research Papers in Science and Archaeology. Drink and be merry!: Infrared spectroscopy and ancient Near Eastern wine. In: Biers WR, McGovern PE, editors. Organic Contents of Ancient Vessels: Materials Analysis and Archaeological Investigation. Vol 7. Philadelphia: MASCA, University of Pennsylvania Museum, University of Pennsylvania; 1990. pp. 25–36. [Google Scholar]
- 17.Beck CW, Stout E, Lee KC, Chase AA, DeRosa N. Analysis of organic remains in the fabric of Minoan and Mycenaean pottery sherds by gas chromatography-mass spectrometry. In: Tzedakis Y, Martlew H, Jones MK, editors. Archaeology Meets Science: Biomolecular Investigations in Bronze Age Greece. Oxford: Oxbow Books; 2008. pp. 12–47. [Google Scholar]
- 18.McGovern PE. Wine of Egypt's golden age: An archaeochemical perspective. J Egypt Archaeol. 1997;83:69–108. [Google Scholar]
- 19.Guasch-Jané MR, Ibern-Gómez M, Andrés-Lacueva C, Jáuregui O, Lamuela-Raventós RM. Liquid chromatography with mass spectrometry in tandem mode applied for the identification of wine markers in residues from ancient Egyptian vessels. Anal Chem. 2004;76:1672–1677. doi: 10.1021/ac035082z. [DOI] [PubMed] [Google Scholar]
- 20.McGovern PE. A ceramic sequence for northern Jordan: An archaeological and chemical perspective. In: Bisheh G, Zaghloul M, Kehrberg I, editors. Studies in the History and Archaeology of Jordan VI. Amman, Egypt: Department of Antiquities; 1997. pp. 421–425. [Google Scholar]
- 21.Hamm S, Bleton J, Connan J, Tchapla A. A chemical investigation by headspace SPME and GC-MS of volatile and semi-volatile terpenes in various olibanum samples. Phytochemistry. 2005;66:1499–1514. doi: 10.1016/j.phytochem.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 22.Seidel A, editor. Kirk-Othmer Encyclopedia of Chemical Technology. Hoboken, NJ: Wiley-Interscience; 2004. Available at http://mrw.interscience.wiley.com/emrw/9780471238966/home/ [Google Scholar]
- 23.Clarke RJ, Bakker J. Wine Flavour Chemistry. Oxford: Blackwell; 2004. [Google Scholar]
- 24.Feinbrun-Dothan N. In: Flora Palaestina, Part 3: Ericaceae to Compositae. Zohary M, editor. Jerusalem: Israel Academy of Sciences and Humanities; 1978. [Google Scholar]
- 25.Zohary D, Hopf M. Domestication of Plants in the Old World: The Origin and Spread of Cultivated Plants in West Asia, Europe, and the Nile Valley. Oxford: Oxford Univ Press; 2000. pp. 205–206. [Google Scholar]
- 26.McGovern PE. The origins of the tomb U-j Syro-Palestinian type jars as determined by neutron activation analysis. In: Hartung U, editor. Umm el-Qaab II: Importkeramik aus dem Friedhof U in Abydos (Umm el-Qaab) und die Beziehungen ägyptens zu Vorderasien im 4. Jahrtausend v. Chr. Mainz, Germany: P von Zabern; 2001. pp. 407–416. [Google Scholar]
- 27.Davis PH. Flora of Turkey and the East Aegean Islands. Vol 7. Edinburgh: Edinburgh Univ Press; 1982. [Google Scholar]
- 28.Mills JS, White R. The Organic Chemistry of Museum Objects. Oxford: Butterworth-Heinemann; 1994. [Google Scholar]
- 29.Majno G. The Healing Hand: Man and Wound in the Ancient World. Cambridge, MA: Harvard Univ Press; 1975. [Google Scholar]
- 30.Owen T, translator. Geoponika: Agricultural Pursuits. London: 1805–1806. printed for the author. [Google Scholar]
- 31.Grapow H. Grundriss der Medizin der alten Ägypter. Berlin: Akademie; 1954–1973. [Google Scholar]
- 32.Germer R. Die Pflanzenmaterialien aus dem Grab des Tutanchamun. Hildesheim, Germany: Gerstenberg; 1989. [Google Scholar]
- 33.Germer R. Ancient Egyptian pharmaceutical plants and the eastern Mediterranean. In: Jacob I, W, editors. The Healing Past: Pharmaceuticals in the Biblical and Rabbinic World. Leiden, Germany: E.J. Brill; 1993. pp. 69–80. [Google Scholar]
- 34.Juan-Tresserras J. La cerveza prehistórica: investigaciones arqueobotánicas y experimentales. In: Maya JL, Cuesta F, Lôpez Cachero J, editors. Genó: un poblado del Bronce Final en el Bajo Segre (Lleida) Barcelona: University of Barcelona; 1998. pp. 239–252. [Google Scholar]
- 35.Nelson M. The Barbarian's Beverage: A History of Beer in Ancient Europe. London: Routledge; 2005. [Google Scholar]
- 36.Dalby A. Siren Feasts: A History of Food and Gastronomy in Greece. London: Routledge; 1996. p. 190. n. 21. [Google Scholar]
- 37.Lai PK, Roy J. Antimicrobial and chemopreventive properties of herbs and spices. Curr Med Chem. 2004;11:1451–1460. doi: 10.2174/0929867043365107. [DOI] [PubMed] [Google Scholar]
- 38.Aggarwal MM, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.