Abstract
Intracellular signaling by which pancreatic β-cells synthesize and secrete insulin in control of glucose homeostasis is not fully understood. Here we show that Shp2, a cytoplasmic tyrosine phosphatase possessing 2 SH2 domains, coordinates signaling events required for insulin biosynthesis in β-cells. Mice with conditional ablation of the Shp2/Ptpn11 gene in the pancreas exhibited defective glucose-stimulated insulin secretion and impaired glucose tolerance. Consistently, siRNA-mediated Shp2-knockdown in rat insulinoma INS-1 832/13 cells resulted in decreased insulin production and secretion despite an increase in cellular ATP. Shp2 modulates the strength of signals flowing through Akt/FoxO1 and Erk pathways, culminating in control of Pdx1 expression and activity on Ins1 and Ins2 promoters, and forced Pdx1 expression rescued insulin production in Shp2-knockdown β-cells. Therefore, Shp2 acts as a signal coordinator in β-cells, orchestrating multiple pathways controlling insulin biosynthesis to maintain glucose homeostasis.
Keywords: Pdx1, signal transduction, diabetes, insulin secretion, gene expression
Pancreatic β-cell dysfunction contributes to the development of all forms of diabetes (1). Although autoimmune destruction of β-cells is a primary cause of type 1 diabetes, type 2 diabetes is characterized by the relative inability of β-cells to secrete sufficient insulin to compensate for peripheral insulin resistance. However, molecular defects underlying β-cell failure are not fully understood. Genetic analyses of cell type-specific gene-knockout mice suggest that insulin and insulin-like growth factor 1 (IGF1) signaling pathways play critical roles in controlling β-cell mass and functions. Mice lacking the insulin receptor in β-cells (βIRKO) inadequately respond to glucose stimulation, and they show progressive glucose intolerance and reduced β-cell mass with aging (2). Selective deletion of the IGF1 receptor in β-cells leads to glucose intolerance and hyperinsulinemia without affecting the β-cell mass (3). Mice lacking both insulin and IGF1 receptors in β-cells develop severe diabetes shortly after birth due to markedly reduced β-cell mass and insulin content (4).
Several intracellular signaling molecules have been identified as critical players downstream of insulin and IGF1 receptors in β-cells. Mice deficient for insulin receptor substrate 2 (IRS2) develop diabetes due to peripheral insulin resistance and pancreatic β-cell failure (5). Selective deletion of IRS2 in β-cells leads to reduced islet mass, glucose intolerance, and hypothalamic dysfunction, reinforcing the role of IRS2 in β-cell maintenance and functions (6, 7). β-Cell ablation of 3-phosphoinositide-dependent protein kinase 1 (Pdk1) leads to development of diabetes due to loss of islet mass in mice (8). Phospho-Akt levels are reduced in β-cells lacking insulin and IGF1 receptors, accompanied by increased apoptosis (4). Activated Akt can phosphorylate FoxO1, resulting in nuclear exclusion of FoxO1 and derepression of Pdx1 expression (4, 9). Stimulation of β-cells by glucose and insulin also activates Erk1/2 kinases, which phosphorylate and activate Pdx1, MafA, Beta2, E2A, and NFAT transcription factors involved in control of insulin promoters (10). The AMP-activated protein kinase (AMPK) and mTOR pathways are also implicated in glucose and insulin signaling in β-cells (11). β-Cell deletion of calcineurin b1 in mice reveals a critical role of calcineurin/NFAT signaling in β-cell proliferation and functions (12). Therefore, multiple signaling pathways are involved in mediating glucose-stimulated insulin production and secretion in β-cells.
A critical unanswered question is how these different pathways are coordinately regulated in β-cell response to glucose and insulin signals. Shp2 is a widely expressed protein tyrosine phosphatase (PTP) with 2 Src homology 2 (SH2) domains at the N terminus (13, 14). Shp2 is implicated in pathways activated by receptors for growth factors, cytokines, and hormones. In particular, Shp2 binds to tyrosine-phosphorylated IRS proteins and operates in insulin-responsive tissue cells (15, 16). However, whether Shp2 plays a biological role in insulin-producing β-cells is unclear. We generated mutant mice in which Shp2 is selectively deleted in the pancreas (Shp2Panc−/−). Shp2Panc−/− mice exhibit reduced glucose-stimulated insulin secretion and progressively impaired glucose tolerance. We have performed extensive genetic and molecular analyses on Shp2Panc−/− mice and Shp2-knockdown β-cells, which identify Shp2 as a coordinator of multiple signals controlling insulin production in β-cells.
Results
Pancreatic Deletion of Shp2 Induces Glucose Intolerance.
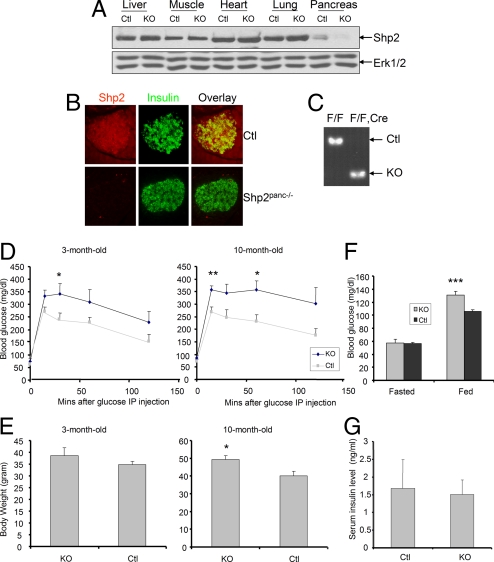
Immunoblot and immunostaining assays detected Shp2 expression in the pancreas (Fig. 1 A and B). Shp2 was abundantly expressed in insulin+ β-cells in pancreatic islets, as revealed by coimmunostaining (Fig. 1B). To determine Shp2 function in the pancreas, we generated a mouse line with the Shp2/Ptpn11 gene deleted in the pancreas (Shp2flox/flox:Pdx1-Cre or Shp2Panc−/−) by crossing Shp2flox/flox mice with a transgenic mouse line expressing Cre recombinase driven by the Pdx1 promoter, which is active in pancreatic progenitor cells and mature β- and δ-cells (17, 18). Notably, Shp2 expression was dramatically reduced in pancreas dissected from Shp2Panc−/− mice, whereas no differences were detected in the liver, muscle, heart, and lung lysates between control (Ctl) and mutant (KO) mice (Fig. 1A). Immunostaining confirmed Shp2 ablation in pancreatic islet (Fig. 1B), and RT-PCR analysis of mRNA extracted from pancreatic islets also showed efficient deletion of Shp2 in islets of Shp2Panc−/− mice (Fig. 1C).
Fig. 1.
Generation and metabolic analysis of pancreas-specific Shp2-knockout mice. (A) Immunoblot of Shp2 protein in pancreas, liver, muscle, heart, and lung dissected from Shp2Panc−/− (KO) or control (Ctl) mice. (B) Immunofluorescence for insulin (green) and Shp2 (red) and overlay in pancreatic sections from Shp2Panc−/− and control mice. (C) RT-PCR analysis of RNA extracted from pancreatic islets of Shp2Panc−/− and control mice. The targeted deletion of Shp2 exon 4 was detected in islets from Shp2Panc−/− mice. (D) Blood glucose levels after i.p. injection of glucose (2 g/kg of body weight) into 3- and 10-month-old male Shp2Panc−/− and control mice (n = 6 per group). *, P < 0.05 versus controls; **, P < 0.01 versus controls (2-tailed Student's t test). (E) Body weights of 3- and 10-month-old male Shp2Panc−/− and control mice (n = 6 per group). (F) Fed ad libitum and fasting blood glucose levels in 3-month-old Shp2Panc−/− and control mice. (n = 18 for fed group; n = 7 for fasting group). ***, P < 0.001 versus controls (2-tailed Student's t test). (G) Serum insulin levels in random-fed, 3-month-old Shp2Panc−/− and control mice (n = 11 per group). Data are shown as means ± SEM.
Homozygous mutant (Shp2Panc−/−) mice were born at the expected Mendelian frequency and survived to adulthood with no obvious defects in pancreatic development. To determine the effect of pancreatic Shp2 deletion on glucose metabolism, we first evaluated glucose handling by Shp2Panc−/− and control mice. Mutant animals displayed age-dependent progressive impairment of glucose tolerance (Fig. 1D). At 3 months of age, Shp2Panc−/− mice exhibited significantly reduced ability to dispose of a glucose load, compared with controls, and glucose intolerance was more severe when examined at 10 months of age. Shp2Panc−/− mice showed mildly elevated body weight at 3 months, an increase that became significant compared with controls at 10 months (Fig. 1E). Significantly increased blood glucose level and mild decrease in serum insulin were detected in Shp2Panc−/− mice, compared with littermate controls (Figs. 1 F and G).
Shp2 Ablation Suppresses Insulin Biosynthesis and Secretion from β-cells in Vivo.
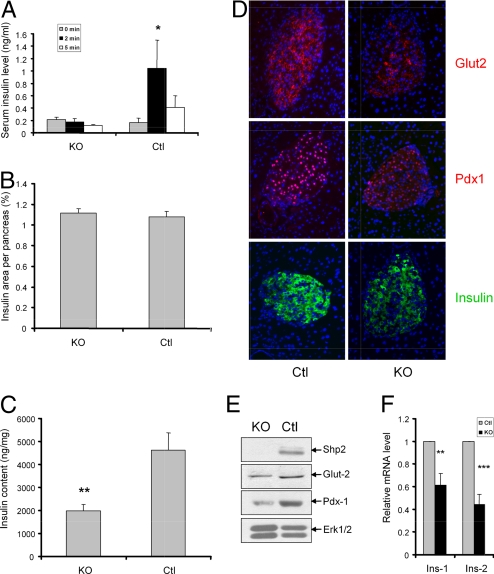
Impaired glucose tolerance seen in Shp2Panc−/− mice indicates potential defects in insulin secretion. Accordingly, we examined first-phase insulin secretion of pancreatic β-cells, and lack of acute insulin response (AIR) elicited by glucose challenge is an early pathological event of type 2 diabetes. A robust increase in serum insulin levels was detected 2 min after glucose injection in control mice, whereas serum insulin levels were not elevated in Shp2Panc−/− mice at the same time point (Fig. 2A), suggesting an ablated acute phase insulin secretion by Shp2-deficent β-cells.
Fig. 2.
Shp2 ablation inhibits glucose-stimulated insulin secretion and insulin biosynthesis in β-cells. (A) Acute-phase insulin secretion after i.p. injection of glucose (3 g/kg of body weight) into 8-month-old Shp2Panc−/− and control mice (n = 7 for Shp2Panc−/− group; n = 4 for control group). All data are presented as means ± SEM. *, P < 0.05 (2-tailed student's t test). (B) Morphometric analysis of insulin-positive area of total pancreatic area from 3-month-old Shp2Panc−/− and control mice (n = 4 per group). (C) Islet insulin content (ng/mg of total protein) in 3-month-old Shp2Panc−/− and control mice (n = 5 per group, **, P < 0.01). (D) Immunofluorescence detection of Glut2, Pdx1, and insulin in pancreatic sections from Shp2Panc−/− and control mice. Blue staining, DAPI. (E) Immunoblot analysis of protein expression of Shp2, Glut2, Pdx1, and Erk in pancreatic islets isolated from Shp2Panc−/− and control mice. (F) Real-time RT-PCR analysis of Ins1 and Ins2 mRNA levels in isolated Shp2Panc−/− and control islets. **, P < 0.01 versus controls; ***, P < 0.001. All data are presented as means ± SEM.
The defect in acute-phase insulin secretion of Shp2Panc−/− mice could result from multiple factors, such as reduced β-cell mass or decreased insulin production in β-cells. Thus, we carried out morphometric quantitation of insulin+ β-cells on paraffin-embedded pancreas sections, but observed no significant difference of β-cell areas between mutant and control mice (Fig. 2B). We next measured insulin contents in isolated islets, and found that islet insulin content was markedly decreased in mutant mice, compared with controls (Fig. 2C). Therefore, impaired insulin biosynthesis in β-cells may contribute to aberrant glucose-stimulated insulin secretion in Shp2Panc−/− animals. We further assessed expression of several β-cell-specific regulators by immunofluorescent staining of pancreatic sections and immunoblotting of isolated islets (Fig. 2 D and E). In Shp2-deficient islets, insulin staining intensity was decreased, consistent with lower insulin content detected by ELISA. Quantitative real-time RT-PCR analysis (qRT-PCR) also indicated significantly reduced Ins1 and Ins2 mRNA levels in Shp2-deficient islets, compared with controls (Fig. 2F). Pdx1 expression, which regulates insulin and Glut2 gene expression in β-cells, was reduced in mutants compared with controls as determined by immunostaining (Fig. 2D). Consistently, expression of Glut2, a glucose-sensing protein, was lower in islets of Shp2Panc−/−, compared with controls.
Shp2 Knockdown Attenuates Insulin Production and Secretion from INS-1 832/13 Cells in Vitro.
Because Shp2 was deleted in all types of pancreatic cells in Shp2Panc−/− mice, we asked whether the observed β-cell defect in insulin production was a cell-autonomous effect. We used siRNA-mediated gene knockdown to suppress Shp2 expression in the glucose-responsive insulinoma cell line INS-1 832/13. Cells were transfected with Shp2-specific or scrambled (control) siRNA by using the Amaxa nucleoporation system, which was found to be the most efficient way to knock down Shp2 gene expression in this cell line (Fig. S1) (19). Shp2 protein levels were decreased by ≈90% after introduction of Shp2-siRNA, compared with controls. Three days after transfection, cells were stimulated with either 3 mM (low) or 15 mM (high) glucose for 2 h, and culture media were collected to measure insulin secretion. Shp2 gene silencing suppressed insulin release from INS-1 832/13 cells after stimulation by both low and high levels of glucose (Fig. S2A), suggesting a positive role of Shp2 in glucose sensing and insulin secretion events in pancreatic β-cells.
Because mitochondrial ATP generation is crucial for coupling glucose metabolism with insulin secretion (20), we measured cellular ATP levels in Shp2-knockdown and control INS-1 832/13 cells. It was previously shown that knockdown of PTPMT1, a dual-specificity tyrosine phosphatase, led to elevated ATP production and increased insulin secretion (19). Surprisingly, in contrast to decreased insulin secretion, cellular ATP content was significantly higher in Shp2-knockdown than in control cells incubated with low or high levels of glucose (Fig. S2B). We further evaluated phosphorylation of AMPK, an indicator of low energy source (19). p-AMPK levels were lower in Shp2-knockdown than in control cells under basal and high glucose amounts (Fig. S2C). These results reveal a previously unknown role for Shp2 in negative regulation of ATP production.
However, the decreased insulin secretion is apparently at odds with the increased ATP generation seen in Shp2-knockdown cells. To address this discrepancy, we measured insulin content directly by acid/ethanol extraction of insulin. Consistent with results from islets isolated from Shp2Panc−/− mice, we detected significantly decreased insulin content in Shp2-knockdown cells (Fig. S2D), a result confirmed by immunostaining (Fig. S2E). Thus, the reduced insulin release in Shp2-knockdown INS-1 832/13 cells is mainly due to impaired insulin production, although a role of Shp2 in insulin exocytosis is not excluded.
Shp2 Deficiency Attenuates Signaling Through Akt/FoxO1 and Erk Pathways in β-Cells.
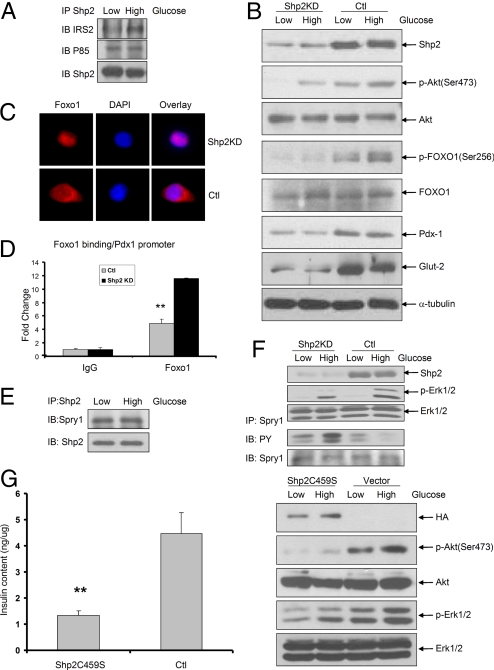
Το determine mechanisms underlying impaired insulin biosynthesis, we checked pathways that are known to operate in β-cells. Using a coimmunoprecipitation assay, we detected physical interaction between Shp2 and IRS2 in INS-1 832/13 cells, and the association was potentiated by exposure to high glucose (Fig. 3A). Shp2 also associated with the p85 subunit of phosphatidylinositol 3-kinase (PI3K) (Fig. 3A), suggesting a possible role for Shp2 in the IRS2-PI3K pathway in β-cells. In support to this idea, we observed decreased levels of p-Y-IRS2 and reduced IRS2/p85 interaction in Shp2-knockdown cells (Fig. S3). Consistently we detected impaired signals of p-Akt (Ser-473) and p-FoxO1 (Ser-256), components downstream of IRS2-PI3K, in Shp2-knockdown cells exposed to both 3 and 15 mM glucose, as compared with controls (Fig. 3B). Thus, Shp2 down-regulation leads to suppression of glucose-induced activation of Akt and FoxO1 in INS-1 832/13 cells. Consistent with the change in p-FoxO1 levels, we detected translocation of FoxO1 to the cytoplasm in control INS-1 832/13 cells, whereas FoxO1 was largely nuclear in Shp2-knockdown cells (Fig. 3C). Moreover, chromatin immunoprecipitation (ChIP) assay detected significantly increased FoxO1 binding to elements within Pdx1 promoter in Shp2-knockdown cells, as compared with controls (Fig. 3D). Previous studies showed that constitutively nuclear localization of a FoxO1 mutant repressed Pdx1 expression (9); thus nuclear localization of FoxO1 in Shp2-knockdown cells likely accounts for Pdx1 repression and impaired insulin gene transcription. Indeed, immunoblot and immunostaining analysis revealed reduced Pdx1 expression after Shp2 gene deletion or silencing (Figs. 2 D and E, 3B, and S2E).
Fig. 3.
Shp2 regulates insulin and glucose signaling in INS-1 832/13 cells. (A) Immunoprecipitation (IP) and immunoblot (IB) analysis showing association of Shp2 with IRS2 and P85. (B) Immunoblot of Shp2, p-Akt (Ser-473), Akt, p-FoxO1 (Ser-256), FoxO1, Pdx1, Glut2, and α-tubulin in Shp2-knockdown and control cells incubated with medium containing 3 or 15 mM glucose for 2 h after a 2-h preincubation with 3 mM glucose. (C) Immunofluorescence detecting FoxO1 (red), DAPI (blue), and overlay in Shp2KD and control cells. (D) ChIP assay was performed by using anti-FoxO1 antibody on Shp2-knockdown and control INS-1 832/13 cells, and analyzed by quantitative PCR to detect association of FoxO1 with Pdx1 promoter. **, P < 0.01 versus controls. (E) Immunoprecipitation and immunoblot analysis of the association of Shp2 with Sprouty 1 in low (3 mM) or high (15 mM) glucose states. (F) Immunoblot analysis of Shp2, p-Erk, and Erk in Shp2-knockdown and control cells in low (3 mM) or high (15 mM) glucose states. Immunoprecipitation and immunoblot analysis of tyrosine phosphorylation of Sprouty 1 in Shp2-knockdown and control cells in low (3 mM) or high (15 mM) glucose states. (G) INS-1 832/13 cells were transfected with vector (pcDNA3.1-HA) control or hemagglutinin epitope (HA)-tagged Shp2C459S expression construct. Cellular insulin contents were determined by ELISA. p-Akt and p-Erk signals were determined by immunoblot analysis.
Parallel with the Akt/FoxO1 pathway, activated Erk1/2 kinases have been shown to transactivate Pdx1 and insulin gene expression in β-cells (10). We detected markedly lower p-Erk1/2 signals after high glucose stimulation of Shp2-knockdown INS-1 832/13 cells, compared with control cells (Fig. 3F). Prior studies have shown that Shp2 promotes receptor tyrosine kinase signaling by inactivating Sprouty 1, a feedback inhibitor of the Erk pathway, in Drosophila (21, 22). To test whether Sprouty 1 is an Shp2 target in INS-1 832/13 cells, we first determined whether Shp2 associated with Sprouty 1 protein by coimmunoprecipitation. A complex of Shp2 with Sprouty 1 was detected in both low and high glucose conditions (Fig. 3E). Furthermore, Shp2 knockdown resulted in elevated tyrosine phosphorylation of Sprouty 1 (Fig. 3F), suggesting that Shp2 can dephosphorylate Sprouty 1 and consequently promote Erk1/2 signaling. Expression of a dominant-negative mutant of Shp2C459S suppressed insulin production, accompanied by impaired p-Erk1/2 and p-Akt signals (Fig. 3G), supporting the notion that the catalytic activity is required for Shp2 function in β-cells.
We further evaluated impacts of Shp2 deficiency on other signaling pathways in β-cells. A recent report suggests a role of Stat5 in coupling nutritional and growth hormone signals controlling insulin secretion from β-cells (23). We and others reported Shp2 promotion of prolactin-stimulated Stat5 activation in mammary gland (24, 25). However, Shp2 knockdown in INS-1 832/13 cells did not have a significant effect on growth hormone-stimulated p-Stat5 and p-Stat3 levels (Fig. S4B). PHIP1, an isoform of a PH domain-interacting protein that is highly expressed in β-cells, is found to promote IRS2-dependent signaling in β-cells (26). We asked whether Shp2 regulated signaling through IRS2-PHIP1, but found no evidence that PHIP1 was tyrosine-phosphorylated in either control or Shp2-knockdown cells exposed to different levels of glucose (Fig. S4C).
Impaired Pdx1 Expression and Activity Is Primarily Responsible for Defective Insulin Biosynthesis in Shp2-Deficient INS-1 832/13 Cells.
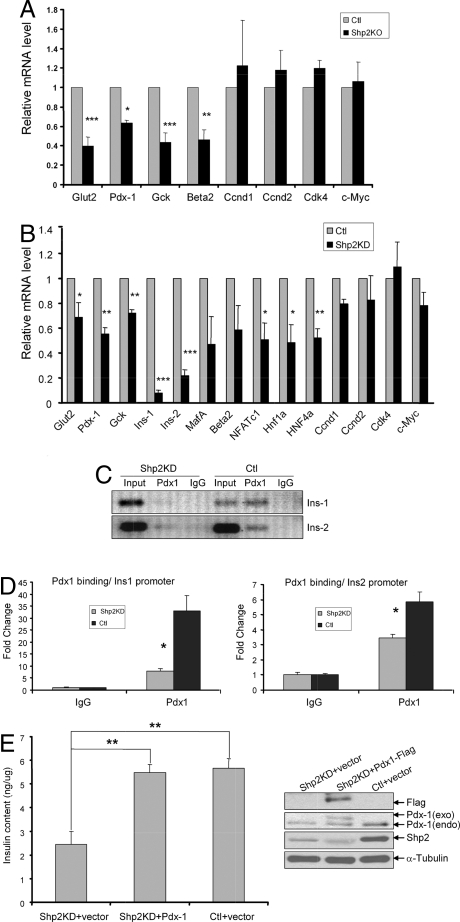
qRT-PCR analysis detected significantly reduced levels of Pdx1, Glut2, Gck, NFATc1, Hnf1a, and Hnf4a mRNAs in Shp2-knockout or knockdown β-cells compared with controls (Fig. 4 A and B), suggesting a critical role for Shp2 signaling in transcriptional regulation of insulin expression. Indeed, both Ins1 and Ins2 gene transcripts were significantly lower in Shp2-knockout or knockdown cells than in controls (Figs. 2F and 4B). We also measured mRNA levels of several cell-cycle regulatory genes, including cyclin D1 (Ccnd1), cyclin D2, cyclin-dependent kinase 4 (Cdk4), and c-myc. Expression of these genes was preserved in Shp2-knockout or knockdown cells (Fig. 4 A and B), consistent with the unaltered insulin+ β-cell area seen in Shp2-deficient pancreas (Fig. 2B) and with the preserved cell proliferation rate in Shp2-knockdown cells (Fig. S4A). In agreement with the qRT-PCR data, immunostaining and immunoblot analyses detected reduced Pdx1 contents in Shp2-knockdown cells (Figs. S2E and S3B).
Fig. 4.
Shp2 deficiency leads to reduced expression and function of Pdx1. (A and B) qRT-PCR analysis of characterized β-cell gene products and cell-cycle regulators in control and Shp2-knockout islets (A) or in control and Shp2-knockdown INS-1 832/13 cells (B). mRNA levels were normalized to cph mRNA. Data are means ± SEM. *, P < 0.05, **, P < 0.01; ***, P <.001. (C) Anti-Pdx-1 ChIP assay performed on Shp2-knockdown and control INS-1 832/13 cells and analyzed by semiquantitative PCR to detect association of Pdx1 with elements in the Ins1 and Ins2 promoters. (D) Quantitative PCR analysis compares association of Pdx1 with promoters of Ins1 and Ins2. (E) (Left) Insulin content (ng/μg of total protein) of INS-1 832/13 cells 72 h after transfection with Shp2-siRNA + empty vector, Shp2-siRNA + Pdx1 cDNA, and scrambled-siRNA + empty vector. (Right) Immunoblot of Flag, Pdx-1, Shp2, and α-tubulin in lysates of these 3 groups of INS-1 832/13 cells.
We next conducted ChIP assays using antibodies against Pdx1 to determine whether Shp2 knockdown altered Pdx1 occupation of target promoters Ins1 and Ins2. As shown in Fig. 4 C and D, ChIP assay detected markedly reduced binding of Pdx1 to elements within Ins1 and Ins2 promoters in Shp2-knockdown cells compared with controls. Therefore, Shp2 modulates signals leading to Pdx1 expression and consequently its association with Ins1 and Ins2 promoters in β-cells. To test the hypothesis that reduced Pdx1 expression and activity is at least partially responsible for suppression of insulin biosynthesis in Shp2-deficient cells, we asked whether restoring Pdx1 expression would rescue insulin production. As shown in Fig. 4E, reduced insulin content by Shp2 gene silencing can be improved to control level by cotransfection with Flag-tagged Pdx1 cDNA. Taken together, our data indicate that Shp2 deficiency suppresses activation of IRS2/PI3K/Akt/FoxO1 and Erk1/2 pathways, leading to decreased expression of Pdx1 and insulin genes and consequently to reduced insulin production in β-cells. On the basis of these experimental results, we propose a model for orchestration by Shp2 of multiple signaling circuits controlling insulin biosynthesis in β-cells (Fig. S5).
Discussion
Full understanding of molecular signaling mechanisms underlying control of insulin biosynthesis in β-cells is a prerequisite to repair β-cell dysfunction in type 2 diabetes. Accumulating data suggest that multiple pathways are involved in regulation of insulin production and secretion from β-cells. This complexity predicts the requirement of 1 or more signaling molecules acting to fine-tune and coordinate a variety of signals in β-cells, although little is known in this regard. Here, we report a critical role of Shp2 as a signal regulator/coordinator in β-cells.
A distinct phenotype of Shp2Panc−/− mice was marked reduction of glucose-stimulated insulin secretion in vivo and age-related, progressively impaired glucose tolerance. Consistent with the β-cell defect in insulin biosynthesis observed in Shp2Panc−/− mice, we show that Shp2 knockdown by siRNA in INS-1 832/13 cells decreased insulin contents and secretion, indicating a β-cell-intrinsic effect of Shp2 in insulin production. qRT-PCR analysis suggests that the decreased insulin production in Shp2-deficient β-cells is mainly due to impaired expression of Ins1 and Ins2. In analyzing this effect, we detected reduced Pdx1 expression and dramatically decreased Pdx1 binding to Ins1 and Ins2 promoters, suggesting that Shp2 deficiency leads to both suppression of Pdx1 expression and decrease in its activity. Exogenous Pdx1 expression in Shp2-knockdown cells partially rescued insulin production. Therefore, decreased Pdx1 expression and activity account at least in part for defective insulin biosynthesis in Shp2-deficient β-cells. Pdx1 was demonstrated to regulate Glut2 and insulin gene transcription (27, 28). Glut2 is a major glucose transport isoform expressed in the plasma membrane of β-cells, and it is essential for glucose sensing (29). Decreased Glut2 expression appears to be the first indication of β-cell dysfunction detected in type 2 diabetic animals (30). Collectively, reduced Pdx1, Glut2, and insulin levels in β-cells may lead to a loss of acute-phase insulin secretion and glucose intolerance in Shp2Panc−/− mice.
In exploring Shp2-modulated signals upstream of Pdx1 in controlling insulin expression, we found that glucose-stimulated Akt/FoxO1 and Erk pathways were altered in Shp2-deficient cells. In previous studies (13–15), we and others detected physical association of Shp2 with IRS1 and other IRS family members, and indeed these adaptor/scaffolding proteins share 2 tyrosyl residues at the C-terminal tail serving as Shp2-docking sites. In this study, we detected physical association of Shp2 with IRS2 and PI3K in INS-1 832/13 cells. Several reports suggest that Shp2 regulates the PI3K-Akt pathway negatively or positively depending on cellular context or the nature of stimulatory signals (31–34). High levels of glucose stimulation can enhance IRS2/Shp2 association, indicating that Shp2 may regulate glucose-stimulated activation of the IRS2/PI3K pathway. Indeed, impaired Akt activation was detected in Shp2-knockdown cells, leading to reduced phosphorylation of FoxO1 and increased nuclear accumulation of FoxO1. FoxO1 acts to repress the Pdx1 promoter, and phosphorylation of FoxO1 by Akt promotes its exclusion from the cytoplasm and loss of its repressor activity. Thus, reduced Akt activity leads to nuclear accumulation of FoxO1 and Pdx1 repression in Shp2-deficient cells. Consistently, transgenic animals expressing a kinase-dead mutant of Akt in β-cells displayed impaired glucose tolerance and defective insulin secretion (35).
Glucose-stimulated Erk1/2 activation was blunted in Shp2-knockdown cells, suggesting a positive role of Shp2 in promoting Erk activation by glucose. How Shp2 promotes the Erk pathway is not fully understood (36), and apparently different mechanisms function in various cell types. Data presented here suggest that Sprouty 1 is a Shp2 substrate in β-cells, because Shp2 was associated with Sprouty 1 in INS-1 832/13 cells, and tyrosine-phosphorylation of Sprouty was enhanced in Shp2-knockdown cells. Sprouty proteins are negative-feedback modulators of receptor tyrosine kinase signaling to the Erk pathway (21). It has not been demonstrated previously that Sprouty is involved in Shp2 regulation of Erk signaling to control insulin synthesis in β-cells. Activated Erk has been shown to phosphorylate and regulate Pdx1 activity in control of insulin gene expression in β-cells (10). Taking these observations together, impaired insulin synthesis seen in Shp2-deficient cells is likely due to reduced expression and activity of Pdx1, resulting from defective PI3K/Akt/FoxO1 and Erk1/2 activation (Fig. S5). However, this model does not exclude the contribution of other transcription factors' deficiency to the phenotype of Shp2-knockout β-cells.
This study also reveals a negative effect of Shp2 in ATP generation in β-cells. One group recently reported detection of Shp2 in purified rat brain mitochondria, although functional analysis was not undertaken (37). Similar to a recent report on mitochondrial PTPMT1 (19), we found that Shp2 gene silencing increased cellular ATP content in response to glucose. These results suggest that Shp2 plays a negative role in energy homeostasis in β-cells. The elevated ATP level would predict increased insulin secretion in Shp2-deficient β-cells after glucose stimulation. However, both Shp2 gene knockout and knockdown severely suppressed insulin production and secretion. Thus, the negative regulation by Shp2 of mitochondrial ATP generation is a minor effect in β-cells, which is offset by a positive role of cytoplasmic Shp2 in promoting signals up-regulating Pdx1 expression and activity required for insulin synthesis in β-cells.
Previous studies showed that βIRKO or βIGFRKO mice display normal β-cell proliferation and development, indicating that insulin/IGF1 is not crucial for early development of islet β-cells (2, 3). Results shown here also suggest that Shp2 has a limited role in pancreatic development but is a critical regulator of β-cell function in adults. However, these studies do not rule out the possibility that a failure to observe developmental defects is partly due to progressive deletion of the floxed target sequence by Pdx1-Cre in conditional gene knockout mouse models. βIRKO mice, rather than βIGFRKO mice, displayed defective islet growth with high-fat diet challenge, indicating that insulin signaling is critical for compensatory β-cell growth (38). Further studies are needed to determine whether β-cell compensatory growth is defective in Shp2Panc−/− animals with increased metabolic demand in status such as pregnancy, obesity, aging, or high-fat diet.
Materials and Methods
Animals.
Mice were maintained in a virus-free, normal light/dark cycle-controlled animal facility. All protocols for animal use and euthanasia were approved by a Burnham institutional animal usage committee. Generation of Shp2flox allele was reported previously (17). Shp2Panc−/− mice were generated by crossing Shp2flox/flox mice with Pdx1-Cre transgenic mice (18). Genotyping was performed by PCR analysis of genomic DNA from the tail.
Physical Studies.
All blood glucose measurements were determined on whole venous blood by using an automated glucose monitor (One Touch Basic, Lifescan) (17). Glucose tolerance tests were performed on mice after 16-hour fasting. Mice were injected intraperitoneally (i.p.) with d-glucose (2 g/kg of body weight) and blood was obtained at indicated time points. For insulin release, glucose (3 g/kg of body weight) was injected i.p., and blood was collected at indicated time points (3). Serum insulin levels were measured by ELISA with a rat insulin standard (Crystal Chem) (4). Statistical analysis was performed by using a 2-tailed unpaired t test.
Islet Isolation and Insulin Content Measurement.
Islets were isolated, cultured, and analyzed by following standard protocol or as described in SI Materials and Methods.
Additional methods are described in SI Materials and Methods. Primers used in qRT-PCR are listed in Tables S1 and S2.
Supplementary Material
Acknowledgments.
We thank Drs. M. Kamps, J. Dixon, C. V. Wright, B. Tyberg, P. Itkin-Ansari, J. Feramisco, R. Henry, and our colleagues for reagents, valuable suggestions, and helpful discussion. This work was supported by National Institutes of Health Grants R01DK73945, R01DK75916, and R01DK075916 to G.-S.F. and R21NS57001 to F.L.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811715106/DCSupplemental.
References
- 1.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni RN, et al. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell. 1999;96:329–339. doi: 10.1016/s0092-8674(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 3.Kulkarni RN, et al. Beta-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter beta-cell mass. Nat Genet. 2002;31:111–115. doi: 10.1038/ng872. [DOI] [PubMed] [Google Scholar]
- 4.Ueki K, et al. Total insulin and IGF-I resistance in pancreatic beta cells causes overt diabetes. Nat Genet. 2006;38:583–588. doi: 10.1038/ng1787. [DOI] [PubMed] [Google Scholar]
- 5.Withers DJ, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- 6.Lin X, et al. Dysregulation of insulin receptor substrate 2 in beta cells and brain causes obesity and diabetes. J Clin Invest. 2004;114:908–916. doi: 10.1172/JCI22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubota N, et al. Insulin receptor substrate 2 plays a crucial role in beta cells and the hypothalamus. J Clin Invest. 2004;114:917–927. doi: 10.1172/JCI21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto N, et al. Ablation of PDK1 in pancreatic beta cells induces diabetes as a result of loss of beta cell mass. Nat Genet. 2006;38:589–593. doi: 10.1038/ng1774. [DOI] [PubMed] [Google Scholar]
- 9.Nakae J, et al. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet. 2002;32:245–253. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence M, Shao C, Duan L, McGlynn K, Cobb MH. The protein kinases ERK1/2 and their roles in pancreatic beta cells. Acta Physiol (Oxford) 2008;192:11–17. doi: 10.1111/j.1748-1716.2007.01785.x. [DOI] [PubMed] [Google Scholar]
- 11.Gleason CE, Lu D, Witters LA, Newgard CB, Birnbaum MJ. The role of AMPK and mTOR in nutrient sensing in pancreatic beta-cells. J Biol Chem. 2007;282:10341–10351. doi: 10.1074/jbc.M610631200. [DOI] [PubMed] [Google Scholar]
- 12.Heit JJ, et al. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006;443:345–349. doi: 10.1038/nature05097. [DOI] [PubMed] [Google Scholar]
- 13.Lai LA, Zhao C, Zhang EE, Feng GS. In: Protein Phosphatases. Arino J, Alexander D, editors. Vol 5. Berlin: Springer; 2004. pp. 275–299. [Google Scholar]
- 14.Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 15.Kuhne MR, Pawson T, Lienhard GE, Feng GS. The insulin receptor substrate 1 associates with the SH2-containing phosphotyrosine phosphatase Syp. J Biol Chem. 1993;268:11479–11481. [PubMed] [Google Scholar]
- 16.White MF, Yenush L. The IRS-signaling system: A network of docking proteins that mediate insulin and cytokine action. Curr Top Microbiol Immunol. 1998;228:179–208. doi: 10.1007/978-3-642-80481-6_8. [DOI] [PubMed] [Google Scholar]
- 17.Zhang EE, Chapeau E, Hagihara K, Feng GS. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc Natl Acad Sci USA. 2004;101:16064–16069. doi: 10.1073/pnas.0405041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 19.Pagliarini DJ, et al. Involvement of a mitochondrial phosphatase in the regulation of ATP production and insulin secretion in pancreatic beta cells. Mol Cell. 2005;19:197–207. doi: 10.1016/j.molcel.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Maechler P, Wollheim CB. Mitochondrial function in normal and diabetic beta-cells. Nature. 2001;414:807–812. doi: 10.1038/414807a. [DOI] [PubMed] [Google Scholar]
- 21.Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: Multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16:45–54. doi: 10.1016/j.tcb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Jarvis LA, Toering SJ, Simon MA, Krasnow MA, Smith-Bolton RK. Sprouty proteins are in vivo targets of Corkscrew/SHP-2 tyrosine phosphatases. Development. 2006;133:1133–1142. doi: 10.1242/dev.02255. [DOI] [PubMed] [Google Scholar]
- 23.Mziaut H, et al. Synergy of glucose and growth hormone signalling in islet cells through ICA512 and STAT5. Nat Cell Biol. 2006;8:435–445. doi: 10.1038/ncb1395. [DOI] [PubMed] [Google Scholar]
- 24.Ke Y, et al. Conditional deletion of Shp2 in the mammary gland leads to impaired lobulo-alveolar outgrowth and attenuated Stat5 activation. J Biol Chem. 2006;281:34374–34380. doi: 10.1074/jbc.M607325200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali S, et al. PTP1D is a positive regulator of the prolactin signal leading to beta-casein promoter activation. EMBO J. 1996;15:135–142. [PMC free article] [PubMed] [Google Scholar]
- 26.Podcheko A, et al. Identification of a WD40 repeat-containing isoform of PHIP as a novel regulator of beta-cell growth and survival. Mol Cell Biol. 2007;27:6484–6496. doi: 10.1128/MCB.02409-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waeber G, Thompson N, Nicod P, Bonny C. Transcriptional activation of the GLUT2 gene by the IPF-1/STF-1/IDX-1 homeobox factor. Mol Endocrinol. 1996;10:1327–1334. doi: 10.1210/mend.10.11.8923459. [DOI] [PubMed] [Google Scholar]
- 28.Chakrabarti SK, James JC, Mirmira RG. Quantitative assessment of gene targeting in vitro and in vivo by the pancreatic transcription factor, Pdx1. Importance of chromatin structure in directing promoter binding. J Biol Chem. 2002;277:13286–13293. doi: 10.1074/jbc.M111857200. [DOI] [PubMed] [Google Scholar]
- 29.Unger RH. Diabetic hyperglycemia: Link to impaired glucose transport in pancreatic beta cells. Science. 1991;251:1200–1205. doi: 10.1126/science.2006409. [DOI] [PubMed] [Google Scholar]
- 30.Thorens B, Wu YJ, Leahy JL, Weir GC. The loss of GLUT2 expression by glucose-unresponsive beta cells of db/db mice is reversible and is induced by the diabetic environment. J Clin Invest. 1992;90:77–85. doi: 10.1172/JCI115858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang SQ, et al. Receptor-specific regulation of phosphatidylinositol 3′-kinase activation by the protein tyrosine phosphatase Shp2. Mol Cell Biol. 2002;22:4062–4072. doi: 10.1128/MCB.22.12.4062-4072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivins Zito C, Kontaridis MI, Fornaro M, Feng GS, Bennett AM. SHP-2 regulates the phosphatidylinositide 3′-kinase/Akt pathway and suppresses caspase 3-mediated apoptosis. J Cell Physiol. 2004;199:227–236. doi: 10.1002/jcp.10446. [DOI] [PubMed] [Google Scholar]
- 33.Kwon M, Ling Y, Maile LA, Badley-Clark J, Clemmons DR. Recruitment of the tyrosine phosphatase Src homology 2 domain tyrosine phosphatase-2 to the p85 subunit of phosphatidylinositol-3 (PI-3) kinase is required for insulin-like growth factor-I-dependent PI-3 kinase activation in smooth muscle cells. Endocrinology. 2006;147:1458–1465. doi: 10.1210/en.2005-1115. [DOI] [PubMed] [Google Scholar]
- 34.Wu CJ, et al. The tyrosine phosphatase SHP-2 is required for mediating phosphatidylinositol 3-kinase/Akt activation by growth factors. Oncogene. 2001;20:6018–6025. doi: 10.1038/sj.onc.1204699. [DOI] [PubMed] [Google Scholar]
- 35.Bernal-Mizrachi E, et al. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet beta cells. J Clin Invest. 2004;114:928–936. doi: 10.1172/JCI20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan RJ, Feng GS. PTPN11 is the first identified proto-oncogene that encodes a tyrosine phosphatase. Blood. 2007;109:862–867. doi: 10.1182/blood-2006-07-028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salvi M, et al. Tyrosine phosphatase activity in mitochondria: Presence of Shp-2 phosphatase in mitochondria. Cell Mol Life Sci. 2004;61:2393–2404. doi: 10.1007/s00018-004-4211-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okada T, et al. Insulin receptors in beta-cells are critical for islet compensatory growth response to insulin resistance. Proc Natl Acad Sci USA. 2007;104:8977–8982. doi: 10.1073/pnas.0608703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.