Abstract
D-serine is a physiological coagonist of N-methyl D-aspartate receptors (NMDARs) that plays a major role in several NMDAR-dependent events. In this study we investigate mechanisms regulating D-serine production by the enzyme serine racemase (SR). We now report that NMDAR activation promotes translocation of SR to the plasma membrane, which dramatically reduces the enzyme activity. Membrane-bound SR isolated from rat brain is not extracted from the membrane by high detergent and salt concentration, indicating a strong association. Colocalization studies indicate that most membrane-bound SR is located at the plasma membrane and dendrites, with much less SR observed in other types of membrane. NMDAR activation promotes translocation of the cytosolic SR to the membrane, resulting in reduced D-serine synthesis, and this effect is averted by blockade of NMDARs. In primary neuronal cultures, SR translocation to the membrane is blocked by a palmitoylation inhibitor, indicating that membrane binding is mediated by fatty acid acylation of SR. In agreement, we found that SR is acylated in transfected neuroblastoma cells using [3H]palmitate or [3H]octanoic acid as precursors. In contrast to classical S-palmitoylation of cysteines, acylation of SR occurs through the formation of an oxyester bond with serine or threonine residues. In addition, we show that phosphorylation of Thr-227 is also required for steady-state binding of SR to the membrane under basal, nonstimulated condition. We propose that the inhibition of D-serine synthesis caused by translocation of SR to the membrane provides a fail-safe mechanism to prevent NMDAR overactivation in vicinal cells or synapses.
Keywords: glutamate, neurotransmission, octanoylation, palmitoylation, synapse
D-serine is a physiological ligand of the coagonist site of NMDARs, mediating several NMDAR-dependent events, including NMDAR neurotransmission (1), neurotoxicity (2, 3), synaptic plasticity (4), and cell migration (5). D-serine is synthesized by serine racemase (SR), an enzyme that directly converts L- into D-serine (6). This enzyme is regulated by interacting proteins, such as the glutamate interacting protein 1 (5), Pick-1 (7), and Golga3 (8), and by nitric oxide produced upon NMDAR activation (9).
Despite the many roles attributed to it, the regulation of D-serine signaling is still largely unknown. Furthermore, many questions remain unresolved regarding the distribution of SR and the roles played by glia vs. neurons in D-serine signaling (10). Although the highest levels of endogenous D-serine were shown to be present in brain astrocytes (11), D-serine has also been detected in neurons (2). Recent data using new antibodies against SR (2) and SR knockout mice as negative controls (12) indicate that SR is abundantly expressed in neurons, with highest levels in the cerebral cortex and the hippocampal formation. Moreover, endogenous D-serine released from neuronal cultures lacking significant levels of astrocytes mediates NMDAR-elicited neurotoxicity (2), suggesting that neuron-derived D-serine may be sufficient to promote NMDAR activation.
In this study we investigate mechanisms regulating neuronal D-serine synthesis. We found that a fraction of serine racemase protein is tightly bound to membrane fractions of rat brain, primary neuronal cultures, and transfected cells. Membrane-bound SR is mostly inactive toward D-serine synthesis. In primary neuronal cultures, SR translocates to the dendritic membrane following NMDAR activation, which leads to prolonged inhibition of the enzyme. Using transfected cells, we found that SR acylation and phosphorylation contributes to membrane binding. Our data provide a new mechanism for regulation of neuronal SR, which may play a role in mediating feedback inhibition of NMDARs.
Results
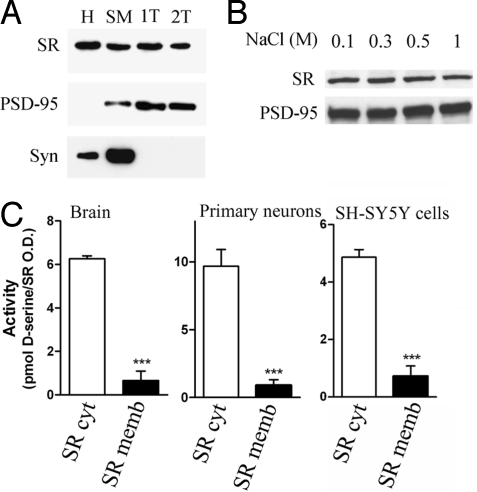
We previously reported that serine racemase (SR) is present in both the cytosolic and the membrane fractions of the rat brain, but the role and characteristics of the membrane-bound SR were not previously investigated (8). To determine the subcellular distribution of SR, we carried out subfractionation of brain homogenates (Fig. 1). We found that a fraction of SR is strongly bound to the purified synaptosomal plasma membrane (SM) fraction. This fraction contains both postsynaptic and presynaptic proteins, such as postsynaptic density 95 (PSD-95) and synaptophysin (Syn), respectively (Fig. 1A). To investigate the strength of interaction of SR to the membrane, SM were washed once (1T) or twice (2T) with 0.5% Triton X-100 (Fig. 1A), a treatment that completely extracts Syn but does not remove either SR or PSD-95 from the membrane (Fig. 1A). Likewise, the membrane-bound SR is not significantly extracted from SM by treatment with up to 1M NaCl, indicating a very strong association with the membrane (Fig. 1B).
Fig. 1.
Association of SR to brain membranes inhibits D-serine synthesis. (A) Western blot analysis showing the presence of SR in rat brain homogenate (H) and synaptosomal plasma membrane (SM). SR is not released from SM by washing once (1T) or twice (2T) with buffer containing 0.5% Triton X-100 (Upper). Middle and lower panels show the postsynaptic density protein 95 (PSD-95) and synaptophysin (Syn) levels. (B) SR is not removed from the membrane by high-salt washing. Fraction 1T was incubated in buffer containing 10 mM Tris-HCl (pH 7.4) and increasing concentrations of NaCl. Membrane-bound SR was recovered by centrifugation and analyzed by Western blot. (C) Membrane-bound SR is mostly inactive. Cytosolic (SR cyt) and membrane-bound (SR memb) SR were separated by centrifugation of cerebral cortex of adult rats (Left), primary neuronal cultures (Middle), and SH-SY5Y neuroblastoma cells overexpressing HA-SR (Right). The values of D-serine synthesis were normalized to the amounts of SR as determined by densitometric analysis of the Western blot with SR antibody. The results in A and B are representative of 3–5 experiments with different preparations. The values in C represent the average ± SEM of 5–6 experiments done in triplicates. ***Different from control at P < 0.001.
To verify whether the membrane-bound SR is functional, we compared the activity of the cytosolic with that of the membrane-bound enzyme from brain. We found that the membrane-bound SR obtained from rat brain is largely inactive, exhibiting only about 10% of the specific activity observed with the cytosolic enzyme (Fig. 1C Left). To ensure complete removal of any loosely bound SR, the membrane fraction was washed twice with buffer containing 0.5% Triton X-100. The lower catalytic activity of the membrane-bound SR was not due to detergent treatment, as the cytosolic SR is not inhibited by Triton X-100, and we obtained identical results when the membranes were washed without detergent (data not shown). A similar inactivation of SR is observed in membranes isolated from rat primary neuronal cultures (Fig. 1C) and SH-SY5Y neuroblastoma cells or HEK-293 cells (Fig. 1C and data not shown) overexpressing HA-SR, confirming that membrane association inactivates SR.
To characterize the biochemical properties of the membranes that SR associates with, we treated purified synaptosomes with 1% Triton X-100 and subjected the membranes to fractionation on a sucrose density gradient (supporting information (SI) Fig. S1). This process separates regular membranes from lipid rafts, which are detergent-resistant membrane microdomains containing cholesterol and gangliosides (13). Contrasting to bona-fide lipid raft markers (Thy1 and flotillin), which are enriched in low-density fractions of the gradient, SR was enriched in high-density fractions, indicating that SR is not a lipid raft protein (Fig. S1).
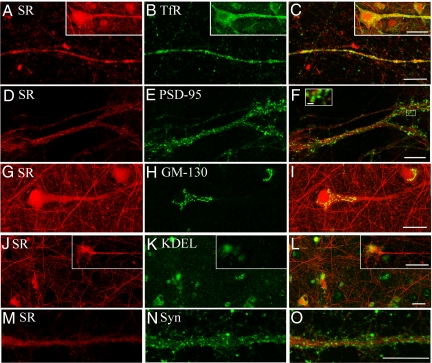
To more precisely determine the subcellular localization of membrane-bound SR, we carried out immunocytochemistry in primary neuronal cultures containing minimal amounts of glia (2). We found that SR is present in dendritic processes, where it largely overlaps with transferrin receptor (TfR) (Fig. 2 A–C). SR did not significantly colocalize with PSD-95, indicating that SR is not significantly present at dendritic spines (Fig. 2 D–F). GM-130, a marker of Golgi membranes, displayed limited overlap with SR in Golgi apparatus at the soma of 30% of the neurons, but not in dendrites (Fig. 2 G–I). Limited colocalization restricted to the soma was also observed with KDEL, an endoplasmic reticulum marker, in only 10% of the neurons (Fig. 2 J–L). We found that SR does not overlap with synaptophysin (Fig. 2 M–O), indicating that SR is not present at significant amounts in early endosomes or at the presynapse in our cultures. The data suggest that membrane-bound SR is predominantly found at the dendritic plasma membrane, with much lower levels in other types of membranes.
Fig. 2.
SR localization at dendritic processes. Localization of SR (A) and transferrin receptor (TfR) (B) in primary neurons. (C) Colocalization of SR and TfR in a dendritic process. Inset shows colocalization in the soma and process of a neuron. SR (D) and PSD-95 (E) do not significantly colocalize (F and Inset). (Scale bar, 2.5 μm [F Inset].) SR (G) and GM130 (H) expression exhibit limited overlap in the soma of 30% of the neurons, but not in dendrites (I). SR (J) and KDEL (K) exhibited limited colocalization with SR in the soma of only 10% of examined cells, but not in dendrites (L Inset). SR (M) and synaptophysin (Syn) (N) display no overlap (O). The pictures are representative of 4–12 different experiments. (Scale bar, 25 μm [A–O].)
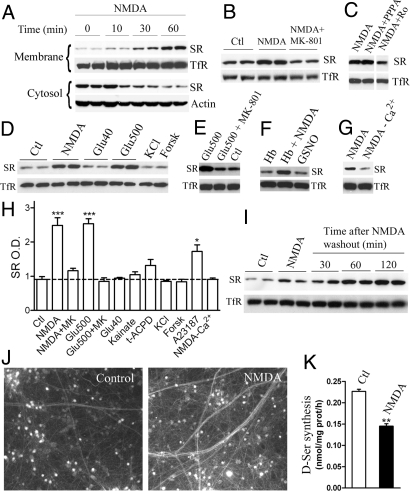
To investigate whether the membrane association of SR is a dynamically regulated process, we monitored the levels of membrane-bound SR after stimulating primary cultured neurons with glutamate receptor agonists. We found that stimulation of NMDARs for 10–60 min promotes a striking increase in the levels of membrane-bound SR (Fig. 3A), which is accompanied by a concomitant decrease in cytosolic SR (Fig. 3A). No changes in total SR levels were observed with NMDAR stimulation (data not shown). The NMDAR-elicited translocation of SR is blocked by addition of MK-801 (Fig. 3 B and H). The selective antagonist of the NR2B subunit of NMDARs, Ro-25–6981, prevents NMDAR-elicited SR translocation to the membrane, whereas PPPA, an antagonist of NR2A-type of NMDARs, has no effect (Fig. 3C). However, addition of a low glutamate concentration (40 μM) or depolarization of the neurons with 20 mM KCl does not induce SR translocation to the membrane (Fig. 3 D and H). Gentle increase in synaptic activity by forskolin treatment (14) does not promote SR translocation to the membrane as well (Fig. 3 D and H). Only incubation of the neurons with a high glutamate concentration (500 μM) increases membrane-bound SR, and this effect is blocked by MK-801 (Fig. 3 D and E), indicating that SR translocation is mainly mediated by NMDAR activation. Accordingly, agonists of other glutamate receptors, such as kainate and t-ACPD, display a much smaller effect on SR translocation (Fig. 3H), confirming that the effect is more specific toward NMDAR activity.
Fig. 3.
NMDAR activation elicits SR translocation to the membrane in neurons. (A) Addition of 100 μM NMDA to primary cortical neurons promotes a time-dependent increase in the levels of membrane-bound SR and a simultaneous decrease in the levels of the cytosolic SR. (B) The increase in the membrane-bound SR upon NMDA activation is blocked by 40 μM MK-801 (Upper) added 20 min before and during the NMDAR stimulation. (C) The increase in the membrane-bound SR upon NMDA activation is blocked by preincubation with 1 μM Ro-25–6981, but not by 0.2 μM PPPA. (D) Levels of membrane-bound SR after a 30-min stimulation with NMDA (100 μM) glutamate (40 and 500 μM), KCl (20 mM), or forskolin (Forsk) (50 μM). (E) Addition of 40 μM MK-801 prevents the increase in membrane-bound SR promoted by 500 μM glutamate. (F) NO scavenging by 20 μM oxyhemoglobin or NO release by 200 μM GSNO do not affect membrane-bound SR levels in the absence or presence of NMDA. (G) Omission of external Ca2+ during NMDAR stimulation inhibits NMDA effect on membrane-bound SR. (H) Densitometric analysis of membrane-bound SR in neurons after a 30-min incubation with 100 μM NMDA, 100 μM NMDA plus 40 μM MK-801, 500 μM glutamate, 500 μM glutamate plus 40 μM MK-801, 40 μM glutamate, 100 μM kainate, 100 μM t-ACPD, 20 mM KCl, 50 μM forskolin, 1 μM A23187, or 100 μM NMDA in the absence of external Ca2+. The results represent the average ± SEM of 4–22 experiments done in duplicates with different primary cultures. (I) Western blot showing lack of reversibility of the NMDAR-elicited SR translocation. After a 30-min stimulation with 100 μM NMDA, the neurons were washed twice and subsequently incubated for up to 2 h in fresh medium containing 100 μM APV to block NMDARs. (J) NMDAR stimulation increases membrane-bound SR levels in neuronal processes. Primary neuronal cultures were incubated with or without 100 μM NMDA for 30 min and subsequently treated for 1 min on ice with HBSS buffer containing 0.5% Triton X-100 before fixation, which removes most cytosolic SR. After fixation with 4% paraformaldehyde, SR localization was revealed by immunocytochemistry. The pictures are representative of 30 fields of different cultures containing approximately the same number of neuronal cell bodies. (K) NMDAR stimulation promotes a prolonged inhibition of D-serine synthesis in primary neurons. D-serine synthesis was assayed for 2 h following NMDA removal. The results represent the average ± SEM of 5 experiments with different culture preparations done in sextuplicate. Loading controls in (A–F) and (G) were monitored with anti-TfR (membrane fraction) or anti-actin (cytosolic fraction). *Different from control at P < 0.05; **Different from control at P < 0.01; ***Different from control at P < 0.001.
Because NMDAR activity is coupled with NO production, we investigated whether NO mediates SR translocation. NMDAR-elicited SR translocation in primary neurons is not affected by scavenging NO by addition of oxyhemoglobin (Fig. 3F). Furthermore, levels of membrane-bound SR in samples treated with the NO donor S-nitrosoglutathione (GSNO) are the same as those observed in samples treated with oxyhemoglobin alone (Fig. 3F) or controls (data not shown), indicating that SR translocation is not mediated by NO.
Addition of the Ca2+ ionophore A23187 significantly increases SR levels in the membrane (Fig. 3H), pointing to the involvement of Ca2+ rise in SR translocation. To confirm the role of internal Ca2+ in SR translocation to the membrane, we preloaded neurons with the intracellular Ca2+ chelator BAPTA-AM (100 μM) and found that it decreases the NMDA effect by about 40% (data not shown). Nevertheless, BAPTA-AM also significantly increases the basal levels of membrane-bound SR in the absence of NMDA, which suggests possible nonspecific effects or unrelated phenomenon. To further investigate the role of Ca2+, we omitted external Ca2+ during the NMDAR stimulation and found that it prevents NMDAR effect, confirming the role of Ca2+ entry in the NMDAR-elicited SR translocation to the membrane (Fig. 3 G and H).
To investigate the reversibility of SR translocation to the membrane, we monitored the levels of membrane-bound SR after NMDA removal. Unexpectedly, membrane-bound SR increases continuously for at least 2 h after NMDA removal and NMDAR blockade (Fig. 3I), with no effect on loading control. The data suggest that membrane translocation of SR is largely irreversible under our experimental conditions and even increases over time following the termination of the NMDA stimuli.
To confirm the NMDAR-elicited SR translocation to the membrane, we carried out immunocytochemistry in primary neuronal cultures treated with NMDA. We observed a clear increase in membrane-bound SR at neuronal processes upon NMDAR stimulation (Fig. 3J), corroborating the biochemical experiments described previously.
To verify whether the NMDAR-elicited SR translocation to the membrane affects SR activity in primary cultures, we monitored D-serine levels after addition of the SR substrate L-serine to the cultures. We took advantage of the fact that the membrane translocation of SR is not reversed within 2 h after NMDA removal (Fig. 3I). Thus, after NMDA treatment, the medium was replaced and L-serine was added to the cultures for 2 h. We found that NMDAR stimulation promotes a significant 40% decrease in intracellular D-serine synthesis (Fig. 3K), indicating that NMDAR is a main regulator of SR activity in primary neuronal cultures. Inhibition of SR activity by membrane binding is not mediated by S-nitrosylation of the enzyme (9), as SR activity from membranes purified from rat brain is not affected by addition of 10 mM DTT (data not shown), which completely reverses SR S-nitrosylation (9).
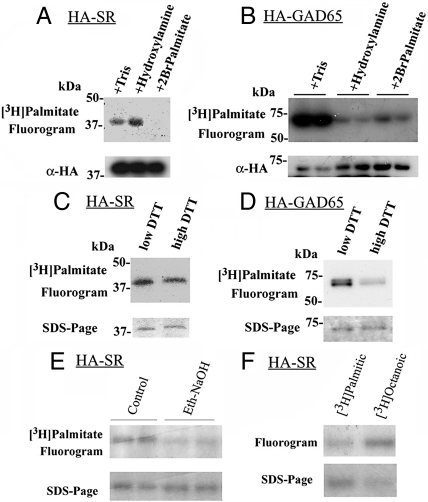
Because SR does not possess any transmembrane region, the strong binding of SR to the membrane suggests a posttranslational modification, such as fatty acid acylation, which increases protein hydrophobicity (15). Prenylation, isoprenylation, or myristoylation require specific amino acid motifs, which are not present in SR. Palmitoylation, however, does not involve a particular amino acid motif. Palmitoylation at cysteine residues (S-palmitoylation) are important for membrane targeting of several proteins, including glutamate decarboxylase-65 (GAD-65) (15). To investigate possible palmitoylation of SR, we labeled SH-SY5Y neuroblastoma cells overexpressing HA-SR with [3H]palmitic acid and carried out immunoprecipitation of SR followed by fluorography. In agreement, we found that SR racemase is acylated in SH-SY5Y neuroblastoma (Fig. 4A) and HEK293 cells (data not shown). To verify the chemical characteristics of the acylation, we incubated the immunoprecipitate for 2 h with 1M hydroxylamine (pH 7.4), which specifically cleaves thioesters and consequently abolishes S-palmitoylation. Unexpectedly, HA-SR acylation is totally resistant to hydroxylamine treatment (Fig. 4A), whereas it is abolished by incubation of the cells with 2-bromopalmitate, a palmitoylation inhibitor (Fig. 4A). By contrast, S-palmitoylation of GAD-65 is clearly sensitive to both hydroxylamine and 2-bromopalmitate under the same conditions (Fig. 4B). Likewise, displacement of possible thioester-bound fatty acids by incubation with 200 mM DTT at 100 °C has no effect on SR (Fig. 4C), and it significantly decreases the GAD-65 acylation (Fig. 4D). Furthermore, mutation of Cys-2 and Cys-6 alone or together with either Cys-46, Cys-113, Cys-128, Cys 217, or Cys-309 does not decrease SR acylation (data not shown), ruling out S-palmitoylation of SR in SH-SY5Y neuroblastoma cells.
Fig. 4.
Acylation of SR by oxyester formation. (A) SH-SY5Y cells overexpressing hemaglutinin-tagged SR (HA-SR) were labeled with 1 mCi/ml [3H]palmitic acid and HA-SR was immunoprecipitated with anti-HA affinity matrix. The immunoprecipitates were treated either with 1 M Tris-HCl (pH 7.4) or 1 M hydroxylamine (pH 7.4) for 2 h, and acylation was monitored by fluorography (Upper) of the SDS-PAGE. Pretreatment of the cell culture with 100 μM 2Br-palmitate abolishes HA-SR acylation, whereas hydroxylamine has no effect (fluorogram, Upper). Lower panel depicts immunoprecipitated HA-SR by analyzing 1/10th of the immunoprecipitate by Western blotting. (B) Acylation of HA-GAD65 in SH-SY5Y cells is sensitive to both hydroxylamine and 2Br-palmitate. Lower panel depicts immunoprecipitated HA-GAD65 by Western blot. (C) Acylation of SR is not affected by treatment with 200 mM DTT for 5 min at 100 °C. Lower panel depicts the loading control in the SDS-PAGE. (D) Acylation of HA-GAD65 is sensitive to DTT. Lower panel depicts the loading control in the SDS-PAGE. (E) HA-SR acylation is decreased by incubating the immunoprecipitate for 1 h with 50 mM NaOH in 90% ethanol. Lower panel depicts the SDS-PAGE, showing equal loading. (F) Comparison of SR acylation by [3H]palmitic acid and [3H]octanoic acid. The experiment was carried out as in (A) except that the cells were incubated with fatty acids using 4× less total radioactivity (250 μCi/ml), which gives a lower signal. Lower panel depicts the loading control in the SDS-PAGE.
S-Palmitoylation is mediated by palmitoyltransferases of the DHHC class (16). Conceivably, SH-SY5Y neuroblastoma cells lack some of the S-palmitoylation enzymes that might be present in the brain, explaining the lack of S-palmitoylation of SR. To discard this possibility, we cotransfected HA-SR with all of the 23 known S-palmitoyltransferases that were previously shown to catalyze S-palmitoylation (Table S1). None of the tested enzymes increased the acylation of SR above the control levels (Fig. S2), confirming that SR acylation does not occur through S-palmitoylation.
Acylation can also occur at hydroxyl groups of serine/threonine residues with the formation of an oxyester bond (O-palmitoylation) or at free amino groups with the formation of an amide bond (N-palmitoylation). O-acylation has been previously reported for only 2 mammalian proteins, accounting for the acylation of Wnt by palmitoleic acid at a serine residue (17) and for the octanoylation of the gastrointestinal peptide ghrelin at serine/threonine residues by membrane-bound O-acyltransferases (18). To investigate possible O-palmitoylation of SR, we carried out a mild base treatment of the immunoprecipitates, which cleaves O-esters but does not affect amide bonds (19). We found that SR acylation is sensitive to NaOH, indicating that SR is acylated at a serine/threonine residue, with the formation of an oxyester bond (Fig. 4E). In addition to palmitic acid, octanoic acid can also acylate serine/threonine residues (18). Interestingly, we found that [3H]octanoic acid was incorporated at levels even higher than [3H]palmitic acid when used at the same specific activity, indicating that SR can be modified by multiple acylation by the formation of oxyester(s) (Fig. 4F).
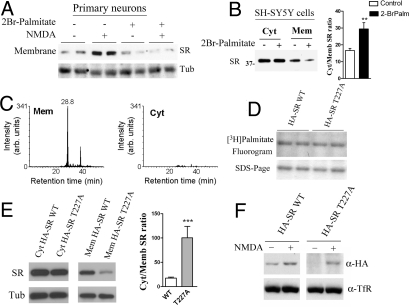
We next investigated whether SR acylation is required for the NMDA-elicited SR translocation to the membrane. Accordingly, we found that the palmitoylation inhibitor 2-bromopalmitate abolishes the NMDA-elicited association of SR to the membrane in primary neuronal cultures (Fig. 5A Upper). 2-Bromopalmitate does not effect β-tubulin levels (Fig. 5A Lower), indicating that palmitoylation is involved in the NMDAR-elicited SR translocation to the membrane.
Fig. 5.
Roles of palmitoylation and phosphorylation in SR binding to the membrane. (A) Palmitoylation inhibition blocks SR translocation in primary neuronal cultures. The neuronal cultures were preincubated for 2.5 h in NB medium containing 7 mg/ml fatty-acid-free albumin and in the presence or in the absence of 100 μM 2-Br-palmitate. Subsequently, 100 μM NMDA was added for 30 min and the levels of membrane-bound SR were monitored by Western blotting (Upper). Lower panel depicts β-tubulin levels in each sample. (B) Treatment with 100 μM 2Br-palmitate for 12 h decreases the amount of membrane-bound SR (Mem) but does not change the cytosolic levels (Cyt) of SR in transfected SH-SY5Y cells. Statistical analysis shows a 2-fold increase in cytosol/membrane SR ratio (Right; n = 6). Cytosolic fractions were analyzed using 5 μg protein, whereas membrane fractions contained 50 μg protein. (C) Phosphorylation of Thr-227 is exclusively found in the membrane-bound SR. Cytosolic and membrane-bound HA-SR from SH-SY5Y cells were purified, in-gel digested with trypsin, and the peptides analyzed by LC-MS (see SI Methods). The extracted ion chromatogram (m/z 737.1 ± 0.1) of the membrane-bound HA-SR shows elution of the monophosphorylated peptide 222–241 after 28.8 min (Left), whereas no corresponding peptide peak was detected in the cytosolic HA-SR (Right). (D) Mutation of Thr-227 to Ala (T227A) does not affect the acylation levels of HA SR by [3H]palmitate in transfected HEK 293 cells (Upper). Lower panel depicts the SDS-PAGE loading control. (E) T227A SR mutant exhibits lower binding to the membrane in transfected HEK293 cells. Statistical analysis shows a 4- to 5-fold increase in cytosol/membrane ratio when T227A is compared with wild-type SR (n = 6). (F) Levels of membrane-bound SR under basal (−NMDA) or stimulated (+NMDA) conditions in primary neuronal culture transduced with recombinant lentivirus containing either HA-SR WT (Left) or HA-SR T227A (Right). HA-SR was detected with anti-HA. Lower panel depicts TfR loading control in the membrane fraction. **Different from control at P < 0.01; ***Different from control at P < 0.001.
In transfected SH-SY5Y (Fig. 5B) or HEK293 cells (data not shown), incubation with 2-bromopalmitate had a partial effect on the levels of membrane-bound SR, leading to a 2-fold increase in the cytosol/membrane SR ratio (Fig. 5B). Thus, palmitoylation may not be the only determinant of membrane binding in nonexcitable cells under basal conditions. Thus, to evaluate additional posttranslational modifications of the membrane-bound SR, we carried out liquid chromatography/mass spectrometry (LC-MS) of immunoprecipitated HA-SR from SH-SY5Y neuroblastoma cells. We did not identify any acylated residues, possibly because of poor recovery of acylated peptides, low stoichiometry, or scarcity of endogenous fatty acids in the culture. However, LC-MS analysis identified phosphorylation of SR at Thr-227, which occurs specifically in the SR, solubilized from the membrane fraction (Fig. 5C and Fig. S3). This was highly significant, as no phosphorylation of Thr-227 was observed in SR immunoprecipitated from the cytosolic fraction, even though the sample analyzed from the cytosol contained a much higher amount of SR than that of the membrane.
To investigate the role of Thr-227 in the membrane-binding ability of SR, we produced a phosphorylation-deficient mutant in which Thr-227 was replaced by alanine (T227A). Interestingly, although the mutation does not change the extent of SR acylation by [3H]palmitate (Fig. 5D), it promotes a significant decrease in SR binding to the membrane (Fig. 5E). Thus, the T227A mutant exhibits a cytosol/membrane SR ratio that is about 5× higher than that observed with the WT SR (Fig. 5E), indicating that phosphorylation also contributes to membrane binding in transfected cells.
To investigate the role of Thr-227 phosphorylation in NMDAR-elicited SR translocation to the membrane, we infected primary neuronal cultures with lentiviruses containing either HA-SR wild-type or HA-SR T227A mutant. When compared with the HA-SR wild-type, the mutation does not prevent NMDAR-elicited SR translocation as revealed by the clear increase in HA-SR T227A at the membrane upon NMDAR stimulation (Fig. 5F). However, levels of membrane-bound SR in the absence of NMDAR stimulation are much lower with the T227A mutant (Fig. 5F), indicating that Thr-227 phosphorylation may regulate the steady-state binding of SR to the membrane under basal, nonstimulated condition.
Discussion
In the present study we show a previously undescribed mechanism regulating SR activity in neurons, linking NMDAR activity and the synthesis of the coagonist D-serine. Our data indicate that the neuronal SR translocates to the plasma membrane at dendritic processes after NMDAR stimulation, leading to inactivation of the enzyme. This provides a mechanism for feedback inhibition of NMDARs by decreasing the synthesis of the coagonist D-serine following NMDAR activation.
We found that NMDAR activation is the main signal mediating SR translocation to the membrane. The molecular mechanism of serine racemase inactivation at the membrane is unclear. It is possible that membrane binding induces conformational changes that block the access of substrates or strongly decrease the turnover rate of the enzyme. An interesting feature of SR binding to the membrane is the apparent lack of reversibility upon NMDA removal and blockade of the NMDARs (Fig. 3H). Once NMDARs are activated, the levels of SR in the membrane keep increasing for at least 2 h. This suggests that NMDAR activation causes prolonged SR inactivation, rather than an easily reversible posttranslational modification. Our NMDA treatment protocol most likely causes NMDARs overactivation, a process that may have drastic consequences for the cells by causing excitotoxicity (20). Because endogenous D-serine is the dominant coagonist for NMDAR-elicited excitotoxicity (3), inactivation of SR might work as a fail-safe mechanism to prevent further NMDAR overactivation in vicinal synapses or cells (21). Furthermore, because about 10% of total SR is bound to membranes acutely isolated from rat brain, it is possible that SR translocation to the membrane also takes place during normal NMDAR neurotransmission.
NMDAR activation has been shown to stimulate the synthesis of NO, which inhibits SR activity by S-nitrosylation (9). We found that NMDAR-mediated NO production is not responsible for SR translocation to the membrane or for its inactivation under our experimental conditions. Contrasting to the translocation of SR to the membrane we observed, S-nitrosylation of SR may be an easily reversible reaction (9). Thus, there may be multiple pathways regulating SR activity under different time scales, strength of NMDAR stimulation, and cellular locations.
In primary neuronal cultures, inhibition of palmitoylation by 2-bromopalmitate prevents SR translocation to the membrane, suggesting a role for fatty acid acylation for the effect. Due to the inherent low signal of [3H]palmitate labeling, it was not possible to detect endogenous SR palmitoylation in primary neuronal cultures. The use of transfected neuroblastoma cells allowed us to obtain high amounts of immunoprecipitated SR and confirm that SR is acylated upon palmitic acid addition. Interestingly, the chemistry differs from classical S-palmitoylation, as SR is acylated through the formation of an oxyester with serine or threonine residues. This type of acylation has been previously detected in the Wnt protein, which is modified at a serine residue by palmitoleic acid, an oxidation product of palmitate (17). Our data do not exclude the possibility that SR racemase acylation is mediated by palmitoleic acid as well. Moreover, because SR is also acylated with octanoic acid, known to modify the peptide ghrelin at serine/threonine residues (18), it is possible that SR is O-acylated by fatty acids of different lengths. Nevertheless, the inhibition of the NMDAR-elicited membrane translocation of SR in primary neuronal cultures by a palmitoylation inhibitor supports the role of palmitoylation in SR translocation to the membrane.
In addition to palmitoylation, we found that the membrane-bound SR isolated from transfected neuroblastoma cells is phosphorylated at Thr-227 and that this phosphorylation is absent from the cytosolic SR. Mutation of Thr-227 to alanine decreases SR binding to the membrane, confirming the role of SR phosphorylation in membrane binding. In primary neurons transduced with lentiviruses, mutation of Thr-227 does not block the NMDAR-elicited SR translocation to the membrane, but it apparently decreases the steady-state binding of SR to the membrane under basal, nonstimulated condition. This suggests that SR binding to the membrane is regulated by multiple mechanisms and that palmitoylation and phosphorylation regulate independent events leading to membrane binding. Analysis of the phosphorylation site of Thr-227 implicates proline-directed kinases, such as MAP kinases, in mediating SR phosphorylation. However, inhibitors of p38 (SB 203580, 10 μM), ERK1/2 (PD98058, 5 μM), and Jnk (SP600125, 10 μM) MAP kinases do not change SR binding to the membrane (V.N.F., L.B., and H.W., unpublished observations). Further characterization of the kinase(s) involved in Thr-227 phosphorylation may give new clues to the signals regulating steady-state SR binding to the membrane.
In sum, we have shown that NMDAR stimulation elicits translocation of SR to the membrane and blocks neuronal D-serine synthesis, providing a fail-safe mechanism to prevent NMDAR overactivation in vicinal cells or synapses. When this manuscript was under review, Mustafa et al. (22) demonstrated that SR is also present in astrocytic membrane by binding to phosphatidylinositol 4,5-bisphosphate, and stimulation of metabotropic glutamate receptors prevents SR binding. The data suggest multiple regulatory roles for attachment of SR to the membrane both in neurons (present study) and in astrocytes (22), and demonstrate a role of glutamate receptors in regulating D-serine synthesis.
Materials and Methods
Primary Neuronal Cultures and Immunocytochemistry.
Neuronal cell cultures were prepared from cortices of E18 Sprague-Dawley rats using Neurobasal Medium with B27 supplement (NB/B27; Invitrogen) as previously described (2), with slight modifications (see SI Methods). The cells were fixed with 100% methanol at −20 °C for 20 min, and colocalization was revealed by using a rabbit anti-SR serum at 1:1,000 (2) against different membrane markers. Laser-scanning confocal microscopy (BioRad Radiance) was carried out using a 60× objective (NA 1.4). Sections of 1 and 0.5 μm were performed at 512 × 512 or 1,024 × 1,024 pixel resolution using 3× zoom. For colocalization of SR with PSD-95 and synaptophysin, raw confocal images from dendritic processes higher than 50 μm from 5–10 neurons were analyzed, and yellow puncta was manually counted.
Treatment of Neuronal Cell Cultures.
Experiments were carried out on DIV12, and drugs were added in NB/B27 medium, unless specified otherwise. In experiments carried out in the absence of external Ca2+, we prepared an NB medium lacking CaCl2. The experiments were terminated by addition of lysis medium consisting of cold HBSS without calcium and magnesium (10 mM Hepes (pH 7.4), 1 mM pyruvate, 5.6 mM glucose, 0.44 mM KH2PO4, 5.4 mM KCl, 0.18 mM NaH2PO4, 4.2 mM NaHCO3, 137 mM NaCl) supplemented with protease inhibitors, 1 μM tetrodotoxin, 10 μM NBQX, 40 μM MK-801 0.2% Triton X-100, and DNase 200 U/ml. To separate membranes from cytosol, the cells were homogenized with 2 up-and-down strokes of a glass homogenizer and rotated for 20 min at 4 °C. The homogenate was subsequently cleared by centrifugation at 1000 × g for 10 min. The supernatant was centrifuged for 30 min at 200,000 × g to obtain membrane and cytosolic fractions. To remove any loosely bound SR, the membranes were further washed by recentrifugation with lysis buffer lacking Triton X-100, DNase, and receptor blockers.
Acylation Experiments.
SH-SY5Y or HEK293 cells overexpressing HA-SR or HA-GAD65 were labeled with 0.25 to 1 mCi/ml [3H]palmitate (Perkin-Elmer) or 0.25 mCi/ml [3H]octanoic acid (American Radiolabeled Chemicals) for 4 h in DMEM (Biological Industries) containing 10 mg/ml fatty acid-free albumin (Sigma) in the absence of serum. The cells were lysed with a solution containing 20 mM Tris-HCl (pH 7.4), 1% SDS, protease inhibitors, and 1 mM EDTA, boiled for 5 min and further sonicated for 20 s. The samples were diluted 10-fold in solution containing 20 mM Tris-HCl (pH 7.4), 2% Triton X-100, 0.5% deoxycholate, and 1 mM EDTA, incubated for 10 min at 4 °C, and cleared by centrifugation at 16,000 × g for 30 min. HA-SR or HA-GAD65 were immunoprecipitated by addition of anti-HA affinity matrix (Sigma). After washing the immunoprecipitates, the samples were boiled for 2 min with SDS sample buffer containing either a low (10 mM) or a high DTT (200 mM) concentration, and analyzed by SDS-PAGE followed by fluorography using Amplify reagent (Amersham Biosciences). In some experiments, the immunoprecipitates were incubated with 1 M hydroxylamine (pH 7.4) for 2 h at room temperature before SDS-PAGE. To evaluate oxyester bonds, the samples were incubated with 180 μl ethanol plus 20 μl NaOH 1 M for 1 h at 37 °C. After neutralization with HCl and concentration by speed vac, the samples were applied to SDS-PAGE gels.
Supplementary Material
Acknowledgments.
This work was supported by a grant from the Israel Science Foundation, Ministry of Health; Dr. R. Lawrence Neuropsychopharmacological Research Fund, Herbert N. Goldman Medial Research Fund, Technion Research Foundation (H.W.), and the European Union under a FP6 Marie Curie Research Training Network (O.N.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809442106/DCSupplemental.
References
- 1.Mothet JP, et al. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA. 2000;97(9):4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kartvelishvily E, Shleper M, Balan L, Dumin E, Wolosker H. Neuron-derived D-serine release provides a novel means to activate N-methyl-D-aspartate receptors. J Biol Chem. 2006;281(20):14151–14162. doi: 10.1074/jbc.M512927200. [DOI] [PubMed] [Google Scholar]
- 3.Shleper M, Kartvelishvily E, Wolosker H. D-serine is the dominant endogenous coagonist for NMDA receptor neurotoxicity in organotypic hippocampal slices. J Neurosci. 2005;25(41):9413–9417. doi: 10.1523/JNEUROSCI.3190-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panatier A, et al. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125(4):775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 5.Kim PM, et al. Serine racemase: Activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proc Natl Acad Sci USA. 2005;102(6):2105–2110. doi: 10.1073/pnas.0409723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolosker H, Blackshaw S, Snyder SH. Serine racemase: A glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci USA. 1999;96(23):13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hikida T, et al. Modulation of D-serine levels in brains of mice lacking PICK1. Biol Psychiatry. 2008;63(10):997–1000. doi: 10.1016/j.biopsych.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumin E, et al. Modulation of D-serine levels via ubiquitin-dependent proteasomal degradation of serine racemase. J Biol Chem. 2006;281(29):20291–20302. doi: 10.1074/jbc.M601971200. [DOI] [PubMed] [Google Scholar]
- 9.Mustafa AK, et al. Nitric oxide S-nitrosylates serine racemase, mediating feedback inhibition of D-serine formation. Proc Natl Acad Sci USA. 2007;104(8):2950–2955. doi: 10.1073/pnas.0611620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolosker H, Dumin E, Balan L, Foltyn VN. D-amino acids in the brain: D-serine in neurotransmission and neurodegeneration. FEBS J. 2008;275(14):3514–3526. doi: 10.1111/j.1742-4658.2008.06515.x. [DOI] [PubMed] [Google Scholar]
- 11.Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: Localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci USA. 1995;92(9):3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miya K, et al. Serine racemase is predominantly localized in neurons in mouse brain. J Comp Neurol. 2008;510(6):641–654. doi: 10.1002/cne.21822. [DOI] [PubMed] [Google Scholar]
- 13.Butchbach ME, Tian G, Guo H, Lin CL. Association of excitatory amino acid transporters, especially EAAT2, with cholesterol-rich lipid raft microdomains: Importance for excitatory amino acid transporter localization and function. J Biol Chem. 2004;279(33):34388–34396. doi: 10.1074/jbc.M403938200. [DOI] [PubMed] [Google Scholar]
- 14.Beaumont V, Zucker RS. Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic Ih channels. Nat Neurosci. 2000;3(2):133–141. doi: 10.1038/72072. [DOI] [PubMed] [Google Scholar]
- 15.Linder ME, Deschenes RJ. Palmitoylation: Policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8(1):74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 16.Fukata M, Fukata Y, Adesnik H, Nicoll RA, Bredt DS. Identification of PSD-95 palmitoylating enzymes. Neuron. 2004;44(6):987–996. doi: 10.1016/j.neuron.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Galli LM, Barnes TL, Secrest SS, Kadowaki T, Burrus LW. Porcupine-mediated lipid-modification regulates the activity and distribution of Wnt proteins in the chick neural tube. Development. 2007;134(18):3339–3348. doi: 10.1242/dev.02881. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132(3):387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Stanley SL, Jr, Tian K, Koester JP, Li E. The serine-rich Entamoeba histolytica protein is a phosphorylated membrane protein containing O-linked terminal N-acetylglucosamine residues. J Biol Chem. 1995;270(8):4121–4126. doi: 10.1074/jbc.270.8.4121. [DOI] [PubMed] [Google Scholar]
- 20.Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 21.Boehning D, Snyder SH. Novel neural modulators. Annu Rev Neurosci. 2003;26:105–131. doi: 10.1146/annurev.neuro.26.041002.131047. [DOI] [PubMed] [Google Scholar]
- 22.Mustafa AK, et al. Glutamatergic regulation of serine racemase via reversal of PIP2 inhibition. Proc Natl Acad Sci USA. 2009;106(8):2921–2926. doi: 10.1073/pnas.0813105106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.