Abstract
Cortical GABAergic interneurons, most of which originate in the ganglionic eminences, take distinct tangential migratory trajectories into the developing cerebral cortex. However, the ligand–receptor systems that modulate the tangential migration of distinct groups of interneurons into the emerging cerebral wall remain unclear. Here, we show that netrin-1, a diffusible guidance cue expressed along the migratory routes traversed by GABAergic interneurons, interacts with α3β1 integrin to promote interneuronal migration. In vivo analysis of interneuron-specific α3β1 integrin, netrin-1–deficient mice (α3lox/−Dlx5/6-CIE, netrin-1−/−) reveals specific deficits in the patterns of interneuronal migration along the top of the developing cortical plate, resulting in aberrant interneuronal positioning throughout the cerebral cortex and hippocampus of conditional α3lox/−Dlx5/6-CIE, netrin-1−/− mice. These results indicate that specific guidance mechanisms, such as netrin-1–α3β1 integrin interactions, modulate distinct routes of interneuronal migration and the consequent positioning of groups of cortical interneurons in the developing cerebral cortex.
The development of the six-layered cerebral cortex, composed predominantly of glutamatergic projection neurons and GABAergic interneurons, depends on the appropriate migration of neurons from ventricular zones of the dorsal and ventral telencephalon to their final position in the emerging cortical plate. Neuronal progenitors from the dorsal telencephalon migrate radially as cohorts along the radial glial scaffold, pass their predecessors in the developing cortical plate, and coalesce into layers, giving rise to glutamatergic projection neurons (1). In contrast, neuronal precursors from the ventral telencephalon, principally the medial ganglionic eminence (MGE), migrate tangentially through the cortical marginal zone and intermediate zone and enter the cortical plate, using the radial glial scaffold, before differentiating into GABAergic interneurons (2–4). A subset of these interneurons initially migrates radially inwards, toward the cortical ventricular zone, before turning back up toward the cortical plate (4). These different modes of neuronal migration are coordinated temporally to achieve the “inside-out” laminar organization of neurons in the neocortex.
Specific cell adhesion interactions between neurons, glia, and the surrounding ECM are critical for the appropriate migration and placement of cortical neurons. These adhesive interactions are mediated in large part by integrins, heterodimeric cell-surface ECM receptors, which serve as structural links between extracellular ligands and the internal cytoskeleton. The functional significance of integrin signaling for cerebral cortical development is demonstrated by the distinct types of cortical malformations exhibited by mice deficient in αv, α3, α6, β1, and β4 integrins (1, 5–8). However, the selective adhesive interactions needed to coordinate the migration of distinct groups of interneurons and projection neurons into the developing cerebral cortex remain poorly understood.
The prominent expression of netrin-1 in the ganglionic eminence (GE), where interneurons originate (9), as well as the expression of α3β1 integrin in migrating cortical neurons (5), suggests that netrin-1–α3β1 integrin interactions may influence specific aspects of the tangential migration of GABAergic interneurons. Recent evidence that α3β1 integrin serves as a receptor for netrin-1 in epithelial cells and evidence for cross-talk between integrins and classical netrin receptors have opened an avenue for investigating this possibility (10). Netrins serve as secreted guidance cues, regulating axonal outgrowth and neuronal migration by binding to Deleted in Colon Cancer (DCC), Down's Syndrome Cell Adhesion Molecule (DSCAM), or UNC5 receptors (11, 12). Netrin-1 interacts with DCC and DSCAM to induce growth cone attraction (12, 13), whereas its association with UNC5 receptors promotes axonal repulsion (14). Recently, Yebra and colleagues (10) have demonstrated that α6β4 and α3β1 integrins regulate the migration of epithelial cells on netrin-1 through a direct interaction of these integrins with the C-terminal domain of netrin-1, although the significance of these interactions is yet to be determined in vivo. Here, we have investigated the contribution of netrin-1–α3β1 integrin–mediated signal transduction to the migration of GABAergic interneurons from the MGE into the developing cerebral wall.
Results
Netrin-1 Is Localized Along the Migratory Route of Cortical GABAergic Interneurons.
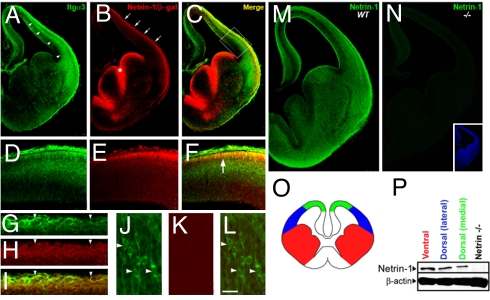
To evaluate the potential involvement of netrin-1–α3β1 integrin signaling in interneuronal migration, we mapped the expression pattern of netrin-1 and α3β1 integrin in embryonic cortex during interneuronal migration (Fig. 1 and Fig. S1). Sections from netrin-1 gene trap indicator mice (Netrin-1LacZ/+), in which β-galactosidase expression faithfully indicates endogenous netrin-1 gene expression (15), were co-labeled with β-galactosidase and α3 integrin antibodies. Prominent netrin-1–LacZ expression is evident in the ventricular zone of the GE and in the marginal zone of the developing cortex (Fig. 1 B, E, and H). α3 integrin is present in radially and tangentially oriented neurons in the cerebral wall (Fig. 1 A, J, and L and Fig. S1). Both α3 integrin and netrin-1 are prominently co-expressed in the marginal zone region (Fig. 1 C, F, and I).
Fig. 1.
Expression of netrin-1 and α3β1 integrin in the developing cortex. (A–C) Coronal sections of the forebrain at E13.5 labeled with α3 integrin (A) and β-galactosidase (B) antibodies demonstrate that tangentially migrating interneurons express α3β1 integrin (arrowheads in A) and indicate that netrin-1 is present at the cortical marginal zone (arrows in B) and ventricular zone of the GE (asterisk in B). C is a merge of A and B. (D–F) High magnification of the developing cerebral wall boxed in C shows colocalization (arrow in F) of netrin-1 (E) and α3β1 integrin (D) at the marginal zone. (G–I) Tangentially migrating interneurons at the marginal zone express α3β1 integrin (arrowheads in G and I) and netrin-1 (arrowheads in H and I). (J–L) Radially migrating neurons in the intermediate zone express α3 integrin (arrowheads in J and L), but not netrin-1 (K and arrowheads in L). (M) Coronal section of E13.5 cortex labeled with netrin-1 antibodies demonstrates that secreted netrin-1 is expressed in the developing forebrain during tangential neuronal migration. (N) Netrin-1 expression was not detected in the forebrain of E13.5 netrin-1–null mice by immunohistochemistry. (O–P) Immunoblot of netrin-1 in the GE, lateral pallium, and dorsal pallium of E14.5 forebrain (P) indicate that netrin-1 is expressed in each of these regions (schematized in O). (Scale bar, 15 μm.)
Although LacZ expression is indicative of netrin-1 gene expression patterns, it may not reveal fully the localization of secreted netrin-1 in the developing cortex. To examine the expression pattern of functional, secreted netrin-1 in the developing telencephalon during tangential migration, embryonic day 13.5 (E13.5) cortices were immunolabeled with netrin-1 antibodies. Expression of secreted protein was detected in marginal zone and intermediate zone regions traversed by tangentially migrating interneurons (Fig. 1M). This immunolocalization pattern was not detected in netrin-1–null mice (Fig. 1 N and P). The presence of netrin-1 protein in the ventral and dorsal telencephalon was verified further by immunoblotting performed on microdissected portions of the telencephalon (Fig. 1P). Together, these results reveal α3β1 integrin expression in interneurons and the presence of netrin-1 in the migratory route of these interneurons in the developing cerebral cortex.
Netrin-1–α3 Integrin Interactions.
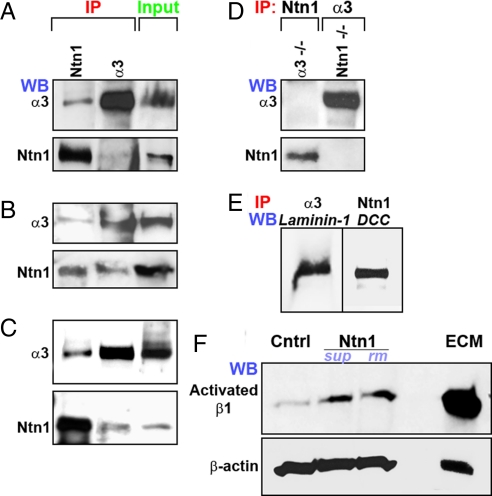
To determine if α3 integrin and netrin-1 interact with each other in the developing cerebral cortex, we first performed co-immunoprecipitations of netrin-1 and α3 integrin. Immunoprecipitation of E13.5 forebrain lysates with anti-netrin-1 antibodies and immunoblotting with anti-α3 integrin antibodies or immunoprecipitation of lysates with anti-α3 integrin antibodies and immunoblotting with anti-netrin-1 antibodies demonstrates that α3 integrin co-immunoprecipitates with netrin-1 in vivo (Fig. 2A). Previously known ligands for α3 integrin (i.e., laminin-1) or other known receptors for netrin-1 (i.e., DCC) were co-immunoprecipitated in these assays and served as positive controls (Fig. 2E). Netrin-1 or α3 integrin was not co-immunoprecipitated when α3 integrin-deficient or netrin-1–deficient forebrain extracts were used to co-immunoprecipitate netrin-1 or α3 integrin, respectively (Fig. 2D). To verify further the interaction between α3 integrin and netrin-1, we performed co-immunoprecipitations of netrin-1 and α3 integrin using netrin-1–expressing 293 cells transfected with α3 integrin. Immunoprecipitation of these cell lysates with anti-netrin-1 antibodies and immunoblotting with anti-α3 integrin antibodies or immunoprecipitation of lysates with anti-α3 integrin antibodies and immunoblotting with anti-netrin-1 antibodies shows that α3 integrin co-immunoprecipitates with netrin-1 in vitro (Fig. 2B). To establish whether netrin-1 and α3β1 integrin associate directly, netrin-1–containing supernatants from stably transfected 293 cells were incubated with purified α3β1 integrin protein. Netrin-1 or α3 integrin bound to each other was analyzed following immunoprecipitation with either anti-α3 integrin or netrin-1 antibodies, respectively. α3 integrin co-immunoprecipitates with netrin-1 in these assays, thus further indicating a direct association between α3 integrin and netrin-1 (Fig. 2C).
Fig. 2.
Netrin-1–α3β1 integrin interactions. To verify netrin-1–α3β1 integrin interactions, E13.5 forebrain extracts (A), netrin-1–expressing 293 cell lysates (B), or a mixture of purified netrin-1 (ntn1) and α3β1 integrin proteins (C) were immunoprecipitated (IP) with α3 integrin or netrin-1 antibodies and Western blotted (WB) with antibodies to α3 integrin or netrin-1. (A–C) In each assay, netrin-1 co-immunoprecipitated with α3 integrin and vice versa. (D) Netrin-1 or α3 integrin was not detected when α3 integrin-deficient or netrin-1–deficient forebrain extracts were used to co-immunoprecipitate netrin-1 or α3 integrin. (E) The co-immunoprecipitation of α3 integrin and laminin-1, a known α3 integrin ligand, and of netrin-1 and DCC, a known netrin-1 receptor, from E14.5 forebrain lysates serves as positive controls. (F) Robust β1 integrin receptor activation is observed following netrin-1 supernatant (sup), recombinant mouse netrin-1 (rm), and ECM stimulation of MGE neurons.
Netrin-1 Activates β1 Integrin Receptors.
To establish whether the physical interaction between netrin-1 and α3β1 integrin has functional relevance, we examined the effects of netrin-1 on β1 integrin receptor activation. E14.5 MGE cells were incubated with netrin-1–containing supernatant, recombinant mouse netrin-1 (50 ng/ml), medium alone, or ECM (10 mg/ml). The medium-alone condition serves as a negative control, and ECM stimulation functions as a positive control for β1 integrin receptor activation. The activation of β1 integrin in netrin-1–treated MGE cells was assessed by application of a β1 integrin antibody (9EG7), which specifically recognizes its active conformation. Supernatants containing secreted netrin-1 or mouse recombinant netrin-1 markedly increased β1 integrin receptor activation in MGE cells when compared with controls (Fig. 2F), thus demonstrating a functional, physiologically relevant interaction between netrin-1 and α3β1 integrin.
Disruption of Netrin-1–α3β1 Integrin Interactions Reduces the Number of Interneurons Migrating Through the Cortical Marginal Zone.
In vitro observations suggest that retrin-1–modulated interneuronal migration from the MGE involves α3 integrin function (see Fig. S2 and SI Text). To evaluate the significance of netrin-1–α3 integrin interactions during interneuronal migration in vivo, we generated interneuron-specific α3 integrin-deficient mice in a netrin-1–null background. Dlx5/6-Cre-IRES-EGFP (Dlx5/6-CIE) mice, in which interneurons generated from the ganglionic eminence express Cre and EGFP, were used to inactivate α3 integrin in interneurons. Generation of mice with a floxed and null Itga3 allele (α3lox/−) in a netrin-1–null background, which also contain the Dlx5/6-Cre-IRES-EGFP transgene, enables the inactivation of netrin-1–α3 integrin interactions in interneurons. Dlx5/6-Cre induces recombination throughout the GE and ventral telencephalon from E12.5 onwards (16), and loss of α3 integrin is evident in the interneurons of α3lox/−Dlx5/6-CIE mice (Fig. S1). We compared the patterns of tangential interneuronal migration in double-mutant mice with α3 integrin (α3lox/−Dlx5/6-CIE, netrin-1+/+) or netrin-1 (α3+/+Dlx5/6-CIE, netrin-1−/−) single-mutant mice and in control (α3lox/+Dlx5/6-CIE, netrin-1+/+) mice. Migration patterns were evaluated first at E13.5, during early stages of cortical tangential migration, immediately following Dlx5/6-Cre–mediated inactivation of α3 integrin. We determined the extent of interneuronal migration in the dorsal telencephalon. Because tangential migration through the marginal zone precedes migration through the intermediate zone or subventricular zone, we measured the extent of migration as the distance from the corticostriatal junction to the tip of the leading process of the foremost tangentially migrating neuron in the cortical marginal zone. α3 integrin (α3lox/−Dlx5/6-CIE, netrin-1+/+) and netrin-1 (a3+/+Dlx5/6-Cre, netrin-1−/−) single mutants as well as double-mutant (a3lox/−Dlx5/6-Cre, netrin-1−/−) mice displayed an 8% (SD±1.6), 9% (SD±1.7), and 10% (SD±1.3) reduction in the extent of migration through the marginal zone, respectively, compared with controls (Fig. S3 A–E). Further, binning analysis of interneurons across the developing cerebral wall (i.e., from pial to ventricular surface) indicates a 50% (SD±0.02) reduction in interneurons in the marginal zone of double mutants (Fig. S3 A–D and F-I). We also examined the number of interneurons migrating into the cortex by dividing the dorsal telencephalon, from the corticostriatal junction to the top of the dorsal telencephalon, into 10 equal bins and quantifying interneurons in each of these bins. A significant decrease in the number of EGFP+ interneurons migrating into the dorsal telencephalon in single- and double-mutant mice was evident (data not shown). These results suggest that netrin-1 and α3β1 integrin may regulate very early stages of tangential neuronal migration through the cortical marginal zone.
To explore further the relevance of netrin-1–α3 integrin interactions to cortical tangential migration, we examined migration patterns at E16.5, at the height of interneuronal migration into the developing cerebral wall. Quantification of interneuron distribution within distinct compartments of the cerebral wall indicates that, compared with control mice, α3lox/−Dlx5/6-CIE, netrin-1−/− double-mutant mice exhibit a substantial reduction in EGFP+ interneurons migrating through the marginal zone region of the cortical plate (control, 27% [SD±1.06]; α3lox/−Dlx5/6-CIE, netrin-1−/−, 19% [SD±0.52]) (Fig. S4 A and C). Correspondingly, double mutants display an increased number of EGFP+ cells ectopically migrating through the ventricular zone (control, 28% [SD±1.04]; α3lox/−Dlx5/6-CIE, netrin-1−/−, 37% [SD±0.47]) (Fig. S4 B and D). Both α3 integrin and netrin-1 single-mutant mice exhibited an increase in EGFP+ interneurons ectopically placed in the ventricular zone (control, 28% [SD±1.04]; α3lox/−Dlx5/6-CIE, netrin-1+/+, 32% [SD±0.58]; α3+/+Dlx5/6-CIE, netrin-1−/−, 34% [SD±0.91]) (Fig. S4 B and D). The ratio of interneurons in the marginal zone to those in the ventricular zone indicates the significance of this misplacement and reveals that a substantial number of interneurons in netrin-1–α3 integrin–deficient mice are misrouted (Fig. S4E). These data suggest that netrin-1–α3 integrin interactions may modulate distinct patterns of interneuronal migration within the developing cerebral cortex, especially the interneuronal migration through the marginal zone region of the developing cerebral cortex.
Real-Time Assessment of Interneuronal Migration Patterns Through the Cortical Marginal Zone Reveals Abnormal Migration in α3lox/−Dlx5/6-Cre, Netrin-1−/− Double Mutants.
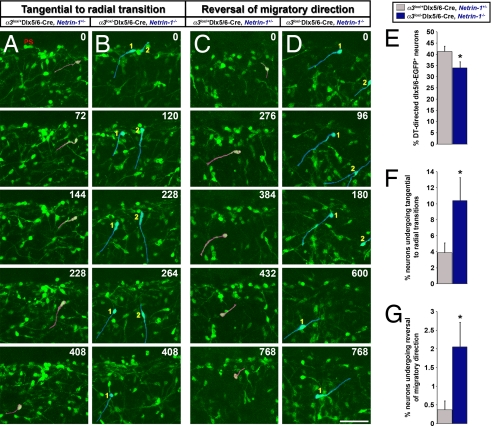
To characterize further these altered patterns of interneuronal migration in vivo, real-time imaging of interneuron migration was performed on control and double-mutant E15.5 embryonic cortices. In the cerebral wall, the leading processes of migrating interneurons are oriented toward the direction of their movement. We found a significant decrease in the number of interneurons migrating toward the dorsal cortex, away from the GE, in α3lox/−Dlx5/6-CIE, netrin-1−/− double mutants when compared with α3lox/+Dlx5/6-CIE, netrin-1+/− controls (control, 41% [SD±2.3]; α3lox/−Dlx5/6-CIE, netrin-1−/−, 34% [SD±2.7]) (Fig. 3E and Movies S1 and S2). Furthermore, compared with controls, a 2.7-fold increase in the number of interneurons switching from tangentially oriented migration along the marginal zone to ventricular surface–directed radial migration is evident in double-mutant mice (Fig. 3 A, B, and F and Movies S3 and S4). This increase in ventricular surface-directed radial migration at the cortical marginal zone may underlie the reduction of interneurons at the marginal zone of α3lox/−Dlx5/6-CIE, netrin-1−/− double mutants. Last, double-mutant mice also displayed a 5.6-fold increase in the number of interneurons undergoing local reversals in the orientation of tangential migration (i.e., interneurons migrate tangentially toward the dorsal cortex, then turn around and move in the opposite direction) in the marginal zone region (Fig. 3 C, D, and G; Movies S5 and S6). These results indicate that netrin-1–α3 integrin interactions are required for interneurons to maintain appropriate, dorsally directed tangential migration along the cortical marginal zone region.
Fig. 3.
Real-time assessment of interneuronal migration patterns through the cortical marginal zone reveals abnormal migration in α3lox/−Dlx5/6-CIE, netrin-1−/− double mutants. (A–D) Live imaging of E15.5 cortices reveals an increase in the number of interneurons transitioning from tangential migration along the marginal zone to ventricular surface-directed radial migration in α3lox/−Dlx5/6-CIE, netrin-1−/− double mutants (B, cells highlighted in blue) compared with α3lox/+Dlx5/6-CIE, netrin-1+/− controls (A, cells highlighted in red). The number of interneurons reversing their tangential migratory orientations in the marginal zone region is increased in α3lox/−Dlx5/6-CIE, netrin-1−/− double-mutant cortices (D, cells highlighted in blue) compared with controls (C, cells highlighted in red). (E) Quantification of the number of interneurons migrating toward the dorsal telencephalon in controls and double-mutant mice. (F–G) Quantification of the number of interneurons transitioning from tangential to radial migration (F) and reversing tangential trajectories (G) through the marginal zone region. Time elapsed since the onset of observations is indicated in minutes. (Scale bar, 50 μm.) Data shown are mean ± SEM (n = 5); *, significant when compared with controls at P < 0.05 (Student's t test). ps, pial surface.
To investigate this deficit further, we performed a slice co-culture assay in which E14.5 MGE explants from control or conditional α3 integrin-mutant mice were placed over the ganglionic eminences of E14.5 wild-type or netrin-1–mutant coronal brain slices. Neuronal migration from the explants onto distinct domains of cortical substrates was assessed 72 h later. α3 integrin-deficient interneurons displayed significant deficits in their ability to migrate onto cortical slices in the absence of netrin-1 (Fig. S5 A, D, E, H, and I). A 25% (SD±0.06) reduction in interneurons traversing the upper cortical plate was noticed (Fig. S5 A, D, E, H, and J). Neuronal migration from wild-type explants onto netrin-1–deficient slices was not affected (Fig. S5 A, C, E, G, and I). Together, these real-time interneuronal migration analyses and slice co-culture assays suggest that netrin-1–α3 integrin signaling facilitates interneuronal migration in the developing cerebral cortex, specifically the subset of interneurons migrating through the top of the cortical plate.
Loss of Netrin-1–α3β1 Integrin Interactions Decrease the Number and Alter the Positioning of Interneurons in Cerebral Cortex.
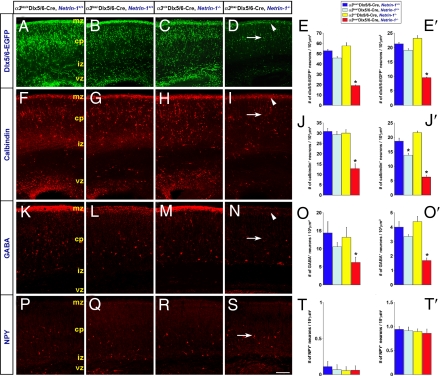
To evaluate the outcome of disrupted netrin-1–α3 integrin interactions on interneuronal organization in vivo, we analyzed the positioning of interneurons in the postnatal day 0 (P0) cerebral cortex, when active interneuronal migration comes to an end. Netrin-1–α3 integrin double-null mice do not survive beyond P0. The migration deficits observed in α3lox/−Dlx5/6-CIE, netrin-1−/− double-mutant mice in vivo predict disruptions in the number and positioning of interneurons in the α3lox/−Dlx5/6-CIE, netrin-1−/− postnatal cortex. Analysis of P0 cortices revealed that the number of EGFP+ interneurons in the upper cortical plate region in double-mutant mice indeed was reduced by 64% compared with α3lox/+Dlx5/6-CIE, netrin-1+/+ controls (Fig. 4A, D, and E). Surprisingly, the total number of EGFP+ interneurons in α3lox/−Dlx5/6-CIE, netrin-1−/− double-mutant cortices also was reduced substantially, to 45% of that observed in controls (Fig. 4 A, D, and E′). This reduction in the number of interneurons in α3lox/−Dlx5/6-CIE, netrin-1−/− double-mutant cortices at P0 may in part be the result of increased apoptosis of misplaced interneurons, because we detected an increase in cleaved caspase-3+ cells in double-mutant cortices compared with controls (Fig. S6). The decrease in cortical interneurons in double mutants is unlikely to be caused by reduced neurogenesis. We did not detect any significant changes in mitotically active progenitors, as assessed by phospho-histone H3 labeling, in the GE ventricular zone of double-mutant forebrains compared with controls (Fig. S6).
Fig. 4.
Loss of netrin-1–α3β1 integrin interactions decreases the number and alters the positioning of interneurons in cortex at P0. (A–D, F–I, K–N) Compared with controls (A, F, K), α3 integrin mutants (B, G, L), or netrin-1 mutants (C, H, M), α3lox/−Dlx5/6-CIE, netrin-1−/− double-mutant mice (D, I, N), display a significant reduction in the number of EGFP+, calbindin+, and GABA+ cells at the marginal zone (D, I, N, arrowhead) and throughout the P0 cortex (D, I, N, arrow). (E-E′, J-J′, O-O′) Quantification of the number of EGFP+, calbindin+, and GABA+ cells in the marginal zone (E, J, O) and cortex (E′, J′, O′). (P–S) No significant changes in the number or positioning of NPY+ interneurons were noticed in the absence of netrin-1–α3β1 integrin interactions (S, arrow). (T-T′) Quantification of the number of NPY+ cells in the marginal zone (T) and cortex (T′). Data shown are mean ± SEM; *, significant when compared with controls at P < 0.05 (one-way ANOVA, Tukey-Kramer posthoc test). cp, cortical plate; ; iz, intermediate zone; mz, marginal zone; vz, ventricular zone. (Scale bar, 170 μm in A–D, 105 μm in F–I, 65 μm in K–N, and 100 μm in P–S.)
To evaluate this disruption in interneuronal organization further, we immunolabeled subpopulations of interneurons with calbindin, calretinin, GABA, somatostatin, neuropeptide Y (NPY), and distal-less homeobox 2 (Dlx2) antibodies. The cerebral wall was divided into 10 equal bins, and interneurons in each of these bins were quantified (Figs. 4 and Fig. S7). Selective changes in interneuronal positioning in the absence of netrin-1–α3 integrin interactions are evident. Strikingly, interneuronal placement in the upper cortical plate is reduced consistently (Fig. 4 D, I, and N, arrowhead). The number of calbindin+ interneurons at the marginal zone (i.e., bin 10, Fig. S7) and throughout the cerebral wall were decreased by 58% and 66%, respectively, in α3lox/−Dlx5/6-CIE, netrin-1−/− brains, compared with controls (Fig. 4 F, I, J, and J′, arrowhead and arrow). Similarly, a substantial decrease in GABA+ interneurons is evident in α3lox/−Dlx5/6-CIE, netrin-1−/− double mutants, particularly at the upper cortical plate region. The number of GABA+ cells at the marginal zone and throughout the cerebral cortex was reduced by 57% and 58%, respectively, in α3lox/−Dlx5/6-CIE, netrin-1−/− brains, compared with controls (Fig. 4 K, N, O, and O′, arrowhead and arrow). Furthermore, similar reductions in Dlx2+ interneurons of 40% and 35% were noticed at the marginal zone (Fig. S7) and throughout the cerebral cortex, respectively, in α3lox/−Dlx5/6-CIE, netrin-1−/− double mutants (Fig. S8 A and D–F). Calretinin+ interneurons in α3lox/−Dlx5/6-CIE, netrin-1−/− brains did not exhibit deficits at the upper cortical plate region but displayed 44% and 36% reductions in number at the ventricular surface and throughout the cerebral wall, respectively, compared with controls (Fig. S7). No significant changes in the number or positioning of NPY+ interneurons were observed in double mutants (Fig. 4 P–T′). Both the conditional α3 integrin single mutants and netrin-1 single mutants displayed deficits in the positioning of interneurons as well. α3 integrin single-mutant mice displayed a 26% reduction in the number and ectopic positioning of calbindin+ interneurons (Fig. 4 F, G, and J′). In netrin-1–deficient mice, a general dispersal of calbindin+ interneurons in the cortical plate was noticed (Fig. 4H). Somatostatin+ interneurons were detected only in the very lateral piriform cortex, where their numbers were decreased in α3lox/−Dlx5/6-CIE, netrin-1−/− double nulls (data not shown).
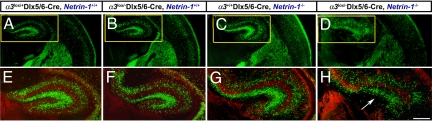
Because the hippocampus represents the end point of long-distance migration from the ventral telencephalon through the marginal zone region of the cerebral cortex (17, 18), analysis of interneuronal positioning in the hippocampal cortex provides another reliable measure of defects in long-distance interneuronal migration through the marginal zone region. We find a significant reduction in the number and organization of EGFP+ interneurons throughout the hippocampus in α3lox/−Dlx5/6-CIE, netrin-1−/− double mutants compared with controls (Fig. 5A, D, E, and H). In sum, these analyses of interneuronal organization indicate that netrin-1–α3 integrin interactions are necessary for the appropriate migration and positioning of distinct subsets of interneurons within the developing cerebral cortex.
Fig. 5.
Disorganization and reduced numbers of hippocampal interneurons in α3lox/−Dlx5/6-CIE, netrin-1−/− mutants at P0. (A–H) A reduction in the number and organization of EGFP+ interneurons throughout the hippocampal subfields of α3lox/−Dlx5/6-CIE, netrin-1−/− double mutants is evident (D and H, arrow) compared with controls (A and E). (Scale bar, 500 μm.)
Discussion
In this study, we provide genetic evidence for the significance of netrin-1–α3 integrin interactions in distinct aspects of interneuronal migration in the developing cerebral cortex. α3β1 integrin is expressed by tangentially migrating interneurons, and netrin-1 is present along their migratory route. In vitro, netrin-1 activates β1 integrin and modulates the migration of interneurons in an α3β1 integrin signaling-dependent manner. In vivo, interneuron-specific α3β1 integrin, netrin-1–deficient mice display deficits in the migration of interneurons in the upper cortical plate region. Significant and selective disruptions in the number and positioning of subsets of interneurons, especially calbindin+, GABA+, Dlx2+, and calretinin+ interneurons, but not NPY+ interneurons, were observed in the absence of netrin-1–α3β1 integrin interactions. Thus, netrin-1–α3β1 integrin interactions guide the appropriate navigation of subsets of GABAergic interneuronal populations through the developing cerebral wall and are necessary for the proper placement of interneurons in the cerebral cortex.
Altered Migration and Positioning of Interneurons in the Absence of Netrin-α3β1 Integrin Signaling.
The heterogeneity of cortical interneuron origins and the differential distribution of interneuron subtypes in the cerebral cortex suggest that cotical interneuron subtypes may take distinct migratory routes into the developing cerebral cortex (see SI Text). Our data show that netrin-1–α3 integrin interactions modulate interneuronal migration through the cortical marginal zone from the beginning at E13.5. Deficits in this migration become progressively more severe in double-mutant mice. By E16.5, the migration of GABAergic interneurons through the upper cortical plate is significantly retarded in α3lox/−Dlx5/6-CIE, netrin-1−/− double mutants (Fig. S4 A and C). Correspondingly, α3lox/−Dlx5/6-CIE, netrin-1−/− double-mutant mice exhibited a significant increase in the number of interneurons aberrantly migrating through the ventricular zone, a region largely devoid of netrin-1 expression (Fig. S4 B and D). Therefore, in the absence of netrin-1–α3β1 integrin interactions, cortical interneurons may take alternate, aberrant routes into the developing cortex. Real-time imaging analysis of tangential migration in E15.5 α3lox/−Dlx5/6-CIE, netrin-1−/− double-mutant mice reveals, at the marginal zone, an increase in tangential to radial transitions directed toward the ventricular zone (Fig. 3 B and F and Movie S4). This increase in ventricular surface-directed radial migration at the cortical marginal zone provides an explanation for the decrease in the number of interneurons at the marginal zone of E16.5 α3lox/−Dlx5/6-CIE, netrin-1−/− double-mutant mice and underscores the relevance of netrin-1–α3β1 integrin interactions for appropriate, dorsal cortex directed tangential migration in this region. Furthermore, the absence of netrin-1–α3β1 integrin interactions also disrupts the local migration of interneurons within the cortical plate (Fig. 3 D and G and Movie S6). It remains to be determined if the aberrant tangential to radial transition of interneurons in double mutants affects the areal-specific distribution of interneurons in the cerebral cortex. By P0, a substantial change in the number and positioning of interneurons within the marginal zone and cerebral wall is observed. No significant changes were evident in interneuronal progenitor proliferation in the GE (Fig. S6). Aberrant neuronal migration patterns resulting in abnormal neuronal positioning may lead to apoptosis of misplaced neurons. Consistently, we observed increased apoptosis in the developing cerebral cortex (Fig. S6). In addition, the presence of a large ectopic aggregation of interneurons in the ventral telencephalon (data not shown) suggests that apoptosis and misrouting of cortical interneurons in the absence of netrin-1–α3β1 integrin interactions may contribute to the deficiency in the number of cortical interneurons in double-mutant mice. Together, these data suggest that netrin-1–α3β1 integrin interactions are necessary for interneurons to sustain directed tangential migration along the upper cortical plate and for the appropriate positioning of interneurons in the developing telencephalon.
Clearly, netrin-1 and α3β1 integrin do not have an exclusive ligand-receptor relationship and the less severe deficits seen in single mutants are suggestive of redundancies in these interactions (see SI Text). Furthermore, how the loss of netrin1-α3β1 integrin interactions may lead to non-cell-autonomous changes in other signaling pathways (e.g., CXCL12/CXCR4; 19, 20) capable of influencing interneuronal also needs to be deciphered.
Deficits in the timing of cortical plate invasion by GABAergic interneurons results in aberrant laminar and regional distribution of cortical interneurons. Specifically, disrupted CXCL12/CXCR4 signaling results in premature migration of GABAergic interneurons into the cortical plate and, consequently, aberrant laminar and regional positioning of cortical interneurons (19, 20). Furthermore, GABAergic interneurons migrating through the marginal zone, including calbindin+ interneurons, exhibit slow multidirectional movement, a local positioning phase, before descent and integration into the cortical plate (21, 22). Disruption of netrin-1–α3β1 integrin–mediated cell migration through the cortical marginal zone may lead to temporal and spatial alterations in the local positioning phase of interneurons and subsequent deficits in the integration of interneurons within the cortical plate. Indeed, the calbindin+ cell distribution of α3lox/−Dlx5/6-CIE, netrin-1−/− double-mutant mice is altered significantly compared with controls, indicating that netrin-1–α3β1 integrin interactions at the cortical marginal zone may modulate the laminar positioning of calbindin+ interneurons within the cortical plate. In contrast to the established “inside-out” gradient of cortical plate invasion, calretinin+ interneurons populate the cortex in an “outside-in” gradient (30). Therefore, netrin-1–α3β1 integrin interactions may differentially influence the guidance of calretinin+ interneurons into the cortical plate compared with other interneuron subpopulations. The reduction of calretinin+ interneurons at the ventricular zone instead of the marginal zone, in the absence of netrin-1–α3β1 integrin interactions, supports this idea. Furthermore, the calbindin+ and calretinin+ cell distribution is affected more severely than the NPY+ cell distribution of α3lox/−Dlx5/6-CIE, netrin-1−/− double-mutant mice, suggesting that netrin-1–α3β1 integrin interactions influence the migration and placement of specific interneuronal subpopulations. Whether the reduced expression of Dlx2+ interneurons in the cerebral wall also is indicative of potential changes in oligodendrocyte cell fate in α3lox/−Dlx5/6-CIE, netrin-1−/− double-mutant mice remains to be determined (31).
Disruptions in the placement and development of cortical interneuron subtypes may underlie the pathogenesis of neurodevelopmental disorders such as epilepsy, schizophrenia, and autism (see SI Text). This emerging evidence for interneuronal subtype–specific dysfunction in a variety of neurodevelopmental disorders highlights the importance of understanding the mechanisms underlying interneuron subtype development. Our study demonstrates that the differential regulation of interneuronal subtype migration by directional guidance molecules, such as netrin-1, ultimately may modulate the placement and connectivity of interneuronal subpopulations in distinct regions of the cerebral cortex and contribute to normal cortical function.
Materials and Methods
Mutant Mouse Strains.
The generation and characterization of α3 integrin floxed alleles is described in Liu et al. (35). See SI Text for details on the generation and characterization of α3lox/−Dlx5/6-CIE, netrin-1−/− double mutants and related controls.
Quantification of Interneuronal Cell Distribution in Cerebral Cortex.
Interneuronal cell counts in the E16.5 and P0 cortex were performed in sections corresponding to the somatosensory cortex as described previously (36). See SI Text for details.
β1 Activation Assay.
Netrin-1–mediated β1-integrin activation in MGE cells was assayed using methods described in SI Text.
Explant Co-cultures.
Explant co-cultures were prepared as described previously (14). For details, see SI Text.
Cortical Slice-MGE Explant Co-cultures.
Slice co-cultures were prepared as described previously, with minor alterations (3). For details, see SI Text.
Real-Time Imaging.
Coronal slices (200 μm) of E15.5 α3lox/+Dlx5/6-CIE, netrin-1+/− control and littermate α3lox/−Dlx5/6-CIE, netrin-1−/− double-mutant brains were imaged repeatedly and analyzed as described earlier (4). For details, see SI Text.
Immunohistochemical, immunoprecipitation, and immunoblotting methods are described in the SI Text.
Supplementary Material
Acknowledgments.
We thank P. Maness and A. Lamantia for helpful comments. This research was supported by National Institutes of Health Grant MH060929 and by a Staglin Award from NARSAD to E.A.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811343106/DCSupplemental.
References
- 1.Ayala R, Shu T, Tsai LH. Trekking across the brain: The journey of neuronal migration. Cell. 2007;128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 2.Lavdas AA, Grigoriou M, Pachnis V, Parnavelas JG. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci. 1999;19:7881–7888. doi: 10.1523/JNEUROSCI.19-18-07881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polleux F, Whitford KL, Dijkhuizen PA, Vitalis T, Ghosh A. Control of cortical interneuron migration by neurotrophins and PI3-kinase signaling. Development (Cambridge, UK) 2002;129:3147–3160. doi: 10.1242/dev.129.13.3147. [DOI] [PubMed] [Google Scholar]
- 4.Yokota Y, et al. Radial glial dependent and independent dynamics of interneuronal migration in the developing cerebral cortex. PLoS ONE. 2007;2:e794. doi: 10.1371/journal.pone.0000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anton ES, Kreidberg JA, Rakic P. Distinct functions of alpha3 and alpha (v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron. 1999;22:277–289. doi: 10.1016/s0896-6273(00)81089-2. [DOI] [PubMed] [Google Scholar]
- 6.Schmid RS, Anton ES. Role of integrins in the development of the cerebral cortex. Cereb Cortex. 2003;13:219–224. doi: 10.1093/cercor/13.3.219. [DOI] [PubMed] [Google Scholar]
- 7.McCarty JH, et al. Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- 8.Graus-Porta D, et al. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31:367–379. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 9.Métin C, Deléglise D, Serafini T, Kennedy TE, Tessier-Lavigne M. A role for netrin-1 in the guidance of cortical efferents. Development. 1997;124:5063–5074. doi: 10.1242/dev.124.24.5063. [DOI] [PubMed] [Google Scholar]
- 10.Yebra M, et al. Recognition of the neural chemoattractant Netrin-1 by integrins alpha6beta4 and alpha3beta1 regulates epithelial cell adhesion and migration. Developmental Cell. 2003;5:695–707. doi: 10.1016/s1534-5807(03)00330-7. [DOI] [PubMed] [Google Scholar]
- 11.Yee KT, Simon HH, Tessier-Lavigne M, O'Leary DM. Extension of long leading processes and neuronal migration in the mammalian brain directed by the chemoattractant netrin-1. Neuron. 1999;24:607–622. doi: 10.1016/s0896-6273(00)81116-2. [DOI] [PubMed] [Google Scholar]
- 12.Ly A, et al. DSCAM is a netrin receptor that collaborates with DCC in mediating turning responses to netrin-1. Cell. 2008;133:1241–1254. doi: 10.1016/j.cell.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serafini T, et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 14.Hong K, et al. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–941. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- 15.Skarnes WC, Moss JE, Hurtley SM, Beddington RS. Capturing genes encoding membrane and secreted proteins important for mouse development. Proc Natl Acad Sci USA. 1995;92:6592–6596. doi: 10.1073/pnas.92.14.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stenman J, Toresson H, Campbell K. Identification of 2 distinct progenitor populations in the lateral ganglionic eminence: Implications for striatal and olfactory bulb neurogenesis. J Neurosci. 2003;23:167–174. doi: 10.1523/JNEUROSCI.23-01-00167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pleasure SJ, et al. Cell migration from the ganglionic eminences is required for the development of hippocampal GABAergic interneurons. Neuron. 2000;28:727–740. doi: 10.1016/s0896-6273(00)00149-5. [DOI] [PubMed] [Google Scholar]
- 18.Yozu M, Tabata H, Nakajima K. The caudal migratory stream: A novel migratory stream of interneurons derived from the caudal ganglionic eminence in the developing mouse forebrain. J Neurosci. 2005;25:7268–7277. doi: 10.1523/JNEUROSCI.2072-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G, et al. Regional distribution of cortical interneurons and development of inhibitory tone are regulated by Cxcl12/Cxcr4 signaling. J Neurosci. 2008;28:1085–1098. doi: 10.1523/JNEUROSCI.4602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.López-Bendito G, et al. Chemokine signaling controls intracortical migration and final distribution of GABAergic interneurons. J Neurosci. 2008;28:1613–1624. doi: 10.1523/JNEUROSCI.4651-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ang ES, Jr, Haydar TF, Gluncic V, Rakic P. Four-dimensional migratory coordinates of GABAergic interneurons in the developing mouse cortex. J Neurosci. 2003;23:5805–5815. doi: 10.1523/JNEUROSCI.23-13-05805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka D, Nakaya Y, Yanagawa Y, Obata K, Murakami F. Multimodal tangential migration of neocortical GABAergic neurons independent of GPI-anchored proteins. Development. 2003;130:5803–5813. doi: 10.1242/dev.00825. [DOI] [PubMed] [Google Scholar]
- 23.Rymar VV, Sadikot AF. Laminar fate of cortical GABAergic interneurons is dependent on both birthdate and phenotype. J Comp Neurol. 2007;501:369–380. doi: 10.1002/cne.21250. [DOI] [PubMed] [Google Scholar]
- 24.Petryniak MA, Potter GB, Rowitch DH, Rubenstein JL. Dlx1 and Dlx2 control neuronal versus oligodendroglial cell fate acquisition in the developing forebrain. Neuron. 2007;55:417–433. doi: 10.1016/j.neuron.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- 26.Robbins RJ, et al. A selective loss of somatostatin in the hippocampus of patients with temporal lobe epilepsy. Ann Neurol. 1991;29:325–332. doi: 10.1002/ana.410290316. [DOI] [PubMed] [Google Scholar]
- 27.Powell EM, et al. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J Neurosci. 2003;23:622–631. doi: 10.1523/JNEUROSCI.23-02-00622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, et al. Coordinate integrin and c-Met signaling regulate Wnt gene expression during epithelial morphogenesis. Development. 2009;136:843–853. doi: 10.1242/dev.027805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yokota Y, Ring C, Cheung R, Pevny L, Anton ES. Nap1-regulated neuronal cytoskeletal dynamics is essential for the final differentiation of neurons in cerebral cortex. Neuron. 2007;54:429–445. doi: 10.1016/j.neuron.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.