Abstract
The proepicardium (PE) is a transient structure that forms at the venous pole of the embryonic vertebrate heart. This cardiac progenitor cell population gives rise to the epicardium, coronary vasculature, and fibroblasts. In the chicken embryo, the PE displays left-right (L-R) asymmetry and develops only on the right side, while on the left only a vestigial PE is formed, which subsequently gets lost by apoptosis. In this study, we analyzed how the L-R asymmetry pathway affects PE formation. Experimental manipulation of left-side determinants such as Shh, Nodal, and Cfc as well as forced expression of Pitx2 had no effect on the sidedness of PE development. In contrast, inhibition of early-acting regulators of L-R axis formation such as H+/K+-ATPase or primitive streak apoptosis affected the sidedness of PE development. Experimental interference with the right-side determinants Fgf8 or Snai1 prevented PE formation, whereas ectopic left-sided expression of Fgf8 or Snai1 resulted in bilateral PE development. These data provide novel insight into the molecular control of asymmetric morphogenesis suggesting that also the right side harbors an instructive signaling pathway that is involved in the control of PE development. This pathway might be of general relevance for setting up L-R asymmetries at the venous pole of the heart.
Keywords: left-right asymmetry, Tbx18, Pitx2, venous pole morphogenesis
The left-right (L-R) axis controls asymmetric organogenesis in vertebrates (1–3). In lower chordates, Xenopus, and chick, breaking of the initial bilateral body symmetry involves the asymmetric distribution of ion channels and transporters, resulting in the development of membrane potential differences between the left and right body side, which may drive an asymmetric transport of small molecules through gap junctions (4, 5). In mammals, Xenopus, and zebrafish, ciliated cells in the organizer generate a fluid flow to the left that transports L-R determinants (6). In mammals, this nodal flow is believed to be the sole mechanism by which L-R asymmetry is initiated (7). In the chicken embryo, asymmetric gene expression in Hensen's node of gastrula stage embryos is an important intermediate step of establishing L-R asymmetry (2). Shh, for example, is asymmetrically expressed on the left side of Hensen's node and induces Nodal expression in a small left-sided domain adjacent to Hensen's node (8). Nodal establishes a large expression domain in the left lateral plate mesoderm (LPM) and induces the expression of Pitx2, a homeobox gene of the bicoid class (7). Pitx2 determines morphological L-R asymmetries in most organ systems (9–12) via modulation of cell–cell adhesion, cell morphology, extracellular matrix composition, spindle orientation, and cell proliferation (13–15).
A right-sided regulatory cascade of gene expression is also initiated in Hensen's node including Bmp4 that induces asymmetric expression of Fgf18 and Fgf8 on the right side (16–18). It is believed that the right side does not initiate its own side-specific morphogenetic program but that Fgf8 induces Snai1, a zinc finger repressor that prevents the right lateral plate mesoderm from assuming left-sided identity through inhibition of Pitx2 (19).
At the venous pole of the embryonic heart, a progenitor cell population is formed, which is termed the proepicardium (PE). The PE cells colonize the developing heart via the formation of a secondary tissue bridge and differentiate into a variety of cell types including the epicardium, the cardiac interstitium, and the coronary vasculature (20). In the chick and frog embryo, the PE only forms on the right side of the embryo (21–23). Therefore, in these species several PE-marker genes normally display a bilaterally asymmetric expression, being strongly expressed on the right and only weakly on the left side (23). The vestigial PE that forms on the left body side ultimately undergoes apoptosis and never establishes contact to the heart (22).
In this study we have tested whether the bilaterally asymmetric development of the chicken PE is controlled by the L-R pathway. In contrast to many other aspects of asymmetric organ formation, we have found that PE development is not affected by the left-sided Nodal/Pitx2 pathway. However, interfering with earlier acting processes of L-R determination, such as asymmetric ion-flux or preventing cell death during early primitive streak formation, randomizes sidedness of PE development. Therefore, we reasoned that a Nodal/Pitx2-independent pathway exists that determines asymmetric PE formation in avians. We show here that this morphogenetic pathway involves Fgf8 and Snai1. Interfering with right-sided Fgf8 and Snai1 prevented PE development, while ectopic expression of Fgf8 or Snai1 on the left side induced bilateral PE formation. These data suggest that both the left and the right body side generate morphogenetic signaling pathways important for asymmetric organ morphogenesis.
Results
Tbx18 Normally Displays Bilateral L-R Asymmetric Expression During PE Development in the Chicken Embryo.
Tbx18 expression was used throughout our experiments as a PE-specific marker gene (22, 23). At Hamburger Hamilton (HH) stage 12 and 13, a Tbx18 expression domain was found on the right sinus horn but was absent on the left sinus horn [supporting information (SI) Fig. S1; (23)]. At HH stage 15, the Tbx18 expression domain on the right side had increased in size, while on the left side a small Tbx18 expression domain became visible (Fig. S1). A 3D reconstruction analysis revealed that the volume of Tbx18 positive PE cells on the right vs. left side differed by more than 3-fold (Fig. 2 E and F). At subsequent stages, the right PE makes contact to the ventricle resulting in the transfer of epicardial cells to the surface of the myocardium (22). The left side, on the other hand, never makes contact to the heart and gets lost through apoptosis. Thus, in normally developing chick embryos there are temporal, quantitative, and qualitative differences in PE development between the left and right body sides.
Fig. 2.
Inhibitors of H+/K+-ATPase and apoptosis randomizes laterality of proepicardial Tbx18 expression. (A-D) Whole mount in situ hybridization analysis of Pitx2 (red color) and Tbx18 (blue color) expression in cultured embryos that were treated with omeprazole. Only the heart region is shown in a ventral view, anterior end up. (A) Embryo with right-sided Tbx18 and left-sided Pitx2 expression domains. (B) Embryo with left-sided Tbx18 and right-sided Pitx2 expression domains. (C) Embryo with bilateral Pitx2 and loss of Tbx18. (D) Embryo with bilateral Tbx18 expression domains and loss of Pitx2. Red and blue arrowheads point to the Pitx2 and Tbx18 expression domains, respectively. (E-H) Fluorescent in situ hybridization of Tbx18 in embryos that were treated with the apoptosis inhibitor Z-VAD-FMK and cultured until HH stage 15. The inflow tract region of (E) control, and (G) inhibitor-treated embryo are shown. Green and red arrowheads point to PE anlagen with right or left identity, respectively. (F and H) 3D reconstructions of the Tbx18 expression domains in the inflow tract region shown in (E and G).
The Nodal-Pitx2 Pathway Does Not Control L-R Asymmetric PE Development.
Pitx2 is expressed in the left sinuatrial region but is absent from the right (24) and was used in most of our experiments to identify tissues with left-sided molecular identity. To analyze the signaling pathways that govern asymmetric development of the chicken PE, we first asked, whether side-specific PE development is the result of left-sided suppression by the Nodal-Pitx2 pathway. In a first set of experiments, we induced ectopic right-sided expression of the Nodal-Pitx2 pathway by implantation of Shh-expressing cells on the right side of Hensen's node at HH stage 4 (Fig. 1A). A total number of 61 embryos underwent implantation at HH stage 4 and were subjected to double-color whole mount in situ hybridization for Pitx2 and Tbx18 at HH stage 12. Nine (15%) of the 61 experimental embryos had bilateral Pitx2 expression domains and all of them displayed the normal right-sided Tbx18 expression pattern (Fig. 1 B–D and L; Table S1).
Fig. 1.
Misexpression of Shh, Nodal, and Pitx2 on the right side does not affect sidedness of Tbx18 expression. (A, E, and I) Schematic depiction of the site of implantation of (A) Shh-expressing cells, (E) beads soaked in Nodal protein, or (I) Pluronic gel containing antisense Cfc oligonucleotides. Embryos were cultured until HH stage 12/13 and subjected to whole mount in situ hybridization analysis of (B-D, J, K, and P) Pitx2 (red color) and Tbx18 (blue color) expression. Red and blue arrowheads point to Pitx2 and Tbx18 expression domains, respectively. Only the heart region is shown in a ventral view, anterior end up. Embryos were treated on the right side of Hensen's node at HH stage 4 with (B-D) Shh-expressing cells, or (J and K) Cfc antisense oligonucleotides. (F and G) Whole mount in situ hybridization analysis of Nodal expression in HH stage 8 embryos after (F) control treatment or (G) implantation of Nodal protein at HH stage 6/7. (H) Tbx18 expression in a Nodal-treated embryo cultured until HH stage 12. (L) Proportion (%) of experimental embryos displaying aberrant Tbx18 or Nodal expression domains subsequent to Shh, Nodal, or antisense CFC treatment. Red bars indicate the percentage of embryos with aberrant Pitx2 expression, and yellow bars indicate the amount of normal left-sided expression. In the case of Nodal implantation, Nodal expression within LPM was analyzed. Blue bars display percentage of embryos with right-sided Tbx18 expression. (M-P) Electroporation of eGFP and Pitx2-RCAS constructs. (M-O) GFP fluorescent signal of an electroporated embryo at (M) HH stage 4, (N) HH stage 7, and (O) HH stage 12. (P) Forced expression of Pitx2 did not affect Tbx18 expression in the right sinus horn.
We next implanted beads soaked with Nodal protein (1 μg/μl) into the right LPM of cultured embryos at HH stage 6/7. To confirm the effectiveness of this procedure, a first set of 24 Nodal-treated embryos was scored for Nodal expression at HH stage 8/9. From these embryos, 10 (42%) displayed an ectopic right-sided Nodal expression domain, while 14 (58%) embryos displayed a left-sided Nodal domain (Fig. 1 E–G, and L; Table S1). A second set of 21 Nodal-treated embryos then was scored for Tbx18 expression at HH stage 12. All of these 21 embryos showed the normal right-sided Tbx18 expression pattern (Fig. 1 H and L; Table S1).
Loss of Cfc, a chick member of the EGF-CFC family of competence factors for Nodal signaling, disrupts the midline barrier formation due to a transient loss of Lefty expression resulting in bilateral expression of Nodal and Pitx2 (25, 26). We thus decided to manipulate the Nodal-Pitx2 pathway additionally by treating embryos with antisense Cfc oligonucleotides. From 48 antisense Cfc-treated embryos, 16 (33%) displayed bilateral Pitx2 expression at HH stage 12 (Fig. 1 I–L, Table S1). However, despite this effect on Pitx2 expression, all of the antisense Cfc oligonucleotide-treated embryos showed a normal right-sided Tbx18 expression pattern.
To finally rule out any direct contribution of Pitx2 to asymmetric PE development, a chick Pitx2a-RCAS construct was electroporated into cardiac progenitor cells residing at HH stage 3 in the primitive streak. To follow the fate of the transfected cells during further development, a CAG-GFP reporter gene was coinjected (Fig. 1 M–O). Due to this manipulation Pitx2 was bilaterally expressed in the entire inflow region; however Tbx18 expression was not affected (n = 14; Fig. 1P, Table S1). We conclude that a left-sided suppression of PE development via the Nodal-Pitx2 pathway is therefore unlikely to be the mechanism that is responsible for the generation of L-R asymmetry of PE development.
Asymmetric PE Development Depends on Regulators of L-R Axis Formation That Act Upstream of the Nodal-Pitx2 Pathway.
In the primitive streak of early gastrulating chick embryos, an asymmetric membrane potential difference is present, which is involved in establishing L-R asymmetry (4). To test whether asymmetric PE development depends on L-R determining processes upstream of the Nodal-Pitx2 pathway, chick embryos were treated at HH stage 3 with omeprazole, an antagonist of the H+/K+-ATPase pump, which is involved in the generation of the asymmetric membrane potential (4). From a total number of 64 treated embryos, 38 (59%) showed normal left-sided Pitx2 expression at HH stage 12 (Table S2). Of the remaining 36 embryos, 7 (11%) displayed right-sided Pitx2 expression, 9 (14%) bilateral, and 10 (16%) complete absence of Pitx2 expression (Fig. 2 A–D, Table S2). Omeprazole treatment also affected Tbx18 expression, which was complementary to that of Pitx2 (Fig. 2 A–D; Table S2). Thus, those embryos with an absence of Pitx2 expression displayed bilateral Tbx18 expression, while embryos with a right-sided Pitx2 expression domain had a left-sided Tbx18 domain. Although we have shown that Pitx2 does not control Tbx18 expression, and that Pitx2 expression can be experimentally altered without affecting Tbx18, in this particular set of experiments the sidedness of both expression domains appeared to be coupled, suggesting the presence of a shared regulatory input that is active during early primitive streak formation.
The primitive streak demarcates the midline border between the left and right side of the early gastrulating embryo and cells do not mingle between sides (27). It has been shown that this midline border functions as a signaling barrier important for the establishment of L-R asymmetries (28). Extensive apoptosis of cells residing in the primitive streak probably contributes to the establishment of this signaling barrier. Experimental inhibition of primitive streak apoptosis results in aberrant L-R development (28). We studied the effects of blocking midline apoptosis at the primitive streak stage on Tbx18 expression at the venous pole of the heart. A total of 22 embryos were treated with the Caspase family inhibitor benzyloxycarbonyl-val-ala-asp (OMe) fluoromethylketone (z-VAD-FMK) during primitive streak formation (HH stages 3–4) and cultured until HH stage 15 when they were subjected to fluorescent in situ hybridization analysis of Tbx18 expression (Fig. 2 E–H, Table S2). Thirteen embryos (59%) showed normal right-sided dominant Tbx18 expression while 9 (41%) displayed bilateral symmetric Tbx18 expression. Since at HH stage 15 the left side also displays a small Tbx18 expression domain (Fig. S1D), the volume of the right and left Tbx18 expression domain was determined after 3-D reconstruction (Fig. 2 F and H). In control embryos (n = 3), the right Tbx18 expression domain had a volume of 3 × 10−4 mm3 and that of the left side was 9 × 10−5 mm3. In embryos with bilateral Tbx18 expression domains (n = 3), equally sized expression domains of 2.8 × 10−4 mm3 volume were found. Thus, we conclude that midline apoptosis is an important determinant for generating asymmetric PE development.
FGF8 Induces PE Development on the Right Side of the Embryo.
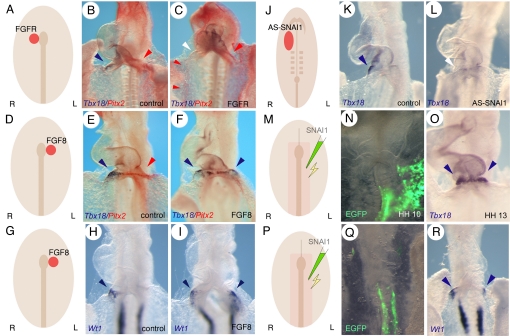
The above-mentioned data showed that early acting determinants of the L-R pathway were involved in the control of asymmetric PE formation, while the later acting left-sided determinants were not. We therefore asked whether right-sided signals might control PE formation. Fgf8 is asymmetrically expressed on the right side of Hensen's node at HH stage 5 in the chicken embryo (16). RCAS virus-infected cells that secreted the ligand binding regions of FGFR1, -2, and -4 were implanted on the right side of Hensen's node at HH stage 4 to interfere with Fgf8 signaling. This treatment resulted in the induction of bilateral Pitx2 expression domains at HH stage 12/13 (n = 11 out of 19, 58%; Fig. 3 A–C, Table S3). Corresponding to omeprazole treated embryos, a complementary expression pattern of Pitx2 and Tbx18 was observed: i.e., those embryos with bilateral Pitx2 expression domains displayed an absence of Tbx18, whereas embryos with left-sided Pitx2 expression displayed right-sided Tbx18 expression. To further corroborate the concept that Fgf8 is involved in the induction of right-sided PE development, we implanted beads soaked in FGF8 protein on the left side of Hensen's node of embryos at HH stage 4/5. It has been previously reported that ectopic Fgf8 represses the expression of Nodal and Pitx2 (16). In our experiments ectopic left-sided FGF8 repressed Pitx2 expression in 10 out of 16 (62%) embryos, while 6 out of 16 (38%) embryos retained normal left-sided expression. (Fig. 3 D–F, Table S3). Those embryos with a loss of Pitx2 displayed bilateral Tbx18 expression and specimen that retained a left-sided Pitx2 domain displayed right-sided Tbx18 expression. We additionally analyzed the expression of Wt1, another PE marker gene (23). Fgf8 induced ectopic left-sided Wt1 expression in 9 out of 14 embryos (64%), while only 5 out of 14 embryos maintained the normal right-sided expression domain (Fig. 3 G–I, Table S3). These data suggest the existence of a right-sided signaling cascade involving Fgf8 that is upstream of asymmetric PE formation in the chicken embryo.
Fig. 3.
FGF8 and Snai1 are essential for right-sided PE development. (A, D, G, J, M, and P) Schematic depiction of the experiments. (B, E, H, and K) are controls and (C, F, I, L, N, O, Q, and R) experimental embryos. Only the heart region is shown in a ventral view, anterior end up of embryos subjected to whole mount in situ hybridization analysis of (B, C, E, and F) Pitx2 (red color) and Tbx18 (blue color), (H, I, R) Wt1, or (K, L, O) Tbx18 expression. (N and Q) GFP signal of electroporated embryos. (A-C) Embryos implanted with an aggregate of FGFR-expressing cells to the right of Hensen's node. (D-I) Embryos implanted with beads soaked in FGF8 protein to the left of Hensen's node. (J-L) Embryos treated with (K) control or (L) antisense Snai1 oligonucleotides. (M-R) Embryos electroporated with Snai1 and eGFP encoding plasmids. Blue arrowheads point to the endogenous and ectopic Tbx18 or Wt1 expression domains. Red arrowheads point to the endogenous and ectopic Pitx2 expression domains. White arrowheads in (C and L) point to the loss of endogenous Tbx18 expression.
Snai1 Induces PE Development on the Right Side of the Embryo.
It has been previously shown that in the chicken embryo Fgf8 induces the transcriptional repressor Snai1, which represses Pitx2 in the right lateral plate mesoderm (16, 19). Starting at HH stage 6, Snai1 is expressed in the right lateral plate mesoderm (29). Expression in the right inflow tract region persists until HH stage 11, and thus expression disappears only shortly before PE marker gene expression starts to appear (29) (Fig. S2). To test the role of Snai1 in PE formation we treated embryos with antisense Snai1 oligonucleotides. Expression of Tbx18 was lost by antisense treatment in 17 out of 24 (71%) embryos demonstrating that Snai1 indeed plays a role in asymmetric PE development (Fig. 3 J–L, Table S3). The role of Snai1 was also tested by a gain-of-function experiment employing electroporation of Snai1 into the left lateral plate mesoderm. We analyzed the expression of Tbx18 and Wt1 in the electroporated embryos (Fig. 3 M–R, Table S3). Both Tbx18 [13 out of 18 (72%) embryos] and Wt1 [7 out of 12 (72%) embryos] were ectopically induced by Snai1, suggesting that a right-sided Fgf8-Snai1 pathway controls asymmetric PE development in the chicken embryo.
Discussion
The PE is a bilaterally paired structure that undergoes asymmetric development in the chicken embryo (21–23). Our present data show that the bilaterally asymmetric PE formation in the chick is a process that is controlled by the L-R pathway. We have shown here that this process depends on early-acting determinants of L-R axis development such as the establishment of membrane potential differences in the primitive streak and on the establishment of a signaling barrier between the right and left epiblast halves through midline apoptosis (Fig. 4). Subsequent to these early-acting processes, Hensen's node becomes asymmetric and side-specific expression domains of Shh on the left and Fgf8 on the right are established. Our data show that the right side-specific genes Fgf8 and Snai1 are required for PE formation, whereas the left side-specific factors Shh, Nodal, or Pitx2 are not required. The Fgf8-Snai1 pathway induces directly or indirectly genes that demarcate PE formation (Tbx18, Wt1, and others). Currently we do not know, whether Pitx2, which is expressed in the left sinus horn, is involved in later aspects of PE development such as the induction of apoptosis in the PE anlage that develops on the left body side.
Fig. 4.
Model of the molecular pathway that controls asymmetric PE development. In the early gastrulating chicken embryo a functional midline is established that divides the left from the right side (red line). Endogenous H+/K+-ATPase-dependent difference in membrane voltage potential exists between the left (−) and right (+) sides of the primitive streak. In the midline, cells are fated to undergo apoptosis (green bar). At HH stages 4–6, asymmetric gene expression within Hensen's node is established leading to the induction of the Nodal-Pitx2 pathway on the left side, while FGF8 induces Snai1 on the right. The Nodal-Pitx2 pathway on the left side has no effect on PE-specific marker gene expression. In contrast, Snai1 on the right side is required for Tbx18 and Wt1 expression.
Bilaterally asymmetric PE development is not a peculiar feature of the chicken embryo but appears to be widely distributed among vertebrates and is probably a prerequisite for transferring PE cells via a tissue bridge to the myocardial surface. PE formation in Xenopus is found to be exclusively right-sided (21). In lamprey and dogfish embryos both body sides generate equal-sized PEs; however only the right sided PE establishes contact with the heart surface via a tissue bridge (30). In contrast, PE development in mice appears to proceed in a bilaterally symmetric fashion so that both PEs deliver progenitor cells to the developing heart (22, 31).
The difference in PE formation between mouse and chick embryos might be reflected by differences in the function of Fgf8, which is a determinant of the right side in the chick, whereas Fgf8 mediates left-sided identity in mice (32, 33). Snai1 appears to be an important determinant of left-right asymmetry in both mouse and chick (34). In both species, asymmetric expression in the right lateral plate mesoderm has been observed; however the precise role of Snai1 in either species is poorly defined (19).
At present it is unclear how many intermediate steps are between Snai1 and PE formation. Expression of PE marker genes at the venous pole of the heart are first seen at HH stage 11 (Tbx18) and HH stage 13 (Wt1) (23). However, the fact that Snai1 expression is present in the right sinus horn at HH stage 11, shortly before Tbx18 expression begins, suggests that Snai1 possibly directly influences PE formation. The limited spatial overlap of the expression domains of ectopic or endogenous Snai1 and PE marker genes is however suggestive of a noncell autonomous mechanism. Further work is required to elucidate how Snai1 affects PE marker gene expression.
In several of our above-mentioned experiments, we observed a strictly complementary expression pattern of Tbx18 and Pitx2. However, since the experimentally induced ectopic expression of Pitx2 did not affect Tbx18 expression at the venous pole of the heart, we could rule out that Pitx2 had any direct influence on Tbx18 expression. In a recent study on gut rotation in the chicken embryo, asymmetric right-sided Tbx18 expression with complementary left-sided Pitx2 expression was found in the dorsal gut mesentery (13). In contrast to our data, however, Pitx2 was found to repress Tbx18 expression at this location. The difference between these and our data might reflect differences in the control of asymmetric Tbx18 expression between the developing gut and the venous pole of the heart.
Recent data from mice have shown that bilaterally asymmetric sinus node formation on the right side of the inflow tract is the result of Pitx2-mediated suppression of development of a left-sided sinus node primordium (35). Assuming that sinus node formation in the chick is also under control of Pitx2, while PE formation is not, would suggest that cardiac inflow tract formation is complex and involves independently acting regulatory modules (36). In mice, several aspects of asymmetric morphogenesis are independent of Pitx2 and include heart looping and positioning of the stomach and spleen, as well as embryo turning (37). Interestingly, both heart looping and embryo turning are abnormal in the Snai1 knockout (34) and thus a right-sided signaling cascade may also exist in mammals.
Experimental Procedures
Generation of Cell Aggregates and Bead Preparation.
Cells of the chicken embryonic fibroblast cell line DF1 (ATCC) were transfected with RCAS-BP(A) constructs encoding Shh (38), FGFR1-FC, encoding the extracellular domain of mouse FGFR1 (splice isoform IIIc), and FGFR2-FC, encoding the extracellular domain of mouse FGF-receptor 2 (splice isoform IIIb), both fused to the FC-fragment of mouse IgG (39); FGFR4-FC encoding the extracellular domain of quail FGFR4 fused to the FC-fragment of human IgG (40) or alkaline phosphatase (41). Heparin acrylic beads (Sigma) were washed 3 times in PBS and incubated for 1 h in 1 μg/ml Nodal or 0,5 μg/ml FGF8b (R&D Systems) (16, 42).
Embryo Manipulation.
White Leghorn eggs (Lohmann) were incubated until the desired stage was reached (43). The chicken embryos were explanted and cultured according to the EC culture method (44). Virus-infected cell aggregates and growth factor-loaded beads were implanted adjacent to Hensen's node or into the lateral plate mesoderm. To interfere with FGF signaling, aggregates of DF-1 cells, which coexpressed FGFR1-FC, FGFR2-FC, and FGFR4-FC (39, 40), were implanted on the right side of the node at HH stage 5. The Cfc antisense and control oligonucleotides were diluted in Pluronic Gel (Sigma) to a final concentration of 20 μM and applied onto Hensen's node as previously described (25). Snai1 antisense and control oligonucleotides (19) were diluted in Pluronic Gel to a final concentration of 60 μM and applied to right lateral plate mesoderm. The proton pump inhibitor omeprazole was diluted in egg albumin to a final concentration of 56 μM containing less than 0.2% DMSO. Explanted embryos were incubated in this solution until they had reached HH stage 5. The apoptosis inhibitor Z-VAD-FMK at a final concentration of 10 μM was applied to the embryo via injection onto the blastodisc in ovo as previously described (28). Z-VAD-FMK-treated embryos were explanted at HH stage 5. After being washed with PBS, embryos were transferred to an EC culture plate and incubated until HH stage 12/13 (omeprazole) or HH stage 15 (Z-VAD-FMK). Cells within the primitive streak were electroporated with Pitx2a-RCAS (45) or a chick Snail1-pcDNA3 construct (46). To visualize the electroporated cells, an eGFP reporter construct (pCAGGS-GFP) was used (47). For electroporation of cultured embryos 2–4 μg/μl DNA was injected into the primitive streak or the left-sided mesoderm adjacent to the streak. Subsequently, 4 pulses of 10–30 V with a pulse length of 20–50ms and a pulse interval of 200–500ms using a Tss20 Ovodyne electroporator were applied to the embryo.
Nonradioactive in Situ Hybridization and 3-D Reconstruction.
Whole mount in situ hybridization was performed as previously described (48). Antisense RNA probes for chicken Tbx18, Nodal, Wt1, Snai1, and Pitx2 were generated from full-length cDNA clones (8, 23, 46, 49). Double color whole mount in situ hybridization was performed as previously described (50). BCIP/NBT (Roche) and Fast Red (Roche) were used as substrates. Fluorescent in situ hybridization (FISH) was performed using the Tyramide Signal Amplification (TSA) system (Perkin-Elmer) (51). A 3D reconstruction of gene expression was done with the Amira 3.0 software as previously described (52). Quantitative data were analyzed using Student's t test. Statistical significance was set at P < 0.05.
Supplementary Material
Acknowledgments.
The donation of plasmids by Constanze Cepko, YiPing Chen, Christophe Marcelle, Annette Neubüser, Angela Nieto, Hidesato Ogawa, and Cliff Tabin is gratefully acknowledged. We thank Jörg Männer and Angela Torlopp for carefully reading the manuscript. We thank Anneliese Striewe-Konz for excellent technical assistance. This work was funded by grants from the Deutsche Forschungsgemeinschaft: BR1218/9-4 and BR1218/12-1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811944106/DCSupplemental.
References
- 1.Raya A, Belmonte JC. Left-right asymmetry in the vertebrate embryo: From early information to higher-level integration. Nat Rev Genet. 2006;7:283–293. doi: 10.1038/nrg1830. [DOI] [PubMed] [Google Scholar]
- 2.Schlueter J, Brand T. Left-right axis development: Examples of similar and divergent strategies to generate asymmetric morphogenesis in chick and mouse embryos. Cytogenet Genome Res. 2007;117:256–267. doi: 10.1159/000103187. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Gracia ML, Ros MA. Left-right asymmetry in vertebrate development. Adv Anat Embryol Cell Biol. 2007;188:1–121. [PubMed] [Google Scholar]
- 4.Levin M, et al. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell. 2002;111:77–89. doi: 10.1016/s0092-8674(02)00939-x. [DOI] [PubMed] [Google Scholar]
- 5.Levin M, Palmer AR. Left-right patterning from the inside out: Widespread evidence for intracellular control. BioEssays. 2007;29:271–287. doi: 10.1002/bies.20545. [DOI] [PubMed] [Google Scholar]
- 6.Hirokawa N, Tanaka Y, Okada Y, Takeda S. Nodal flow and the generation of left-right asymmetry. Cell. 2006;125:33–45. doi: 10.1016/j.cell.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Shiratori H, Hamada H. The left-right axis in the mouse: From origin to morphology. Development. 2006;133:2095–2104. doi: 10.1242/dev.02384. [DOI] [PubMed] [Google Scholar]
- 8.Levin M, et al. A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell. 1995;82:803–814. doi: 10.1016/0092-8674(95)90477-8. [DOI] [PubMed] [Google Scholar]
- 9.Gage P, Hoonkyo S, Camper S. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999;126:4643–4651. doi: 10.1242/dev.126.20.4643. [DOI] [PubMed] [Google Scholar]
- 10.Liu C, et al. Regulation of left-right asymmetry by thresholds of Pitx2c activity. Development. 2001;128:2039–2048. doi: 10.1242/dev.128.11.2039. [DOI] [PubMed] [Google Scholar]
- 11.Liu C, et al. Pitx2c patterns anterior myocardium and aortic arch vessels and is required for local cell movement into atrioventricular cushions. Development. 2002;129:5081–5091. doi: 10.1242/dev.129.21.5081. [DOI] [PubMed] [Google Scholar]
- 12.Lu MF, et al. Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature. 1999;401:276–278. doi: 10.1038/45797. [DOI] [PubMed] [Google Scholar]
- 13.Davis NM, et al. The chirality of gut rotation derives from left-right asymmetric changes in the architecture of the dorsal mesentery. Dev Cell. 2008;15:134–145. doi: 10.1016/j.devcel.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurpios NA, et al. The direction of gut looping is established by changes in the extracellular matrix and in cell-cell adhesion. Proc Natl Acad Sci USA. 2008;105:8499–8506. doi: 10.1073/pnas.0803578105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Leon J, et al. Pitx2 regulates gonad morphogenesis. Proc Natl Acad Sci USA. 2008;105:11242–11247. doi: 10.1073/pnas.0804904105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boettger T, Wittler L, Kessel M. FGF8 functions in the specification of the right body side of the chick. Curr Biol. 1999;9:277–280. doi: 10.1016/s0960-9822(99)80119-5. [DOI] [PubMed] [Google Scholar]
- 17.Monsoro-Burq A, Le Douarin NM. BMP4 plays a key role in left-right patterning in chick embryos by maintaining Sonic Hedgehog asymmetry. Mol Cell. 2001;7:789–799. doi: 10.1016/s1097-2765(01)00223-4. [DOI] [PubMed] [Google Scholar]
- 18.Ohuchi H, Kimura S, Watamoto M, Itoh N. Involvement of fibroblast growth factor (FGF)18-FGF8 signaling in specification of left-right asymmetry and brain and limb development of the chick embryo. Mech Dev. 2000;95:55–66. doi: 10.1016/s0925-4773(00)00331-2. [DOI] [PubMed] [Google Scholar]
- 19.Patel K, Isaac A, Cooke J. Nodal signalling and the roles of the transcription factors SnR and Pitx2 in vertebrate left-right asymmetry. Curr Biol. 1999;9:609–612. doi: 10.1016/s0960-9822(99)80267-x. [DOI] [PubMed] [Google Scholar]
- 20.Männer J, Perez-Pomares JM, Macias D, Munoz-Chapuli R. The origin, formation and developmental significance of the epicardium: A review. Cells Tissues Organs. 2001;169:89–103. doi: 10.1159/000047867. [DOI] [PubMed] [Google Scholar]
- 21.Jahr M, Schlueter J, Brand T, Manner J. Development of the proepicardium in Xenopus laevis. Dev Dyn. 2008;237:3088–3096. doi: 10.1002/dvdy.21713. [DOI] [PubMed] [Google Scholar]
- 22.Schulte I, et al. Morphological and molecular left-right asymmetries in the development of the proepicardium: A comparative analysis on mouse and chick embryos. Dev Dyn. 2007;236:684–695. doi: 10.1002/dvdy.21065. [DOI] [PubMed] [Google Scholar]
- 23.Schlueter J, Manner J, Brand T. BMP is an important regulator of proepicardial identity in the chick embryo. Dev Biol. 2006;295:546–558. doi: 10.1016/j.ydbio.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 24.Campione M, et al. Pitx2 expression defines a left cardiac lineage of cells: Evidence for atrial and ventricular molecular isomerism in the iv/iv mice. Dev Biol. 2001;231:252–264. doi: 10.1006/dbio.2000.0133. [DOI] [PubMed] [Google Scholar]
- 25.Schlange T, et al. Chick CFC controls Lefty1 expression in the embryonic midline and nodal expression in the lateral plate. Dev Biol. 2001;234:376–389. doi: 10.1006/dbio.2001.0257. [DOI] [PubMed] [Google Scholar]
- 26.Linask KK, et al. Effects of antisense misexpression of CFC on downstream flectin protein expression during heart looping. Dev Dyn. 2003;228:217–230. doi: 10.1002/dvdy.10383. [DOI] [PubMed] [Google Scholar]
- 27.Levy V, Khaner O. Limited left-right cell migration across the midline of the gastrulating avian embryo. Dev Genet. 1998;23:175–184. doi: 10.1002/(SICI)1520-6408(1998)23:3<175::AID-DVG3>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 28.Kelly KA, Wei Y, Mikawa T. Cell death along the embryo midline regulates left-right sidedness. Dev Dyn. 2002;224:238–244. doi: 10.1002/dvdy.10098. [DOI] [PubMed] [Google Scholar]
- 29.Isaac A, Sargent MG, Cooke J. Control of vertebrate left-right asymmetry by a snail-related zinc finger gene. Science. 1997;275:1301–1304. doi: 10.1126/science.275.5304.1301. [DOI] [PubMed] [Google Scholar]
- 30.Pombal MA, et al. Epicardial development in lamprey supports an evolutionary origin of the vertebrate epicardium from an ancestral pronephric external glomerulus. Evol Dev. 2008;10:210–216. doi: 10.1111/j.1525-142X.2008.00228.x. [DOI] [PubMed] [Google Scholar]
- 31.Serluca FC. Development of the proepicardial organ in the zebrafish. Dev Biol. 2008;315:18–27. doi: 10.1016/j.ydbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Meyers EN, Martin GR. Differences in left-right axis pathways in mouse and chick: functions of FGF8 and SHH. Science. 1999;285:403–406. doi: 10.1126/science.285.5426.403. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka Y, Okada Y, Hirokawa N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature. 2005;435:172–177. doi: 10.1038/nature03494. [DOI] [PubMed] [Google Scholar]
- 34.Murray SA, Gridley T. Snail family genes are required for left-right asymmetry determination, but not neural crest formation, in mice. Proc Natl Acad Sci USA. 2006;103:10300–10304. doi: 10.1073/pnas.0602234103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mommersteeg MT, et al. Molecular pathway for the localized formation of the sinoatrial node. Circ Res. 2007;100:354–362. doi: 10.1161/01.RES.0000258019.74591.b3. [DOI] [PubMed] [Google Scholar]
- 36.Anderson RH, Brown NA, Moorman AF. Development and structures of the venous pole of the heart. Dev Dyn. 2006;235:2–9. doi: 10.1002/dvdy.20578. [DOI] [PubMed] [Google Scholar]
- 37.Shiratori H, Yashiro K, Shen MM, Hamada H. Conserved regulation and role of Pitx2 in situs-specific morphogenesis of visceral organs. Development. 2006;133:3015–3025. doi: 10.1242/dev.02470. [DOI] [PubMed] [Google Scholar]
- 38.Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- 39.Mandler M, Neubuser A. FGF signaling is required for initiation of feather placode development. Development. 2004;131:3333–3343. doi: 10.1242/dev.01203. [DOI] [PubMed] [Google Scholar]
- 40.Marics I, et al. FGFR4 signaling is a necessary step in limb muscle differentiation. Development. 2002;129:4559–4569. doi: 10.1242/dev.129.19.4559. [DOI] [PubMed] [Google Scholar]
- 41.Fekete DM, Cepko CL. Replication-competent retroviral vectors encoding alkaline phosphatase reveal spatial restriction of viral gene expression/transduction in the chick embryo. Mol Cell Biol. 1993;13:2604–2613. doi: 10.1128/mcb.13.4.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tavares AT, Andrade S, Silva AC, Belo JA. Cerberus is a feedback inhibitor of Nodal asymmetric signaling in the chick embryo. Development. 2007;134:2051–2060. doi: 10.1242/dev.000901. [DOI] [PubMed] [Google Scholar]
- 43.Hamburger V, Hamilton H. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 44.Chapman SC, Collignon J, Schoenwolf GC, Lumsden A. Improved method for chick whole-embryo culture using a filter paper carrier. Dev Dyn. 2001;220:284–289. doi: 10.1002/1097-0177(20010301)220:3<284::AID-DVDY1102>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 45.Yu X, et al. Differential expression and functional analysis of Pitx2 isoforms in regulation of heart looping in the chick. Development. 2001;128:1005–1013. doi: 10.1242/dev.128.6.1005. [DOI] [PubMed] [Google Scholar]
- 46.Morales AV, et al. Snail genes at the crossroads of symmetric and asymmetric processes in the developing mesoderm. EMBO Rep. 2007;8:104–109. doi: 10.1038/sj.embor.7400867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Momose T, et al. Efficient targeting of gene expression in chick embryos by microelectroporation. Dev Growth Differ. 1999;41:335–344. doi: 10.1046/j.1440-169x.1999.413437.x. [DOI] [PubMed] [Google Scholar]
- 48.Andrée B, et al. BMP-2 induces ectopic expression of cardiac lineage markers and interferes with somite formation in chicken embryos. Mech Dev. 1998;70:119–131. doi: 10.1016/s0925-4773(97)00186-x. [DOI] [PubMed] [Google Scholar]
- 49.St Amand TR, et al. Cloning and expression pattern of chicken Pitx2: A new component in the SHH signaling pathway controlling embryonic heart looping. Biochem Biophys Res Commun. 1998;247:100–105. doi: 10.1006/bbrc.1998.8740. [DOI] [PubMed] [Google Scholar]
- 50.Stern C. Detection of multiple gene products simultaneously by in situ hybridization and immunohistochemistry in whole mounts of avian embryos. In: Pablo F, Ferrús A, Stern C, editors. Cellular and Molecular Procedures in Developmental Biology. San Diego: Academic; 1998. pp. 223–243. [DOI] [PubMed] [Google Scholar]
- 51.Acloque H, Wilkinson DG, Nieto MA. In situ hybridization analysis of chick embryos in whole-mount and tissue sections. Methods Cell Biol. 2008;87:169–185. doi: 10.1016/S0091-679X(08)00209-4. [DOI] [PubMed] [Google Scholar]
- 52.Soufan AT, et al. Three-dimensional reconstruction of gene expression patterns during cardiac development. Physiol Genomics. 2003;13:187–195. doi: 10.1152/physiolgenomics.00182.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.