Abstract
Neural inhibition within the thalamus is integral in shaping thalamocortical oscillatory activity. Fast, synaptic inhibition is primarily mediated by activation of heteropentameric GABAA receptor complexes. Here, we examined the synaptic physiology and network properties of mice lacking GABAA receptor α3, a subunit that in thalamus is uniquely expressed by inhibitory neurons of the reticular nucleus (nRT). Deletion of this subunit produced a powerful compensatory gain in inhibitory postsynaptic response in nRT neurons. Although, other forms of inhibitory and excitatory synaptic transmission in the circuit were unchanged, evoked thalamic oscillations were strongly dampened in α3 knockout mice. Furthermore, pharmacologically induced thalamocortical absence seizures displayed a reduction in length and power in α3 knockout mice. These studies highlight the role of GABAergic inhibitory strength within nRT in the maintenance of thalamic oscillations, and demonstrate that inhibitory intra-nRT synapses are a critical control point for regulating higher order thalamocortical network activity.
Keywords: benzodiazepine, epilepsy, inhibition, knockout
Neural networks within the thalamocortical system are capable of generating rhythmic activity. These oscillations are correlative to normal behaviors, such as 7–14 Hz spindles displayed during sleep, but can be hallmarks of neurological disorders, such as the bilateral and hypersynchronous 3 Hz spike and wave discharge (SWD) produced during absence epileptic seizures (1, 2). The cellular and molecular basis for both normal and pathological thalamic oscillations has been extensively studied, and demonstrated to rely upon the balance of excitatory and inhibitory inputs between populations of reciprocally interconnected neurons (3). The thalamic reticular nucleus (nRT) contains GABAergic neurons that surround and innervate the dorsal thalamus, providing synaptic inhibition onto excitatory thalamocortical relay neurons (TC) (4). The firing of nRT neurons produces IPSPs on TC neurons, and this hyperpolarization deinactivates low threshold T-Type calcium channels and evokes TC rebound bursting (5). TC neurons, in turn, project collaterals back to the nRT, and reexcite nRT neurons leading to intrathalamic oscillations that integrate with and sustain thalamocortical network oscillations.
An integral component of thalamocortical circuitry is fast inhibition, primarily mediated by activation of postsynaptic GABAA receptors (GABAARs). These receptors are heteropentameric ligand-gated chloride channels formed from an array of 16 identified subunits (α1–6, β1–3, γ1–3, δ, ε, π, and θ) (6). At synapses, GABAARs likely contain 2α's: 2β's: 1γ, and inclusion of different subunits into the pentamer can confer unique biophysical and pharmacological properties, including: conductance, gating, affinity, and sensitivity to allosteric modulators (7). Immunohistochemical localization studies have shown that the thalamus displays nucleus-specific differences in GABAAR subunits (8, 9). Neurons in the nRT primarily express α3, β3, and γ2 subunits, whereas TC neurons, for example those in the ventrobasal nucleus (VB), contain α1, α4, β2 γ2 and δ. These subunits have been proposed to assemble into 3 physiologically distinct receptor subtypes: α3β3γ2 receptors mediate phasic inhibition in nRT neurons (10), α1β2γ2 receptors phasic inhibition in TC neurons, and α4β2δ containing receptors a tonic inhibition (11) in TC neurons. This heterogeneity provides an opportunity to investigate the physiology of inhibition mediated by specific subunits in discrete nuclei in the thalamus, and reveal their corresponding roles in the generation of rhythmic activity.
Recently, a mouse line with the GABAAR α3 subunit gene deleted (α3KO) has been generated and described (12–14). Behaviorally, α3KO mice present a mild phenotype, with no overt disturbances to thalamocortical function, despite a complete loss of α3 subunit protein in the nRT. Because the α3 subunit is the only α subunit detectable in the nRT and is not localized elsewhere in the thalamus (9) we hypothesize that these mice would show disturbances in synaptic inhibition in the nRT and provide a model system to study the physiology of α3 mediated synaptic inhibition within thalamocortical circuitry, as predicted from previous studies in β3 subunit mutants (15).
Results
Inhibitory Postsynaptic Currents in the Thalamus Are Altered in the nRT of α3KO Mice.
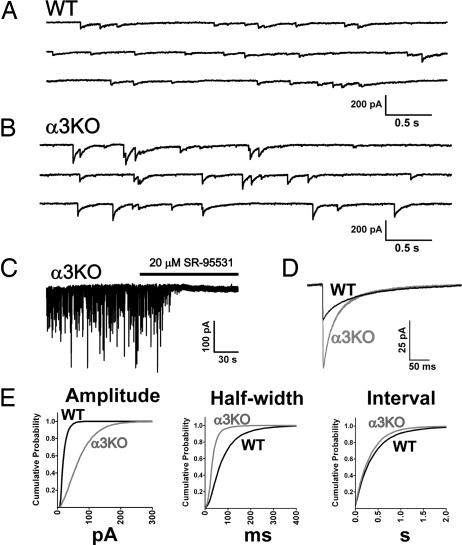
To investigate the impact of deletion of the GABAAR α3 subunit gene on neurotransmission in the thalamus, we performed whole-cell patch clamp recordings on wild-type (WT) and α3KO brain slices. Despite the loss of this major subunit, electrophysiological recordings detected robust spontaneous inhibitory postsynaptic current (IPSC) events in α3KO nRT neurons (representative trace Fig. 1B) in all neurons examined (26/26 cells from 15 animals). Events were clearly GABAAR mediated, as demonstrated by their characteristic chloride conductance and complete blockade by 20 μM the specific GABAAR antagonist SR-95331 (Fig. 1C). The GABAAR antagonists bicuculline and picrotoxin also abolished IPSCs in the nRT of α3KO mice and did not shift the baseline holding current, indicating that there were not compensatory changes in the GABA tonic conductance. This was further examined through analysis of the variance of the baseline noise current, which was similar between genotypes (WT = 2.2 ± 0.2 pA, n = 14; α3KO = 2.4 ± 0.1 pA, n = 14, P > 0.05)
Fig. 1.
Deletion of the GABAA receptor α3 subunit alters synaptic inhibition in the thalamus. Ten seconds of IPSC recordings from representative nRT neurons from (A) WT and (B) α3KO mice. Events in α3KO nRT were GABAA receptor mediated, as demonstrated by (C) complete blockade of events by 20 μM the specific antagonist SR-95331. (D) Ensemble averaged IPSCs from WT (black, n = 25 cells) and α3KO (gray, n = 26 cells) nRT neurons, plotted on the same time scale to illustrate the differences in amplitude and kinetics. (E) Cumulative probability histograms of >5000 isolated events from WT (n = 5 neurons) and α3KO (n = 5) demonstrate changes in amplitude and kinetics across all populations of events.
We next examined the parameters of isolated IPSC events and compared these between WT and α3KO. The kinetics of IPSCs in nRT of WT rodents have been described, and are characterized by a long-lasting, slowly deactivating current (Fig. 1 A, D) (16). We analyzed the mean IPSC response from multiple nRT neurons from WT (n = 25) and α3KO (n = 26). In both genotypes, IPSC events activated in a rapid manner (10–90% rise times: WT = 1.2 ± 0.1 ms; α3KO = 1.2 ± 0.1 ms, P > 0.05). Most significantly, IPSCs in α3KO displayed a large increase in amplitude compared with wild type (WT = 23 ± 1 pA; α3KO = 60 ± 4 pA, P < 0.001). Additionally, decay kinetics were significantly faster in α3KO, measured as both the event half-width (WT = 60 ± 3 ms; α3KO = 32 ± 1 ms, P < 0.001) and the weighted biexponential decay time (WT τD,W = 112 ± 3 ms; α3KO τD,W = 60 ± 2 ms, P < 0.001). These changes in amplitude and kinetics were observed across all populations of events, as demonstrated by the cumulative probability histograms (Fig. 1E). There were no differences in IPSC frequencies between genotypes (WT = 2.4 ± 0.2 Hz; α3KO = 2.9 ± 0.3 Hz, P > 0.05). We also examined the “miniature” IPSC population using bath application of 1 μM tetrodotoxin, and events displayed similar parameters between WT and α3KO (supporting information (SI) Fig. S1) indicating that the observed gain in amplitude in α3KO neurons was not attributable to an increase in the number of action potential mediated events. Additionally, we sorted and analyzed IPSCs according to different ages and sex, and found that the aforementioned alterations in IPSC parameters were not attributable to a transient developmental effect or sexual dimorphism, as we observed the characteristic large-amplitude, faster-decaying type of IPSCs in all ages of α3KO mice tested (postnatal days 12 through 35) and in both males and females.
To further characterize inhibitory neurotransmission in nRT, we examined evoked IPSC events (eIPSCs) in WT and α3KO neurons (Fig. S2). Using a protocol of minimal electrical stimulation within the nRT, we recorded isolated eIPSC events from nRT neurons (WT n = 8; α3KO n = 14). Similar to spontaneous event data, α3KO eIPSCs displayed a significant increase in amplitude (WT = 62 ± 10 pA; α3KO = 296 ± 43 pA, P < 0.01) and faster decay kinetics (WT τD,W = 195 ± 11 ms; α3KO τD,W = 85 ± 8 ms, P < 0.01) compared with wild-type. The charge transfer per event, calculated as the integral area, was also larger in α3KO neurons (WT = 8.5 ± 1.2 pC; α3KO = 17.7 ± 2.5 pC, P < 0.05). Together, these data show that deletion of the α3 subunit produces a paradoxical gain in inhibitory efficacy of synaptic transmission in nRT and increases the total inhibitory current onto nRT neurons.
Changes in Sensitivity to Allosteric Modulators of GABAA Receptors in the nRT of α3KO Mice.
The observed differences in IPSC amplitude and kinetics between genotypes indicate a change in the subunit composition of the postsynaptic receptor in α3KO mice. To investigate this, we used subunit specific pharmacology to probe the identity of GABAARs in nRT. Clonazepem (CZP) is a benzodiazepine and positive allosteric modulator of GABAARs, which invariably requires the presence of a γ subunit in the receptor complex to exert its actions (17). In WT mice, 300 nM CZP robustly increased the amplitude (predrug: 23 ± 1 pA; CZP: 28 ± 1 pA, n = 7, P < 0.01) and decay time (predrug: τD,W = 107 ± 12 ms; CZP: τD,W = 156 ± 17 ms, n = 7, P < 0.05) of IPSCs in the nRT (Fig. 2A), a result that is consistent with postsynaptic currents in this nucleus mediated by GABAARs containing the γ2 subunit (17). In α3KO neurons, CZP retained its modulatory effects on IPSCs in nRT, but the major action was increasing the decay kinetics (predrug: τD,W = 61 ± 2 ms; CZP: τD,W = 83 ± 5 ms, n = 6, P < 0.05), whereas the effects on event amplitude were not significant (predrug: 49 ± 9 pA; CZP: 52 ± 9 pA, n = 6, P > 0.05). These results demonstrate that the γ2 subunit and benzodiazepine binding site remain intact in α3KO GABAARs, but the altered pharmacological effects of CZP on IPSC shape suggest additional changes to the postsynaptic subunit combination.
Fig. 2.
Altered pharmacology at α3KO nRT synapses. The benzodiazepine (A) clonazepam (CZP, 300 nM) and the β2/3 selective modulator (B) loreclezole (LOR, 10 μM) augment the amplitude, decay kinetics, and integral area of IPSCs in WT nRT neurons. Although effects are present in α3KO neurons, the main actions are upon decay kinetics and integral area. Conversely, (C) zolpidem (ZOL, 1 μM) increases the decay and integral area of IPSCs in WT, but has no effect on α3KO currents, suggesting altered α subunit composition of GABAA receptors in α3KO mice. (D) Summaries of the changes in IPSC changed induced by CZP, LOR and ZOL (*, P < 0.05).
Next, we examined the activity of loreclezole (LOR), a modulator of GABAARs that specifically activates receptors containing either β2 or β3 subunit, but not receptors containing β1 (18). 10 μM LOR (Fig. 2B) increased the amplitude (control: 23 ± 1 pA; LOR: 27 ± 1 pA, n = 11, P < 0.05) and decay kinetics (control: τD,W = 112 ± 3 ms; LOR: τD,W = 183 ± 21 ms, n = 11, P < 0.001) of IPSCs in WT neurons, consistent with high levels of β3 subunit expression in nRT. Similar to the effects of CZP, LOR did not alter IPSC amplitude in α3KO nRT neurons (control: 60 ± 4 pA; LOR: 53 ± 4 pA, n = 8, P > 0.05), but did slow decay rate (control: τD,W = 60 ± 2 ms; LOR: τD,W = 132 ± 8 ms, n = 8, P < 0.001). These data indicate that the β3 subunit is likely retained in postsynaptic receptors of α3KO nRT neurons, and interestingly, these receptors appear more sensitive to the effects of LOR, suggesting possible increased β3 subunit dependence.
Next, we examined the effects of the benzodiazepine site agonist zolpidem (ZOL) on IPSCs in the nRT. This compound displays high affinity for GABAARs containing the α1 subunit, intermediate affinity for receptors containing α2 and α3, and no affinity for receptors containing α5 (19). Accordingly, we used 1 μM ZOL, a high concentration that activates α1, α2 and α3 subunits. In WT nRT neurons, ZOL (Fig. 2C) produced no increase in IPSC event amplitude (predrug: 20 ± 2 pA; ZOL: 23 ± 3 pA, n = 5, P > 0.05), but did significantly prolong decay kinetics (predrug: τD,W = 115 ± 7 ms; ZOL: τD,W = 135 ± 12 ms, n = 5, P < 0.05). On α3KO nRT neurons, ZOL had no observable modulatory action, affecting neither amplitude (predrug: 56 ± 7 pA; ZOL: 54 ± 7 pA, n = 7, P > 0.05) nor decay kinetics (predrug: τD,W = 60 ± 4 ms; ZOL: τD,W = 62 ± 5, n = 7, P > 0.05). This result confirms the absence of α3 subunit and indicates that α1 or α2 subunits are not expressed at inhibitory synapses in α3KO nRT neurons, a result that is consistent with immunohistochemical localization studies in these mice (13).
The major GABAAR benzodiazepine binding sites in the brain are formed by the γ2 subunit in combination with α1, α2, α3 or α5 subunits (7, 17). Accordingly, the previous data cannot exclude the possibility that GABAARs in the nRT of α3KO neurons contain α5. To examine this, we used L-655,708, a potent selective inverse agonist for the benzodiazepine binding site, which at low nanomolar concentrations displays high specificity for the α5 subunit (20). However, 20 nM L-655,708 had no effect on IPSCs amplitude or decay kinetics in α3KO nRT neurons (Fig. S3), suggesting that the α5 subunit is not contained in these synaptic GABAARs. Our pharmacological results are consistent with a change in the subunit composition of the postsynaptic receptor of α3KO mice. Although α3KO GABAARs retain sensitivity to benzodiazepines and loreclezole, indicating β and γ are present, their major pharmacological effects were on IPSC decay kinetics, not amplitude. In addition, ZOL and L-655,708, compounds that modulate receptors containing specific α subunits, had no affect in α3KO mice, suggesting an alteration of α subunits in α3KO nRT.
Other Network Features Are Unaltered in α3KO Mice.
A caveat of gene knockout methodology is the potential for multiple in vivo compensatory changes that might obscure the precise phenotype. To determine if deletion of the GABAAR α3 subunit produces other changes within the thalamic circuit, we examined 2 additional types of synaptic transmission and intrinsic excitability of nRT cells. TC relay neurons receive inhibitory input from nRT neurons, and we analyzed isolated IPSCs in VB neurons from WT (n = 9) and α3KO (n = 11) mice (Fig. 3 A–C). The amplitude (WT = 42 ± 5 pA; α3KO = 38 ± 2 pA, P > 0.05), frequency (WT = 5.6 ± 0.8 Hz; α3KO = 5.3 ± 0.8 Hz, P > 0.05), half-width (WT = 15 ± 1 ms; α3KO = 15 ± 1 ms, P > 0.05) and decay kinetics (WT: τD,W = 24 ± 2 ms; α3KO: τD,W = 23 ± 3 ms, P > 0.05) were not statistically significant between genotypes. This result validates the specificity of the α3KO effect for the nRT, because TC neurons in the VB have been demonstrated to only express mRNA and protein for α1 and α4 subunits (8, 9).
Fig. 3.
Other aspects of thalamic synaptic transmission were unaltered in α3KO mice. (A) Representative 10 second recordings showing IPSCs in VB in WT and α3KO, scale bars indicate 100 pA; 500 ms. (B) Ensemble IPSC responses averaged across multiple WT (n = 9) and α3KO (n = 11) VB neurons and (C) cumulative probability histograms of >6000 isolated events demonstrate no changes in amplitude, kinetics or frequency between genotypes. Excitatory neurotransmission in the nRT is also unaffected. (D) Isolated EPSC events from representative 5 second recordings from WT and α3KO nRT neurons, scale bars 50 pA; 200 ms. (E) Ensemble EPSC responses averaged from WT (n = 14) and α3KO (n = 14) nRT neurons and (F) cumulative probability histograms of >4000 isolated EPSC events from each genotype demonstrate no changes in amplitude, kinetics or frequency.
Because neural circuitry requires a precise balance of inhibition and excitation, the increased efficacy of inhibitory neurotransmission in α3KO mice might produce an activity dependent compensatory down-regulation of excitatory postsynaptic current events (EPSCs). To examine this, we recorded isolated sEPSCs from neurons in the nRT of WT (n = 14) and α3KO (n = 14) thalami (Figs. 3 D–F). Again, we observed no significant changes in the amplitude (WT = −21 ± 2 pA; α3KO = −22 ± 2 pA, P > 0.05), frequency (WT = 4.0 ± 0.5 Hz; α3KO = 4.5 ± 0.5 Hz, P > 0.05) or event kinetics (half-width: WT = 1.5 ± 0.1 ms; α3KO = 1.4 ± 0.1 ms, P > 0.05). In addition, we found no changes in intrinsic excitability of nRT neurons (Fig. S4). Overall these results demonstrate the specificity of the gain in inhibitory synaptic strength in nRT in α3KO and show this effect is not accompanied by concomitant changes in intrinsic excitability or excitatory neurotransmission.
Deletion of the GABAAR α3 Subunit Reduces Evoked Thalamic Oscillatory Activity.
The synaptic data show that α3KO mice present a unique neurophysiological phenotype, in which a single component of the thalamocortical circuit has been altered in a gain of function manner, with no apparent changes to other types of synaptic transmission. To investigate the effects of increased nRT inhibitory strength on thalamocortical circuit function, we examined the properties of evoked thalamic oscillations. Delivering a single electrical shock to the internal capsule of thalamic slices elicits rhythmic spiking activity that can be detected via extracellular unit recordings within VB (21, 22). In WT slices (n = 11), evoked thalamic oscillations are robust (Fig. 4A), lasting several seconds (mean duration = 2.20 ± 0.25 s) and producing several hundred spikes (mean spike count = 244 ± 44). By contrast, α3KO thalamic slices (n = 18) displayed a significant decrease in the length of evoked oscillations (mean duration = 1.12 ± 0.14 s, Fig. 4B) and total number of spikes (mean spike count = 126 ± 19, Fig. 4B). These data support the conclusion that thalamic oscillations are modulated by GABAAR mediated inhibitory synaptic transmission within the nRT. Specifically, the duration of network activity is inversely proportional to strength of intra-nRT connectivity, which is consistent with the increased duration and strength of thalamic oscillatory activity in GABAAR β3 knockout mice that have reduced inhibitory transmission in nRT (15).
Fig. 4.
Evoked oscillations are suppressed in thalamic slices from α3KO mice (A) Representative multiunit recordings of intra-thalamic oscillations from WT and α3KO slices elicited in brain slices by single electrical shocks (arrows indicate stimulus artifacts). Insets show a poststimulus time histograms of multiunit spike rates during the oscillations (horizontal scale: 500 ms, vertical scale: 200 Hz). (B) Summary data for evoked thalamic oscillations. The number of spikes and the duration were both significantly reduced in α3KO slices (*, P < 0.05)
α3KO Mice Display a Reduction in Duration and Strength of Induced Absence Seizures.
Absence seizures are electrically characterized by hypersynchronous cortical activity of thalamic origin (23). The drug γ-butyrolactone (GBL) induces thalamocortical seizures characteristic of absence epilepsy, which accompany the behavioral features of seizures, including motor freezing and staring (24). We recorded cortical electroencephalogram (EEG) activity during GBL induced seizures in WT and α3KO mice. During baseline awake conditions, mice produced little or no detectable SWD activity (Fig. 5 A and B), and the peak power in the frequency range of 0.8 to 10 Hz was <1.5 Hz. Subcutaneous injection of 100 mg/kg GBL induced behavioral seizures that accompanied an increase in 2–4 Hz SWD with onset 5–10 min postinjection (Fig. 5 A and B). We analyzed individually isolated SWDs for the period of 60 min after GBL injections from WT (n = 5) and α3KO mice (n = 5). Seizure activity was observed in both genotypes and the occurrence of seizures were similar (WT = 8.7 ± 0.9 SWDs/minute; α3KO = 9.3 ± 0.2 SWDs/minute, P > 0.05), as was the predominant peak spectral frequency (WT = 2.5 ± 0.1 Hz; α3KO = 2.3 ± 0.1 Hz, P > 0.05). However, α3KO mice displayed a significant decrease in the average SWD duration (WT = 2.7 ± 0.2 s; α3KO = 1.9 ± 0.1 s, P < 0.01) and average SWD power (WT = 1.05 ± 0.15 mV2; α3KO = 0.62 ± 0.09 mV2, P < 0.05). To further validate these results, we also examined absence seizures using a second pharmacological model. Pentylenetrazol (PTZ, 25 mg/kg) is a GABAAR antagonist that evokes 3–6 Hz SWD in vivo (25). Analysis of EEG recordings (Fig. S5) showed that PTZ seizures were similarly affected by deletion of α3 as GBL seizures; genotype did not alter the seizure rate or peak spectral frequency, but α3KO mice displayed a reduction in the power and length of PTZ seizure events. Therefore, in 2 pharmacological models of absence seizures, the frequency and characteristic spectral power of SWDs are not affected by deletion of the α3KO subunit, suggesting that pharmacological initiation of SWD seizures is independent of inhibitory strength in nRT. By contrast, seizure duration and power were both affected in the α3KO, consistent with the hypothesis that strength of nRT inhibition strongly regulates seizure duration and severity.
Fig. 5.
Pharmacologically induced absence seizures are reduced in α3KO mice. (A) Representative EEG recordings from right frontal (RF) and left frontal (LF) cortex from a WT mouse during baseline and 15 minutes after injection of GBL (100 mg/kg). SWDs are clearly detectable bilaterally synchronous 2–4 Hz waveform events that emerge 5–10 min after GBL administration. (B) Comparison of 80-min trials of continuous EEG recording from frontal cortex from representative WT and α3KO mice. The arrow marks the GBL injection time point. (C) Summaries of wavelet analysis: SWD power and duration were significantly reduced in α3KO mice (WT n = 5; α3KO n = 5; *, P < 0.05, **, P < 0.01), whereas SWD peak spectral power and SWD rate were not different between genotypes (P > 0.05). (D) Example traces of representative SWD events in WT and α3KO mice.
Discussion
We found that mice lacking the GABAAR α3 subunit display profound changes in the physiology of inhibitory synaptic currents in the nRT. These events are atypically large in amplitude and display faster decay kinetics than those present in wild-type neurons, properties observed in all spontaneous, miniature and evoked synaptic recordings. Measured as a product of the charge transfer per IPSC event and frequency, α3KO nRT neurons receive more inhibitory current than wild-type neurons. At the network level, this specific increase in inhibitory strength in nRT dampens the duration of evoked isolated thalamic oscillations, and decreases the duration and power of pharmacologically induced absence seizures. These data highlight the role of GABAergic inhibition within the nRT as a critical control point for the excitability of the thalamocortical circuit.
Inhibition within thalamic circuitry is established at functionally diverse GABAergic synapses, and this study strongly supports the hypothesis that α3 containing receptors expressed by nRT neurons normally function in an anti-oscillatory capacity. Anatomically, nRT neurons project collaterals onto adjacent nRT neurons that form axo-dendritic and dendro-dendritic synapses, which interconnect a powerful inhibitory network of cells that control the output of the dorsal thalamus to the cortex (26, 27). Previous studies have provided the initial evidence that inhibitory intra-nRT synapses are integral in the oscillatory process. First, it was demonstrated that increasing inhibition within the nRT pharmacologically can dampen thalamic oscillations (28), and genetically engineered knock-in mice, in which the α3 subunit was rendered insensitive to benzodiazepines, failed to display a reduction in evoked thalamic oscillation duration when treated with clonazepam. We can also compare the results of this study to an earlier finding with mice lacking the β3 subunit (15, 29). β3KO mice display a severe phenotype that includes prevalent neonatal lethality, seizures, and powerful, hypersynchronous thalamic oscillations. Synaptic recordings in the nRT of β3KO show a near absence of sIPSCs and a reduction in amplitude and efficacy of eIPSCs, demonstrating the involvement of nRT inhibition in suppressing thalamocortical function. The data in the present study provides complimentary, converse evidence: that while elimination of nRT inhibition produces powerful oscillations and spontaneous seizures, enhancement of nRT inhibition reduces oscillation length and abates induced seizures. We should note that the α3 subunit is also expressed by neurons in cortical layers V and VI (9), and therefore, we cannot exclude that the changes in seizure duration could be partially attributed to alterations in inhibitory synaptic transmission in the cortex. However, the isolated thalamic slice oscillation data shown here provides compelling evidence that implicates a thalamic origin.
An unresolved but interesting issue is the absolute subunit composition of the postsynaptic GABAARs in the nRT of α3KO mice. Although our data suggest that these receptors likely contain β3 and γ2 subunits, pharmacology experiments here, along with immunohistochemical localization studies (13, 14) have failed to detect a complementary α subunit expressed in this nucleus. This result is somewhat confounding, because a preponderance of evidence supports the notion that phasic synaptic inhibition requires α, β and γ subunits to form a functional postsynaptic GABAAR (30–33). There are 2 possible explanations to account for this discrepancy. First, deletion of the α3 subunit might produce up-regulatation of an alternate α subunit that experimental methodology failed to detect. In early development, nRT neurons display a transient expression of the α5 subunit at approximately postnatal day 5 (13). Through maturation, the α5 subunit in nRT is gradually replaced by α3, and it is therefore possible that engineered α3 subunit deletion causes α5 to persist postnatally in this nucleus and form synaptic receptor complexes comprised of α5β3γ2. Contrary to this, the specific α5 subunit inverse agonist L-655,708 has no effect on IPSC shape in α3KO nRT neurons. Additionally, it has been shown that recombinant receptors comprised of α5β3γ2 display smaller peak amplitude currents (34), which is not consistent with the atypically large IPSCs observed in α3KO nRT neurons.
A second possibility is that a modified GABAAR subtype mediates inhibition in α3KO. Ongoing activity in the thalamic circuit in the absence of intra-nRT inhibition might produce a strong compensatory mechanism that could drive expression and assembly of a receptor combination containing α subunits with alternatively spliced mRNA or postranslational modifications to the mature protein, either of which might obscure identification through conventional immunological and pharmacological methods. Nonetheless, the failure to identify the subunit combination in α3KO nRT neurons does not impact the major conclusion of this report — that intra-nRT connection strength is inversely related to strength and duration of pathological thalamocortical network oscillations. We propose further investigation of these inhibitory synapses in nRT, and postulate that α3KO mice might be a promising model system for studying GABAAR subunit modifications or accessory proteins involved in the formation of synaptic GABAARs.
Last, this study provides some insight regarding the relationship between synaptic current kinetics and higher order neural activity. One of the most striking observations was the profound changes in the shape of IPSCs in α3KO mice. In wild-type rodents, nRT IPSCs display a characteristic long-lasting, slowly deactivating current that is attributed to the affinity of postsynaptic α3β3γ2 receptors (35). Because of this unique synaptic phenotype, we believed their role to be integral in the generation of rhythmic thalamocortical activity. However, it is apparent that this WT inhibition can be readily substituted with a higher-amplitude, fast-decaying kinetics with no deleterious consequences (36). On the contrary, the data here show that α3KO mice are resistant to sustained thalamic activity and display of level of resistance to induced seizures. This would suggest that allosteric modulators that augment the amplitude of α3 containing receptors would be effective at terminating or controlling seizures of thalamic origin.
Materials and Methods (See SI Text for Detail)
Thalamic Oscillations.
Slices were placed in an interface chamber at 34 °C and superfused with oxygenated ACSF. Electrical stimuli (20- to 100-V, 40- to 80-μs) were delivered to the internal capsule through a pair of 50–100 KΩ tungsten electrodes (FHC) with a separation of ≈100 μm. Extracellular multiunit recordings were obtained with 50–100 KΩ tungsten electrodes placed in the VB and digitized with a Digidata 1200 and pClamp software (Molecular Devices) and band-pass filtered between 100 Hz and 3 kHz. To detect spikes in these recordings we used analysis methodology described (28). In brief, we detected spikes as steep slope deflections 3x greater that the background noise in each recording. We quantified the duration of oscillatory activity by the last instance of at least 5 spikes in a 50-ms sliding window.
Seizure Models.
Male mice at least 25 days postnatal were anesthetized with ketamine/xylazine (100/10 mg/kg I.P.) and 4 stainless steel electrode screws were surgically implanted on the dural surfaces of the right and left sides of both frontal and parietal cerebral cortex and attached to a connection pedestal glued to the surface of the skull. After at least 7 days of postsurgery recovery, EEG recordings were performed. Mice were placed in the recording area, habituated, and 30 min of baseline EEG activity was monitored on an XLtek acquisition system. Absence seizures were induced by s.c. injection of the drugs γ-butyrolactone (GBL, 100 mg/kg, Sigma) or pentylenetrazol (PTZ, 25 mg/kg, Tocris). EEG activity was recorded simultaneously with video monitoring for 60 min postinjection. Recordings were band passed filtered off-line and wavelet analysis was performed with MatLab.
Supplementary Material
Acknowledgments.
We thank T. Lew (Stanford University) for assistance with animal husbandry, genotyping, and surgery and A. Lagrange (Vanderbilt University) for communicating results with recombinant receptor assays. This work was supported by National Institutes of Health Epilepsy Training Grant NS007280 (to C.M.S.) and the National Institutes of Health Grant NS006477 (to J.R.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811326106/DCSupplemental.
References
- 1.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 2.Crunelli V, Leresche N. Childhood absence epilepsy: Genes, channels, neurons and networks. Nat Rev Neurosci. 2002;3:371–382. doi: 10.1038/nrn811. [DOI] [PubMed] [Google Scholar]
- 3.Huguenard JR, McCormick DA. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci. 2007;30:350–356. doi: 10.1016/j.tins.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Pinault D. The thalamic reticular nucleus: Structure, function and concept. Brain Res Brain Res Rev. 2004;46:1–31. doi: 10.1016/j.brainresrev.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 5.von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993;261:361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]
- 6.Sieghart W. Structure, pharmacology, and function of GABA-A receptor subtypes. Adv Pharmacol. 2006;54:231–263. doi: 10.1016/s1054-3589(06)54010-4. [DOI] [PubMed] [Google Scholar]
- 7.Rudolph U, Mohler H. Analysis of GABA-A receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- 8.Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: Differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 9.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: Immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 10.Browne SH, et al. Kinetic and pharmacological properties of GABA(A) receptors in single thalamic neurons and GABA(A) subunit expression. J Neurophysiol. 2001;86:2312–2322. doi: 10.1152/jn.2001.86.5.2312. [DOI] [PubMed] [Google Scholar]
- 11.Jia F, et al. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- 12.Yee BK, et al. A schizophrenia-related sensorimotor deficit links alpha 3-containing GABAA receptors to a dopamine hyperfunction. Proc Natl Acad Sci USA. 2005;102:17154–17159. doi: 10.1073/pnas.0508752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Studer R, et al. Alteration of GABAergic synapses and gephyrin clusters in the thalamic reticular nucleus of GABAA receptor alpha3 subunit-null mice. Eur J Neurosci. 2006;24:1307–1315. doi: 10.1111/j.1460-9568.2006.05006.x. [DOI] [PubMed] [Google Scholar]
- 14.Winsky-Sommerer R, et al. Normal sleep homeostasis and lack of epilepsy phenotype in GABA A receptor alpha3 subunit-knockout mice. Neuroscience. 2008;154:595–605. doi: 10.1016/j.neuroscience.2008.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huntsman MM, Porcello DM, Homanics GE, DeLorey TM, Huguenard JR. Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science. 1999;283:541–543. doi: 10.1126/science.283.5401.541. [DOI] [PubMed] [Google Scholar]
- 16.Zhang SJ, Huguenard JR, Prince DA. GABAA receptor-mediated Cl- currents in rat thalamic reticular and relay neurons. J Neurophysiol. 1997;78:2280–2286. doi: 10.1152/jn.1997.78.5.2280. [DOI] [PubMed] [Google Scholar]
- 17.Sigel E, Baur R, Trube G, Mohler H, Malherbe P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990;5:703–711. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- 18.Wafford KA, et al. A novel allosteric modulatory site on the GABAA receptor beta subunit. Neuron. 1994;12:775–782. doi: 10.1016/0896-6273(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein PA, et al. Prolongation of hippocampal miniature inhibitory postsynaptic currents in mice lacking the GABA(A) receptor alpha1 subunit. J Neurophysiol. 2002;88:3208–3217. doi: 10.1152/jn.00885.2001. [DOI] [PubMed] [Google Scholar]
- 20.Atack JR, et al. L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for alpha5-containing GABAA receptors. Neuropharmacology. 2006;51:1023–1029. doi: 10.1016/j.neuropharm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Jacobsen RB, Ulrich D, Huguenard JR. GABA(B) and NMDA receptors contribute to spindle-like oscillations in rat thalamus in vitro. J Neurophysiol. 2001;86:1365–1375. doi: 10.1152/jn.2001.86.3.1365. [DOI] [PubMed] [Google Scholar]
- 22.Warren RA, Agmon A, Jones EG. Oscillatory synaptic interactions between ventroposterior and reticular neurons in mouse thalamus in vitro. J Neurophysiol. 1994;72:1993–2003. doi: 10.1152/jn.1994.72.4.1993. [DOI] [PubMed] [Google Scholar]
- 23.Slaght SJ, Leresche N, Deniau JM, Crunelli V, Charpier S. Activity of thalamic reticular neurons during spontaneous genetically determined spike and wave discharges. J Neurosci. 2002;22:2323–2334. doi: 10.1523/JNEUROSCI.22-06-02323.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snead OC., III Basic mechanisms of generalized absence seizures. Ann Neurol. 1995;37:146–157. doi: 10.1002/ana.410370204. [DOI] [PubMed] [Google Scholar]
- 25.Snead OC., III gamma-Hydroxybutyrate model of generalized absence seizures: Further characterization and comparison with other absence models. Epilepsia. 1988;29:361–368. doi: 10.1111/j.1528-1157.1988.tb03732.x. [DOI] [PubMed] [Google Scholar]
- 26.Pinault D, Smith Y, Deschenes M. Dendrodendritic and axoaxonic synapses in the thalamic reticular nucleus of the adult rat. J Neurosci. 1997;17:3215–3233. doi: 10.1523/JNEUROSCI.17-09-03215.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deleuze C, Huguenard JR. Distinct electrical and chemical connectivity maps in the thalamic reticular nucleus: Potential roles in synchronization and sensation. J Neurosci. 2006;26:8633–8645. doi: 10.1523/JNEUROSCI.2333-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sohal VS, Keist R, Rudolph U, Huguenard JR. Dynamic GABA(A) receptor subtype-specific modulation of the synchrony and duration of thalamic oscillations. J Neurosci. 2003;23:3649–3657. doi: 10.1523/JNEUROSCI.23-09-03649.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeLorey TM, et al. Mice lacking the beta3 subunit of the GABAA receptor have the epilepsy phenotype and many of the behavioral characteristics of Angelman syndrome. J Neurosci. 1998;18:8505–8514. doi: 10.1523/JNEUROSCI.18-20-08505.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKernan RM, et al. GABAA receptor subtypes immunopurified from rat brain with alpha subunit-specific antibodies have unique pharmacological properties. Neuron. 1991;7:667–676. doi: 10.1016/0896-6273(91)90379-e. [DOI] [PubMed] [Google Scholar]
- 31.McKernan RM, Whiting PJ. Which GABA-A receptor subunits really occur in the brain? Trends Neuosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 32.Gunther U, et al. Benzodiazepine-insensitive mice generated by targeted disruption of the gamma 2 subunit gene of gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 1995;92:7749–7753. doi: 10.1073/pnas.92.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sur C, et al. Loss of the major GABA(A) receptor subtype in the brain is not lethal in mice. J Neurosci. 2001;21:3409–3418. doi: 10.1523/JNEUROSCI.21-10-03409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caraiscos VB, et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schofield CM, Huguenard JR. GABA affinity shapes IPSCs in thalamic nuclei. J Neurosci. 2007;27:7954–7962. doi: 10.1523/JNEUROSCI.0377-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sohal VS, Huntsman MM, Huguenard JR. Reciprocal inhibitory connections regulate the spatiotemporal properties of intrathalamic oscillations. J Neurosci. 2000;20:1735–1745. doi: 10.1523/JNEUROSCI.20-05-01735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.