Abstract
Organism surfaces represent signaling sites for attraction of allies and defense against enemies. However, our understanding of these signals has been impeded by methodological limitations that have precluded direct fine-scale evaluation of compounds on native surfaces. Here, we asked whether natural products from the red macroalga Callophycus serratus act in surface-mediated defense against pathogenic microbes. Bromophycolides and callophycoic acids from algal extracts inhibited growth of Lindra thalassiae, a marine fungal pathogen, and represent the largest group of algal antifungal chemical defenses reported to date. Desorption electrospray ionization mass spectrometry (DESI-MS) imaging revealed that surface-associated bromophycolides were found exclusively in association with distinct surface patches at concentrations sufficient for fungal inhibition; DESI-MS also indicated the presence of bromophycolides within internal algal tissue. This is among the first examples of natural product imaging on biological surfaces, suggesting the importance of secondary metabolites in localized ecological interactions, and illustrating the potential of DESI-MS in understanding chemically-mediated biological processes.
Keywords: imaging mass spectrometry, macroalga, natural product, surface-associated
Secondary metabolites mediate countless biological interactions including mate recognition, competition for space, prey detection, and defense against adversaries including consumers and pathogens (1, 2). Foulers, pathogens, parasites, and symbionts establish initial physical interactions with hosts via surface contact, and biotic surfaces may represent particularly important sites of chemical signaling (3). In addition to the benefits of maintaining chemical cues throughout tissues (4), it may be advantageous to present such compounds on exterior surfaces where they can effectively and rapidly influence interactions via initial contact with pathogens, consumers, or mutualists.
Despite the apparent advantages of surface-mediated chemical signaling, our understanding of such processes is limited, in large part because of methodological difficulties of compound detection and quantification without tissue damage. In the marine realm, numerous taxa have been suggested to use surface-associated defenses against competitors, foulers, and pathogens (5–10). However, such defenses were often proposed based on inhibitory effects detected in experiments, using whole organism extracts (5, 7), and it is unclear whether target species actually encountered these chemicals in nature. In more ecologically realistic studies, roles of surface-associated molecules were proposed based on experiments employing surface extracts or pure compounds tested at their approximate surface concentration (6, 9–13). Unfortunately, current extraction-based methodologies are limited to certain classes of molecules. Further, these methods do not allow determination of compound distributions on organismal surfaces at lower than centimeter or millimeter scales. Heterogeneous distributions at the submillimeter scale may play important but unexplored roles in mediating biotic interactions. Such fine-scale interactions may be particularly important in governing relationships, whether beneficial or deleterious, between hosts and microorganisms.
Microbe-borne diseases have caused mass mortality among some marine plant and animal species, and epidemics appear to be on the rise (14). Not all organisms are susceptible to infection, and both internal and surface-associated chemical defenses may account for the observed resistance of some species to microbial attack (15, 16). Among marine macroalgae, only a handful of studies have evaluated roles of specific secondary metabolites in defense against deleterious microbes (17–19). Only the 22-membered lactone lobophorolide from the brown alga Lobophora variegata (11), a polybrominated 2-heptanone from the red alga Bonnemaisonia hamifera (9), and furanones from the red alga Delisea pulchra (20) have been proposed as surface-associated antimicrobial defenses of marine algae.
Recent developments in mass spectrometry offer potential for advanced understanding of these and other chemical signaling processes. The Dorrestein and Gerwick groups demonstrated the utility of matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) for pinpointing secondary metabolite locations within marine microbe-invertebrate assemblages (21, 22), and this strategy may prove widely applicable in assigning biosynthetic origins and distributions of chemical defenses within tissues or cell types. Further, time-of-flight secondary ion mass spectrometry (TOF-SIMS) has been recently applied in assessing antibiotic distributions on Streptomyces coelicolor bacterial culture surfaces (23).
Desorption electrospray ionization mass spectrometry (DESI-MS), a recently developed ambient sampling technique (24), has also shown promise for evaluating organic molecules on intact surfaces. In DESI-MS, surfaces are maintained at atmospheric pressure in open air and interrogated with a focused spray of charged microdroplets of polar solvent. Biomolecules are gently desorbed from the surface and delivered as intact ions into the mass spectrometer for analysis (25). Like other soft ionization techniques for mass spectrometry, DESI-MS offers low detection limits and compatibility with molecules ranging from water-soluble to nonpolar, and from low (< 100 Da) to high (> 50,000 Da) molecular mass, but with the inherent advantage of higher analysis speed and capability of performing spatially-resolved measurements with lateral resolutions in the hundreds of micrometers (24). DESI-MS has proven useful in applications such as the analysis of counterfeit drug molecules on intact pharmaceutical tablets (26) and detection of alkaloids from terrestrial plants (27), among others. The potential of DESI-MS imaging has very recently started to be explored in applications including the assessment of lipid and drug spatial distributions in mammalian tissue sections (28–31). These studies suggest that DESI-MS imaging could be used as a powerful tool in exploring the function of surface-associated natural products in ecological interactions.
On the basis of their cytotoxicities toward biomedical targets, we recently discovered 10 unusual C27 diterpene-benzoate macrolides named bromophycolides, from a population of the Fijian red alga Callophycus serratus. Eight C27 diterpene-benzoic acids and 2 C26 diterpene-alcohols, termed callophycoic acids and callophycols, respectively, were isolated from a different population of the same alga (32–34), using traditional methods. In the present study, we merge natural products chemistry and ecological approaches with the capabilities of DESI-MS imaging to provide support for a role of bromophycolides and callophycoic acids as antifungal defenses of whole algae and evidence that bromophycolides are presented heterogeneously on algal surfaces where they may interfere with pathogen attack.
Results
C. serratus Harbors Potent Antifungal Chemical Defenses.
Using standard agar-based growth inhibition assays (SI Methods), chromatographic fractions from extracts of 10 separate collections of C. serratus were evaluated for activity against 2 known pathogens of marine plants: Lindra thalassiae, a widely distributed marine Ascomycete reported to infect diverse hosts ranging from brown algae to seagrasses (35, 36), and Pseudoalteromonas bacteriolytica, the bacterium responsible for red spot disease in kelp (37). At natural volumetric concentrations, fractions containing either bromophycolides or callophycoic acids/callophycols strongly inhibited growth of L. thalassiae relative to no extract controls (P < 0.0001 for n = 4 bromophycolide-containing fractions; P < 0.0001 for n = 6 callophycoic acid/callophycol-containing fractions; 1-way ANOVA with Dunnett's post test), with every fraction inhibiting growth of this fungus by >95%. Neither bromophycolide- nor callophycoic acid/callophycol-containing fractions were significantly inhibitory toward growth of the pathogenic bacterium P. bacteriolytica relative to no extract controls (P = 0.32 for n = 4 bromophycolide fractions; P = 0.29 for n = 6 callophycoic acid/callophycol fractions; 1-tailed paired t test).
Pure Bromophycolides and Callophycoic Acids Are Effective Antifungal Chemical Defenses of C. serratus.
When natural products within algal extracts were quantified by LC-MS, bromophycolides were found to be associated exclusively with 4 algal collections, whereas callophycoic acids and callophycols were observed in 6 other collections (Table S1), consistent with previous reports of 2 chemically-distinct genotypes (“chemotypes”) of C. serratus on Fijian coral reefs (32–34). Sequencing of 18S rRNA (≈1.2 kb) from these algal samples revealed 3 unique haplotypes differing by 1–13 bp. Phylogenetic analysis differentiated 2 highly supported clades of C. serratus, corresponding to the 2 chemotypes (Fig. S1). These algal specimens were all were identified as C. serratus by morphology-based taxonomic analysis (S. Fredericq, personal communication). In some cases, individuals representing different chemotypes were found within a few meters of each other. Together, these genetic and morphological data suggest that this species exists as 2 genetically-distinct chemotypes, 1 containing bromophycolides and the other callophycoic acids and callophycols.
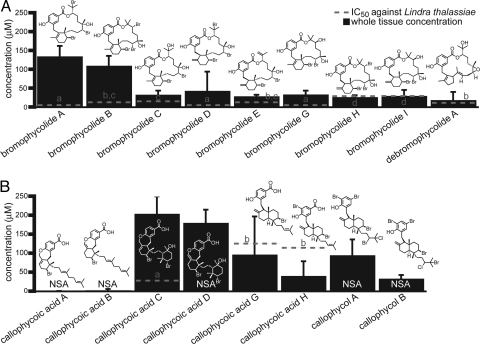
All evaluated natural products from the bromophycolide chemotype of C. serratus significantly suppressed growth of the marine pathogenic fungus L. thalassiae, with average IC50 values for each compound near or below whole tissue natural concentrations (Fig. 1A and Fig. S2). Among compounds of the callophycoic acid and callophycol chemotype, only callophycoic acids C, G, and H were effective <300 μM, and only callophycoic acids C and G were significantly inhibitory near their natural concentration of 100–200 μM (Fig. 1B). The most potent compound evaluated from this chemotype, callophycoic acid C, was 100% inhibitory to L. thalassiae at its average natural whole tissue concentration (n = 3), and may represent the dominant antifungal defense compound for this chemotype. The importance of other callophycoic acids and callophycols in antifungal defense cannot be ruled out, however, because these metabolites might interact additively or synergistically within algal tissues in controlling microbial adversaries.
Fig. 1.
Antifungal IC50 values (dashed lines) and natural whole tissue concentrations (solid bars) of bromophycolides (A) and callophycoic acids/callophycols (B). Natural whole tissue concentrations were determined by LC-MS analysis of extracts from 4 C. serratus collections of the bromophycolide chemotype (A) and 6 collections of the callophycoic acid/callophycol chemotype (B) (Table S1). Error bars denote 1 SD in metabolite concentration. NSA denotes compounds that were not significantly active at the maximum tested concentration of 300 μM (P > 0.05), as determined by 1-way ANOVA with Dunnett's post test comparison of treatments vs. controls. Among compounds within each chemotype, different letters indicate treatments differing significantly in antifungal IC50 values (F test, P ≤ 0.05). Bromophycolide F and callophycoic acids E and F were neither detected in these extracts nor evaluated for antifungal activity.
Evaluated at natural whole tissue concentrations, bromophycolide- and callophycoic acid/callophycol-containing chromatographic fractions did not differ significantly in antifungal activity (P = 0.12; n = 4 bromophycolide-containing fractions; n = 6 callophycoic acid/callophycol-containing fractions; 1-way ANOVA with Dunnett's post test). This suggests that both suites of compounds are effective antifungal chemical defenses of C. serratus. However, most individual bromophycolides were found to be more potently antifungal than individual callophycoic acids or callophycols (Fig. 1 A and B), suggesting that the macrolide molecular architecture confers enhanced antifungal activity.
DESI-MS Reveals Heterogeneous Distribution of Bromophycolides on Algal Surfaces.
With evidence supporting bromophycolides as potent antifungal chemical defenses of whole algal tissues, we next tested the hypothesis that these metabolites are concentrated on algal surfaces, a potentially advantageous site for control of microbial infection. Given the strong antifungal activity and high relative abundance of bromophycolides A and B in whole tissues (Fig. 1A), these metabolites were selected as model compounds for analysis of chemical defenses on C. serratus surfaces.
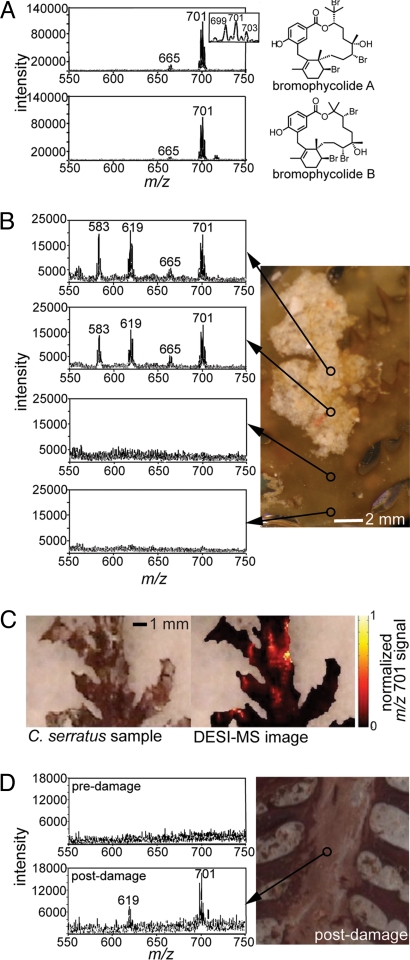
Negative ion mode desorption electrospray ionization mass spectrometry (DESI-MS) analysis of pure bromophycolides on synthetic surfaces revealed a limit of detection of 0.9 pmol/mm2 (signal-to-noise ratio = 6) with an absolute detection limit of 0.3 pmol (2 × 102 pg) for bromophycolide A, supporting the capacity of DESI-MS in assessing this class of secondary metabolites at low concentrations. Mass spectra of isomeric bromophycolides A and B were indistinguishable, each displaying a minor deprotonated molecule cluster, [M−H]−, near m/z 665 and a dominant chloride adduct, [M+Cl]−, centered at m/z 701 (Fig. 2A), both matching expected isotopic splitting patterns and m/z values (DESI spray solution of 100 μM NH4Cl in MeOH). DESI-MS analyses of discrete sites across algal surfaces revealed that these diagnostic bromophycolide A and B signals were associated exclusively with distinct light-colored patches attached to C. serratus surfaces (n = 6 independent algal samples; DESI beam area 200 μm) (Fig. 2B), a finding confirmed by DESI-MS imaging of the bromophycolide A/B chloride adduct ion at m/z 701 (Fig. 2C). Additional patch-associated DESI-MS signals centered at m/z 583 and 619 were assigned to [M−H]− and [M+Cl]− ion clusters, respectively, for bromophycolide E, based on comparison with signals observed for pure standard compounds (Fig. S3) and in agreement with expected m/z values. Bromophycolide signals were not observed on patch-free algal surface sites (Fig. 2 B and C). Light microscopy provided evidence for algal cell integrity both before and after DESI-MS analyses (Fig. S4), supporting this approach as a general, physically nondestructive method for analysis of secondary metabolites and other organic molecules on intact biological surfaces.
Fig. 2.
Negative-ion desorption electrospray ionization (DESI) mass spectra of bromophycolides (using DESI spray solution of 100 μM NH4Cl in MeOH). (A) Mass spectra of pure bromophycolides A and B deposited on synthetic substrates (10 μL, 1 mg/mL solution). Ion clusters centered around m/z 665 and 701 correspond to [bromophycolide A/B − H]− and [bromophycolide A/B + Cl]−, respectively. (B) Typical DESI mass spectra of C. serratus surface, showing that bromophycolides occur on algal surfaces only in association with light-colored patches (n = 40 sites observed on 6 independent algal samples; DESI beam area 200 μm). Ion clusters centered at 583 and 619 represent [bromophycolide E − H]− and [bromophycolide E + Cl]−, respectively (Fig. S3). (C) DESI-MS image (200-μm resolution) of bromophycolide A/B chloride adduct ion m/z 701 on C. serratus surface, indicating that bromophycolide “hot spots” correspond to pale patches. (D) Representative mass spectrum from patch-free algal surface before and after mechanical damage (n = 2 damaged samples).
The combined concentration of bromophycolides A and B on patch surfaces was estimated to be 36 ± 23 pmol/mm2, calculated from integrated DESI-MS [M+Cl]− signals for 3 independent patches, using a standard curve of bromophycolides deposited on patch-free algal surfaces (see Methods). The large standard deviation in bromophycolide surface concentrations suggests high natural variability within these bromophycolide-rich patches, consistent with LC-MS data indicating substantial variation of bromophycolide concentrations among extracts of whole algal tissues (Fig. 1 and Table S1) and extracts of patches removed from algal surfaces. Evaluation of the antifungal activity of combined bromophycolides A and B coated onto nutrient agar substrates revealed a mean IC50 value of 17 pmol/mm2 (log IC50 = 1.2 ± 0.1 SE), suggesting that patch-associated surface concentrations of bromophycolides would inhibit L. thalassiae and other susceptible fungi.
Having found surface-associated bromophycolides A and B among unusual patches (Fig. 2 B and C), we then tested whether these compounds were located internally within algal tissue as well. DESI-MS analysis after physical damage to clean, bromophycolide-free algal surfaces revealed the presence of internal bromophycolides (n = 2, Fig. 2D). The presence of internal bromophycolides within patch-free algal tissues was further confirmed by LC-MS analyses of tissue extracts.
Characterization of Bromophycolide-Containing Algal Surface Patches.
Bromophycolide-containing surface patches seemed random in distribution, not associated with specific morphological features of the algal thallus (Fig. S5). Digital imaging revealed that 4.5 ± 4.3% (± 1 SD) of algal surfaces were covered with these distinctive surface patches (n = 10 algal pieces). Patches were similar in abundance on fresh, frozen, and 1–10% formalin-preserved algal samples and seemed indistinguishable in appearance across these preservation methods.
Patch-associated algal samples were cryosectioned, stained with hematoxylin and eosin, and analyzed by light microscopy in an effort to better characterize these bromophycolide-rich surface regions. This processing resulted in loss of patches from algal samples, but permitted analysis of algal tissue underlying these patches. Large-scale algal cell lysis was not evident in these samples (Fig. S6). However, a variety of cellular structures were observed, some of which could indicate localized sites of damage. Further, epifluorescence microscopy of bromophycolide-rich patches removed from algal surfaces revealed a variety of structures that stained with 4′,6-diamidino-2-phenylindole (DAPI; n = 5). Intact nuclei were not observed, suggesting that these structures may represent prokaryotes or DAPI-stained inorganic material (Fig. S7).
Discussion
Together, bromophycolides and callophycoic acids represent the largest group of algal antifungal chemical defenses reported to date, adding to only a handful of previously identified antimicrobial chemical defenses from macroalgae (9, 11, 17–19). DESI-MS imaging revealed antifungal bromophycolides both within algal tissues and among distinct patches covering only ≈5% of algal surfaces (Fig. 2 B–D), and demonstrated the utility of MS in exploring surface-mediated ecological interactions.
DESI-MS analysis revealed that bromophycolide concentrations on these surface patches were sufficient to suppress growth of L. thalassiae (see Results). These data should be considered semiquantitative, because previous DESI-MS studies have shown that signal intensity is influenced to some extent by surface morphology (38); hence, data are truly quantitative only across identical, homogenous surfaces—a feature not inherent to most biological materials. Despite these limitations, with an antifungal IC50 value of approximately half the measured surface concentration of bromophycolides on patches, it is probable that these compounds would be present at sufficient levels for inhibition of fungi such as L. thalassiae that may encounter this substrate.
The discovery of bromophycolides among heterogeneous patches on algal surfaces and within algal tissues (Fig. 2 B–D) leads to the hypothesis that C. serratus maintains these compounds internally and releases them at sparsely distributed surface sites. In previous work, fluorescence microscopy- and chemical extraction-based investigations of macroalgae Asparagopsis armata and Delisea pulchra revealed secondary metabolites within near-surface gland cells with canals for releasing these metabolites onto algal surfaces, although triggers for release of these compounds and the bromophycolides remain unclear (12, 39).
There are a number of possible explanations for the heterogeneous distribution of bromophycolides across C. serratus surfaces (Fig. 2 B and C). One possibility is that bromophycolide-rich sites represent a targeted response to microbial challenge at these sites. Light and epifluorescence microscopy did not conclusively support the presence of microbes within patches (Fig. S7); however, if bromophycolides are indeed effective antimicrobial chemical defenses in nature, one might expect low microbial abundances in bromophycolide-rich areas. Macroalgae have been reported to up-regulate chemical defenses in response to bites from small grazers (40–42), although it is unclear whether in those studies defenses were induced throughout the alga or exclusively at sites of challenge. Another possibility is that patches are associated with sites of localized algal damage from which bromophycolides have leaked, providing antifungal defenses where tissues are vulnerable to waterborne microbes. In terrestrial systems, antimicrobial defense responses have been linked to tissue wounding (43), presumably because wounding enhances susceptibility to pathogen attack.
Because the biosynthetic origin of bromophycolides has not been established, it is plausible that bromophycolides are not algal natural products, but are instead products of a microbial symbiont present within algal tissues and/or at distinct surface regions. Recent studies have provided convincing evidence that a number of secondary metabolites originally ascribed to sponges, bryozoans, and other macroorganisms are of microbial biosynthetic origin (44–46). Further, microbial metabolites such as 2,3-indolinedione from a bacterium associated with crustacean embryos have been shown to defend hosts against pathogen infection (47). In the case of bromophycolides, however, a microbial origin appears unlikely, because: (i) microorganisms were not obvious within sections of C. serratus (Fig. S6); (ii) epifluorescence microscopy of bromophycolide-containing surface patches revealed DAPI-stained material but no distinct cell-like structures; (iii) chemical analyses of 22 bacterial and fungal isolates from live C. serratus provided no evidence for bromophycolide production (P.R. Jensen, personal communication). Further, biosynthesis of terpenoid and shikimate natural products from red algae has been extensively reported (48), suggesting the capacity of macroalgae such as C. serratus to produce bromophycolide-like metabolites.
Chemically-mediated interactions between C. serratus and associated microbes may be further addressed by cultivation- or genomics-based experiments, and efforts are now underway to culture this macroalga and microorganisms associated with bromophycolide-rich algal surface patches. These approaches may permit further characterization of potential patch-associated microbes and allow direct testing of the effects of bromophycolides on microbes. These experiments may also permit testing of algal tissue cultures for bromophycolide production.
In the present study, desorption electrospray ionization mass spectrometry (DESI-MS) imaging provided an unprecedented ability to map secondary metabolites to distinct surface sites and revealed that bromophycolides are not homogenously distributed across algal surfaces but instead associated with distinct patches. This appears to be the first direct evidence for localization of chemical cues with spatial resolution <200 μm on biological surfaces in concentrations sufficient for targeted antimicrobial defense. The heterogeneous distributions of natural products observed in field-collected algal samples potentially represent essential, but until now, largely overlooked aspects of chemical signaling. Given the inherently small scale of host-microbe interactions, MS imaging technologies applied herein have the potential to revolutionize our understanding of these elusive biological interactions.
Methods
Isolation and Quantification of C. serratus Natural Products.
Bromophycolides, callophycoic acids, and callophycols were isolated and identified from C. serratus as described in refs. 32–34. Pure compounds for DESI-MS, LC-MS, and antimicrobial assays were quantified by 1H NMR spectroscopy, using 2,5-dimethylfuran as internal standard (49).
For antimicrobial assays with chromatographic fractions and LC-MS quantification of metabolites from whole plant extracts, 10 fresh C. serratus collections were extracted exhaustively with methanol and methanol/dichloromethane (2:1 and 1:1); extracts were reduced in vacuo and subjected to fractionation with HP20ss resin (Supelco). Fractions 1 and 2 were eluted with methanol/water (1:1 and 4:1, respectively), and fraction 3 with methanol followed by acetone. Fraction 3 contained all previously reported C. serratus natural products.
Quantitative LC-MS was performed for each chromatographic fraction from all C. serratus collections. For each compound, negative-mode ESI-MS selected ion recordings were integrated for 2–3 m/z values corresponding to isotopic cluster signals; standard curves were prepared by analysis of each compound at 5–7 concentrations (r2 = 0.95–0.99). Concentrations of individual compounds within chromatographic fractions were calculated by interpolation from standard curve data.
Antimicrobial Assays.
Assays with L. thalassiae (ATCC 56663) and Pseudoalteromonas bacteriolytica (ATCC 700679) were adapted from methods described in ref. 11 (see SI Methods), and data were analyzed with standard statistical methods (50, 51) (SI Methods).
DESI-MS Analyses.
DESI-MS was performed with a custom-built DESI ion source as described in ref. 52. Experiments were performed by subjecting targeted surface sites to a DESI spray solution of 100 μM NH4Cl (Sigma–Aldrich) in MeOH at a flow rate of 5 μL/min. Alternative spray solvents that provided lower sensitivity included 100% ACN, 100% MeOH, and aqueous mixtures of these solvents (53). The nebulizer gas pressure was set at 110 psi and the spray solution was electrically charged to −3 kV. All experiments were performed on an LCQ DECA XP+quadrupole ion trap mass spectrometer (Thermo Finnigan) operated in negative ion mode. The ion transfer capillary was held at 300 °C, and data were collected in full scan mode (m/z 550–750), using Xcalibur software version 2.0 (Thermo Finnigan).
To determine the limit of detection for pure bromophycolide A on a model substrate, serially diluted solutions of this compound in MeOH were deposited on measured areas of polytetrafluoroethylene (PTFE, 52). The MeOH was allowed to air dry before analysis, and the detection limit was recorded as the surface concentration at which S/n = 6. Algal samples (≈1.0–1.5 cm length; 0.2–1.0 cm width), preserved with 10% formalin in seawater, were affixed to PTFE substrates as an inert support for DESI-MS analysis and samples kept moist with seawater; no additional sample pretreatment was completed. Algal cell integrity was verified before and after DESI-MS experiments by evaluation under a light microscope. For each of 6 evaluated patch-containing algal samples, 6–8 independent sites were targeted with the DESI spray beam. These sites comprised both patch-covered areas and areas of clean alga representing all surface morphological features.
For DESI-MS experiments comparing bromophycolide levels on the intact surface of clean, patch-free alga with those found within damaged tissue, 2 intact C. serratus pieces (≈1.0–1.5 cm length; 0.2–1.0 cm maximum width) were first evaluated for bromophycolides by rastering, or continuously probing the algal surface with DESI spray while gradually moving the spray jet along the entire length of the sample. These intact algal pieces were then wounded by scraping with a sterile scalpel and evaluated again at approximately the same sites as before.
Concentrations of bromophycolides A + B for individual sites on patch surfaces were estimated by comparing integrals from chloride adduct DESI-MS signals with a standard curve developed by depositing known concentrations of bromophycolides A and B (2:1) on intact patch-free algal surfaces of known surface area (r2 = 0.97, n = 4 standards analyzed in triplicate). The 2:1 ratio of bromophycolides A and B represented a reasonable approximation based on the average 2.2:1 ratio observed by LC-MS for these compounds in extracts from patches.
DESI-MS imaging experiments were conducted with the above mass spectrometer, equipped with a joystick and software-controlled motorized microscope x–y stage (Prior Scientific). The algal sample was prepared as before. Mass spectra were acquired in profile mode with automatic gain control (AGC) turned off. The ion injection time was set at 40 ms, and 2 microscans were summed for each pixel in the image. Imaging data were acquired in looped stage scanning mode, controlled by LabVIEW software (National Instruments). The sample was scanned shuttlewise. The stage scan speed in both dimensions was set to 80 μm/s and the step size in the y-dimension was set to 200 μm. Mass spectra were collected in negative ion, full-scan mode, over the m/z range of 550–800. The DESI sprayer emitter was mounted ≈2 mm above the sampling surface at a 55° angle. The spray solvent was set at 3 μL/min and the nebulizer gas pressure at 110 psi. Mass spectral scans were assembled into an image with an in-house MATLAB (version R2008a, MathWorks) script.
Microscopy of Bromophycolide-Containing Surface Patches.
The percentage of C. serratus surfaces covered with distinctive patches was estimated by randomly clipping segments from 10 collections of C. serratus (≈1.0–1.5 cm length; 0.2–1.0 cm width), digitally photographing under a dissection microscope (≈25×), and analyzing images with the area calculator feature of ImageJ software (National Institutes of Health) to compare the number of pixels covered in distinctive patches to pixels containing clean, patch-free alga. To better understand the nature of these surface regions, 5 groups of 2–4 patches were removed from algal surfaces with a sterile scalpel and/or forceps, pulverized, and observed under a light microscope at magnifications ranging from 100× to 1000×. Samples were stained with DAPI and observed at the DAPI excitation wavelength. Sections of C. serratus with bromophycolide-containing patches were cryosectioned into 5-μm-thick pieces with a microtome. Resulting sections were stained with hematoxylin and eosin and examined by light microscopy.
Supplementary Material
Acknowledgments.
This work was supported by a National Science Foundation-Integrative Graduate Education and Research Traineeship predoctoral fellowship (to A.L.L.), National Institutes of Health International Cooperative Biodiversity Groups Grant U01-TW007401-01 (to M.E.H. and J.K.), National Science Foundation Grant OCE-0726689 (to J.K.), National Science Foundation CAREER Grant 0645094 (to F.M.F.), and the Bio-Imaging Mass Spectrometry Center at the Georgia Institute of Technology. Additional acknowledgments are available in SI Methods.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 7269.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812020106/DCSupplemental.
References
- 1.Paul VJ, Ritson-Williams R. Marine chemical ecology. Nat Prod Rep. 2008;25:662–695. doi: 10.1039/b702742g. [DOI] [PubMed] [Google Scholar]
- 2.Hay ME. Marine chemical ecology: Chemical signals and cues structure marine populations, communities, and ecosystems. Annu Rev Mar Sci. 2009 doi: 10.1146/annurev.marine.010908.163708. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinberg PD, de Nys R, Kjelleberg S. In: Chemical Mediation of Surface Colonization. McClintock JB, Baker BJ, editors. Boca Raton, FL: CRC; 2001. pp. 355–387. [Google Scholar]
- 4.Hay ME. Marine chemical ecology: What's known and what's next? J Exp Mar Biol Ecol. 1996;200:103–134. [Google Scholar]
- 5.Kelly SR, Jensen PR, Henkel TP, Fenical W, Pawlik JR. Effects of Caribbean sponge extracts on bacterial attachment. Aquat Microbial Ecol. 2003;31:175–182. [Google Scholar]
- 6.Nylund GM, Gribben PE, de Nys R, Steinberg PD, Pavia H. Surface chemistry versus whole-cell extracts: Antifouling tests with seaweed metabolites. Mar Ecol Progr Ser. 2006;329:73–84. [Google Scholar]
- 7.Nylund GM, Pavia H. Inhibitory effects of red algal extracts on larval settlement of the barnacle Balanus improvisus. Mar Biol. 2003;143:875–882. [Google Scholar]
- 8.Nylund GM, Cervin G, Hermansson M, Pavia H. Chemical inhibition of bacterial colonization by the red alga Bonnemaisonia hamifera. Mar Ecol Progr Series. 2005;302:27–36. [Google Scholar]
- 9.Nylund GM, et al. Seaweed defence against bacteria: A poly-brominated 2-heptanone from the red alga Bonnemaisonia hamifera inhibits bacterial colonisation. Mar Ecol Progr Ser. 2008;369:39–50. [Google Scholar]
- 10.Schmitt TM, Hay ME, Lindquist N. Constraints on chemically mediated coevolution - Multiple functions for seaweed secondary metabolites. Ecology. 1995;76:107–123. [Google Scholar]
- 11.Kubanek J, et al. Seaweed resistance to microbial attack: A targeted chemical defense against marine fungi. Proc Natl Acad Sci USA. 2003;100:6916–6921. doi: 10.1073/pnas.1131855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dworjanyn SA, De Nys R, Steinberg PD. Localisation and surface quantification of secondary metabolites in the red alga Delisea pulchra. Mar Biol. 1999;133:727–736. [Google Scholar]
- 13.Kubanek J, et al. Multiple defensive roles for triterpene glycosides from two Caribbean sponges. Oecologia. 2002;131:125–136. doi: 10.1007/s00442-001-0853-9. [DOI] [PubMed] [Google Scholar]
- 14.Harvell CD, et al. Emerging marine diseases—Climate links and anthropogenic factors. Science. 1999;285:1505–1510. doi: 10.1126/science.285.5433.1505. [DOI] [PubMed] [Google Scholar]
- 15.Engel S, Jensen PR, Fenical W. Chemical ecology of marine microbial defense. J Chem Ecol. 2002;28:1971–1985. doi: 10.1023/a:1020793726898. [DOI] [PubMed] [Google Scholar]
- 16.Lane AL, Kubanek J. In: Secondary Metabolite Defenses Against Pathogens and Biofoulers. Amsler CD, editor. Berlin: Springer; 2008. pp. 229–243. [Google Scholar]
- 17.Puglisi MP, Tan LT, Jensen PR, Fenical W. Capisterones A and B from the tropical green alga Penicillus capitatus: Unexpected anti-fungal defenses targeting the marine pathogen Lindra thallasiae. Tetrahedron. 2004;60:7035–7039. [Google Scholar]
- 18.Paul NA, de Nys R, Steinberg PD. Chemical defence against bacteria in the red alga Asparagopsis armata: Linking structure with function. Mar Ecol Progr Ser. 2006;306:87–101. [Google Scholar]
- 19.Jiang RW, et al. Structures and absolute configurations of sulfate conjugated triterpenoids including an antifungal chemical defense of the marine green alga Tydemania expeditionis. J Nat Prod. 2008;71:1616–1619. doi: 10.1021/np800307h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maximilien R, et al. Chemical mediation of bacterial surface colonisation by secondary metabolites from the red alga Delisea pulchra. Aquat Microbial Ecol. 1998;15:233–246. [Google Scholar]
- 21.Simmons TL, et al. Biosynthetic origin of natural products isolated from marine microorganism-invertebrate assemblages. Proc Natl Acad Sci USA. 2008;105:4587–4594. doi: 10.1073/pnas.0709851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esquenazi E, et al. Visualizing the spatial distribution of secondary metabolites produced by marine cyanobacteria and sponges via MALDI-TOF imaging. Mol Biosyst. 2008;4:562–570. doi: 10.1039/b720018h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaidyanathan S, et al. Subsurface biomolecular imaging of Streptomyces coelicolor using secondary ion mass spectrometry. Anal Chem. 2008;80:1942–1951. doi: 10.1021/ac701921e. [DOI] [PubMed] [Google Scholar]
- 24.Takats Z, Wiseman JM, Gologan B, Cooks RG. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 2004;306:471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- 25.Cooks RG, Ouyang Z, Takats Z, Wiseman JM. Ambient mass spectrometry. Science. 2006;311:1566–1570. doi: 10.1126/science.1119426. [DOI] [PubMed] [Google Scholar]
- 26.Nyadong L, Green MD, De Jesus VR, Newton PN, Fernandez FM. Reactive desorption electrospray ionization linear ion trap mass spectrometry of latest-generation counterfeit antimalarials via noncovalent complex formation. Anal Chem. 2007;79:2150–2157. doi: 10.1021/ac062205h. [DOI] [PubMed] [Google Scholar]
- 27.Talaty N, Takats Z, Cooks RG. Rapid in situ detection of alkaloids in plant tissue under ambient conditions using desorption electrospray ionization. Analyst. 2005;130:1624–1633. doi: 10.1039/b511161g. [DOI] [PubMed] [Google Scholar]
- 28.Wiseman JM, Ifa DR, Song QY, Cooks RG. Tissue imaging at atmospheric pressure using desorption electrospray ionization (DESI) mass spectrometry. Angew Chem Int Ed. 2006;45:7188–7192. doi: 10.1002/anie.200602449. [DOI] [PubMed] [Google Scholar]
- 29.Wiseman JM, Ifa DR, Venter A, Cooks RG. Ambient molecular imaging by desorption electrospray ionization mass spectrometry. Nat Protocols. 2008;3:517–524. doi: 10.1038/nprot.2008.11. [DOI] [PubMed] [Google Scholar]
- 30.Wiseman JM, et al. Desorption electrospray ionization mass spectrometry: Imaging drugs and metabolites in tissues. Proc Natl Acad Sci USA. 2008;105:18120–18125. doi: 10.1073/pnas.0801066105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kertesz V, et al. Comparison of drug distribution images from whole-body thin tissue sections obtained using desorption electrospray ionization tandem mass spectrometry and autoradiography. Anal Chem. 2008;80:5168–5177. doi: 10.1021/ac800546a. [DOI] [PubMed] [Google Scholar]
- 32.Lane AL, et al. Callophycoic acids and callophycols from the Fijian red alga Callophycus serratus. J Org Chem. 2007;72:7343–7351. doi: 10.1021/jo071210y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kubanek J, et al. Bromophycolides C-I from the Fijian red alga Callophycus serratus. J Nat Prod. 2006;69:731–735. doi: 10.1021/np050463o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubanek J, et al. Antineoplastic diterpene-benzoate macrolides from the Fijian red alga Callophycus serratus. Org Lett. 2005;7:5261–5264. doi: 10.1021/ol052121f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohlmeyer J, Kohlmeyer E. Marine Mycology: The Higher Fungi. New York: Academic; 1979. [Google Scholar]
- 36.Kohlmeyer J. Fungi from the Sargasso Sea. Mar Biol. 1971;8:344–350. [Google Scholar]
- 37.Sawabe T, et al. Pseudoalteromonas bacteriolytica sp. nov., a marine bacterium that is the causative agent of red spot disease of Laminaria japonica. Int J Syst Bacteriol. 1998;48:769–774. doi: 10.1099/00207713-48-3-769. [DOI] [PubMed] [Google Scholar]
- 38.Nyadong L, Late S, Green MD, Banga A, Fernandez FM. Direct quantitation of active ingredients in solid artesunate antimalarials by noncovalent complex forming reactive desorption electrospray ionization mass spectrometry. J Am Soc Mass Spectrom. 2008;19:380–388. doi: 10.1016/j.jasms.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 39.Paul NA, Cole L, de Nys R, Steinberg PD. Ultrastructure of the gland cells of the red alga Asparagopsis armata (Bonnemaisoniaceae) J Phycol. 2006;42:637–645. [Google Scholar]
- 40.Pavia H, Toth GB. Inducible chemical resistance to herbivory in the brown seaweed Ascophyllum nodosum. Ecology. 2000;81:3212–3225. [Google Scholar]
- 41.Cronin G, Hay ME. Induction of seaweed chemical defenses by amphipod grazing. Ecology. 1996;77:2287–2301. [Google Scholar]
- 42.Taylor RB, Sotka E, Hay ME. Tissue-specific induction of herbivore resistance: Seaweed response to amphipod grazing. Oecologia. 2002;132:68–76. doi: 10.1007/s00442-002-0944-2. [DOI] [PubMed] [Google Scholar]
- 43.Aneja M, Gianfagna T. Induction and accumulation of caffeine in young, actively growing leaves of cocoa (Theobroma cacao L. ) by wounding or infection with Crinipellis perniciosa. Physiol Mol Plant Pathol. 2001;59:13–16. [Google Scholar]
- 44.Schmidt EW, et al. Patellamide A and C biosynthesis by a microcin-like pathway in Prochloron didemni, the cyanobacterial symbiont of Lissoclinum patella. Proc Natl Acad Sci USA. 2005;102:7315–7320. doi: 10.1073/pnas.0501424102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piel J, et al. Antitumor polyketide biosynthesis by an uncultivated bacterial symbiont of the marine sponge Theonella swinhoei. Proc Natl Acad Sci USA. 2004;101:16222–16227. doi: 10.1073/pnas.0405976101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sudek S, et al. Identification of the putative bryostatin polyketide synthase gene cluster from “Candidatus endobugula sertula,” the uncultivated microbial symbiont of the marine bryozoan Bugula neritina. J Nat Prod. 2007;70:67–74. doi: 10.1021/np060361d. [DOI] [PubMed] [Google Scholar]
- 47.Gil-Turnes MS, Hay ME, Fenical W. Symbiotic marine bacteria chemically defend crustacean embryos from a pathogenic fungus. Science. 1989;246:116–118. doi: 10.1126/science.2781297. [DOI] [PubMed] [Google Scholar]
- 48.Moore BS. Biosynthesis of marine natural products: Macroorganisms (part B) Nat Prod Rep. 2006;23:615–629. doi: 10.1039/b508781n. [DOI] [PubMed] [Google Scholar]
- 49.Gerritz SW, Sefler AM. 2,5-dimethylfuran (DMFu): An internal standard for the “traceless” quantitation of unknown samples via H-1 NMR. J Comb Chem. 2000;2:39–41. doi: 10.1021/cc990041v. [DOI] [PubMed] [Google Scholar]
- 50.Zar JH. Biostatistical Analysis. Upper Saddle River, NJ: Prentice Hall; 1999. [Google Scholar]
- 51.Motulsky HJ, Christopoulos A. Fitting Models to Biological Data Using Linear and Nonlinear Regression. A Practical Guide to Curve Fitting. San Diego: GraphPad Software; 2003. [Google Scholar]
- 52.Nyadong L, et al. Desorption electrospray ionization reactions between host crown ethers and the influenza neuraminidase inhibitor oseltamivir for the rapid screening of Tamiflu. Analyst. 2008;133:1513–1522. doi: 10.1039/b809471c. [DOI] [PubMed] [Google Scholar]
- 53.Nyadong L, et al. Desorption electrospray ionization mass spectrometry (DESI MS) of natural products of a marine alga. Anal Bioanal Chem. 2009 doi: 10.1007/s00216-009-2674-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.