Summary
Over the past decade it has become clear that there is significant overlap in the clinical spectrum of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. The identification of TDP-43 as the major disease protein in the pathology of both frontotemporal lobar degeneration with ubiquitin inclusions and amyotrophic lateral sclerosis provides the first molecular link for these diseases. Pathological TDP-43 is abnormally phosphorylated, ubiquitinated, and cleaved to generate carboxy-terminal fragments in affected brain regions. The normal nuclear expression of TDP-43 is also reduced leading to the hypothesis that sequestration of TDP-43 in pathological inclusions contributes to disease pathogenesis. Thus, TDP-43 is the newest member of the growing list of neurodegenerative proteinopathies, but unique in that it lacks features of brain amyloidosis.
Introduction
A wide variety of neurodegenerative diseases are characterized pathologically by the accumulation of intracellular or extracellular protein aggregates composed of amyloid fibrils [1]. For example, the pathology of Alzheimer's disease (AD) is defined by senile plaques and neurofibrillary tangles composed of β-amyloid and microtubule-associated protein tau, respectively, and Lewy bodies composed of α-synuclein are the disease-defining lesions of Parkinson's disease. Until recently, the neuropathology of both frontotemporal lobar degeneration with ubiquitin inclusions (FTLD-U) [2], the most common phenotype associated with the FTLD syndrome, and amyotrophic lateral sclerosis (ALS) [3] were defined by non-amyloidogenic ubiquitinated inclusions (UBI).
FTLD, the second most common form of presenile dementia, refers to a heterogeneous group of neurodegenerative disorders that have in common behavioral and/or language dysfunction [2]. Some affected individuals manifest a movement disorder such as parkinsonism or motor neuron disease (MND). While the designation FTLD reflects the prominent frontal and temporal lobe degeneration, multiple neuropathological abnormalities are identified in these patients [4]. Two broad pathological subdivisions of FTLD are recognized: brains with tau-positive inclusions (i.e., tauopathies) and brains with UBI that are not detected with antibodies to tau, α-synuclein, and β-amyloid (i.e., FTLD-U). Up to 40% of FTLD show a familial pattern of inheritance with three different genetic abnormalities associated with FTLD-U pathology including mutations in progranulin (PGRN) [5,6] and valosin-containing protein (VCP) [7-9] as well as linkage to a novel locus on chromosome 9p [10-12].
ALS, the most common adult-onset MND, is characterized by rapidly progressive weakness, muscular wasting, and spasticity resulting in death within a few years [13]. There is loss of both upper and lower motor neurons with UBI, typically filamentous skeins or compact round bodies, in the surviving motor cells. Familial forms of ALS (fALS) with Mendelian inheritance account for ∼10% of cases and are associated with numerous genetic loci including mutations in five genes: Cu/Zn superoxide dismutase (SOD1), alsin, senataxin, vesicle-/synaptobrevin-associated membrane protein B, and dynactin. Mutations in SOD1 gene are the most common accounting for ∼20% of fALS.
Until recently, it was unclear whether the ubiquitin pathology in both FTLD-U and ALS was associated with the aggregation of a specific protein or through a generalized defect in protein ubiquitination and degradation. However, this past year, the transactive response (TAR)-DNA binding Protein with a molecular weight of 43 KDa (TDP-43) was identified as the major disease protein in the UBI of FTLD-U and ALS [14]. The identification of TDP-43 pathology in both of these disorders provided a mechanistic link for the following: 1) a large proportion of ALS patients manifest a range of behavioral and cognitive changes that lie on the spectrum of FTLD [15]; 2) MND is commonly observed in FTLD-U patients [16]; 3) there is significant overlap in the ubiquitin pathology observed in ALS and FTLD-U [17]; and 4) identification of genetic loci and mutations in specific genes in families with co-segregation of both ALS and FTLD [18]. In this review, we highlight work over the past twelve months on TDP-43 and its role in the pathogenesis of FTLD-U and ALS.
Identification of TDP-43 as a major disease protein in FTLD-U & ALS
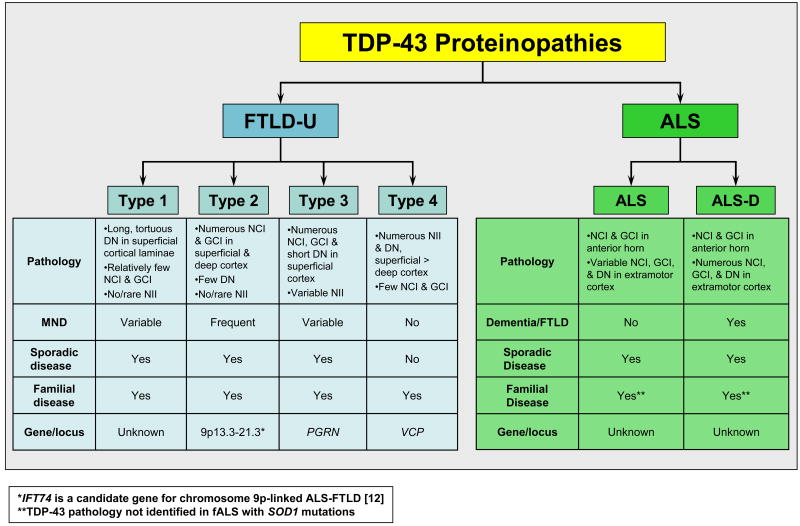
Characterization of the biochemical composition of the UBI in FTLD-U and ALS was complicated by the relatively low abundance and uneven distribution of the pathology. Unlike the amyloidogenic inclusions composed of β-amyloid, tau, and α-synuclein, the UBI were not clearly fibrillar; they were not detected using amyloid binding dyes such as Congo red, thioflavin S or silver stains. This observation suggested that FTLD-U and ALS are unique proteinopathies characterized by protein misfolding in the absence of brain amyloidosis, a signature of many neurodegenerative diseases. Moreover, the description of subtypes of FTLD-U pathology (Fig. 1) [4,19,20] raised the possibility of multiple disease proteins or pathways. To address this issue, novel monoclonal antibodies (Mab) were generated to high molecular weight insoluble protein extracts prepared from FTLD-U brains of distinct subtypes [20]. These Mab immunolabeled the UBI in the FTLD-U subtype from which they were generated while a subset of the Mab also immunoblotted disease-specific insoluble proteins extracted from affected FTLD-U brain tissue. These Mab facilitated an extensive analysis of protein extracts from FTLD-U brains and led to the identification of TDP-43 as the major component of the UBI [14]. Despite the pathological heterogeneity among FTLD-U subtypes (Fig. 1), immunohistochemistry with commercially available antibodies demonstrated TDP-43 in the UBI of all FTLD-U subtypes as well as sporadic ALS. Furthermore, biochemical analysis of TDP-43 demonstrated a signature profile of TDP-43 in detergent-insoluble, protein extracts from affected FTLD-U and ALS tissue [14]. Thus, pathological TDP-43 was abnormally phosphorylated, ubiquitinated and N-terminally truncated. The identification of these biochemical modifications suggested a specific role for TDP-43 in the pathogenesis of FTLD-U and ALS, rather than simply representing a non-specific entrapped protein within UBI.
Figure 1. Proposed classification scheme for TDP-43 proteinopathies.
Despite the significant clinical, genetic, and neuropathologic heterogeneity within FTLD and ALS, TDP-43 is a common pathological substrate linking FTLD-U and ALS caused by different genetic alterations. This observation supports the hypothesis that FTLD and ALS represent two extremes of a clinicopathological spectrum of one disease, TDP-43 proteinopathies. FTLD-U is subclassified based on distinct morphological, genetic, and clinical parameters while dementia is reported in a significant subset of ALS patients. ALS, amyotrophic lateral sclerosis; DN, dystrophic neurites; fALS, familial amyotrophic lateral sclerosis; FTLD, frontotemporal lobar degeneration; FTLD-U, frontotemporal lobar degeneration with ubiquitin inclusions; GCI, glial cytoplasmic inclusions; NCI, neuronal cytoplasmic inclusions; NII, neuronal intranuclear inclusions; MND, motor neuron disease; PGRN, progranulin; SOD1, Cu/Zn superoxide dismutase; VCP, valosin-containing protein.
Biology of TDP-43
TDP-43, a 414 amino acid nuclear protein encoded by the TARDBP gene on chromosome 1, was initially cloned from a genomic screen for cellular factors that bind to the TAR-DNA element of HIV where it acts as a transcriptional repressor [21]. It is highly conserved and ubiquitously expressed in all tissues including brain [22,23]. The expressed protein contains two RNA-recognition motifs as well as a glycine-rich C-terminal sequence. It was also independently identified as part of a complex involved in the splicing of the cystic fibrosis transmembrane conductance regulator [24] and apolipoprotein A2 genes [25]. The glycine-rich domain in TDP-43 is required for the exon skipping and splicing inhibitory activity [26,27], an observation consistent with the finding that the C-terminal domain binds to several proteins of the heterogeneous nuclear ribonucleoprotein (hnRNP) family involved in the biogenesis of mRNA [27]. TDP-43 was also recently shown to bind to the proximal promoter of the mouse SP-10 gene (acrosomal vesicle protein 1) involved in spermatogenesis and to regulate its expression [28]. Finally, TDP-43 may also act as scaffold for nuclear bodies called ‘GEMS’ through interaction with survival motor neuron (SMN) protein [29]. Thus, the physiological function(s) of TDP-43 are diverse and incompletely characterized but likely involve the regulation of multiple biological processes through its binding to single stranded DNA, RNA, and/or proteins.
TDP-43 pathology in FTLD-U and ALS
As demonstrated in our initial report [14] and rapidly confirmed in several follow up studies, TDP-43 is a specific and sensitive marker to detect the UBI in both FTLD-U [30-34] and ALS [32,35,36], including neuronal cytoplasmic inclusions (NCI), dystrophic neurites (DN), and neuronal intranuclear inclusions (NII). Notably, while physiological TDP-43 is detectable in the nuclei of unaffected neurons and some glial cells, TDP-43 pathology is associated with a dramatic reduction of normal nuclear TDP-43 staining, raising the possibility that an essential function of TDP-43 is lost in FTLD-U and ALS. Immunohistochemistry for TDP-43 also facilitated the detection of white matter pathology with numerous oligodendroglial cytoplasmic inclusions in a subset of FTLD-U and ALS cases that was not previously appreciated [32,36,37].
FTLD-U pathology is heterogeneous with respect to morphology, laminar distribution of pathological inclusions and relative proportion of intranuclear and cytoplasmic inclusions leading to the description of four distinct subtypes (Fig. 1) [8,14,19,20,30,38]. The relevance of the distinct patterns of pathology with respect to disease pathogenesis remains unclear. While some cases of FTLD-U do not fit neatly into a specific category, a correlation of distinct histologic subtypes was observed with familial forms of FTLD-U thereby supporting the significance of this classification [30]. For example, mutations in PGRN, a secreted growth factor associated associated with cell cycle progression and cell motility, were associated with type 3 pathology. Nearly all of the mutations in PGRN are predicted to cause premature termination of the coding sequence by nonsense mediated decay of mutant mRNAs leading to haploinsufficiency. By contrast, mutations in the gene VCP are characterized by type 4 pathology. VCP, a member of the AAA-ATPase gene family, associates with a number of protein adaptors to perform a plethora of cellular processes including ubiquitin-dependent protein degradation, stress responses, programmed cell death, nuclear envelope reconstruction, and Golgi and endoplasmic reticulum assembly. The mechanism whereby VCP gene mutations cause neurodegeneration remains unclear although disruption of ubiquitin-dependent protein degradation pathways has been implicated. Lastly, the recently identified locus on chromosome 9p is associated with type 2 FTLD-U pathology. As yet, no genetic alterations have been associated with FTLD-U type 1.
The role of TDP-43 in sporadic ALS versus fALS has also been evaluated. TDP-43 was detected in the round and skein-like NCI as well as glial inclusions in affected brain regions from ALS patients with and without dementia [14,32,35,36]. Remarkably, while pathological TDP-43 was a consistent feature in non-SOD1-fALS, TDP-43 was not detected in the UBI of any patients with SOD1 mutations [35,36]. Consistent with these findings is the reported absence of TDP-43 immunoreactivity in inclusions in mutant SOD1 (G93A) transgenic mice [39]. In contrast, in Guam ALS and parkinsonism-dementia complex (PDC), a disease of unknown etiology affecting the Chamorro populations and characterized by extensive tau pathology, TDP-43 inclusions were detected in the spinal cord of both ALS and PDC cases but not in controls [40]. These TDP-43 inclusions were distinct from the tau pathology in the spinal cord. Interestingly, TDP-43 pathology was also detected in cortical and limbic regions from Guam-PDC cases, inclusions that were not detected with antibodies to the tau protein [40,41]. Thus, these results support the hypothesis that ALS and FTLD-U represent a clinical spectrum of neurodegenerative disease characterized by TDP-43 pathology (Fig. 1). However, the absence of TDP-43 in SOD1-fALS implies that motor neuron degeneration in these cases results from a different disease pathway that also affects motor neurons. However, this hypothesis is highly controversial [42].
The specificity of TDP-43 as a marker for FTLD-U lesions now permits the investigation of FTLD-U pathology in the setting of concurrent ubiquitin-positive pathology in other neurodegenerative diseases (Box 1) [14,30-32,43-45]. Surprisingly, additional TDP-43 pathology similar to that found in FTLD-U was reported in several other neurodegenerative diseases. This observation raised the possibility that amyloid deposition in the brain (i.e., neurofibrillary tangles and Lewy bodies) predisposes TDP-43 to misfold and aggregate to form non-fibrillar inclusions. However, the clinical significance of concomitant TDP-43 pathology in these other diseases is unknown.
Box 1. Spectrum of TDP-43 pathology in neurodegenerative disease.
The initial report identifying TDP-43 in the UBI of FTLD-U and ALS suggested that TDP-43 is a specific marker for these diseases [14]. However, a follow-up study identified TDP-43 not only in the UBI of FTLD-U and ALS but also in tau inclusions including the majority of Pick bodies in Pick's disease, as well as a subset of the neurofibrillary tangles in AD and tangle-predominant senile dementia and threads and coiled bodies in corticobasal degeneration [32]. TDP-43 was not detected in the tau pathology of progressive supranuclear palsy or the α-synuclein pathology in Lewy body disease and multiple system atrophy. While subsequent studies did not detect TDP-43 in the tau inclusions of both familial and sporadic tauopathies [30,31], these findings prompted further investigation into the specificity of TDP-43 pathology.
Amador-Ortiz and colleagues detected TDP-43 pathology in 71% of hippocampal sclerosis (n = 65) cases [45]. While this result is not surprising in light of the high prevalence of hippocampal sclerosis in FTLD-U, they also detected TDP-43 pathology in 23% of AD cases (n = 167). However, double-labeling for TDP-43 and phospho-tau demonstrated that the TDP-43-immunoreactive pathology was largely distinct from the neurofibrillary tangles. In a related study on Lewy body disease, co-morbid TDP-43 pathology was identified in 29% of cases with Dementia with Lewy body and AD pathology, 19% of Parkinson's disease dementia, and 7% of Parkinson's disease [44]. TDP-43 pathology was also a consistent feature in affected Chamorrans with Guam-PDC, but not in controls [40,41]. Whether TDP-43 pathology represents concomitant FTLD-U pathology in these cases or is analogous to co-localization of α-synuclein pathology in AD remains to be determined. Nonetheless, these studies expand the concept of TDP-43 proteinopathies by implication of TDP-43 in a variety of neurodegenerative diseases characterized by the aggregation of fibrillar amyloid deposits.
Pathobiology of TDP-43
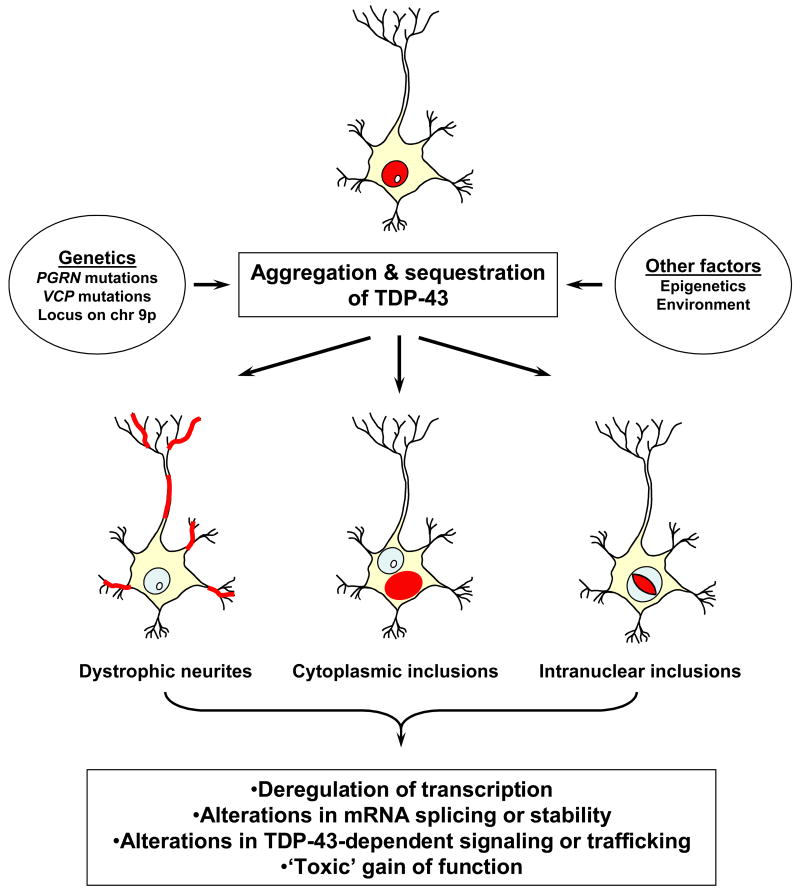
The identification of TDP-43 in the UBI of FTLD-U and ALS implicates a role for TDP-43 in disease pathogenesis. However to date, the proverbial ‘smoking gun’, i.e., genetic variation in the TARDBP leading to increased risk for disease, is lacking [46]. Although its functions are reported as a transcriptional repressor and splicing regulator [21-23], the mechanism whereby TDP-43 contributes to neuron degeneration is unknown (Fig. 2). Nonetheless, based on this functional data, a number of hypotheses have been generated. The sequestration of TDP-43 in inclusions could cause a loss of function defect and thereby result in transcriptional deregulation and aberrant splicing of pre-mRNA. For example, TDP-43 was recently demonstrated to stabilize low molecular weight neurofilament mRNA via a direct interaction with the 3′UTR [47]. Loss of TDP-43 activity could destabilize low molecular weight neurofilament mRNA thereby altering the stoichiometry of neurofilament subunits and leading to the formation of neurofilament aggregates as observed in ALS. The sequestration of TDP-43 could also alter the cellular distribution of SMN and hnRNP; however, changes in the expression and posttranslational modification of hnRNP were not observed in FTLD-U and ALS, and hnRNP were not detected in the UBI [48]. Alternatively, the C-terminal domain of TDP-43 that aggregates in the inclusions and is implicated in its splicing regulatory function [23,27], may have aberrant biological activities (i.e., toxic ‘gain of function’). It has also been hypothesized that PGRN might be a protein binding partner of TDP-43, involved in its trafficking to and from the nucleus [49]. Thus, dysfunction or dysregulation of PGRN could contribute to the abnormal compartmentalization of TDP-43. Finally, the abnormal phosphorylation of TDP-43 may disrupt important signaling pathways or directly affect the trafficking of TDP-43 itself, thereby leading to neuronal dysfunction. The development of cell culture and murine model systems will be critical to testing these hypotheses and elucidating the role of TDP-43 in the pathogenesis of FTLD-U. Furthermore, the development of genetic models will be essential to our understanding of the link between TDP-43 and mutations in multiple different genes including PGRN and VCP.
Figure 2. Model of TDP-43 disease pathogenesis.
The aggregation of TDP-43 (depicted in red) in neurons as well as glia leads to its sequestration in cytoplasmic and intranuclear inclusions as well as dystrophic neurites. The sequestration of TDP-43 may be toxic due to loss of normal function. Alternatively, the aggregation of TDP-43, in particular the C-terminal fragment(s) may lead to a toxic gain of function.
Conclusions
Despite the significant clinical, genetic, and neuropathologic heterogeneity within FTLD and ALS, TDP-43 is a common pathological substrate linking FTLD-U and ALS caused by different genetic alterations. This observation supports the hypothesis that FTLD and ALS represent two extremes of a clinicopathological spectrum of TDP-43 proteinopathies. An understanding of the role of TDP-43 in the pathogenesis of FTLD-U and ALS will have to integrate the biology of multiple distinct genetic elements. However, the absence of pathological TDP-43 in fALS with SOD1 mutations implies that MND in these cases is not the familial counterpart of sporadic ALS.
While these are still early days in the understanding of the pathobiology of TDP-43, it is evident that a new classification of neurodegenerative disorders has emerged (Fig. 1). However, the TDP-43 proteinopathies are distinct from other protein misfolding neurodegenerative diseases because of the lack of amyloid fibrils and will likely lead to unique challenges. Nonetheless, the identification of TDP-43 in the pathological inclusions of FTLD-U and ALS will have significant implications for the diagnosis and treatment of FTLD and ALS. For example, the development of assays to monitor levels of normal and pathological TDP-43 in cerebrospinal fluid could be used as a diagnostic tool to distinguish TDP-43 proteinopathies from other clinically similar neurodegenerative disorders. Further, the development of imaging ligands that enable the detection of TDP-43 neuropathology in living patients will provide a tool not only for diagnosis but also for following the response of patients with a neurodegenerative TDP-43 proteinopathy to disease-modifying therapies. Finally, the recognition that TDP-43 pathology underlies and links FTLD-U and ALS will be a significant driver of efforts to develop mechanistically-based therapies for these disorders.
Acknowledgments
The authors would like to thank the patients and their families who make the research described in this review possible. This review summarizes research funded by the National Institutes of Health (AG09215, AG10124, AG17586). VMYL is the John H. Ware III Chair of Alzheimer's Research. JQT is the William Maul Measey-Truman G. Schnabel, Jr., M.D. Professor of Geriatric Medicine and Gerontology.
Footnotes
Ethics & Conflicts of Interest: The authors report full compliance with the ‘code of conduct’ as outlined in the ‘Author Guidelines’. The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mark S Forman, Email: formanm@mail.med.upenn.edu.
John Q Trojanowski, Email: trojanow@mail.med.upenn.edu.
References & Annotations
- 1.Forman MS, Trojanowski JQ, Lee VMY. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10:1055–1063. doi: 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- *2.Kumar-Singh S, Van BC. Frontotemporal lobar degeneration: current concepts in the light of recent advances. Brain Pathol. 2007;17:104–114. doi: 10.1111/j.1750-3639.2007.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Outstanding review summarizing recent advances in genetics, biochemistry and pathology in FTLD research.
- 3.Xiao S, McLean J, Robertson J. Neuronal intermediate filaments and ALS: a new look at an old question. Biochim Biophys Acta. 2006;1762:1001–1012. doi: 10.1016/j.bbadis.2006.09.003. [DOI] [PubMed] [Google Scholar]
- *4.Cairns NJ, Bigio EH, Mackenzie IRA, Neumann M, Lee VMY, Hatanpaa KJ, White CL, III, Schneider JA, Grinberg LT, Halliday G, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;1145:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Consensus paper on revised criteria for the neuropathological diagnosis of FTLD that incorporates recent advances in molecular genetics, biochemistry, and neuropathology including the identification of TDP-43 as the major disease protein in FTLD-U. The proposed classification scheme includes an algorithm for the neuropathological diagnosis of FTLD and is compared with the previous classification system described in 2001 [16].
- **5.Cruts M, Gijselinck I, van der ZJ, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin JJ, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]; Identification of PGRN mutations in patients with frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17) and FTLD-U pathology. Interestingly, PGRN is located on chromosome 17q21, 1.7 Mb centromeric of MAPT, in which mutations are associated with FTDP-17 with tau pathology. All of the mutations in PGRN were predicted to cause premature termination of the coding sequence by nonsense mediated decay of mutant mRNAs. In one large Belgian kindred, mutations were identified in the splice donor site of intron 0 causing loss of mutant transcript by nuclear degradation.
- **6.Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]; First identification of PGRN mutations in patients with FTDP-17 and FTLD-U pathology. Interestingly, PGRN is located on chromosome 17q21, 1.7 Mb centromeric of MAPT, and encodes for 68-kDa secreted growth factor. All of the mutations in PGRN were predicted to cause premature termination of the coding sequence by nonsense mediated decay of mutant mRNAs. The authors propose that the mutations cause disease by haploinsufficiency.
- 7.Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, Pestronk A, Whyte MP, Kimonis VE. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–381. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- *8.Forman MS, Mackenzie IR, Cairns NJ, Swanson E, Boyer PJ, Drachman DA, Jhaveri BS, Karlawish JH, Pestronk A, Smith TW, et al. Novel ubiquitin neuropathology in frontotemporal dementia with valosin-containing protein gene mutations. J Neuropathol Exp Neurol. 2006;65:571–581. doi: 10.1097/00005072-200606000-00005. [DOI] [PubMed] [Google Scholar]; First comprehensive description of the neuropathology of ‘Inclusion body myopathy with Paget's disease of bone and frontotemporal dementia’ (IBMPFD) caused by mutations in VCP. The CNS pathology in IBMPFD is characterized by a novel pattern of ubiquitin pathology distinct from sporadic and familial FTLD-U, i.e., type 4 (Fig. 1).
- *9.Schröder R, Watts GD, Mehta SG, Evert BO, Broich P, Fliessbach K, Pauls K, Hans VH, Kimonis V, Thal DR. Mutant valosin-containing protein causes a novel type of frontotemporal dementia. Ann Neurol. 2005;57:457–461. doi: 10.1002/ana.20407. [DOI] [PubMed] [Google Scholar]; First description of the ubiquitin pathology in IBMPFD. The CNS pathology was characterized by ubiquitin- and VCP-positive intranuclear inclusions. The presence of VCP in the inclusions has not been confirmed in subsequent publications (see Forman et al [8].
- *10.Morita M, Al-Chalabi A, Andersen PM, Hosler B, Sapp P, Englund E, Mitchell JE, Habgood JJ, de BJ, Xi J, et al. A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology. 2006;66:839–844. doi: 10.1212/01.wnl.0000200048.53766.b4. [DOI] [PubMed] [Google Scholar]; Genetic linkage analysis was performed in a large Scandinavian family with FTLD and ALS assuming autosomal dominant inheritance of a single neurodegenerative disease manifesting as either FTLD or ALS. A new FTLD-ALS locus was identified on chromosome 9p21.3-p13.3 with a maximum multipoint LOD score of 3.00. This is a similar locus to that identified by Vance et al [11].
- *11.Vance C, Al-Chalabi A, Ruddy D, Smith BN, Hu X, Sreedharan J, Siddique T, Schelhaas HJ, Kusters B, Troost D, et al. Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.2-21.3. Brain. 2006;129:868–876. doi: 10.1093/brain/awl030. [DOI] [PubMed] [Google Scholar]; Genome-wide linkage study using Affymetrix 10K GeneChip microarrays in a large Dutch kindred with both ALS and FTLD. Analysis of single nucleotide polymorphism data showed linkage to chromosome 9p13.2-21.3 with a maximal LOD score of 2.4. This is a similar locus to that identified by Morita et al [10].
- 12.Momeni P, Schymick J, Jain S, Cookson MR, Cairns NJ, Greggio E, Greenway MJ, Berger S, Pickering-Brown S, Chio A, et al. Analysis of IFT74 as a candidate gene for chromosome 9p-linked ALS-FTD. BMC Neurol. 2006;6:44. doi: 10.1186/1471-2377-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *13.Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]; Outstanding review on recent advances in our understanding of the molecular genetics of amyotrophic lateral sclerosis.
- **14.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]; The authors generated novel monoclonal antibodies to the ubiquitin pathology of FTLD-U leading to the identification of TDP-43 as the major disease protein. Antibodies to TDP-43 did not detect the pathology in a variety of other neurodegenerative characterized by inclusion pathology. However, TDP-43 was also detected in the UBI of ALS, providing the first molecular link between these two neurodegenerative disorders. Pathological TDP-43 was abnormally phosphorylated, ubiquitinated and cleaved to generate C-terminal fragments from affected CNS regions.
- 15.Murphy J, Henry R, Lomen-Hoerth C. Establishing subtypes of the continuum of frontal lobe impairment in amyotrophic lateral sclerosis. Arch Neurol. 2007;64:330–334. doi: 10.1001/archneur.64.3.330. [DOI] [PubMed] [Google Scholar]
- 16.McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 17.Mackenzie IR, Feldman H. Ubiquitin immunohistochemistry suggests classic motor neuron disease, motor neuron disease with dementia, and frontotemporal dementia of the motor neuron disease type represent a clinicopathologic spectrum. J Neuropathol Exp Neurol. 2005;64:730–739. doi: 10.1097/01.jnen.0000174335.27708.0a. [DOI] [PubMed] [Google Scholar]
- 18.Talbot K, Ansorge O. Recent advances in the genetics of amyotrophic lateral sclerosis and frontotemporal dementia: common pathways in neurodegenerative disease. Hum Mol Genet. 2006;15(Spec No 2):R182–R187. doi: 10.1093/hmg/ddl202. [DOI] [PubMed] [Google Scholar]
- *19.Mackenzie IR, Baborie A, Pickering-Brown S, Du PD, Jaros E, Perry RH, Neary D, Snowden JS, Mann DM. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol. 2006;112:539–549. doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of two studies published in 2006 in which three distinct histological patterns of FTLD-U pathology were described in a large pathological series based on ubiquitin immunohistochemistry (see Sampathu et al for comparison [20]). In this study, the histological type correlated strongly with clinical phenotype.
- *20.Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, Bruce J, Grossman M, Trojanowski JQ, Lee VMY. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol. 2006;169:1343–1352. doi: 10.2353/ajpath.2006.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of two studies published in 2006 in which three distinct histological patterns of FTLD-U pathology were described in a large pathological series based on ubiquitin immunohistochemistry (see Mackenzie et al for comparison [19]). Novel monoclonal antibodies were generated that immunolabeled distinct subtypes of FTLD-U pathology. These antibodies were utilized in a subsequent study to identify TDP-43 as the major disease protein in all subtypes of FTLD-U pathology [14].
- 21.Ou SH, Wu F, Harrich D, Garcia-Martinez LF, Gaynor RB. Cloning and characterization of a novel cellular protein, TDP-43, that binds to human immunodeficiency virus type 1 TAR DNA sequence motifs. J Virol. 1995;69:3584–3596. doi: 10.1128/jvi.69.6.3584-3596.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buratti E, Baralle FE. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J Biol Chem. 2001;276:36337–36343. doi: 10.1074/jbc.M104236200. [DOI] [PubMed] [Google Scholar]
- *23.Ayala YM, Pantano S, D'Ambrogio A, Buratti E, Brindisi A, Marchetti C, Romano M, Baralle FE. Human, Drosophila, and C.elegans TDP43: nucleic acid binding properties and splicing regulatory function. J Mol Biol. 2005;348:575–588. doi: 10.1016/j.jmb.2005.02.038. [DOI] [PubMed] [Google Scholar]; To investigate the function of TDP-43, this study describes the cloning and characterization of TDP-43 homologs from Drosophila melanogaster and Caenorhabditis elegans. The proteins from human, fly, and worm showed striking similarities in their nucleic acid binding specificity. However, differences in the structure of the glycine-rich C-terminal domain between human, fly, and worm implicated this domain in the splicing regulatory function of the protein.
- 24.Buratti E, Dork T, Zuccato E, Pagani F, Romano M, Baralle FE. Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J. 2001;20:1774–1784. doi: 10.1093/emboj/20.7.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mercado PA, Ayala YM, Romano M, Buratti E, Baralle FE. Depletion of TDP 43 overrides the need for exonic and intronic splicing enhancers in the human apoA-II gene. Nucleic Acids Res. 2005;33:6000–6010. doi: 10.1093/nar/gki897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang HY, Wang IF, Bose J, Shen CK. Structural diversity and functional implications of the eukaryotic TDP gene family. Genomics. 2004;83:130–139. doi: 10.1016/s0888-7543(03)00214-3. [DOI] [PubMed] [Google Scholar]
- *27.Buratti E, Brindisi A, Giombi M, Tisminetzky S, Ayala YM, Baralle FE. TDP-43 binds heterogeneous nuclear ribonucleoprotein A/B through its C-terminal tail: an important region for the inhibition of cystic fibrosis transmembrane conductance regulator exon 9 splicing. J Biol Chem. 2005;280:37572–37584. doi: 10.1074/jbc.M505557200. [DOI] [PubMed] [Google Scholar]; The study demonstrated that the C-terminal domain of TDP-43 is capable of binding directly to several proteins of the hnRNP family with well-defined splicing inhibitory activity including hnRNP A2/B1 and hnRNP A1. TDP-43 protein lacking the C-terminal region did not inhibit splicing because hnRNP-rich complexes were not formed at the earliest stages of spliceosomal assembly.
- 28.Acharya KK, Govind CK, Shore AN, Stoler MH, Reddi PP. cis-requirement for the maintenance of round spermatid-specific transcription. Dev Biol. 2006;295:781–790. doi: 10.1016/j.ydbio.2006.04.443. [DOI] [PubMed] [Google Scholar]
- 29.Wang IF, Reddy NM, Shen CK. Higher order arrangement of the eukaryotic nuclear bodies. Proc Natl Acad Sci U S A. 2002;99:13583–13588. doi: 10.1073/pnas.212483099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *30.Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL, III, Schneider JA, Kretzschmar HA, et al. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol. 2007;171:227–240. doi: 10.2353/ajpath.2007.070182. [DOI] [PMC free article] [PubMed] [Google Scholar]; Large survey study on 193 cases of familial and sporadic FTLD. The study confirmed the specificity of TDP-43 for the ubiquitin pathology in FTLD-U but not in other pathologies associated with FTLD including Pick's disease, corticobasal degeneration, progressive supranuclear palsy, and FTDP-17 due to MAPT gene mutations. The study also validated the classification scheme of FTLD-U and correlated the pathological subtypes with specific genetic abnormalities.
- *31.Davidson Y, Kelley T, Mackenzie IR, Pickering-Brown S, Du PD, Neary D, Snowden JS, Mann DM. Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein, TDP-43. Acta Neuropathol. 2007;113:521–533. doi: 10.1007/s00401-006-0189-y. [DOI] [PubMed] [Google Scholar]; Study confirming the presence of TDP-43 in the UBI of 37 cases of FTLD-U. TDP-43 was not detected in other pathologies associated with FTLD including Pick's disease and FTDP-17 due to MAPT tau gene mutations. This study also validated the classification scheme for FTLD-U proposed by Mackenzie et al [19]
- *32.Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]; Study confirming the presence of phosphorylated TDP-43 in the UBI associated with FTLD-U and ALS. The study also reports the detection of TDP-43 in tau pathological inclusions including Pick bodies in Pick's disease, neurofibrillary tangles in AD and tangle-predominant senile dementia, and threads and coiled bodies in corticobasal degeneration. However, the presence of TDP-43 in tau inclusions has not been confirmed in other studies [30,31].
- 33.Seelaar H, Schelhaas HJ, Azmani A, Kusters B, Rosso S, Majoor-Krakauer D, de Rijik MC, Rizzu P, ten BM, van Doorn PA, et al. TDP-43 pathology in familial frontotemporal dementia and motor neuron disease without Progranulin mutations. Brain. 2007;130:1375–1385. doi: 10.1093/brain/awm024. [DOI] [PubMed] [Google Scholar]
- 34.Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, Togo T, Katsuse O, Uchikado H, Furukawa Y, et al. Appearance pattern of TDP-43 in Japanese frontotemporal lobar degeneration with ubiquitin-positive inclusions. Neurosci Lett. 2007;419:213–218. doi: 10.1016/j.neulet.2007.04.051. [DOI] [PubMed] [Google Scholar]
- *35.Tan CF, Eguchi H, Tagawa A, Onodera O, Iwasaki T, Tsujino A, Nishizawa M, Kakita A, Takahashi H. TDP-43 immunoreactivity in neuronal inclusions in familial amyotrophic lateral sclerosis with or without SOD1 gene mutation. Acta Neuropathol. 2007;113:535–542. doi: 10.1007/s00401-007-0206-9. [DOI] [PubMed] [Google Scholar]; The brains and spinal cords from four cases of fALS due to SOD1 gene mutations was examined by TDP-43 immunohistochemistry and compared with sporadic ALS and fALS without SOD1 mutations. The UBI in both sporadic ALS and non-SOD1 fALS were TDP-43 immunoreactive. In contrast, the ubiquitin pathology in fALS with SOD1 gene mutations was not detected with antibodies to TDP-43. These data support the hypothesis that ALS due to SOD1 mutations reflects a disease pathway distinct from the sporadic disease counterpart.
- **36.Mackenzie IR, Bigio EH, Ince PG, Geser F, Neumann M, Cairns NJ, Kwong LK, Forman MS, Ravits J, Stewart H, et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol. 2007;61:427–434. doi: 10.1002/ana.21147. [DOI] [PubMed] [Google Scholar]; Survey study investigating TDP-43 using both biochemistry and immunohistochemistry in a large series of ALS cases (n = 111) including fALS with and without SOD1 gene mutations. The UBI of all cases of sporadic ALS, ALS with dementia, and SOD1-negative fALS were immunoreactive with antibodies to TDP-43 including the detection of oligodendroglial inclusions that were not appreciated with immunostaining for ubiquitin. In contrast, the UBI of fALS with SOD1 gene mutations were not detected with TDP-43 immunohistochemistry. These findings implicate TDP-43 in the pathogenesis of ALS and support the hypothesis that fALS caused by SOD1 gene mutations may result from a different disease pathway that also affects motor neurons.
- *37.Neumann M, Kwong LK, Truax AC, Vanmassenhove B, Kretzschmar HA, Van D V, Clark CM, Grossman M, Miller BL, Trojanowski JQ, et al. TDP-43-positive white matter pathology in frontotemporal lobar degeneration with ubiquitin-positive inclusions. J Neuropathol Exp Neurol. 2007;66:177–183. doi: 10.1097/01.jnen.0000248554.45456.58. [DOI] [PubMed] [Google Scholar]; This study demonstrates abundant TDP-43 immunoreactive white matter pathology as a characteristic disease feature in 38 FTLD-U cases including 3 with PGRN mutations. The white matter inclusions are most likely oligodendrocytes based on morphology and double-labeling studies. These findings expanded the spectrum of TDP-43 pathology in FTLD-U and implicated white matter disease in the neurodegenerative process.
- *38.Neumann M, Mackenzie IR, Cairns NJ, Boyer PJ, Markesbery WR, Smith CD, Taylor JP, Kretzschmar HA, Kimonis VE, Forman MS. TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP gene mutations. J Neuropathol Exp Neurol. 2007;66:152–157. doi: 10.1097/nen.0b013e31803020b9. [DOI] [PubMed] [Google Scholar]; This study analyzed the ubiquitin pathology in IBMPFD associated with VCP gene mutations. The NII and DN were labeled with antibodies to TDP-43 leading to the hypothesis that VCP mutations lead to a dominant-negative loss or alteration of VCP function culminating in impaired degradation of TDP-43. This study supports the central role of TDP-43 as a common pathological substrate linking a variety of distinct patterns of FTLD-U pathology caused by different genetic alterations.
- 39.Robertson J, Sanelli T, Xiao S, Yang W, Horne P, Hammond R, Pioro EP, Strong MJ. Lack of TDP-43 abnormalities in mutant SOD1 transgenic mice shows disparity with ALS. Neurosci Lett. 2007;420:128–132. doi: 10.1016/j.neulet.2007.03.066. [DOI] [PubMed] [Google Scholar]
- 40.Geser F, Winton MJ, Kwong LK, Xu Y, Xie SX, Igaz LM, Garruto RM, Perl DP, Galasko D, Lee VMY, et al. Pathological TDO-43 in parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Am J Pathol. 2007 doi: 10.1007/s00401-007-0257-y. in press. [DOI] [PubMed] [Google Scholar]
- 41.Hasegawa M, Arai T, Akiyama H, Nonaka T, Mori H, Hashimoto T, Yamazaki M, Oyanagi K. TDP-43 is deposited in the Guam parkinsonism-dementia complex brains. Brain. 2007;130:1386–1394. doi: 10.1093/brain/awm065. [DOI] [PubMed] [Google Scholar]
- *42.Rothstein JD. TDP-43 in amyotrophic lateral sclerosis: Pathophysiology or patho-babel? Ann Neurol. 2007;61:382–384. doi: 10.1002/ana.21155. [DOI] [PubMed] [Google Scholar]; This very interesting commentary questions whether the TDP-43 inclusions in ALS represent a pathological curiosity or have “real pathophysioloigcal relevance”. Time will tell.
- 43.Leverenz JB, Yu CE, Montine TJ, Steinbart E, Bekris LM, Zabetian C, Kwong LK, Lee VMY, Schellenberg GD, Bird TD. A novel progranulin mutation associated with variable clinical presentation and tau, TDP43 and alpha-synuclein pathology. Brain. 2007;130:1360–1374. doi: 10.1093/brain/awm069. [DOI] [PubMed] [Google Scholar]
- 44.Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE, Arnold SE, Siderowf A, Grossman M, Leverenz JB, et al. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Am J Pathol. 2007 doi: 10.1007/s00401-007-0261-2. in press. [DOI] [PubMed] [Google Scholar]
- *45.Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified TDP-43 pathology in 71% of cases with pure hippocampal sclerosis and 23% of AD, although the TDP-43 pathology did not co-localize with the majority of neurofibrillary tau pathology in AD as previously reported [32]. These findings expand the pathological spectrum of TDP-43 pathology. However, the identification of TDP-43 pathology in AD has not been consistently observed [30].
- 46.Rollinson S, Snowden JS, Neary D, Morrison KE, Mann DM, Pickering-Brown SM. TDP-43 gene analysis in frontotemporal lobar degeneration. Neurosci Lett. 2007;419:1–4. doi: 10.1016/j.neulet.2007.03.044. [DOI] [PubMed] [Google Scholar]
- *47.Strong MJ, Volkening K, Hammond R, Yang W, Strong W, Leystra-Lantz C, Shoesmith C. TDP43 is a human low molecular weight neurofilament (hNFL) mRNA-binding protein. Mol Cell Neurosci. 2007;35:320–327. doi: 10.1016/j.mcn.2007.03.007. [DOI] [PubMed] [Google Scholar]; This study demonstrated that TDP-43 stabilizes low molecular weight neurofilament mRNA through a direct interaction with the 3′UTR. Thus, the sequestration of TDP-43 in cytosolic inclusions could contribute to the formation of neurofilament aggregates by destabilizing low molecular weight neurofilament message thereby altering the stoichiometry of neurofilament subunits in neurons.
- 48.Neumann M, Igaz LM, Kwong LK, Nakashima-Yasuda H, Kolb SJ, Dreyfuss G, Kretzschmar HA, Trojanowski JQ, Lee VMY. Absence of heterogeneous nuclear ribonucleoproteins and survival motor neuron protein in TDP-43 positive inclusions in frontotemporal lobar degeneration. Acta Neuropathol (Berl) 2007;113:543–548. doi: 10.1007/s00401-007-0221-x. [DOI] [PubMed] [Google Scholar]
- 49.Ahmed Z, Mackenzie IR, Hutton ML, Dickson DW. Progranulin in frontotemporal lobar degeneration and neuroinflammation. J Neuroinflammation. 2007;4:7. doi: 10.1186/1742-2094-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]