Abstract
Background
Methamphetamine (MA) abuse is associated with neurotoxicity to frontostriatal brain regions with concomitant deleterious effects on cognitive processes. Deficits in behavioral control are thought to be one contributing factor to the sustainment of addictive behaviors in chronic MA abuse.
Methods
In order to examine patterns of behavioral control relevant to addiction, we employed a fast-event related fMRI design to examine trial to trial reaction time (RT) adjustments in 12 chronic MA abusers who met DSM-IV criteria for MA dependence and 16 non-substance abusing controls. A variant of the Stroop task was employed to contrast the groups on error rates, RT Stroop conflict effect and the level of trial-to-trial adjustments seen after incongruent trials.
Results
The MA abusers exhibited reduced RT adjustments along with reduced activation in the right prefrontal cortex compared to controls on conditions that measured the ability to use exposure to conflict situations (i.e., conflict trials) to regulate behavior. MA abusers did not differ from controls on accuracy rates or within-trial Stroop conflict effects.
Conclusions
The observed deficits in trial to trial RT adjustments suggest that the ability to adapt a behavioral response based on prior experience may be compromised in MA abusers. Such adjustments are critical to everyday functioning and deficits in modifying behavior based on prior events may reflect a key deficit that contributes to maladaptive drug seeking behavior.
Keywords: Methamphetamine, prefrontal, attention, fMRI, imaging
Introduction
In the past decade the use of the stimulant methamphetamine (MA) has increased in the general population, with worldwide abuse of amphetamines surpassing that of cocaine and opiates combined (1). There is an established literature in both animals (2) and humans (3) that documents damage to regions following MA abuse within neural pathways that are integral to addiction (4, 5). Proton MR spectroscopy (MRS) (6–8) and positron emission tomography (PET) imaging studies (9, 10) suggest that fronto-cingulate regions of the brain are affected by MA abuse. Consistent with abnormalities in brain structure and function, cognitive impairments have also been observed in MA abusers on tasks that require the suppression of task irrelevant information (8, 11), decision-making (12, 13), and working memory (14). Any combination of the cognitive impairments described above may contribute and promote maintenance of the maladaptive actions associated with drug-seeking behavior (15).
Study Rationale
Neurobiological models of addiction propose that ventral brain regions (i.e., orbital frontal cortex and nucleus accumbens) contribute to the impulse to seek drugs, whereas the recruitment of fronto-cingulate regions may be critical to control those prepotent impulses (5). In the context of addiction, cognitive control can be interpreted as the inhibition of a prepotent response (e.g., habitual drug use) in order to carry out behaviors associated with long-term rewards and positive outcomes (e.g., abstaining from drug use). In order to selectively examine the neural substrates of cognitive control relevant to addiction, we conducted a fast-event related fMRI study in which we examined trial to trial reaction time (RT) adjustments using a variant of the single-trial Stroop task in 12 chronic MA abusers and 16 controls. This version of the Stroop task creates conditions in which performance (RT and accuracy) reflects the ability to recognize and resolve conflict at the time of selection (i.e., within a trial), as well as trial sequences in which performance is influenced by the ability to use exposure to conflict situations (i.e., conflict trials) to regulate behavior (i.e., trial to trial adjustments) (16). Since previous imaging studies have shown that this variant of the Stroop task elicits activity in both the Anterior Cingulate Cortex (ACC) and PreFrontal Cortex (PFC) (16–18), these brain regions were the focus of our fMRI imaging analysis. Given the association between PFC activity and cognitive control processes relevant to addiction we hypothesized that the MA abusers would show abnormal patterns of trial to trial adjustments (i.e., conflict adaptation) associated with reduced PFC activity.
Methods
Subjects
Two groups were studied: 12 MA abusing subjects and 16 age-matched non-substance abusing control subjects. The MA abusers met DSM-IV criteria for lifetime methamphetamine dependence determined from the Structured Clinical Interview (SCID) (19) but were currently drug abstinent a minimum of 3 weeks. See Table 1.
Table 1.
Demographic and clinical characteristics of 12 methamphetamine (MA) abusers and 16 control subjects.
| Methamphetamine Abusers (n =12) |
Control Subjects (n = 16) |
|
|---|---|---|
| Demographic Variables | ||
| Age, y, mean (SD) | 35.7 (7.7) | 30.2 (8.9) |
| Range | 24 to 44 years | 20 to 48 years |
| Females | 7 | 8 |
| Subject’s education, y, mean | 12.3 (1.4) †† | 15.0 (1.2) |
| Parental education, y, mean | 13.4 (2.7) | 13.8 (2.7 ) |
| NART | 107.8 (7.8) † | 110.3 (4.5 ) |
| Clinical Variables | ||
| Methamphetamine use | -- | |
| Duration, y, mean (SD) | 13.9 (5.7) | -- |
| Range | 5 to 22 years | |
| Months Abstinent, mean (SD) | 4.1 (2.8) | -- |
| Range | 2 to 12 months | |
| Age of first use, y, mean (SD) | 20.7 (5.9) | -- |
| History of Cannabis Abuse | 9 | -- |
Significantly different from control group, p < .05
Significantly different from control group, p < .01
Procedure
A single-trial Stroop task was administered during the scanning session that employed both Incongruent (conflict) and Congruent (non-conflict) trials (see Supplemental Material for detailed task description). Behavioral analyses contrasted the groups on mean error rates, RT Stroop conflict effect (Conflict minus non-conflict), and the level of trial-to-trial adjustments seen after conflict trials. Analysis of variance procedures (ANOVA) for repeated measures were used to analyze the data in a 2 × 2 mixed ANOVA with group as a between subjects factor (patients vs controls) and wordtype (incongruent vs congruent) as within subjects variables. Incorrect responses were not included within the analysis of variance for RT. Analyses were carried out to examine trial to trial adjustments to conflict-conflict (iI) compared to non-conflict-conflict (cI) sequences.
Imaging
Functional MRI data were collected on a 3T Siemens TRIO scanner (see Supplemental Material for scanning parameters and image preprocessing details). Analyses were preformed using a General Linear Model (GLM) as implemented in SPM5. In a first-level analysis, individual subject GLMs were built and fitted to each subject’s functional data. The statistical models included regressors coding for 7 covariates (cC, iC, iI, cI, Errors, Post-Errors and Non-Responses). Parameter estimates obtained from this first level analysis were used to compute maps of the contrasts of interest for effects of conflict and trial-to-trial effects (I-C for RT conflict, and iI-cI for trial to trial RT adjustments). Maps of the contrasts of interest for effects of error conflict were also computed. Hypotheses put forth within this proposal regarding activity in specific regions of interest (ROIs) including ACC and PFC were tested in a second level random-effects analysis of these contrast maps. The volume of search was restricted to areas for which we had specific hypotheses by using an explicit mask of lateral and medial prefrontal cortical areas. The mask was built using the AAL atlas in the SPM5 toolbox (see supplemental material for details). Clusters of active voxels reported were corrected for multiple comparisons at a set level of p < 0.05 (20).
Results
Behavioral Data
Reaction Time Analyses
Analyses revealed main effects of Stroop wordtype [F (1, 26) = 65.09, p< .001] as well as an interaction between group, trial to trial adjustment and wordtype [F (1, 26) =4.04, p = .05]. Analyses revealed that the trial to trial adjustment RT effect (cI-iI) differed significantly between the MA abusers and controls [F (1, 26) = 6.54; p =.04, bonferroni corrected for multiple comparisons). While the controls showed an RT advantage (13 msec benefit) to conflict trials that were preceded by conflict trials (iI) the MA abusers showed no advantage and were actually slower (21 msec cost). Virtually identical results were obtained when the trials that included exact stimulus repetitions (color and word) were included (p=.04). These group differences in Stroop endured with age, education, NART scores as covariates. No group differences were observed on within-trial Stroop conflict effects (F < 1).
Error Analyses
Analyses revealed a main effect of wordtype [F (1,26) = 18.97, p =. 0001] with both groups making significantly more errors in the incongruent condition (7%) than in the congruent condition (4%). No other effects were significant. There was no evidence of a speed-accuracy trade-off for both groups (MA abusers; r=.264; p =.41, controls: r=.287; p =.28].
Imaging Results
We first examined whether activity within the ACC and PFC was associated with within-trial conflict monitoring (I-C) and trial-to-trial adjustments (iI-cI). Using ANOVA procedures with subject as a random variable, significant activation to conflict contrasts (I-C) was observed in the ACC in both groups with no differences observed between groups. In contrast, when we examined the pattern of activation for the trial to trial RT adjustments we found that controls exhibited increased PFC activity, most notably in BA6 on iI sequences compared to the cI sequences. In contrast the MA abusers showed little or no activation within the PFC region. (See Figure 1). We then ran a second-level random-effects analysis to explore group differences across the contrasts of interest. Between group differences emerged in activation in the right middle gyrus centered in Brodmann’s area 6. (p < .05, corrected) (See figure 2). This region showed lower levels of activation in the MA abusers after iI trial sequences relative to cI trial sequences. Table 3 summarizes the data for frontal regions with significantly different activation between the MA abusers and controls.
Figure 1.
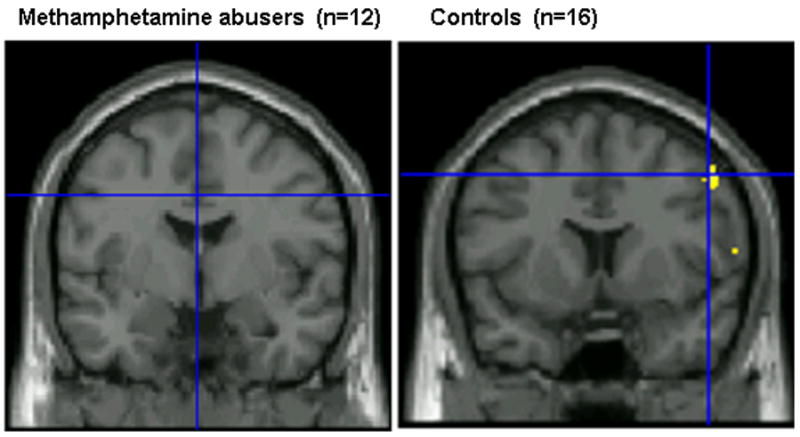
Regions of Increased Activation Associated with Trial to Trial Adjustments (iI– cI) in Methamphetamine Abusers and Controls
Figure 2.
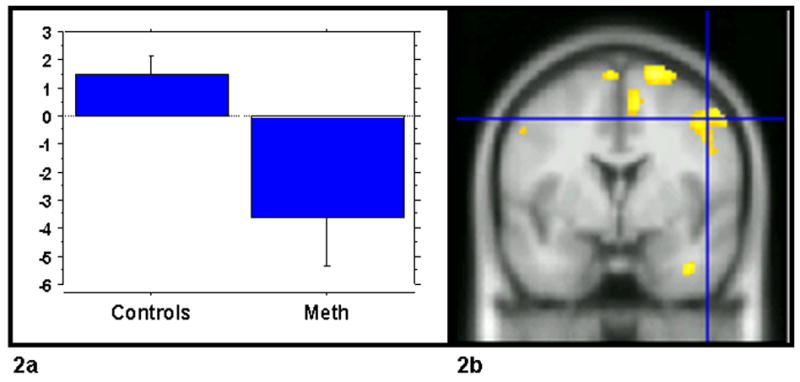
DLPFC region showing increased activity related to trial to trial adjustments (iI-cI) in control subjects relative to methamphetamine (MA) abusers. 2a) Graph of the average coefficients for the iI-cI statistical contrast in the two groups, averaged within group for the voxels of the DLPFC activation showed in panel 2a.
Table 3.
Brain regions with significant group differences associated with trial to trial adjustments in 12 methamphetamine (MA) abusers and 16 control subjects.
| Region | Brodmann’s Area | # of voxels | MNI Coordinates | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Right Cingulate | 32 | 44 | 8 | 14 | 44 |
| Right Superior | 6 | 128 | 18 | 0 | 72 |
| Mid Frontal | 6 | 404 | 44 | 0 | 46 |
| Left Superior | 6 | 30 | −14 | −6 | 72 |
| Right Superior | 8 | 41 | 14 | 44 | 42 |
| Right Precentral | -- | 14 | 62 | 12 | 10 |
| Left Precentral | 6 | 17 | −52 | −2 | 40 |
Conclusion
Reduced right PFC activation, most notably in the right mid-frontal cortex (BA6), was observed in MA abusers compared to non-substance abusing controls when contrasting conditions that measured how prior exposure to conflict modulated subsequent behavior. In contrast, no significant group differences were observed in conflict related activation in the ACC (17, 18, 21). These data provide preliminary evidence that MA abuse is associated with deficits in behavioral regulation associated with abnormal PFC activation and to the best of our knowledge constitute the first published data of abnormal trial to trial adjustments in MA abusers. Given that frontally-mediated behavioral regulation assists with maintenance of goal-directed behavior, it is not surprising that the pattern of deficits in the MA abusers involve reduced activation in frontal regions.
These data cannot speak to the acute effects of MA use as our subjects were MA abstinent at the time of study nor can the findings rule out pre-existing vulnerabilities that could have predated the MA abuse. One limitation of the current study is that no significant correlations were observed between trial to trial behavioral RTs and beta coefficients within the PFC, thus future studies in larger groups of MA abusers are needed to confirm brain-behavior relationships. Studies that include at risk populations are needed to determine how deficits in conflict adaptation and abnormal behavioral control may contribute to the development and sustainment of addictive behaviors. Functional imaging techniques, such as those employed in the current study, are powerful tools to probe these questions and to test the validity of neurobiological models as they apply to human addiction (5).
Supplementary Material
Table 2.
Behavioral results from 12 methamphetamine (MA) abusers and 16 control subjects.
| Methamphetamine Abusers (n =12) |
Control Subjects (n = 16) |
|
|---|---|---|
| Within-Trial Stroop Effects, mean (SD)s (msec) | ||
| Incongruent, mean (SD) | 719.7 (88.1) | 725.2 (115.5) |
| Congruent, mean (SD) | 628.5 (67.9) | 649.6 (97.5) |
| Stroop Conflict Effect | 91.2 | 75.6 |
| Conflict Errors, mean (SD) | .05 (.04) | .09 (.10) |
| Between-Trial Stroop Effects mean (SD)s (msec) | ||
| Congruent-Incongruent (cI) | 709.2 (88.0 ) | 731.5 (125.1) |
| Incongruent-Incongruent (iI) | 730.1 (91.1) | 718.8 (115.7) |
| Trial to Trial Adjustment (cI-iI) | − 20.9 | 12.7 † |
Significantly different from control group, p < .05
Acknowledgments
We would like to thank Jerry Sonico for his support and technical assistance with MR data collection. We are also very appreciative of the support of Thomas E. Nordahl, MD, PhD.
Footnotes
Disclosure/Conflict of Interest
The authors declare that this work was funded by NIDA grant DA16293-01 to RS. The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U Nations. The world drug problem: A status report. In: Crime UNOoDa., editor. 2004 World Drug Report. Vol. 1. Vienna: United Nations; 2004. pp. 25–26. [Google Scholar]
- 2.Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain research. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- 3.Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koob GF, Moal L. Neurobiology of Addiction. London: Academic Press; 2006. [Google Scholar]
- 5.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 6.Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology. 2000;54:1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- 7.Nordahl TE, Salo R, Natsuaki Y, Galloway GP, Waters C, Moore CD, et al. Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study . Archives of general psychiatry. 2005;62:444–452. doi: 10.1001/archpsyc.62.4.444. [DOI] [PubMed] [Google Scholar]
- 8.Salo R, Nordahl TE, Natsuaki Y, Leamon MH, Galloway GP, Waters C, et al. Attentional control and brain metabolite levels in methamphetamine abusers. Biological psychiatry. 2007;61:1272–1280. doi: 10.1016/j.biopsych.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 9.London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers . Archives of general psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- 10.Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. The American journal of psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 11.Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug and alcohol dependence. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Kalechstein AD, Newton TF, Green M. Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. The Journal of neuropsychiatry and clinical neurosciences. 2003;15:215–220. doi: 10.1176/jnp.15.2.215. [DOI] [PubMed] [Google Scholar]
- 13.Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA. Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biological psychiatry. 2003;53:65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- 14.McKetin R, Mattick RP. Attention and memory in illicit amphetamine users: comparison with non-drug-using controls. Drug and alcohol dependence. 1998;50:181–184. doi: 10.1016/s0376-8716(98)00022-2. [DOI] [PubMed] [Google Scholar]
- 15.Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature. 1999;398:567–570. doi: 10.1038/19208. [DOI] [PubMed] [Google Scholar]
- 16.Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 17.Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, et al. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America; 2000. pp. 1944–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 19.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Archives of general psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 20.Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. NeuroImage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- 21.Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


