Abstract
The present series of studies provide validation of a new paradigm that uniquely combines the assessment of the propensity to engage in social investigation with measures of (nonsocial) exploratory activity in rats. Assessment of this social investigation paradigm indicated that (a) rats showed a robust preference for social investigation over nonsocial exploratory activity, (b) female rats showed a greater preference for social investigation than male rats, (c) no signs of habituation in these responses were observed when rats were tested once daily for 4 consecutive days, (d) the preference for social investigation was stable and robust in both the dark and light periods of the daily light cycle for 5 consecutive days, and (e) testing under bright light conditions suppressed social investigation. In addition, acute administration of opiate drugs, low dose morphine (1.0 mg/kg) and naltrexone (1.0 mg/kg) produced a more robust attenuation of social investigation than nonsocial exploratory activity. Amphetamine increased both forms of investigation and haloperidol had the opposite effect, but the overall preference for social investigation over exploratory activity remained largely intact after both amphetamine and haloperidol injection. Together, these findings validate the use of this behavioral task to assess changes in social-motivation and general exploratory activity. Importantly, the task is bi-directionally sensitive to subject characteristics (i.e., sex), drug manipulations which modulate social motivation, and environmental manipulations.
Keywords: social investigation, social preference, exploration, morphine, naltrexone, amphetamine, haloperidol, rats
Introduction
Most laboratory animals such as rats are gregarious species and prefer social interaction over individual isolation [1-3]. The propensity to engage in social behavior varies significantly across the lifespan [4, 5] and is potently modulated by a wide variety of individual subject characteristics such as sex (eg., [6, 7]) as well as environmental circumstances such as living conditions [8-10]. Indeed, the importance of social interaction for evolutionary survival and organismic health has been the subject of critical concern in behavioral neurobiology in contemporary reviews (see [11] for an excellent example), and there is currently a major push towards understanding the basic neurobiology of social behavior and its relevance to human clinical disorders such autism spectrum disorders [12-14].
The assessment of social interaction more broadly (including play behavior in adolescents) has been used for purposes other than delineating neural substrates of social behavior, particularly for the measurement of anxiety [15] and acute illness [16, 17]. For instance, anxiogenic drugs and conditions that promote anxiety, such as bright test significantly reduce social interaction and test conditions that promote anxiety, such as a bright test chamber and testing in an unfamiliar environment, do the same [18]. Anxiety produced by withdrawal from drugs of abuse such as nicotine [19] and alcohol [20, 21] profoundly suppress social interaction, as does prior exposure to certain stressors such as the odor of a cat [22, 23]. Importantly, the social interaction test appears to be bi-directionally sensitive; normal expression of social interaction can be facilitated by anxiolytic treatment or reinstated by anxiolytics after manipulations that provoke anxiety in this test. As a result, tests of social interaction have become a valuable tool for the assessment of anxiety and/or the disruption of normal behavioral status incurred by a diverse range of challenges.
At the neurochemical level, a wide variety of systems have been examined for their role in the normal expression of social behavior [24, 25]. Among these systems, there is a growing body of evidence to suggest that opioid tone is a critical component for the expression of normal social behavior (see [26] for a review). For instance, low doses of morphine produce a robust facilitation of social contacts including play behavior, while higher doses (more typical of the analgesic range) potently suppress it [27, 28]. Similarly, opiate antagonism with drugs such as naltrexone suppresses social interaction [29] and playful interaction [30], an effect that is thought to result from inhibition of social reinforcement in both rodents [30] and primates [31]. These pharmacological effects have been buttressed by data implicating the release of endogenous opioids during even brief bouts of social interaction as indicated by displacement of 3H-diprenorphine binding [32, 33] as well as site-specific changes in opioid receptor expression [34].
One notorious problem in assessing the impact of drug manipulations or other perturbations on social behavior has been identifying the specificity of the effects to the social-motivational aspect of the task. For example, amphetamine administration has been shown to decrease the time spent in social interaction [15] and suppress playful interaction [9, 35], an effect that appears to be independent of catecholamine release [36]. Interestingly, haloperidol produces a similar decrement in social interaction [37] and play behavior, with a robust suppression of pinning observed in a dose-dependent manner [36]. Even more problematic is the finding that haloperidol fails to reverse the social interaction-suppressing effects of amphetamine, even though it effectively reverses the effects of amphetamine in other behavioral tasks quite well [36]. Although these findings are somewhat counterintuitive given the locomotor stimulating properties of amphetamine (eg., [38]), suppression of social interaction by amphetamine suggests that the regulation of social behavior and locomotor activity may be independent constructs that are governed by separate neural substrates. It would therefore be advantageous to develop a behavioral task that could potentially dissociate the effects of drugs and other manipulations on general exploratory/locomotor activity from more specific effects on social motivational processes within the same task.
In light of this, the present series of studies provide critical validation of a new paradigm that uniquely combines the assessment of the propensity to engage in social behavior with measures of (nonsocial) exploratory activity [39]. This simple paradigm involves placing rats into adjacent chambers where interaction is physically limited between holes in the chamber. Importantly, this simple task has already been established as highly sensitive to the effects of social isolation and oxytocin administration [26], both of which increased social investigation, and has been used a highly sensitive measure of teratogenic consequences of toxicant exposure [40, 41]. The goals of the following series of experiments, therefore, were to (i) provide a more comprehensive description of behavior in this task across both individual and repeated days of testing; (ii) assess the impact of several drugs on social investigation; and (iii) establish the sensitivity of this task to individual subject characteristics (sex differences in social interaction) as well as environmental manipulations (daily light/dark cycling and lighting condition).
Materials & Methods
Subjects
The subjects for Experiments 1-3 were 46 Blue Spruce-Long Evans male and female rats (male n=26, Female n=20), born and bred at the animal facility of Bowling Green State University, Psychology Department. For Experiments 1-3, rats were housed in individual, suspended wire-bottom cages (23×10×13 cm) for 2-3 weeks prior to behavioral testing. Subjects for Experiment 4-5 were adult male Sprague Dawley rats purchased from Harlan and housed in the animal facility at SUNY-Binghamton. Rats were housed in pairs using Plexiglas tubs with pine shavings until 48 hr prior to behavioral testing to prevent adverse consequences of long-term social isolation, after which they were isolate housed (also in Plexiglas tubs with pine shavings) for the remainder of the experiment. Housing at both facilities was maintained on a 12:12 light:dark cycle (light from 0800 hours to 2000 hours) and subjects were permitted free access to food and water at all times except during the behavioral testing. All procedures were conducted in accordance with PHS policy for the humane use of laboratory animals and with the IACUC approvals from the appropriate institutions where the work was conducted.
Assessment of social investigation
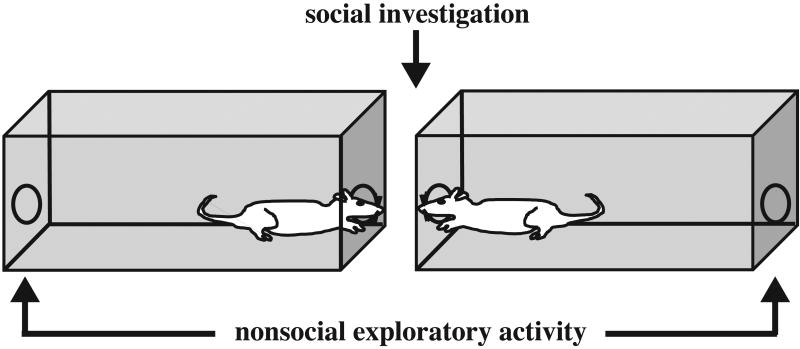
The social investigation apparatus consisted of two clear Plexiglas boxes measuring 65 cm × 24 cm × 15 cm sitting 12 cm apart, end to end (see Figure 1 for a diagram, and [40, 41]). In both ends of each box there was a 3.2 cm hole surrounded by photoelectric cells to detect movement through the hole. This permitted each animal to investigate the hole facing a conspecific or the hole that does not, thus yielding a measure of social interaction on one end and a measure of exploratory activity at the other. Each animal was placed in one of the social proximity boxes while its pair-mate was placed in the adjacent box for a total of thirty minutes. Testing occurred in a dimly lit room in the absence of an experimenter. The sawdust beddings in the proximity boxes were changed after every trial and the apparatus was wiped with a damp towel. The frequency and duration of photobeam breaks, indicative of head pokes through a hole, were automatically recorded at each of the social and nonsocial holes. Additionally, the average duration of head pokes at each of the holes was calculated. Head pokes were scored separately for each animal in the pair, and were divided into three consecutive epochs of ten minutes for the 30 min test session, though in some cases data are cumulated across the entire 30 min session (as noted below).
Figure 1.
Diagram of the apparatus employed for the assessment of social investigation and exploratory activity. Boxes are separated by 12 cm and illuminated by a dim light above the apparatus.
Drug Treatment
A 2×2 drug administration schedule was used for 4 drug conditions in experiment 2 (vehicle-vehicle, vehicle-morphine, vehicle-naltrexone, and morphine-naltrexone), and 4 drug conditions in experiment 3 (vehicle-vehicle, vehicle-amphetamine, vehicle-haloperidol, and amphetamine-haloperidol). Intraperitoneal (i.p.) injections were made at volumes of 1ml/kg body weight. The vehicle was physiological saline. Drug doses were 1.0 mg/kg morphine, 1.0 mg/kg naltrexone, 0.25 mg/kg d-amphetamine, and 0.25 mg/kg haloperidol. In all cases, both animals in a pair received the same two injections on any given test day with a 20 minute delay between the first and second injections. Twenty minutes after the final injection, rats were transferred to a testing room for the play session. Each dyad was tested in all 4 drug conditions over 4 separate days, and the order of drug treatment was thoroughly counterbalanced to eliminate any possible order effects.
Procedure
Experiment 1: Baseline testing of social investigation
The goal of this experiment was to provide an initial characterization of behavioral responding in a relatively new model of social interaction. Specifically, the goals were to (a) determine whether rats would show a clear preference for the hole with the potential for social reinforcement, (b) determine whether any sex differences would emerge, and (c) establish patterns of behavioral habituation in this task. Thus, young adult male and female rats (46 rats total; 72 days of age) were assessed in the social investigation apparatus described above for 30 min on each of 4 consecutive days. Rats were always tested with the same-sex, identical partner and behavioral testing was performed at approximately the same time each day during the light period of the light/dark cycle.
Experiment 2: Modulation of investigatory behavior by morphine and/or naltrexone
Behavioral responding in the social investigation apparatus was examined using a 2×4 (Gender × Drug) design. Adult male and female rats (n=23 pairs) were injected with the same doses and combinations of morphine and naltrexone as described previously. Since behavioral responding in the social investigation task was stable across 4 days of testing (see Experiment 1), each pair of rats was tested in all four drug conditions using a repeated measures design.
Experiment 3: Modulation of investigatory behavior by amphetamine and/or haloperidol
The final experiment examined whether behavioral responding in the social investigation apparatus would be affected by administration of amphetamine and haloperidol (alone or in combination). Adult male and female rats (n=23 pairs) were injected with the same doses and combinations of d-amphetamine and haloperidol as described previously. As before, each pair of rats was tested in all four drug conditions in counterbalanced fashion.
Experiment 4: The effect of light versus dark cycle on social investigation
The goal of this experiment was to determine whether behavioral responding in this task varied as a function of time of day. This is particularly important because rats naturally engage in social interaction during the dark phase (subjective waking period) yet our prior experiments were all conducted during the light phase. To do this, rats (n=8 per group) were tested 3 hr after lights on or at an equivalent time after lights-off on 5 consecutive days. Groups tested during the light cycle were always tested during the light cycle (i.e., the light versus dark comparison was a between subjects variable). Because Experiment 4-5 was conducted at a separate institution using a different strain of rats, subjects were tested on 5 consecutive days to further assess the stability of the behavioral task with once-daily testing.
Experiment 5: The impact of illumination on social investigation
The goal of this experiment was to determine whether the social investigation task would be sensitive to standard manipulations of the environment that are known to influence social behavior. To do this, we chose the simple method of testing rats (n=6 per group) under dim lighting conditions (approximately 2-3 lux) or using normal ambient room lighting (provided by 2, 100-watt bulbs in wall-mounted fixtures a few feet from the testing apparatus). We hypothesized that testing during bright light conditions would reduce social investigation to a much greater extent than nonsocial exploratory activity. Rats were placed into the chambers under the dim or bright light conditions for a period of 30 min and behavior was recorded as previously described.
Statistics
All data were analyzed using an Analysis of Variance (ANOVA) design appropriate for each given experiment (described in respective sections below). Criterion for rejection of the null hypothesis was always p<.05. Post hoc analyses were performed using the Student-Newman-Keuls method in cases where an overall significant main effect or interaction was observed.
Results
Experiment 1: Baseline testing of social investigation
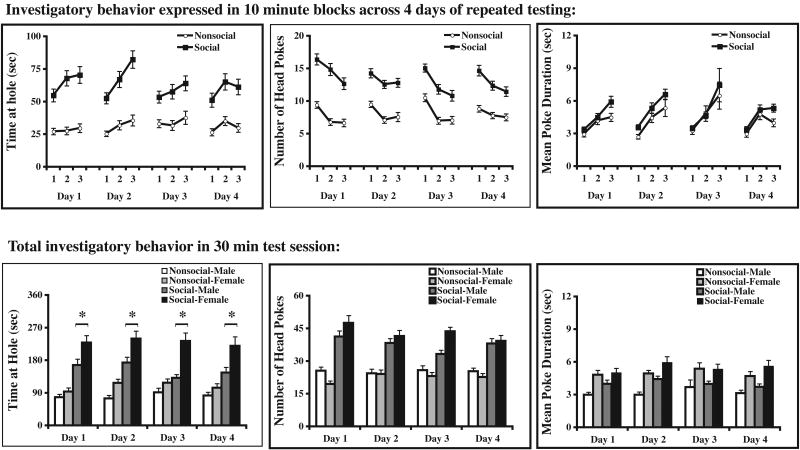
The goal of this experiment was to provide an expanded characterization of a relatively novel social investigation paradigm. In order to highlight the key features of behavioral responding during this behavioral task, the data were analyzed in two different ways. The first analysis (presented in Figure 2, top row of graphs) examined (a) how behavioral responding varied across the 30 min test session on each of 4 consecutive test days, and (b) whether a clear and stable preference to investigate the social reinforcement hole would emerge. In this first set of analyses, all rats demonstrated a clear preference for investigation at the social reinforcement hole with substantially greater time spent at the social hole relative to the nonsocial hole (F(1,45)=63.8, p<0.0001; Figure 2, top left), and a greater number of head pokes through the social hole relative to the nonsocial hole (F(1,45)=73.18, p<0.0001; Figure 2, top middle). While frequency of head pokes differed, the mean poke duration between the holes did not differ significantly (F(1,45)=1.27, p>0.05; Figure 2, top right). When data were examined over the 30 min test session (in 10 min blocks), rats tended to spend more time investigating at the holes evidenced by a main effect of [F(2,45)=3.11, p<0.05], while the frequency of head pokes decreased [F(2,45)=15.01, p<0.0001] and the average duration of an individual head poke lengthened [F(2,45)=28.19, p<0.0001] as the 30 min session progressed. The result was greater mean poke duration in the third 10 min block relative to the first. This pattern of behavioral responding was evident on all 4 days of repeated testing, and lack of effect of test day on time spent at hole [F(3,45)=2.05, p>0.05], frequency of head pokes [F(3,45)=0.549, p>0.05] and mean poke duration [F(3,45)=1.90, p>0.05] indicated that little if any habituation occurred across 4 days of behavioral testing.
Figure 2.
Baseline investigatory behavior in the apparatus depicted in Figure 1. The top panel of graphs shows that in a 30 min test session, the time spent investigating at the social hole increases (top left) while the number of head pokes decreases (top middle), yielding an overall increase in mean poke duration (top right). Moreover, no habituation was observed in behavioral responding across 4 successive days of testing, and a robust preference to explore the hole where social reinforcement is possible was observed on all test days. In the bottom panel of graphs, data are cumulated across the entire 30 min test session. Although a significant preference for social investigation was observed in both sexes, female rats spent significantly more time in social investigation than male rats. All data are expressed as means ± SEM; an asterisk (*) indicates a significant difference between male and female rats at the social hole (p<.05).
In the second analysis, data were cumulated across the 30 min test session so that (a) the possibility of sex differences could be more readily examined, and (b) the potential for habituation in behavioral responding across repeated days of testing could be more directly evaluated. Data were analyzed using a 2×4 (sex × days of testing) ANOVA. A significant main effect of sex was observed on the total amount of time spent at both nonsocial [F(1,44)=5.04, p<.05] and social [F(1,44)=17.86, p<.0001] holes. Post hoc analysis revealed that females spent significantly more time at both holes than male rats, but that the magnitude of the preference for social investigation relative to nonsocial investigation was much more pronounced in female rats than in male rats (Figure 2, bottom left). Similarly, the mean poke duration (Figure 2, bottom right) for females was substantially longer (nearly twice the duration) than male rats irrespective of which hole was examined [nonsocial hole: F(1,44)=14.79, p<.001; social hole: F(1,44)=12.22, p<.005]. When the total number of head pokes was examined, a main effect of sex was once again observed (Figure 2, bottom middle). However, this difference was only observed at the social hole where females had more head pokes than males. [F(1,44)=4.09, p<.05]. No significant main effects or interactions were observed for repeated testing across the 4 test days, so the effect of repeated testing was not considered further. Together, these data indicate a clear sex difference whereby female rats seem to show a greater propensity to investigate at the social reinforcement hole than at the nonsocial hole, and that behavioral responding in this task is highly consistent across 4 days of repeated testing for both sexes.
Experiment 2: Modulation of investigatory behavior by morphine and/or naltrexone
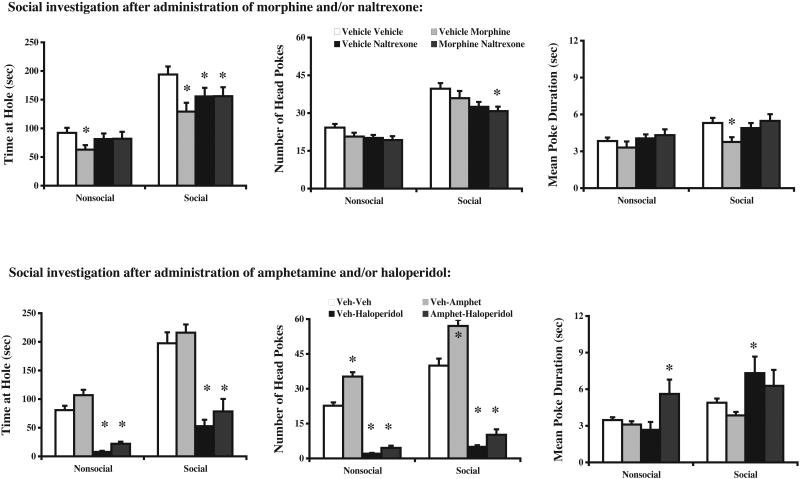
Given the putative role for endogenous opioids in the expression of social behavior, the effect of opiate agonist and antagonist treatment on behavior in this model of social investigation was investigated. Data were analyzed using a single factor ANOVA design with 4 levels (each corresponding to one drug condition) as a repeated measures variable since all rats were tested in each drug condition. In all cases, preliminary analyses revealed no drug × sex interactions so the sex factor was removed from the analysis and was not considered further. There was a significant main effect of drug treatment on time spent at the nonsocial [F(3,135)=4.26, p<.01] and social holes [F(3,135)=6.00, p<.001]. Post hoc analyses revealed that time spent investigating at the nonsocial hole was reduced by morphine alone and unaffected by any other manipulations. However, time spent at the social hole was significantly reduced by morphine alone, naltrexone alone and the combination of drugs (see Figure 3, top left). The number of head pokes and mean poke duration were affected to a much lesser extent by drug injections (Figure 3, top middle and right, respectively). Specifically, the number of head pokes at the social hole was significantly reduced by co-injection of morphine and naltrexone while pokes at the nonsocial hole were largely unaffected. Mean poke duration at the social hole was significantly reduced by morphine injection [main effect: F(3,135)=5.02, p<.005], while no effect of injection condition was observed on mean poke duration at the nonsocial hole [F(3,135)=1.66, n.s.]. Overall, these data suggest a more robust influence of opiate drugs on the social aspects of the task while investigatory behavior at the nonsocial hole was much less affected by these treatments.
Figure 3.
Modulation of investigatory behavior by several drugs. The top panel of graphs shows that peripheral administration of morphine (1 mg/kg i.p.) or naltrexone (1 mg/kg i.p.) alone or in combination reduced the amount of time spent in social investigation. Overall, the impact of opioid drugs was much greater on social investigation than on nonsocial exploratory activity. The bottom panel shows that peripheral administration of amphetamine (0.25 mg/kg i.p.) produced a robust facilitation of both social investigation and exploratory activity, but that the overall preference for the social hole was relatively intact. Haloperidol treatment (0.25 mg/kg i.p.) dramatically reduced activity at both holes irrespective of whether it was administered alone or in combination with amphetamine. All data are expressed as means ± SEM; an asterisk (*) indicates a significant difference between the groups indicated (p<.05).
Experiment 3: Amphetamine/haloperidol modulation of investigatory behavior
In order to further characterize this new behavioral task, sensitivity to the locomotor activating effects of amphetamine and suppressing effects of haloperidol were examined. Data were analyzed using the same design described above for Experiment 2. As expected, a significant main effect of drug treatment was observed on the total time spent at both the nonsocial [F(3,135)=65.69, p<.0001] and social holes [F(3,135)=32.63, p<.0001]. Post hoc analyses revealed that this effect was primarily due to a robust suppression of total time spent investigating in both drug conditions in which haloperidol was administered relative to vehicle-vehicle controls (Figure 3, bottom left). Although there was a trend for amphetamine to reverse the locomotor-suppressing effects of haloperidol, this effect failed to achieve statistical significance. A similar pattern of results was observed on the number of head pokes at both the nonsocial [F(3,135)=175.19, p<.0001] and social [F(3,135)=142.28, p<.0001] holes, except that amphetamine significantly increased the number of pokes at both locations relative to vehicle-vehicle control conditions (Figure 3, bottom middle). Mean poke duration was the least affected parameter of all; mean poke duration was significantly increased at the nonsocial hole when amphetamine and haloperidol were co-injected, while mean poke duration was significantly increased at the social hole in haloperidol alone and haloperidol + amphetamine conditions (Figure 3, bottom right). Together, these data indicate that this new behavioral task is sensitive to the locomotor properties of dopaminergic drugs, but that not all behavioral measures scored in this task are equally sensitive. Specifically, we found that the number of head pokes and the total time at hole were the most sensitive to drug manipulation, whereas mean poke duration was the least sensitive.
Experiment 4: The effect of light versus dark cycle on social investigation
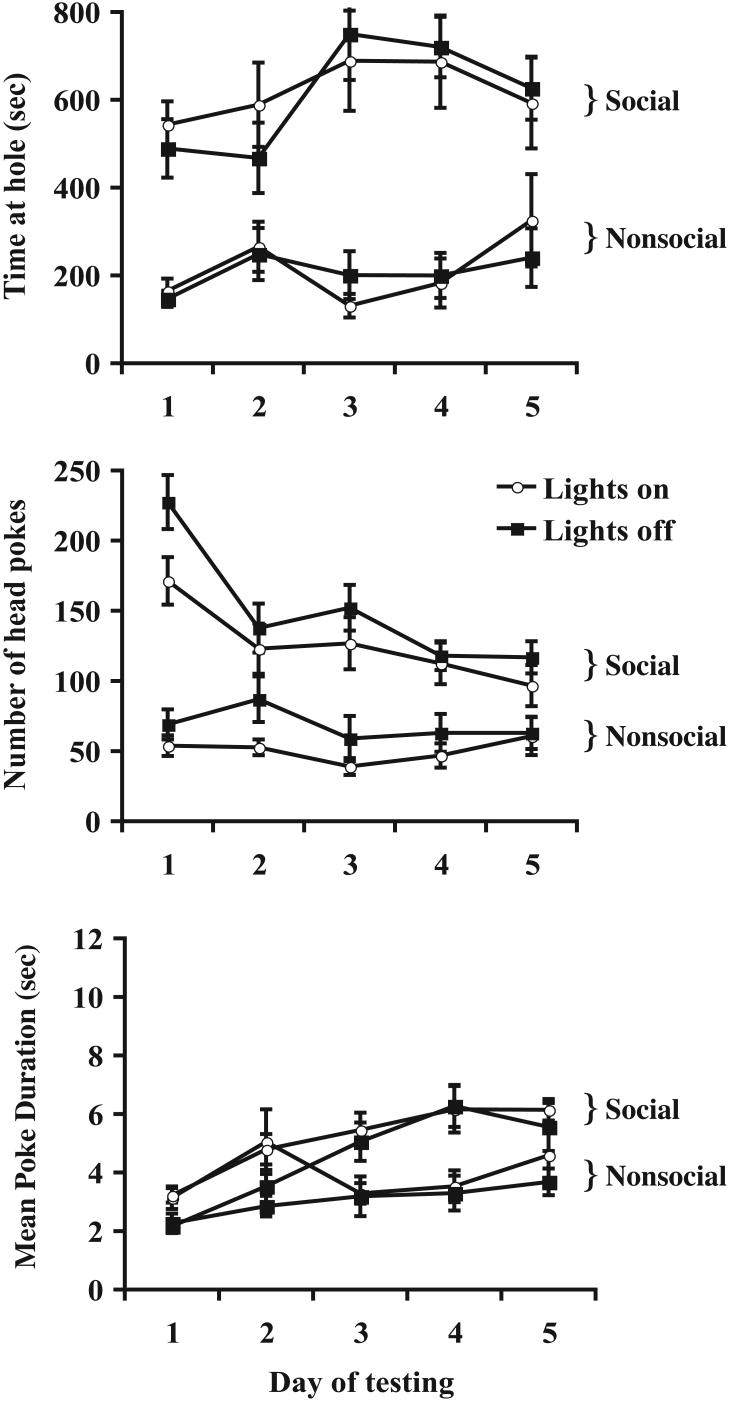
These data were analyzed using a 2×5 (time of day × repeated days) mixed ANOVA. The primary goal of the experiment was to determine whether testing in the light versus dark phase would alter the amount of social investigation. No differences were found between groups tested at differing times of the circadian cycle as evidenced by lack of a main effect on total time spent at the social hole [F(1,14)=0.01, n.s.] or the nonsocial hole [F(1,14)=0.02, n.s.]. Similar null effects of light versus dark testing were observed in the number of head pokes at the social [F(1,14)=2.05, n.s.] and nonsocial holes [F(1,14)=1.84, n.s.]. These data are all depicted in Figure 4. Together, these findings suggest that behavioral responding during the light and dark cycle is comparable and stable across the circadian rhythm.
Figure 4.
The impact of light/dark cycle on investigatory behavior. During the 30 min test sessions, the time spent investigating (top panel), the number of head pokes (middle panel), and mean poke duration (bottom panel) at both the social and non-social holes were measured in both light and dark phases of daily light cycling for 5 successive days. No effects of light/dark cycling were found; Time and number of social hole were stably greater than those of non-social hole in both light and dark phases on all test sessions. All data are expressed as means ± SEM.
The second question addressed with this experiment was whether behavioral responding would show differential adaptation when animals were tested in the light cycle versus when they were tested in the dark cycle across repeated says of testing. This effect is best represented by the interaction between the two variables, and in no case were significant interactions between time of testing and repeated days of testing observed. However, in contrast to data obtained in Experiment 1, we observed a small but reliable increase in the amount of time spent at the social hole across repeated days of testing [F(4,40)=4.73, p<0.01]. Post hoc analyses revealed that time spent at the social hole was significantly elevated on days 3 & 4 of testing relative to all other test days (p-values <0.05), suggesting a somewhat inverted-U shape curve in behavioral responding. No such effects were observed in time spent at the nonsocial hole [F(4,40)=0.60, n.s.]. When the number of head pokes at the social hole were analyzed, a main effect of test day was observed [F(4,40)=19.073, p<0.001] and post hoc analyses revealed that the number of pokes was significantly higher on the first day of testing relative to all other days (p-values <.05).
When the derived parameter of mean poke duration was analyzed, we found that the average bout at the social hole significantly increased across repeated days of testing as evidenced by the significant main effect of repeated days [F(4,40)=16.917, p<0.001], while no such effect was observed at the nonsocial hole [F(4,40)=1.771, n.s.]. Post hoc analyses indicated that the increase in mean poke duration was significant by the third day of testing, but did not change further after that (p-values <.05). Together, these findings indicate that, across repeated days of testing, the average length of time for each poke at the social hole increased in the first few days of testing.
Experiment 5: The impact of illumination on social investigation
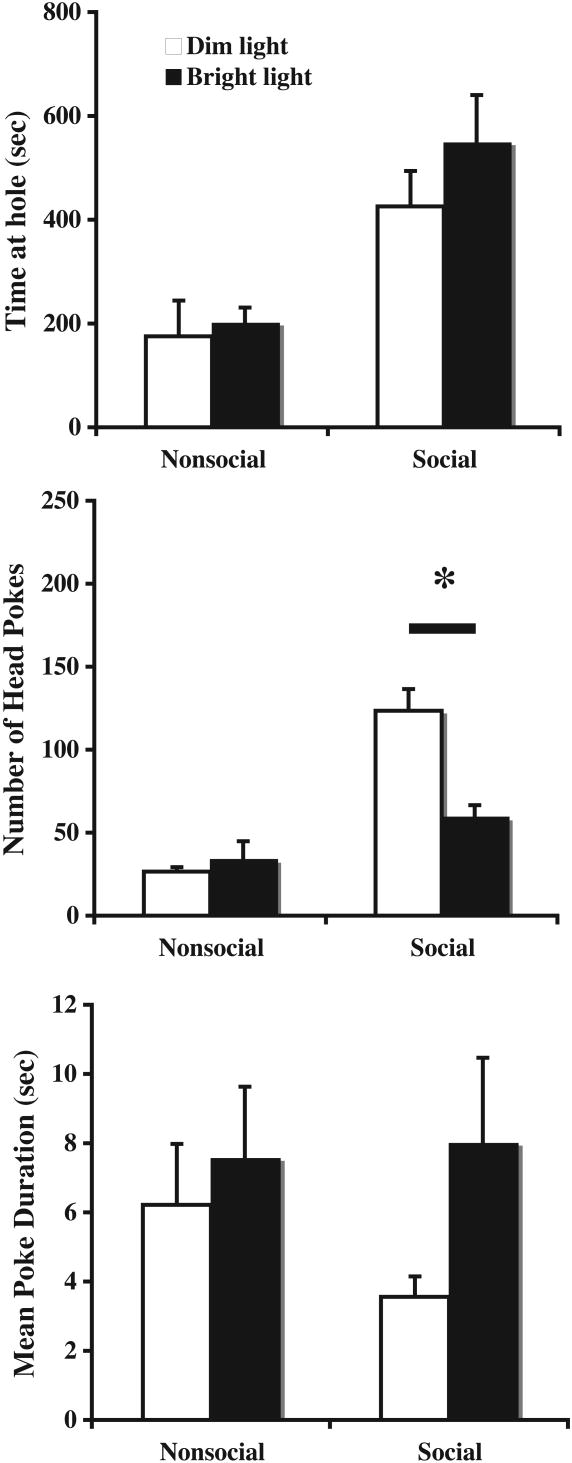
The goal of this experiment was to determine whether lighting conditions at the time of behavioral testing would influence behavioral responding in the social investigation task. Analysis revealed that testing under bright light conditions significantly reduced the number of pokes at the social hole [F(1,10)=7.1, p<0.05], while the number of pokes at the nonsocial hole was unaffected. No significant effects of illumination conditions were observed on the amount of time spent at the hole [F(1,10)=1.848, n.s.]. Though mean poke duration tended to be lower at the social hole under dim lighting conditions, this effect approached but did not achieve statistical significance [F(1,10)=3.145, n.s.]. These data are depicted in Figure 5. Together, these data suggest that testing under bright light conditions has more substantial influence on social investigation than nonsocial exploratory activity.
Figure 5.
The effect of illumination on investigatory behavior. The time spent investigating (top panel), the number of head pokes (middle panel), and mean poke duration (bottom panel) at both the social and non-social holes were scored under dimly lit and bright lit conditions. The number of head pokes at social hole, but not non-social hole, was significantly lower under bright lit condition compared to dim lit condition. All data are expressed as means ± SEM; an asterisk (*) indicates a significant difference between the conditions indicated (p<.05).
Discussion
In this series of experiments, we sought to characterize a new model of social investigation in which rats may choose to explore at a hole with the potential for social reinforcement or one where no social interaction is available. We hypothesized that young adult rats would spend significantly more time investigating at a social hole than at the nonsocial hole. Data from all 5 experiments employing this task support this hypothesis, with rats spending nearly double the amount of time at the social hole and poking their heads at the social hole twice as much as at the nonsocial hole. Across a single 30 min test session, rats appeared to spend progressively more time investigating outside the chamber; head pokes decreased across the 30 minute session while amount of time at the hole increased, resulting in increased mean poke duration as the 30 min session progressed. There was remarkably little variability in these measures over 4 consecutive days of repeated exposure to the test apparatus and during both the light and dark periods of daily lighting cycle, suggesting that behavioral responding in this task does not readily habituate, but instead remains consistent through the daily continuous exposure and the daily lighting cycle even in a nocturnal species. In line with earlier findings [39] we also found pronounced sex differences in this task where females consistently explored the social hole more often and for longer periods of time than the nonsocial hole. Thus, this task may be a sensitive paradigm for the assessment of the propensity to engage in social investigation, and it has the advantage that repeated testing of the same rats during both the light and dark periods is possible without the problem of behavioral habituation.
Interestingly, there are significant sex differences for social interaction task in that the social interaction scores of the female rats were lower and did not increase the duration of social interaction as in response to increasing familiarity with the test chamber as those of the male rats [42]. This difference between social investigation and social interaction paradigms may be due to actual physical contacts and territorial tendency that develops in male adult rats. The familiarization to the apparatus develops territorial-related behaviors in males but not female rats, which involves offensive aggression such as anogenital sniffing, aggressive grooming, lateral displays, and on-top-posture [1, 43-46]. These specific behavioral components related to aggressiveness could influence the scores of social interaction. On the other hand, the present social investigation paradigm allows animals to sniff the holes adjacent to the holes of the chamber in which counterparts were exposed, but did not allow actual contacts (beyond the snout) or social interaction, indicating that animals are prevented from expressing actual territorial behaviors and sexual behaviors in the social investigation paradigm. This may explain the difference in sexual dimorphism between the two paradigms, and a significant advantage in the social investigation paradigm as a task that more purely assesses the propensity toward social investigation, viz. the motivation for social contacts in animals.
Relevant to the point above, it is important to note that in all 5 of these studies, pairs of rats received the identical treatment. This approach raises some question about how manipulation of one rat in the pair (pharmacologically or otherwise) influences the behavior of the pair-mate. In this apparatus, animals are able to detect and investigate the stimulus animals predominantly via odorant cues through the hole, and to a much lesser extent via auditory and visual cues. If treatment conditions have a specific effect on odor cues released by the rat, then it would be expected to influence the opponent's behavior significantly and we have some very exciting studies under way examining this issue. With this in mind, one might consider additional validation studies where one rat in the pair remains unmanipulated, serving only as a stimulus rat for the experimentally treated rat.
The familiarity of individuals is measured by the differences in investigation time in investigation-based tasks such as social recognition task (habituation-dishabituation paradigm) and sociability task (three-chamber test). In both cases, the inter-trial interval for memory retention is very short; about 2 hr for social recognition task (if animals are exposed to the same animal with more than 2 hr interval, they would show similar amount of investigation to the previously exposed animal). In the sociability test, mice are exposed to subsequent stimulus animals immediately after the first trial. These data suggest that in the present social investigation paradigm, if animals are exposed to the same animal with a short interval, they would show a reduced investigation. Thus, the present data that animals continuously show similar (high) levels of investigation between the same pairs of animals in 4 consecutive days is consistent with previous studies concerning social recognition and sociability tasks as well.
We performed two additional experiments using drugs that induce a facilitation or inhibition of social interaction, in order to assess the sensitivity to pharmacological treatment. Given the well-documented role for the opioid system in the expression of social behavior [26], we hypothesized that administration of opiate drugs would have a relatively selective effect – particularly when low doses are administered – on social investigation, with little or no effect on nonsocial exploratory activity. These expected outcomes held true for some measures (time spent at hole, mean poke duration), while others (frequency of head pokes) appeared to be less sensitive to the influence of opioid manipulations. Specifically, there was a modest reduction in the amount of time spent at the nonsocial hole after acute morphine injection, which may reflect previous observations of rats monitored in open field situations [47] and play behavior [48] where overall activity was decreased by low doses of morphine. The fact that morphine and naltrexone (alone or in combination) reduced time spent at the social hole probably illustrates the importance of ‘opioid tone’ in the normal expression of social behavior as has been described elsewhere [26]. Alternatively, one might speculate that individual measures in this task may be differentially affected by opioid manipulations. Future studies examining dose-response relationships with selective opiate drugs may help clarify this issue.
Larger effects resulted from treatment with low doses of amphetamine and haloperidol. In this case, however, the impact of drug treatment did not appear to be selective for the social aspects of the task because robust and equivalent changes in behavior were observed on investigation of both the social and nonsocial hole. These effects were observed on both total amount of time spent at the hole and the number of head pokes. Specifically, amphetamine injection led to increased time spent at both holes with a robust increase in the frequency of head pokes, whereas in both conditions where haloperidol was administered investigatory behavior dropped off precipitously. These effects could be due to a direct locomotor-impairing action of dopaminergic antagonism by haloperidol consistent with prior work [37] [49-51]. However, the overall preference for social investigation over nonsocial exploratory activity was largely intact after amphetamine and relatively intact after haloperidol administration. Evidence demonstrated that haloperidol did reduce the time spent in social interaction in male rat pairs using a social interaction paradigm [37, 49, 50] seems to be inconsistent to the present finding. An important property of haloperidol is to reduce aggressive behavior [51, 52], suggesting that an inhibition of increment in aggression by haloperidol injection results in a decreased time spent in social interaction in the social interaction paradigm. This, again, suggests a validation that the social investigation paradigm could dissociate the propensity to engage in social investigation from other related factors involving aggression, sexuality, and exploratory activity.
In the final 2 experiments, we once again examined stability of the task across repeated days of testing during both light and dark phases of the circadian cycle. The outcome of this experiment reported no significant differences when tested during light and dark phases, indicating that testing can be performed without the use of a reverse-phase light cycle. The final Experiment demonstrated subtle yet reliable changes in behavior in response to bright illumination conditions, where number of head pokes, but not time spent at the central hole, was significantly reduced under bright illumination. Similar effects were observed in mean poke duration, a parameter derived from the other two measures. Though it is not clear why time at hole and number of pokes differed in this case, the key point to be made is that bright light conditions only affected behavior at the social hole, with no effects whatsoever at the nonsocial hole. Future studies examining whether the effect of bright illumination can be reversed by anxiolytic agents are clearly warranted.
To briefly summarize, we now report that acute administration of morphine or naltrexone both significantly impair the expression of social investigation in a behavioral task where rats may choose to engage in investigation of a conspecific and nonsocial exploratory activity. These findings extend our knowledge of opioid involvement specifically with respect to social investigation, viz. social motivation. It is well known that the social interaction test is a useful and sensitive measure for anxiety in detecting both anxiolytic and anxiogenic effects of drugs [53]. An important advance for the future will be to address the effects of anoxiogenic/anxiolytic drugs on behavior in the social investigation paradigm to provide valuable information to this task as well as neural circuitry relevant to social investigation. In addition, this paradigm has the added advantage of automated scoring, allowing for rapid data acquisition and processing, greater objectivity and apparent sensitivity to sex differences and pharmacological manipulations as well as environmental manipulations.
Acknowledgments
This work was supported in part by a research grant from the Hope For Depression Research Foundation (HDRF grant # 6-008) to T.D. Special thanks to Marc Washington for his technical assistance in a subset of these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grant EC, Mackintosh JH. A comparison of the social postures of some common laboratory rodents. Behaviour. 1963;21:246–295. [Google Scholar]
- 2.Serra M, et al. Social isolation stress and neuroactive steroids. Eur Neuropsychopharmacol. 2007;17:1–11. doi: 10.1016/j.euroneuro.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Shewin CM. Social context affects the motivation of laboratory mice, mus musculus, to gain access to resources. Animal Behaviour. 2003;66:649–655. [Google Scholar]
- 4.Panksepp J. The ontogeny of play in rats. Developmental Psychobiology. 1981;14(4):327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- 5.Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Developmental Psychobiology. 1983;16(2):83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- 6.Hole G. Temporal features of social play in the laboratory rat. Ethology. 1988;78:1–20. [Google Scholar]
- 7.Meaney MJ, Stewart J. Environmental factors influencing the affiliative behavior of male and female rats. Animal Learning & Behavior. 1979;7:397–405. [Google Scholar]
- 8.Panksepp J, Beatty WW. Social deprivation and play in rats. Behavioral and Neural Biology. 1980;30:197–206. doi: 10.1016/s0163-1047(80)91077-8. [DOI] [PubMed] [Google Scholar]
- 9.Beatty WW, et al. Psychomotor stimulants, social deprivation and play in juvenile rats. Pharmacology, Biochemistry, and Behavior. 1982;16:417–422. doi: 10.1016/0091-3057(82)90445-2. [DOI] [PubMed] [Google Scholar]
- 10.Van den Berg CL, Van Ree JM, Spruijt BM. Morphine attenuates the effects of juvenile isolation in rats. Neuropharmacology. 2000;39:969–976. doi: 10.1016/s0028-3908(99)00216-6. [DOI] [PubMed] [Google Scholar]
- 11.Burghardt GM. The genesis of animal play: testing the limits. Cambridge, MA: The MIT Press; 2005. [Google Scholar]
- 12.Arakawa H, Blanchard DC, Blanchard RJ. Colony formation of C57BL/6J mice in visible burrow system: identification of eusocial behaviors in a background strain for genetic animal models of autism. Behavioural Brain Research. 2007;176:27–39. doi: 10.1016/j.bbr.2006.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikemoto S, Panksepp J. The effects of early social isolation on the motivation for social play in juvenile rats. Developmental Psychobiology. 1992;25(4):261–274. doi: 10.1002/dev.420250404. [DOI] [PubMed] [Google Scholar]
- 14.Young LJ. The neurobiology of social recognition, approach, and avoidance. Biological Psychiatry. 2002;51:18–26. doi: 10.1016/s0006-3223(01)01268-9. [DOI] [PubMed] [Google Scholar]
- 15.File SE, Hyde JRG. Can social interaction be used to measure anxiety? British Journal of Pharmacology. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bluthe R, et al. Central injection of IL-10 antagonizes the behavioural effects of lipopolysaccharide in rats. Psychoneuroendocrinology. 1999;24:301–11. doi: 10.1016/s0306-4530(98)00077-8. [DOI] [PubMed] [Google Scholar]
- 17.Deak T, Bellamy C, Bordner KA. Protracted increases in core body temperature and interleukin-1 following acute administration of lipopolysaccharide. Physiology & Behavior. 2005;85(3):296–307. doi: 10.1016/j.physbeh.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 18.File SE, Hyde JRG. A test of anxiety that distinguishes between the actions of benzodiazepines and those of other minor tranquilizers and of stimulants. Pharmacology, Biochemistry, and Behavior. 1979;11:65–69. doi: 10.1016/0091-3057(79)90298-3. [DOI] [PubMed] [Google Scholar]
- 19.Irvine EE, et al. Different treatment regimens and the development of tolerance to nicotine's anxiogenic effects. Pharmacology, Biochemistry, and Behavior. 2001;68(4):769–776. doi: 10.1016/s0091-3057(01)00469-5. [DOI] [PubMed] [Google Scholar]
- 20.Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcoholism: Clinical and Experimental Research. 2002;26(10):1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- 21.Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol Clin Exp Res. 2004;28(1):40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- 22.Short K, Maier S. Stressor controllability, social interaction, and benzodiazepine systems. Pharmacology, Biochemistry, and Behavior. 1993;45:827–835. doi: 10.1016/0091-3057(93)90128-g. [DOI] [PubMed] [Google Scholar]
- 23.Siviy S, Harrison KA, McGregor IS. Fear, risk assessment, and playfulness in the juvenile rat. Behavioral Neuroscience. 2006;120(1):49–59. doi: 10.1037/0735-7044.120.1.49. [DOI] [PubMed] [Google Scholar]
- 24.Panksepp J, et al. Psychopharmacology of social play. In: Olivier B, Mos J, Brain BF, editors. Ethnopharmacology of social behavior. Dordrecht: Martinus Nijhoff; 1987. pp. 132–144. [Google Scholar]
- 25.Vanderschuren LJ, Niesink RJM, Van Ree JM. The neurobiology of social play behavior in rats. Neuroscience and Biobehavioral Reviews. 1997;21(3):309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- 26.Nelson E, Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neuroscience and Biobehavioral Reviews. 1998;22(3):437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- 27.Panksepp J, et al. Endogenous opioids and social behavior. Neuroscience and Biobehavioral Reviews. 1980;4:473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- 28.Panksepp J, et al. Opiates and play dominance in juvenile rats. Behavioral Neuroscience. 1985;99(3):441–453. doi: 10.1037//0735-7044.99.3.441. [DOI] [PubMed] [Google Scholar]
- 29.File SE, Guardiola-Lemaitre BJ. L-Fenfluramine in tests of dominance and anxiety in the rat. Neuropsychobiology. 1988;20:205–211. doi: 10.1159/000118500. [DOI] [PubMed] [Google Scholar]
- 30.Siegel MA, Jensen RA, Panksepp J. The prolonged effects of naloxone on play behavior and feeding in the rat. Behavioral and Neural Biology. 1985;44:509–514. doi: 10.1016/s0163-1047(85)91024-6. [DOI] [PubMed] [Google Scholar]
- 31.Guard HJ, Newman JD, Roberts R Lucille. Morphine administration selectively facilitates social play in common marmosets. Developmental Psychobiology. 2002;41:37–49. doi: 10.1002/dev.10043. [DOI] [PubMed] [Google Scholar]
- 32.Panksepp J, Bishop P. An autoradiographic map of (3H)Diprenorphine binding in rat brain: effects of social interaction. Brain Research Bulletin. 1981;7:405–410. doi: 10.1016/0361-9230(81)90038-1. [DOI] [PubMed] [Google Scholar]
- 33.Vanderschuren LJ, et al. Social play alters regional brain opioid receptor binding in juvenile rats. Brain Research. 1995;680:148–156. doi: 10.1016/0006-8993(95)00256-p. [DOI] [PubMed] [Google Scholar]
- 34.Van den Berg CL, et al. Effects of juvenile isolation and morphine treatment on social interactions and opioid receptors in adult rats: behavioural and autoradiographic studies. European Journal of Neuroscience. 1999;11(9):3023–3032. doi: 10.1046/j.1460-9568.1999.00717.x. [DOI] [PubMed] [Google Scholar]
- 35.Sutton ME, Raskin LA. A behavioral analysis of the effects of amphetamine on play and locomotor activity in the post-weanling rat. Pharmacology, Biochemistry, and Behavior. 1986;24:455–461. doi: 10.1016/0091-3057(86)90541-1. [DOI] [PubMed] [Google Scholar]
- 36.Beatty WW, Costello KB, Berry SL. Suppression of play fighting by amphetamine: effects of catecholamine antagonists, agonists and synthesis inhibitors. Pharmacology, Biochemistry, and Behavior. 1984;20:747–755. doi: 10.1016/0091-3057(84)90194-1. [DOI] [PubMed] [Google Scholar]
- 37.Becker A, Grecksch G. Haloperidol and clozapine affect social behaviour in rats postnatally lesioned in the ventral hippocampus. Pharmacology, Biochemistry, and Behavior. 2003;76:1–8. doi: 10.1016/s0091-3057(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 38.Swerdlow NR, et al. The neural substrates for the motor-activating properties of psychostimulants: a review of recent findings. Pharmacology, Biochemistry, and Behavior. 1986;25(1):233–248. doi: 10.1016/0091-3057(86)90261-3. [DOI] [PubMed] [Google Scholar]
- 39.Panksepp J, Nelson E, Bekkedal MYV. Brain systems for the mediation of social separation-distress and social reward: Evolutionary antecedents and neuropeptide intermediaries. Annals of the New York Academy of Science. 1997;807:78–100. doi: 10.1111/j.1749-6632.1997.tb51914.x. [DOI] [PubMed] [Google Scholar]
- 40.Bekkedal MYV, Panksepp J, Rossi J., III Long term changes in rat social behavior following treatment with trimethylolpropane. Neurotoxicology & Teratology. 1998;20:307–316. doi: 10.1016/s0892-0362(97)00089-5. [DOI] [PubMed] [Google Scholar]
- 41.Bekkedal MYV, Rossi J, III, Panksepp J. Fetal and neonatal exposure to trimethylolpropane phosphate alters rat social behavior and emotional responsivity. Neurotoxicology & Teratology. 1999;21:435–443. doi: 10.1016/s0892-0362(99)00002-1. [DOI] [PubMed] [Google Scholar]
- 42.Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiology & Behavior. 1991;49:245–250. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- 43.Blanchard RJ, et al. Conspecific aggression in the laboratory rat. J Comp Physiol Psych. 1975;89:1204–1209. doi: 10.1037/h0077177. [DOI] [PubMed] [Google Scholar]
- 44.Blanchard RJ, et al. Attack and defensive behaviour in the albino rat. Animal Behaviour. 1977;25:622–634. doi: 10.1016/0003-3472(77)90113-0. [DOI] [PubMed] [Google Scholar]
- 45.Panksepp J, Siviy S, Normansell L. The psychobiology of play: theoretical and methodological perspectives. Neuroscience and Biobehavioral Reviews. 1984;8:465–492. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- 46.Pellis SM. Agonistic versus amicable targets of attack and defense: consequences for the origin, function and descriptive classification of play fighting. Aggressive Behavior. 1988;14:85–104. [Google Scholar]
- 47.Panksepp J, Najam N, Soares F. Morphine reduces social cohesion in rats. Pharmacology, Biochemistry, and Behavior. 1979;11:131–134. doi: 10.1016/0091-3057(79)90002-9. [DOI] [PubMed] [Google Scholar]
- 48.Vanderschuren LJ, et al. Effects of morphine on different aspects of social play in juvenile rats. Psychopharmacology. 1995;117(2):225–231. doi: 10.1007/BF02245191. [DOI] [PubMed] [Google Scholar]
- 49.Holloway WR, Jr, Thor DH. Interactive effects of caffeine, 2-chloroadenosine and haloperidol on activity, social investigation and play fighting of juvenile rats. Pharmacology, Biochemistry, and Behavior. 1985;22:421–426. doi: 10.1016/0091-3057(85)90043-7. [DOI] [PubMed] [Google Scholar]
- 50.Panksepp J. Affective Neuroscience. New York: Oxford University Press; 1998. [Google Scholar]
- 51.Pucilowski O, Eichelbaum B. Nicardipine protects against chronic ethanol- or haloperidol-induced supersensitivity to apomorphine-induced aggression. Neuropsychopharmacology. 1991;5:55–60. [PubMed] [Google Scholar]
- 52.Skrebuhhova-Malmros T, et al. The serotonin 5-HT(2A) receptor subtype does not mediate apomorphine-induced aggressive behaviour in male Wistar rats. Pharmacology, Biochemistry, and Behavior. 2000;67:339–343. doi: 10.1016/s0091-3057(00)00368-3. [DOI] [PubMed] [Google Scholar]
- 53.File SE, Seth P. A review of 25 years of the social interaction test. European Journal of Pharmacology. 2003;463:33–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]