Abstract
The prognosis for patients with primary central nervous system (CNS) lymphoma (PCNSL) who relapse after the initial response is usually poor. A standard treatment for relapsed PCNSL has not yet been identified because of the heterogeneity of the therapies employed and the lack of large, prospective clinical trials. We describe a 46-year-old relapsed PCNSL patient who was successfully treated with intraventricular applications of rituximab to minimize neurotoxicity, 2 cycles of salvage chemotherapy with etoposide, ifosfamide, and cytarabine (VIA) regimen and high-dose chemotherapy with autologous stem cell rescue. The high-dose chemotherapy consisted of bischloroethylnitrosourea, etoposide, cytarabine, and melphalan (BEAM) regimen. Partial remission was detected after intraventricular rituximab therapy and the patient has been in complete remission without evidence of neurotoxicity for 28 months after high-dose chemotherapy with autologous stem cell rescue. This case indicates a new appropriate treatment guideline in relapsed PCNSL patient after initial intensive chemo-radiotherapy.
Keywords: Primary central nervous system lymphoma, salvage therapy, rituximab, high-dose chemotherapy with autologous stem cell rescue
INTRODUCTION
Primary central nervous system lymphoma (PCNSL), a rare form of extranodal non-Hodgkin's lymphoma (NHL), occurs in the brain, leptomeninges, spinal cord, or eyes, and typically remains confined to the CNS.1
The prognosis of immunocompetent patients diagnosed with PCNSL has improved during the last decade with the introduction of methotrexate-based regimens and cranial radiotherapy.2,3 However, failure after first-line therapy has been reported in 35-60% of patients with PCNSL.2,4 Patients who are refractory to primary therapy or relapse after an initial response have a poor prognosis, with median survival of 2 months without further treatment. The information on salvage therapies is limited and, in most published series of patients with PCNSL treated initially with a uniform regimen, the therapies given for relapse have been heterogeneous.4,5
Here, we report a relapsed PCNSL patient who was successfully treated with intraventricular applications of rituximab to minimize neurotoxicity, 2 cycles of chemotherapy with etoposide, ifosfamide, and cytarabine (VIA) regimen and high-dose chemotherapy with autologous stem cell rescue.
CASE REPORT
A 46-year-old Korean woman presented in April 2003 with headache and dizziness that had continued for 2 weeks. The patient's physical examination demonstrated no focal neurological abnormalities. The Eastern Cooperative Oncology Group (ECOG) performance status was 1 and she had no B symptoms.6 MRI-contrast enhanced images showed 4 cm sized homogeneously and highly enhanced masses at left basal ganglia with right-sided subfalcine herniation. The stereotactic brain biopsy was performed on April 10, 2003. Histopathology confirmed diffuse large B-cell lymphoma. There was no evidence of systemic lymphadenopathy or ocular involvement and no evidence of bone marrow or cerebrospinal fluid (CSF) involvement. CSF protein concentration was within normal ranges. The biochemical profile revealed lactic dehydrogenate (LDH) levels of 322 IU/L (normal range, 101-202 IU/L) and β2-microglobulin level of 0.5 mg/dL (normal range, 0-2.7 mg/dL). The results of serologic tests for HIV, hepatitis B and C virus, and Ebstein-Barr virus were negative.
The patient received chemotherapy with CHOD/BVAM regimen [CHOD = cyclophosphamide (750 mg/m2 on day 1), doxorubicin (50 mg/m2 on day 1), vincristine (1.4 mg/m2 on day 1), dexamethasone (4 mg orally on days 1 through 7); BVAM (= 2×42-day cycles) = carmustine (100 mg/m2 on days 8 and 50), vincristine (1.4 mg/m2 On days 15, 29, 43, 57, 71, 85), methotrexate (1.5 g/m2 on days 15, 29, 43, 57, 71, 85), cytarabine (3 g/m2 on days 16, 30, 44, 58, 72, 86)] and achieved partial remission. After chemotherapy, the patient was treated with radiotherapy, 45 Gy whole-brain irradiation in 25 fractions during a 5-week period, plus a boost 10 Gy in five fractions in 1-week, and she achieved complete remission (CR).
She visited the emergency room in July 2006 with left-right postural sway and transient aphasia. The PCNSL relapsed and the first CR was maintained for 34 months. Brain MRI and a staging workup revealed newly appearing lesions in the right thalamus and splenium of the corpus callosum without any identifiable systemic tumor mass or bone marrow involvement (Fig. 1). The patient received the 1st salvage chemotherapy. Rituximab alone without concomitant systemic steroid or other chemotherapeutic drugs was administered at a dose of 20 mg twice a week for 2 weeks via Ommaya reservoir whose tip was located in the left lateral ventricle. A partial remission of the parenchymal tumor was detectable after 2 weeks of intraventricular rituximab applications, total dose of 80 mg (Fig. 2). The patient was treated with 2 courses of the VIA (etoposide, ifosfamide, cytarabine) chemotherapy, and peripheral blood stem cells were harvested at the end of the 1st cycle of VIA chemotherapy. VIA included etoposide 100 mg/m2 on days 1 through 3, ifosfamide 1 g/m2 on days 1 through 5, and cytarabine 2 g/m2/12 h on day 1.
Fig. 1.
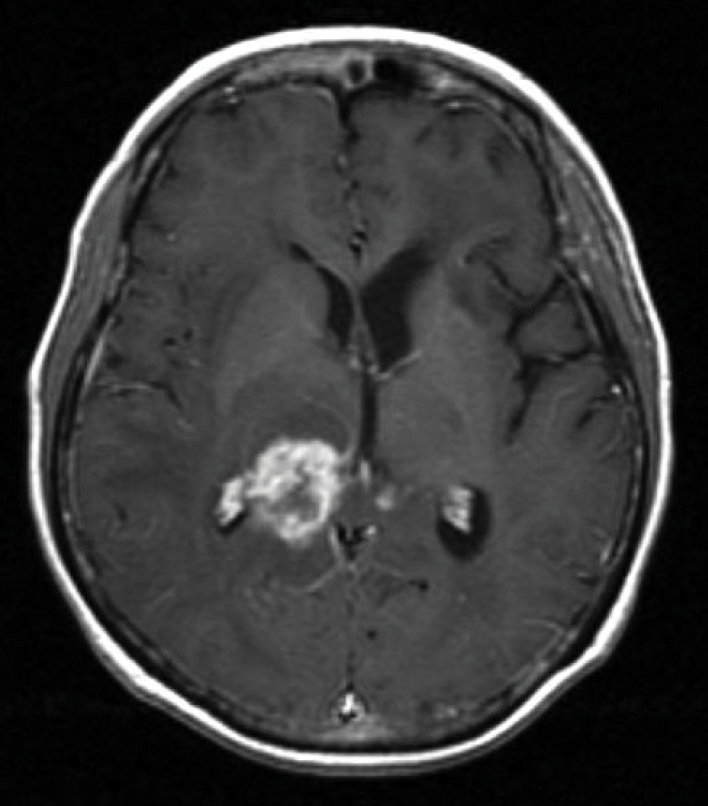
Gadolinium-enhanced, T1-weighted, MRI shows an enhancing lesion involving the right thalamus and splenium of the corpus callosum at relapse.
Fig. 2.
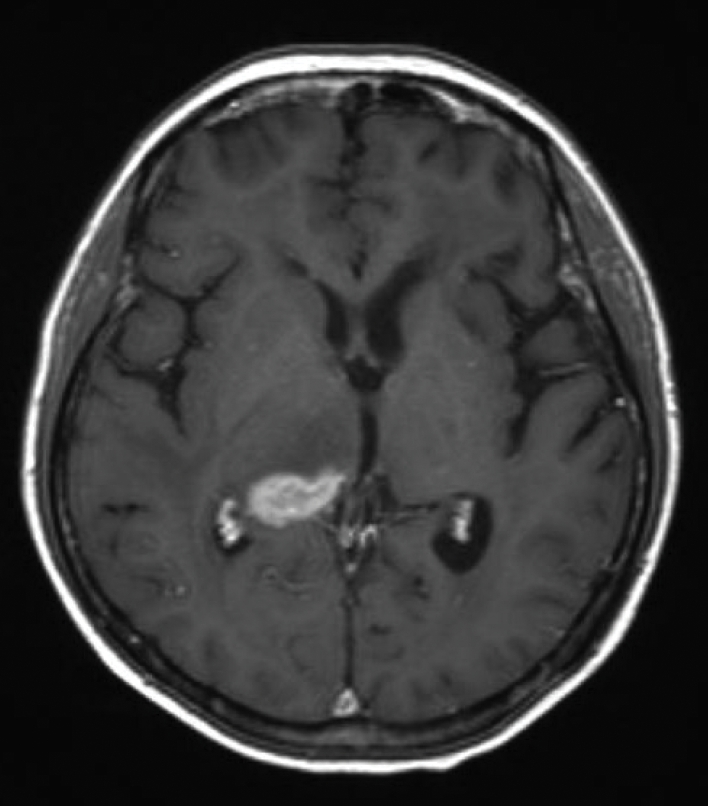
About 50% reduction of the lesion size in the right thalamus after treatment of intraventricular rituximab.
The patient received high-dose chemotherapy with a BEAM regimen (bischloroethylnitrosourea 300 mg/m2 on day -7, etoposide 200 mg/m2 on days -6 through -3, cytarabine 200 mg/m2 on days -6 through -3, and melphalan 140 mg/m2 on day -2), and autologous peripheral blood stem cells (6.04×106 CD34+ cells/kg on day 0 and 6.93×106 CD34+ cells/kg on day 1) were infused on November 2006. The treatment was well-tolerated, and a MRI scan performed by the end of January 2007 showed full regression of lymphoma infiltration (Fig. 3). The patient has been in remission for 28 months after high-dose chemotherapy with autologous stem cell rescue.
Fig. 3.
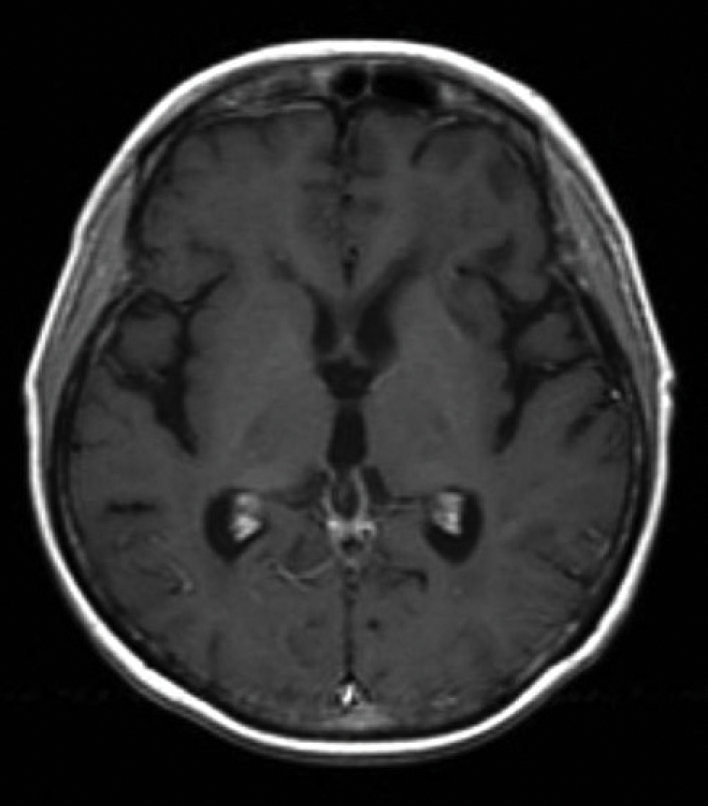
Full regression of lymphoma infiltration after 2 cycles of VIA chemotherapy and high-dose chemotherapy with autologous stem-cell rescue.
DISCUSSION
The prognosis for patients with PCNSL refractory to initial treatment or those who relapse after initial response is usually poor. Without treatment, median survival is usually less than 3 months.5 The most appropriate therapeutic approach of refractory or relapsed PCNSL has not yet been established because of the heterogeneity of the therapies employed and the lack of large, prospective clinical trials. However, second-line treatment for PCNSL is probably beneficial for those patients. A retrospective analysis of salvage treatment in 173 patients cited in 24 papers reports the results of upfront treatment for PCNSL and showed that the median survival after relapse or progression was 14 months in the group of patients who receive second-line treatment compared with only 2 months for those who were treated by only supportive therapy.5
We treated the patient with relapsed PCNSL with intraventricular rituximab as initial salvage regimen to minimize neurotoxicity. Neurotoxicity is a potentially devastating complication that can occur in response to antineoplastic therapies. This complication can manifest in a variety of ways, including impaired cognition, ataxia, and incontinence, and is associated with a significant decline in the quality of life. Among the different therapeutic strategies for PCNSL, whole-brain radiotherapy followed by methotrexate-based chemotherapy seems to produce the highest incidence of neurotoxicity, followed by whole-brain radiotherapy alone, and then chemotherapy alone.1 This patient may be a high-risk patient who will easily develop neurotoxicity if she received repeated salvage chemotherapy because she received high-dose methotrexate-based combination chemotherapy and whole-brain radiotherapy (total 55 Gy) as the initial treatment. Therefore, intraventricular rituximab therapy instead of repeated salvage chemotherapy as an initial salvage regimen may maximize response rate and minimize neurotoxicity.
Rituximab is a chimeric monoclonal antibody against the B-cell specific CD20-antigen that has proven to be highly effective in the treatment of B-cell NHL. When administered intravenously, rituximab concentrations in the CSF are very low and they are not increased significantly upon repeated intravenous administration. The high molecular weight of rituximab (146 kDa) might limit its uptake into brain parenchyma after systemic application.7 Because of that reason, there are several case reports of the relapsed PCNSL treated with intrathecal rituximab application. Although the clinical experience reported so far is very limited.8-11 the safety of intrathecal rituximab is in accordance with experimental studies, which showed that there was no clinical evidence of neurotoxicity after seven intrathecal administrations in cynomolgus monkeys.12 Recently, phase I study investigating intraventricular dose intensification, toxicity, and pharmacokinetics of rituximab in patients with PCNSL was performed, and the result suggested that intrathecal rituximab at the doses from 10 to 25 mg is feasible and effective. Intrathecal rituximab at 50 mg was associated with grade 3 infusion-related hypertension, nausea, vomiting, and abdominal cramping.13 The estimated elimination half-life averaged 34.9 hours on multiple dosing twice weekly at 25 mg, and serum concentrations rose steadily and were still increasing at the time of the last intraventricular dose, but were still far below CSF concentrations.13 Therefore, intraventricular rituximab at a dose of 20 mg twice a week in this case might be a safe and effective schedule in the treatment of relapsed PCNSL patients.
Intraventricular rituximab alone seems to be not sufficient to achieve and maintain CR for a long time. Schulz et al.9 reported a series of six patients in whom rituximab was used as intrathecal or intraventricular and systemically. CSF tumor cells were almost completely cleared in most of the patients, however, parenchymal disease did not respond.9 Rubenstein et al.13 also reported poor response in parenchymal lesions in phase I study. According to Akyuz et al.,11 there was only one case of PCNSL responding completely after systemic and intraventricular rituximab application. In our patient, the size of the relapsed tumor was reduced by about 50% after intraventricular rituximab application, but could not achieve CR as we expected. She needed additional therapy, nevertheless she received only 2 cycles of chemotherapy with VIA regimen before high-dose chemotherapy with autologous stem cell rescue. Although it is difficult to expect CR with intraventricular rituximab application alone in relapsed PCNSL, it should be considered for salvage treatment to minimize neurotoxicity before additional therapy such as systemic chemotherapy or high-dose chemotherapy with autologous stem cell rescue.
Arellano-Rodrigo et al.14 reported the results in terms of response and survival to the VIA regimen as a salvage treatment for 16 relapsed PCNSL patients after the CHOD/BVAM regimen and radiotherapy. Six patients (37%) achieved CR. After median follow-up of 15 months for surviving patients, two have relapsed with a median failure-free survival of 5 months. Twelve patients have died from progression of PCNSL with a 12-month overall survival of 41%.14
High-dose chemotherapy followed by hematopoietic stem cell rescue is now the standard treatment for patients with relapses of chemo-sensitive, aggressive, systemic NHL without CNS involvement.15 The importance of high-dose chemotherapy with autologous stem cell rescue also should be emphasized in the patients with relapsed and chemo-sensitive PCNSL. Soussain et al.16 reported 80% CR rate and a 3-years probability of 63.7% survival in relapsed PCNSL patients who were treated with high-dose chemotherapy together with autologous stem cell rescue. Our patient also achieved CR after this procedure, and the present case report suggests that high-dose chemotherapy and autologous stem cell rescue are feasible and effective in patients with recurrent PCNSL.
Although the standard salvage treatment for relapsed PCNSL has not yet been established, intraventricular rituximab application followed by short courses of systemic chemotherapy and high-dose chemotherapy with autologous stem cell rescue is a feasible and effective treatment plan in patients with relapsed PCNSL. Intraventricular rituximab application should be considered for salvage treatment to minimize neurotoxicity.
References
- 1.Batchelor T, Loeffler JS. Primary CNS lymphoma. J Clin Oncol. 2006;24:1281–1288. doi: 10.1200/JCO.2005.04.8819. [DOI] [PubMed] [Google Scholar]
- 2.Ferreri AJ, Reni M, Villa E. Therapeutic management of primary central nervous system lymphoma: lessons from prospective trials. Ann Oncol. 2000;11:927–937. doi: 10.1023/a:1008376412784. [DOI] [PubMed] [Google Scholar]
- 3.Ferreri AJ, Reni M, Pasini F, Calderoni A, Tirelli U, Pivnik A, et al. A multicenter study of treatment of primary CNS lymphoma. Neurology. 2002;58:1513–1520. doi: 10.1212/wnl.58.10.1513. [DOI] [PubMed] [Google Scholar]
- 4.Reni M, Ferreri AJ. Therapeutic management of refractory or relapsed primary central nervous system lymphomas. Ann Hematol. 2001;80(Suppl 3):B113–B117. doi: 10.1007/pl00022772. [DOI] [PubMed] [Google Scholar]
- 5.Reni M, Ferreri AJ, Villa E. Second-line treatment for primary central nervous system lymphoma. Br J Cancer. 1999;79:530–534. doi: 10.1038/sj.bjc.6690083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 7.Harjunpää A, Wiklund T, Collan J, Janes R, Rosenberg J, Lee D, et al. Complement activation in circulation and central nervous system after rituximab (anti-CD20) treatment of B-cell lymphoma. Leuk Lymphoma. 2001;42:731–738. doi: 10.3109/10428190109099335. [DOI] [PubMed] [Google Scholar]
- 8.Pels H, Schulz H, Manzke O, Hom E, Thall A, Engert A. Intraventricular and intravenous treatment of a patient with refractory primary CNS lymphoma using rituximab. J Neurooncol. 2002;59:213–216. doi: 10.1023/a:1019999830455. [DOI] [PubMed] [Google Scholar]
- 9.Schulz H, Pels H, Schmidt-Wolf I, Zeelen U, Germing U, Engert A. Intraventricular treatment of relapsed central nervous system lymphoma with the anti-CD20 antibody rituximab. Haematologica. 2004;89:753–754. [PubMed] [Google Scholar]
- 10.Watanabe N, Takahashi T, Sugimoto N, Tanaka Y, Kurata M, Matsushita A, et al. Excellent response of chemotherapy-resistant B-cell-type chronic lymphocytic leukemia with meningeal involvement to rituximab. Int J Clin Oncol. 2005;10:357–361. doi: 10.1007/s10147-005-0496-7. [DOI] [PubMed] [Google Scholar]
- 11.Akyuz C, Aydin GB, Cila A, Akalan N, Soylemezoglu F, Cengiz M, et al. Successful use of intraventricular and intravenous rituximab therapy for refractory primary CNS lymphoma in a child. Leuk Lymphoma. 2007;48:1253–1255. doi: 10.1080/10428190601142831. [DOI] [PubMed] [Google Scholar]
- 12.Rubenstein JL, Combs D, Rosenberg J, Levy A, McDermott M, Damon L, et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003;101:466–468. doi: 10.1182/blood-2002-06-1636. [DOI] [PubMed] [Google Scholar]
- 13.Rubenstein JL, Fridlyand J, Abrey L, Shen A, Karch J, Wang E, et al. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol. 2007;25:1350–1356. doi: 10.1200/JCO.2006.09.7311. [DOI] [PubMed] [Google Scholar]
- 14.Arellano-Rodrigo E, López-Guillermo A, Bessell EM, Nomdedeu B, Montserrat E, Graus F. Salvage treatment with etoposide (VP-16), ifosfamide and cytarabine (Ara-C) for patients with recurrent primary central nervous system lymphoma. Eur J Haematol. 2003;70:219–224. doi: 10.1034/j.1600-0609.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 15.Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. 1995;333:1540–1545. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 16.Soussain C, Suzan F, Hoang-Xuan K, Cassoux N, Levy V, Azar N, et al. Results of intensive chemotherapy followed by hematopoietic stem-cell rescue in 22 patients with refractory or recurrent primary CNS lymphoma or intraocular lymphoma. J Clin Oncol. 2001;19:742–749. doi: 10.1200/JCO.2001.19.3.742. [DOI] [PubMed] [Google Scholar]


