Abstract
Malignant obstruction develops frequently in advanced gastric cancer. Although it is primarily the gastric outlet that is obstructed, there are occasional reports of colonic obstruction. Treating intestinal obstruction usually requires emergency surgery or stent insertion. There are several kinds of complications with stent insertion, such as bowel perforation, stent migration, bleeding, abdominal pain and reobstruction. Nevertheless, endoscopic stent insertion could be a better treatment than emergency surgery in cases of malignant bowel obstruction in cancer patients with poor performance status. We report a case of advanced gastric cancer with carcinomatosis in which a recurrent colonic stent was inserted at the same site because of cancer growth into the stent. The patient maintained a good condition for chemotherapy, thus improving their chances for survival.
Keywords: Gastric cancer, carcinomatosis, colonic stent, benefit
INTRODUCTION
More than 50% of patients with gastric cancer are inoperable at the time of diagnosis.1,2 The resectability rate ranges from 31 to 66%, and the 5-year survival rate ranges from 5 to 15%.2 Malignant obstruction develops frequently in advanced gastric cancer, mainly in the gastric outlet and duodenum.3 However, colonic obstruction has occasionally been reported in cases of advanced gastric cancer with carcinomatosis or colonic invasion.4,5
Terminally ill patients with malignant bowel obstruction often present with nausea, vomiting and constipation, resulting in reduced oral intake and a negative impact on the patient's quality of life.6 Therefore, intestinal obstruction usually requires treatment by emergency surgery or stent insertion. Decompressive surgery is the first-line therapy for the palliation of symptomatic patients. However, palliative surgery is associated with high morbidity and mortality, and the results of surgery are not always satisfactory.7
Gastrointestinal stent insertion is performed as a palliative effort to improve the quality of life for inoperable cancer patients with gastrointestinal obstruction. Endoscopic stent placement has been reported as a feasible, safe, and effective alternative in patients with an especially short life expectancy, advanced disease stage, and poor general condition.8
Endoscopic stenting was introduced 40 years ago for the treatment of malignant obstruction using polyethylene prostheses.9 In recent years, self-expandable metal stents (SEMS) have emerged as a simple therapeutic option for non-resectable malignant intestinal obstruction. The ideal stent should have a large internal diameter to ensure the passage of a normal diet, and should be flexible and nontraumatic when fully expanded. It should also inhibit tumor overgrowth into the stent and remain fixed in its position.10
On the other hand, there are several complications associated with stent insertion, including bowel perforation, stent migration, bleeding, abdominal pain, and reobstruction. The causes of reobstruction include tumor growth, stent migration and fecal impaction, with the main cause of obstruction due to cancer growth blocking the stent lumen or growing around the lumen.11
We report a case of advanced gastric cancer with carcinomatosis, in which colonic reobstruction was treated by recurrent colonic stent insertion at the same site of transverse colon. This patient maintained a good general condition, allowing for the continuation of chemotherapy.
CASE REPORT
A 67-year-old woman was admitted with constipation and abdominal pain lasting for 5 days and a history of large bowel obstruction and two previous transverse colonic stent insertions. A plain abdominal radiograph showed dilated large bowel loops. The patient had undergone radical subtotal gastrectomy with gastrojejunostomy 5 years earlier.
The patient's pathological stage was T3N0M0 (stage II). After the operation, 12 cycles of 5-floururacil and adriamycin were administrated for adjuvant chemotherapy. After 3 years, the patient was diagnosed with cancer recurrence in the transverse colon and carcinomatosis, along with the development of colonic obstruction. On colonoscopy, two uncovered colonic stents (Niti-S D type, Taewoong Inc., Seoul, Korea) were inserted in the obstructed transverse colon, followed by a palliative treatment of 10 cycles of 5-floururacil and oxaliplatin chemotherapy.
Ten months after the first stent insertion, the patient showed signs of intestinal reobstruction. Upon examination of the previous stent, we observed on colonoscopy that the malignant tumor had grown into the stent, therefore, we inserted a 10 cm-sized Nitinol covered stent (Niti-S Comvi, Taewoong Inc., Seoul, Korea). The patient's disease progressed, and we changed the chemotherapy regimen to paclitaxel.
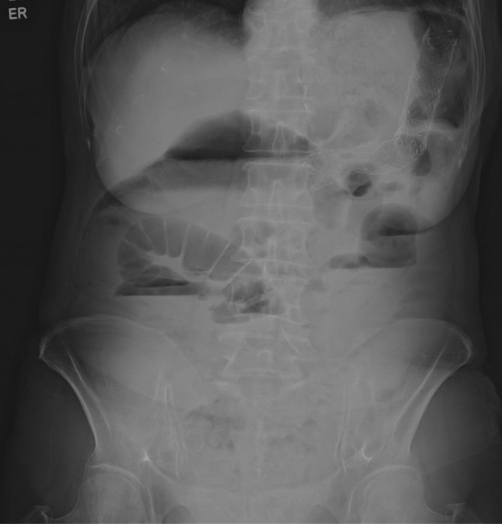
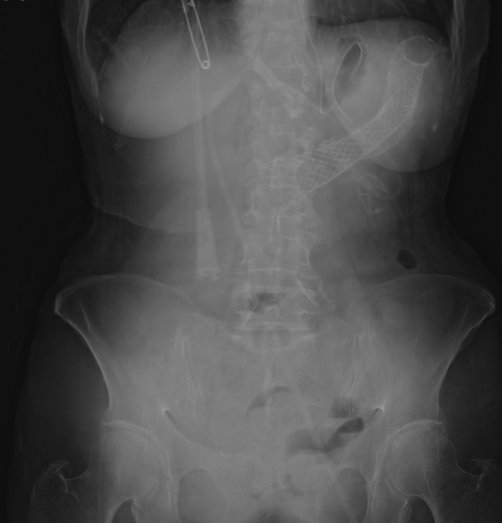
Two months following the reinsertion of the colonic stent, the patient again developed increasing abdominal pain and constipation. Nasogastric Levin tube decompression was administered for 1 week, but the bowel obstruction was not improved. On colonoscopy, the new stent was found to remain in place. However, there was a tumor growth into the stent. Finally, a 9 cm-sized Wallflex uncovered colonic stent (Boston Scientific, MA, USA) was inserted at the same site (Figs. 1 and 2).
Fig. 1.
Abdominal X-ray before 3rd colonic stent.
Fig. 2.
Abdominal X-ray after 3rd colonic stent.
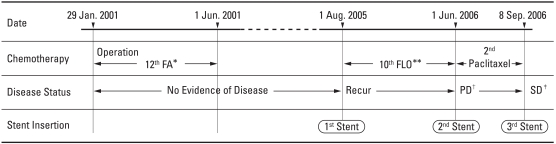
The procedure decompressed the bowels, and the general condition of the patient improved, allowing her to continue with chemotherapy after 6 months. Although the prognosis of recurrent gastric cancer with carcinomatosis is extremely poor, this case demonstrates that supportive care, such as three recurrent colonic stent insertions, may help maintain a generally good condition to allow for the continuation of chemotherapy. (Fig. 3)
Fig. 3.
Chemotherapy Schedule. *5-Flourouracil/Adriamycin, **5-Flourouracil/Oxaliplatin, †Disease Progression, ‡Stable Disease.
DISCUSSION
For malignant obstructive cancer patients, the board of directors of the European Association for Palliative Care (EAPC) recommends surgery in patients with poor prognostic criteria such as intra-abdominal carcinoma, poor performance status, and massive ascites.6
Endoscopic intervention is an effective and relatively safe palliative modality for patients with inoperable gastrointestinal cancer. In 1991, Dohmoto first described palliative stenting for colorectal obstruction.12,13 Colonic stenting as a palliative aim or a bridge to surgery for malignant colorectal obstruction is less invasive, and is associated with lower morbidity than emergency surgery. A recent study showed that palliative malignant colonic stenting, when compared to surgical decompression, offers not only shorter times for oral intake and hospital stay, but also less frequent complications. Furthermore, there was no procedure-associated mortality.4 In contrast, the morbidity and mortality rates for colonic surgical bypass ranges from 9% to 39.5% and 7.5% to 20.4%, respectively.14
The use of SEMS could be the treatment of choice in malignant obstructive cancer patients who are in a generally poor condition. Self-expandable stents are made of metal alloys, can be covered or uncovered, and have varying sizes and shapes. An uncovered stent is limited by tumor ingrowth through the gaps in the stent mesh. Covered stents provide several advantages; they prevent tumor ingrowth, and have no need for predilation with a balloon, and are easy to insert. However, higher incidence of migration with a covered stent has been reported.7
A systematic review, including 15 comparative studies, showed stenting to relieve malignant colorectal obstruction: the median rate of technical success was 96.2%, ranging from 66.6% to 100%, that of clinical success was 92%, ranging from 46% to 100%, and complication rated up to 30%. In the palliative population, the median duration of stent patency was 106 days (range, 68-288 days).15,16 Another study reported a median survival of 1.6 months after stent insertion in 35 malignant cancer patients with bowel obstruction.6
Our patient showed improved conditions for 2 years after the recurrence of cancer as carcinomatosis. At first, our patient was disease-free for 3 years after the postoperative adjuvant chemotherapy of 12 cycles of 5-floururacil and adriamycin. At that time, the patient was tolerant to chemotherapy. Then, her disease recurred and the first colonic stents were inserted. Subsequently, she started with palliative chemotherapy, including oxaliplatin, and presented with nausea, vomiting, peripheral neuropathy and anorexia as side effects. No major adverse effects were observed. Nevertheless, her disease progressed 10 months after the initiation of palliative chemotherapy with oxaliplatin. The chemotherapy regimen was changed to paclitaxel and a second colonic stent was reinserted. Although the patient experienced mild dizziness during the Paclitaxel treatment, she was still very tolerant of chemotherapy. Until the reobstruction of the previously inserted second colonic stent, her Eastern Cooperative Oncology Group (ECOG) performance status was grade 1. When colonic obstruction developed again at the same site after the second time, the patient's performance status decreased to grade 3 on the ECOG scale. A third stent was inserted into the obstructed colon. Fortunately, the patient never experienced complications, such as bleeding, perforation, or severe abdominal pain from the three stent insertions. Finally, the patient recovered from the malignant obstruction and her status improved to grade 1 on the ECOG performance scale. Thereafter, she continued to being treated by paclitaxel chemotherapy for 4 months.
This case shows that colonic stenting relieves malignant colonic obstruction and can help a patient maintain a tolerable condition for chemotherapy, ultimately improving their chances for survival.
References
- 1.Eickhoff A, Knoll M, Jakobs R, Weickert U, Hartmann D, Schilling D, et al. Self-expanding metal stents versus plastic prostheses in the palliation of malignant dysphagia: long-term outcome of 153 consecutive patients. J Clin Gastroenterol. 2005;39:877–885. doi: 10.1097/01.mcg.0000180631.61819.4a. [DOI] [PubMed] [Google Scholar]
- 2.De Vivo R, Pignata S, Palaia R, Parisi V, Daniele B. The role of chemotherapy in the management of gastric cancer. J Clin Gastroenterol. 2000;30:364–371. doi: 10.1097/00004836-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Maetani I, Akatsuka S, Ikeda M, Tada T, Ukita T, Nakamura Y, et al. Self-expandable metallic stent placement for palliation in gastric outlet obstructions caused by gastric cancer: a comparison with surgical gastrojejunostomy. J Gastroenterol. 2005;40:932–937. doi: 10.1007/s00535-005-1651-7. [DOI] [PubMed] [Google Scholar]
- 4.Ptok H, Meyer F, Marusch F, Steinert R, Gastinger I, Lippert H, et al. Palliative stent implantation in the treatment of malignant colorectal obstruction. Surg Endosc. 2006;20:909–914. doi: 10.1007/s00464-005-0594-7. [DOI] [PubMed] [Google Scholar]
- 5.Song HY, Kim JH, Shin JH, Kim HC, Yu CS, Kim JC, et al. A dual-design expandable colorectal stent for malignant colorectal obstruction: results of a multicenter study. Endoscopy. 2007;39:448–454. doi: 10.1055/s-2007-966270. [DOI] [PubMed] [Google Scholar]
- 6.Okorie MI, Hussain SA, Riley PL, McCafferty IJ. The use of self-expandable metal stents in the palliation of malignant bowel obstruction. Oncol Rep. 2004;12:67–71. [PubMed] [Google Scholar]
- 7.Park KB, Do YS, Kang WK, Choo SW, Han YH, Suh SW, et al. Malignant obstruction of gastric outlet and duodenum: palliation with flexible covered metallic stents. Radiology. 2001;219:679–683. doi: 10.1148/radiology.219.3.r01jn21679. [DOI] [PubMed] [Google Scholar]
- 8.Mosler P, Mergener KD, Brandabur JJ, Schembre DB, Kozarek RA. Palliation of gastric outlet obstruction and proximal small bowel obstruction with self-expandable metal stents: a single center series. J Clin Gastroenterol. 2005;39:124–128. [PubMed] [Google Scholar]
- 9.Atkinson M, Ferguson R. Fiberoptic endoscopic palliative intubation of inoperable oesophagogastric neoplasms. Br Med J. 1977;1:266–267. doi: 10.1136/bmj.1.6056.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siersema PD, Marcon N, Vakil N. Metal stents for tumors of the distal esophagus and gastric cardia. Endoscopy. 2003;35:79–85. doi: 10.1055/s-2003-36418. [DOI] [PubMed] [Google Scholar]
- 11.Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg. 2002;89:1096–1102. doi: 10.1046/j.1365-2168.2002.02148.x. [DOI] [PubMed] [Google Scholar]
- 12.Parker MC. Colorectal stenting. Br J Surg. 2006;93:907–908. doi: 10.1002/bjs.5494. [DOI] [PubMed] [Google Scholar]
- 13.Dohomoto M. New method-endoscopic implantation of rectal stent in palliative treatment of malignant stenosis. Endosc Dig. 1991;3:1507–1512. [Google Scholar]
- 14.Vandervoort J, Tham TC. Colonic stents for malignant obstruction--not a bridge too far? Gastrointest Endosc. 2006;64:921–924. doi: 10.1016/j.gie.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 15.Watt AM, Faragher IG, Griffin TT, Rieger NA, Maddern GJ. Self-expanding metallic stents for relieving malignant colorectal obstruction; a systematic review. Ann Surg. 2007;246:24–30. doi: 10.1097/01.sla.0000261124.72687.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Athreya S, Moss J, Urquhart G, Edwards R, Downie A, Poon FW. Colorectal stenting for colonic obstruction: the indications, complications, effectiveness and outcome--5-year review. Eur J Radiol. 2006;60:91–94. doi: 10.1016/j.ejrad.2006.05.017. [DOI] [PubMed] [Google Scholar]