Abstract
This study aimed to identify the association between gamma-aminobutyric acid-A (GABA-A) receptor subunit β3 (GABRB3) gene and autism spectrum disorders (ASD) in Korea. Fifty-eight children with ASD [47 boys (81.0%), 5.5 ± 4.1 years old], 46 family trios, and 86 healthy control subjects [71 males (82.6%), 33.6 ± 9.3 years old] were recruited. Transmission disequilibrium test revealed that, 183 bp long allele in GABRB3 gene was preferentially transmitted in families with ASD (p = 0.025), whereas a population-based case-control study, however, showed no association between ASD and GABRB3 microsatellite polymorphism. Our data provide preliminary evidence that GABRB3 gene is associated with ASD in Korea.
Keywords: Autism spectrum disorders, genetics, transmission disequilibrium test, microsatellite, gamma-aminobutyric acid-A receptor subunit β3
INTRODUCTION
Autism spectrum disorders (ASD) are childhood-onset neurodevelopmental disorders characterized by impairments in social interaction, communication, and stereotypic behaviors. Genetic factors may contribute to the pathogenesis of ASD,1,2 and diverse biological studies show that gamma-aminobutyric acid (GABA) is associated with ASD.3-7 Cook et al.8 initially presented evidence of linkage disequilibrium in the GABA type-A receptor subunit gene in chromosome 15q11-13 which includes the Prader-Willi and Angelman syndrome critical region (PWACR). Since then, several studies have replicated the positive results on the association of GABRB3 gene with autistic disorder.9-11 In contrast, however, negative results have also been reported.12,13 Several researchers reported the association of a microsatellite marker, simply known as GABRB3, 3' to the GABRB3 gene, with autistic disorder.10,11 Since no genetic studies have so far been conducted in Korean subjects with ASD, we employed both family-based and population-based analyses to investigate the association of GABRB3 microsatellite marker of the GABRB3 gene.
Fifty-eight Korean children with ASD [47 boys (81.0%), mean age, 5.5 ± 4.1 years old] were enrolled from the outpatient clinic at a general hospital in Seoul, Korea. Biological parents of 46 patients among those 58 patients with ASD were also recruited. Eighty-six healthy control subjects [71 males (82.6%), mean age, 33.6 ± 9.3 years old] were also recruited through advertisements in the local newspapers. The study protocol was approved by the Institutional Review Boards. After a complete description of the study to participants, written informed consent was obtained.
A trained child psychiatrist diagnosed patients according to criteria in the Diagnostic and Statistical Manual of Mental Disorder-fourth edition (DSM-IV). Twenty children (34.5%) were diagnosed with autistic disorders, 5 (8.6%) with Asperger's disorder, and 33 (56.9%) with pervasive developmental disorder not otherwise specified (PDD NOS). To assess healthy control subjects within the normal range of intelligence, the Structured Clinical Interview for DSM-IV14 and the Wechsler Adult Intelligence Scale-Revised15 were administered. Children with chromosomal abnormalities or a history of definite head trauma were excluded.
Genomic DNA was extracted from peripheral leukocytes of subjects using a standard phenol/chloroform method (Invitrogen Easy-DNA™ Kit, Boehringer Mannheim, San Diego, California, USA), according to the manufacturer's instructions. Genotyping was performed with an investigator blinded to the status of the probands and family information. Polymerase chain reaction (PCR) was performed with sense primer 5'-CTCTTGTTCCTGTTGCTTTCAATACAC-3'and anti-sense primers 5'-CACTGTGCTAGTAGATTCAGCTC-3' for analyzing the GABRB3 marker located approximately 60 kb beyond the 3' end of the GABRB3 gene.16 The amplified products were analyzed by electrophoresis on 8% acrylamidebis (19 : 1) 40% urea sequencing type gel on which subject samples were loaded.
Transmission disequilibrium test (TDT) was applied to analyze the preferential intergenerational transmission of GABRB3 microsatellite in 46 complete trios. We used the χ2 test for comparison of allele and genotype frequencies between probands and healthy controls. Statistical significance was defined at an alpha of < 0.05, two-tailed. All analyses of genotype distribution were performed using the SNP Alyze software package V 5.0 (Dynacom Co. Yokohama, Japan).
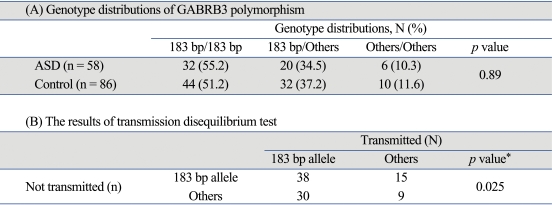
Nine alleles (179, 181, 183, 185, 187, 191, 193, 195, 197 bp long) were observed in Korean subjects: the allele with 183 bp long was the most frequent allele (72.4%) in children with ASD, while the other eight alleles had frequencies less than 10%. When we divided the alleles into two subclasses (allele with 183 bp long and others), no deviation from Hardy-Weinberg equilibrium was seen in ASD (p = 0.30) and normal controls (p = 0.27). TDT result disclosed that allele with 183 bp long in GABRB3 was significantly preferentially transmitted in families with autism spectrum disorders (McNemar χ2 value = 5.000, df = 1, p = 0.025). However, no differences were observed between the allele and genotype frequencies of ASD probands and healthy control subjects (Table 1).
Table 1.
Genotype Distributions of GABRB3 Polymorphism and the Results of Transmission Disequilibrium Test (TDT)
GABRB3, gamma-aminobutyric acid A receptor, subunit β3; ASD, autism spectrum disorders; Others, all alleles except 183 bp allele.
*p value for TDT test, McNemar χ2 value = 5.000, df = 1.
To our best knowledge, this is the first report on the association between the GABRB3 microsatellite polymorphism of GABRB3 gene and ASD in Korea, although Kim et al.17 have reported the association of single nucleotide polymorphism (SNP) of GABRB3 gene with ASD in Korean trios. The allele frequencies of GABRB3 microsatellite markers in this Korean population differed from those of a previous foreign study.10,11 Martin et al.11 reported 187 bp long allele that was transmitted less often than expected, whereas another two alleles, 191 and 197 bp long alleles, were transmitted with increased frequencies. The allele with 191 bp long was also reported to be overtransmitted to the children in Curran et al.10 study. However, we observed that 183 bp long allele was overtransmitted to children with ASD when we considered 8 other alleles (179, 181, 185, 187, 191, 193, 195, 197 bp long) together as one category of rare alleles because of the less than 10% of allele frequencies. The data indicated clear ethnic differences in polymorphism of GABRB3 microsatellite marker of Korean subjects.
In the present study, both family-based and population-based association studies were conducted in parallel, and the result showed that positive association was evident only in TDT analysis. Similarly, previous studies12,13 also found no correlation between GABRB3 and ASD. The present finding, therefore, suggests that GABRB3 gene may be not associated with ASD in the general population where the possibility of crossovers is higher than that in a family group. An isolated group with less frequent inflow of population may be more useful for genetic association studies, since the linkage between the etiological gene and other alleles in the vicinity is more consistent.18 In ASD, a rare disorder, the frequency of casual genes of ASD would also be low. A considerable number of study subjects have to be recruited to obtain sufficient gene frequency for statistical power. Therefore, TDT analysis can be a more useful association study of disorders with rare prevalence, such as autism.19 Moreover, TDT can overcome the stratification bias that occurs due to different sample compositions from the general population, a crucial weak feature of case-control genetic association studies.20
This study provides preliminary evidence that GABRB3 gene could be associated with ASD in Korea, and additionally supports the functional roles for genetic variants within the GABA receptor gene complex in Korean ASD. However, this study has the limitation of a relatively small sample size for population-based association study which may influence type II error. Furthermore, we did not apply the objective diagnostic tools for ASD such as the Autism Diagnostic Interview and the Autism Diagnostic Observation Schedule on healthy comparison subjects as well as children with ASD. Further studies with larger sample size and application of objective diagnostic tools are required to confirm our findings.
ACKNOWLEDGEMENTS
This study was supported by a research grant from the Clinical Research Institute of Seoul National University Hospital (04-2005-067-0).
References
- 1.Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- 2.Bolton P, Macdonald H, Pickles A, Rios P, Goode S, Crowson M, et al. A case-control family history study of autism. J Child Psychol Psychiatry. 1994;35:877–900. doi: 10.1111/j.1469-7610.1994.tb02300.x. [DOI] [PubMed] [Google Scholar]
- 3.Aldred S, Moore KM, Fitzgerald M, Waring RH. Plasma amino acid levels in children with autism and their families. J Autism Dev Disord. 2003;33:93–97. doi: 10.1023/a:1022238706604. [DOI] [PubMed] [Google Scholar]
- 4.Blatt GJ, Fitzgerald CM, Guptill JT, Booker AB, Kemper TL, Bauman ML. Density and distribution of hippocampal neurotransmitter receptors in autism: an autoradiographic study. J Autism Dev Disord. 2001;31:537–543. doi: 10.1023/a:1013238809666. [DOI] [PubMed] [Google Scholar]
- 5.Chugani DC, Muzik O, Juhász C, Janisse JJ, Ager J, Chugani HT. Postnatal maturation of human GABAA receptors measured with positron emission tomography. Ann Neurol. 2001;49:618–626. [PubMed] [Google Scholar]
- 6.Dhossche D, Applegate H, Abraham A, Maertens P, Bland L, Bencsath A, et al. Elevated plasma gamma-aminobutyric acid (GABA) levels in autistic youngsters: stimulus for a GABA hypothesis of autism. Med Sci Monit. 2002;8:PR1–PR6. [PubMed] [Google Scholar]
- 7.Moreno-Fuenmayor H, Borjas L, Arrieta A, Valera V, Socorro-Candanoza L. Plasma excitatory amino acids in autism. Invest Clin. 1996;37:113–128. [PubMed] [Google Scholar]
- 8.Cook EH, Jr, Courchesne RY, Cox NJ, Lord C, Gonen D, Guter SJ, et al. Linkage-disequilibrium mapping of autistic disorder, with 15q11-13 markers. Am J Hum Genet. 1998;62:1077–1083. doi: 10.1086/301832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buxbaum JD, Silverman JM, Smith CJ, Greenberg DA, Kilifarski M, Reichert J, et al. Association between a GABRB3 polymorphism and autism. Mol Psychiatry. 2002;7:311–316. doi: 10.1038/sj.mp.4001011. [DOI] [PubMed] [Google Scholar]
- 10.Curran S, Roberts S, Thomas S, Veltman M, Browne J, Medda E, et al. An association analysis of microsatellite markers across the Prader-Willi/Angelman critical region on chromosome 15 (q11-13) and autism spectrum disorder. Am J Med Genet B Neuropsychiatr Genet. 2005;137B:25–28. doi: 10.1002/ajmg.b.30126. [DOI] [PubMed] [Google Scholar]
- 11.Martin ER, Menold MM, Wolpert CM, Bass MP, Donnelly SL, Ravan SA, et al. Analysis of linkage disequilibrium in gamma-aminobutyric acid receptor subunit genes in autistic disorder. Am J Med Genet. 2000;96:43–48. doi: 10.1002/(sici)1096-8628(20000207)96:1<43::aid-ajmg9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Maestrini E, Lai C, Marlow A, Matthews N, Wallace S, Bailey A, et al. Serotonin transporter (5-HTT) and gamma-aminobutyric acid receptor subunit beta3 (GABRB3) gene polymorphisms are not associated with autism in the IMGSA families. The International Molecular Genetic Study of Autism Consortium. Am J Med Genet. 1999;88:492–496. doi: 10.1002/(sici)1096-8628(19991015)88:5<492::aid-ajmg11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.Salmon B, Hallmayer J, Rogers T, Kalaydjieva L, Petersen PB, Nicholas P, et al. Absence of linkage and linkage disequilibrium to chromosome 15q11-q13 markers in 139 multiplex families with autism. Am J Med Genet. 1999;88:551–556. [PubMed] [Google Scholar]
- 14.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Version (SCID-P) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 15.Wechsler E. Wechsler Adult Intelligence Scale-Revised (WAIS-R) manual. New York: The Psychological Corporation; 1981. [Google Scholar]
- 16.Mutirangura A, Ledbetter SA, Kuwano A, Chinault AC, Ledbetter DH. Dinucleotide repeat polymorphism at the GABAA receptor beta 3 (GABRB3) locus in the Angelman/Prader-Willi region (AS/PWS) of chromosome 15. Hum Mol Genet. 1992;1:67. doi: 10.1093/hmg/1.1.67. [DOI] [PubMed] [Google Scholar]
- 17.Kim SA, Kim JH, Park M, Cho IH, Yoo HJ. Association of GABRB3 polymorphisms with autism spectrum disorders in Korean trios. Neuropsychobiology. 2006;54:160–165. doi: 10.1159/000098651. [DOI] [PubMed] [Google Scholar]
- 18.Sham P. Genetic epidemiology. Br Med Bull. 1996;52:408–433. doi: 10.1093/oxfordjournals.bmb.a011557. [DOI] [PubMed] [Google Scholar]
- 19.Karayiorgou M, Gogos JA. Dissecting the genetic complexity of schizophrenia. Mol Psychiatry. 1997;2:211–223. doi: 10.1038/sj.mp.4000271. [DOI] [PubMed] [Google Scholar]
- 20.Murphy KC, Cardno AG, McGuffin P. The molecular genetics of schizophrenia. J Mol Neurosci. 1996;7:147–157. doi: 10.1007/BF02736794. [DOI] [PubMed] [Google Scholar]