Abstract
Rates of skin cancer continue to increase despite the improved use of traditional sunscreens to minimize damage from ultraviolet radiation. The public perception of tanned skin as being healthy and desirable, combined with the rising demand for treatments to repair irregular skin pigmentation and the desire to increase or decrease constitutive skin pigmentation, arouses great interest pharmaceutically as well as cosmeceutically. This review discusses the intrinsic biochemistry of pigmentation, details mechanisms that lead to increased or decreased skin pigmentation, and summarizes established and potential hyper- and hypo-pigmenting agents and their modes of action.
Keywords: skin, pigmentation, ultraviolet, hypopigmentation, hyperpigmentation
Introduction
Despite the increased use of traditional sunscreens, the incidence of malignant melanoma has tripled over the past 40 years. In addition to the progressive thinning of the ozone layer, increased recreational sun exposure of the population and the cosmetically motivated desire to tan are held at least in part responsible. The widely accepted view that sunscreens protect against melanoma has been challenged by epidemiological and experimental studies that revealed no or even an inverse association between the use of sunscreens and melanoma development [1,2]. In addition, there is a rising demand for remedies to treat irregular hyperpigmentation or, especially in Asian cultures, to obtain a general whitening of the skin. In light of these facts, the modification of skin pigmentation, whether by hyper-or hypo-pigmenting agents, arouses great interest pharmaceutically as well as cosmeceutically. This review discusses the intrinsic biochemistry of pigmentation, details mechanisms that lead to increased or decreased pigmentation and summarizes established and potential hyper- and hypopigmenting agents and their modes of action.
Biochemistry of melanogenesis
Skin color is determined by a mixture of four biochromes: oxyhemoglobin (red), reduced hemoglobin (blue), carotenoids (yellow) and, most importantly, the amounts and types of melanins (brown) produced and their distribution in the skin. Although the number of melanocytes (specialized cells in the epidermis that produce the pigment) does not differ between subjects from different racial/ethnic backgrounds [3], there are intra-individual differences in melanocyte density at different sites of the body (e.g. the palms and soles contain only 10–20% the density of melanocytes found elsewhere) [4]. Melanocytes account for only 1% of epidermal cells and occur at an approximate ratio of 1:10 among keratinocytes in the basal skin layer. Via their elongated dendrites, melanocytes transport their ovoid membrane-bound organelles (termed melanosomes), in which melanin is synthesized and stored, to neighboring keratinocytes [5], where melanosomes form a critical barrier as supranuclear “caps” to shield DNA from ultraviolet radiation (UVR) [6]. Proliferating keratinocytes in the suprabasal epidermal layers gradually ascend towards the skin surface along with their ingested melanin to contribute to photoprotection.
The regulation of pigmentation is a complex process and there are currently more than 125 genes known to be directly or indirectly involved [7]. Many genes (>25) regulate melanosomal biogenesis or function, which requires a number of specific enzymatic and structural proteins for effective melanin production [8,9].
Three different kinds of melanin are produced by melanocytes: the lighter red/yellow, alkali soluble sulfur-containing pheomelanin (which is predominant in the red hair/freckles phenotype) and two types of eumelanin, dark brown/black insoluble pigments found in dark skin and black hair. Human skin normally contains a mixture of all 3 types of melanin, but the ratio varies greatly and determines the color of the skin [10].
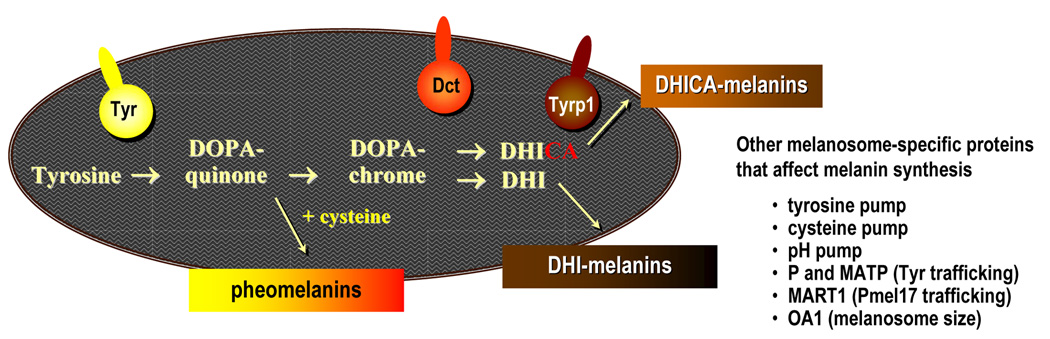
Within melanosomes, at least 3 enzymes are indispensable for the synthesis of different types of melanin (Figure 1). Tyrosinase (TYR) catalyzes the initial rate-limiting step in melanogenesis, the hydroxylation of tyrosine to β-3,4-dihydroxyphenylalanine (DOPA) and the subsequent oxidation of DOPA to DOPAquinone. In the presence of cysteine, DOPAquinone is converted stochiometrically into 3- or 5-cysteinylDOPA which oxidizes and polymerizes to pheomelanins [11,12]. After depletion of cysteine, DOPAquinone spontaneously cyclizes to form DOPAchrome. Dark brown/black 5,6-dihydroxyindole (DHI) melanins are generated after DOPAchrome spontaneously loses its carboxylic acid group and oxidizes and polymerizes. However, in the presence of the enzyme DOPAchrome tautomerase (DCT), the carboxylic acid group is retained, and DOPAchrome is converted into DHI-2-carboxylic acid (DHICA), which finally results in the formation of DHICA-melanin, a moderately soluble lighter brown product of intermediate size [13].
Figure 1. Schematic of the melanin synthetic pathway and enzymes involved that occur specifically in melanosomes.
Three enzymes are indispensable for the synthesis of melanin (eu- and pheo-melanogenesis as discussed in the text). Tyrosinase (TYR) is the rate-limiting enzyme for initial melanogenesis; DOPAchrome tautomerase (DCT) and tyrosinase-related protein 1 (TYRP1) are also involved in modulating eumelanogenesis. So far, no specific enzymes related to pheomelanogenesis have been detected. Other melanosome-specific proteins that help modulate melanin biosynthesis are listed.
Tyrosinase-related protein 1 (TYRP1) is a critical enzyme for the correct trafficking of tyrosinase to melanosomes [14], and DCT also seems to play an important role in detoxification processes within melanosomes [15]. Mutations in these enzymes lead to dramatic changes in the quantity and quality of synthesized melanin. Tyrosinase is responsible for the critical rate-limiting steps of melanogenesis; mutations that affect TYR function result in albinism. Mutations in proteins that are involved in sorting/trafficking of proteins to melanosomes or in melanosome structure result in inherited hypopigmentary disorders [16]. Proteins regulating the uptake of substrates needed for melanin production, for example tyrosine and cysteine transporters, as well as those regulating the pH within melanosomes, for example proton pumps, are also critical in regulating melanin production.
Regulation of constitutive pigmentation
The determination of pigmentary phenotype is genetically complex and at a physiological level is quite complicated. Genes determining a number of rare Mendelian disorders of pigmentation, such as albinism, have been identified, but one gene, the melanocortin-1 receptor (MC1R), has been found to explain much of the variation of skin color in the normal population. The MC1R is a G protein coupled receptor expressed on the surface of melanocytes that regulates the quality and quantity of melanin production [17,18]. MC1R is regulated by agonists (α-melanocyte stimulating hormone (αMSH) and adrenocorticotropic hormone (ACTH)) as well as by an antagonist (agouti-signaling protein (ASP)). Binding of an agonist to MC1R triggers the melanogenic cascade that starts with the activation of adenylate cyclase that results in the stimulation of cyclic adenosine monophosphate (cAMP) synthesis (Figure 2), activation of the cAMP-dependent protein kinase A (PKA) and then a series of reactions (many of them are yet unknown) that finally result in the increased synthesis of eumelanin. If the MC1R signal is blocked by ASP, the eumelanin synthesis switches to pheomelanin production. The switch to synthesize eu- versus pheomelanin is controlled by MC1R, but the mechanism(s) remain obscure. The high polymorphism of MC1R accounts for the diversity of constitutive pigmentation from extremely fair to extremely dark skin while defective MC1R variants that lead to decreased function of the receptor that have been associated with red hair and fair skin [19] and high sun sensitivity [19,20] as well as an increased risk of melanoma [21]. Beyond MC1R control, various environmental factors such as UVR and factors secreted by surrounding keratinocytes and fibroblasts in the skin can influence melanocyte proliferation and differentiation significantly. DKK1, a factor secreted by dermal fibroblasts in the palms/soles is an inhibitor of the Wnt/β-catenin pathway and therefore able to suppress melanocyte growth and function dramatically [4,22]. Not only does DKK1 inhibit the expression of transcription factors (e.g. MITF) and melanogenic proteins, but it also impairs the ability of keratinocytes for melanin uptake.
Figure 2. Regulatory processes involved in melanin synthesis.
Melanogenesis is regulated by complex processes that involve extrinsic effects of UVR as well as the intrinsic release of factors produced by keratinocytes, melanocytes and fibroblasts. Based on and modified from Yamaguchi et al (127).
There are other factors that influence melanin production, such as P and MATP proteins; both are melanocyte-specific 12 membrane-transporters that are key factors in the sorting/trafficking of tyrosinase to melanosomes. The exact mechanisms of P and MATP remain to be elucidated, but it has been hypothesized that they act as transporters/pumps that control intracellular ion transport and in this way regulate tyrosinase sorting and melanin synthesis. As shown by population studies, polymorphisms of the P [23] and MATP [24] genes (in combination with polymorphisms of the MC1R gene) make major contributions to determine the normal range of pigmentation in hair, skin and eyes. Recently, an important role of SLC24A5, a member of a family of potassium-dependent sodium-calcium exchangers, in the regulation of pigmentation was reported. A close association was found between a polymorphism in SLC24A5 (Ala111Thr) and cutaneous melanin content in an intermixed group [25]. On the basis of the HapMap database (www.hapmap.org), the fixed Ala111Thr polymorphism of SLC24A5 was found in Europeans (with lighter skin color) while the ancestral allel (Ala111) was conserved in African-Americans and in African-Caribbeans (with darker skin). Dysfunctions of TYR and/or other proteins of the melanogenic pathway lead to the disruption of skin, hair and eye pigmentation, a condition termed albinism [26]. So far, 5 types of albinism have been described that are associated with 5 distinct pigment-related loci. Mutations in those genes result in direct or indirect impairment of TYR acitivity. Proteasomal degradation of TYR is characteristic for oculocutaneous albinism (OCA) type 1 (TYR) and OCA3 (TYRP1), while OCA2 (P) and OCA4 (MATP) lead to disrupted sorting of functional TYR to melanosomes. Ocular albinism type 1 (OA1) causes a disruption of melanin synthesis, primarily in the eye, by an as yet unknown mechanism [27].
In sum, constitutive pigmentation depends on: (a) melanocyte number, (b) the expression and function of melanosomal enzymes and structural proteins, (c) the amount of eu- and pheomelanin synthesized, (d) the transport of melanosomes to dendrites, (e) their transfer to keratinocytes and, (f) last but not least, the distribution of melanin in suprabasal skin layers.
Regulation of facultative pigmentation
An increase of skin pigmentation above the basal constitutive level induced by physiological factors (for example, UVR) is called facultative pigmentation, or more commonly ‘tanning’ [28]. The relationship between MC1R and UVR is complex and is regulated at many levels [29]. Microphthalmia-associated transcription factor (MITF - the master regulator of pigmentation) is the most important factor involved in the MC1R regulation of melanocyte function. Levels of MITF are quickly increased by UVR which then stimulates the expression of downstream melanogenic proteins, including Pmel17, MART1, TYR, TYRP1 and DCT [30,31]. The sum of those factors eventually leads to significant increases in melanin content after several weeks.
UVR also leads to increased melanocyte density, melanin synthesis and melanocyte dendricity, which play important roles in the transfer of melanin to keratinocytes. UV also enhances levels of proteinase-activated receptor 2 (PAR2) which stimulates melanosome uptake and distribution by epidermal keratinocytes [32]. Melanocytes, keratinocytes and fibroblasts react to UVR by releasing a wide variety of melanogenic factors. Following exposure to UVR, melanocytes as well as keratinocytes express higher amounts of proopiomelanocortin (POMC), the precursor of the melanocortins αMSH and ACTH, which stimulates MC1R function and consequently boosts melanocyte responses to those melanocortins. Keratinocytes also respond to UV with increased expression of other factors, including endothelin-1 (ET-1) which stimulates melanocyte function in a paracrine manner [33], interleukin-1 (IL-1) which enhances autocrine secretion of ACTH, αMSH, ET-1 and basic fibroblast growth factor (bFGF), stem cell factor (SCF) which is involved in the regulation of proliferation and melanogenesis of melanocytes, and nerve growth factor (NGF) which is involved in regulating melanogenesis and/or dendritogenesis of melanocytes [34]. UV-induced activation of p53 in keratinocytes leads to increased expression of the POMC gene which in turn results in increased levels of αMSH and stimulation of MC1R function in melanocytes [35]. Fibroblast activation by UVR results in the secretion of growth factors, including hepatocyte growth factor (HGF), bFGF and SCF all of which stimulate pigmentation via their receptors on melanocytes [36]. Hormones also represent important factors that regulate skin pigmentation. It was hypothesized that vitamin D3, which is synthesized in the skin upon UV exposure, might play a role in the melanogenic effects of UV, as human melanocytes also express vitamin D3 receptors, but so far, the underlying mechanism(s) remains to be elucidated. For example, topical application of 1,25-(OH)2-vitamin D3 leads to an increase in the number of melanocytes and augments the melanogenic effect of UV on mouse skin, while melanocytes in vitro respond to 1,25-(OH)2-vitamin D3 by decreasing TYR activity [37]. Sex steroid hormones also play an important role in skin pigmentation. In response to β-estradiol, melanocytes increase TYR activity in a dose-dependent manner although there is no correlation of this response with constitutive pigmentation [38,39].
Although the mechanisms involved in pigmentation are similar in skin of different racial/ethnic groups, melanin transfer from the basal level of the epidermis upwards is more effective in dark skin than in fair skin resulting in a better protection of the lower epidermis in dark skin [40,41].
Induction of hyperpigmentation – specific mechanisms
In the face of the complex mechanisms that regulate human skin pigmentation, disorders that result from exogenous or endogenous influences are not uncommon. As a vast variety of factors exists that can lead to hyper- or hypo-pigmentation, this review will focus on the more common and well-investigated stresses that can lead to acquired pigmentation disorders. Increased levels of melanin in the epidermis result in a state known as hypermelanosis. Two types of changes exist: a) increased numbers of melanocytes in the epidermis followed by increased production of melanin, which is called melanocytic hypermelanosis (e.g. lentigo), and b) no increase of melanocyte number but increased melanin production only, termed melanotic hypermelanosis (e.g. melasma). Hypermelanosis of both types can be the result of genetic, hormonal (increase in circulating pituitary melanotropic hormones) and environmental (UVR) factors.
UV is one of the most powerful agents that induces hyperpigmentation of the skin. Lentigines solares (LS) (also termed age spots, sun spots and actinic lentigines) are circumscribed, pigmented macules, usually brown in color, that range in size from a few millimeters to a few centimeters in diameter and may coalesce into even more extended lesions [42]. They typically appear on sun-exposed areas of the skin such as the neck, face and forearms [43] and increase in number with age, affecting more than 90% of the Caucasian population older than 50 years [44]. LS result from increased amounts of melanin in the basal and suprabasal layers of the epidermis. The mechanisms underlying this type of hyperpigmentation process have recently been elucidated by Imokawa and coworkers. Besides a two-fold increase of TYR-positive melanocytes in lesional skin compared to perilesional skin [45], they demonstrated the existence of a molecular network in which increased expression of the ET-1/ET(B)R cascade and higher expression of SCF in lesional skin as well as cross-talk between these two signaling pathways following UV exposure play an important role in the mechanisms underlying LS [46,47].
A wide variety of drugs and substances has been reported to induce hyperpigmentation, including antibiotics (mainly tetracyclines), chemotherapeutics, heavy metals and antiepileptic medications. Diffuse muddy brown discolorations in sun-exposed areas of the skin (type III reaction) induced by minocyclin, a tetracycline-derivative, are well documented side-effects presumably resulting from increased melanin production by minocyclin-stimulated melanocytes that can lead, among other things, to deposits of melanin or minocyclin/melanin-complexes at the epidermal basal membrane and in the dermis [48]. Chemotherapeutics such as bleomycin, daunorubicin, doxorubicin, cyclophosphamide and 5-fluoruracil are able to cause hyperpigmentation, supposedly by stimulation of melanogenesis via direct toxic effects on melanocytes, although the underlying mechanisms are unknown. Based on the observation that fragments of nucleic acids can stimulate melanin synthesis [49], chemotherapy-induced damage to DNA in skin cells could induce signals that promote melanogenesis [50]. Heavy metals such as gold, silver, arsenic or bismuth can enhance melanin synthesis [51]. It is believed that such metals complex with sulfhydryl compounds in the skin that normally block TYR activity and thus resulting in the stimulation of melanogenesis. Antiepileptic drugs (e.g. hydantoins) are also known to increase pigmentation and might do so by a direct stimulatory action on melanocytes [52]. Tricyclic depressants (desipramine and imipramine) are associated with slate-gray pigmentation in sun-exposed areas caused both by increased melanin in the dermis as well as by electron-dense inclusions within dermal cells [53,54].
Melasma is a hyperpigmentation disorder that presents with arcuate or polycyclic hyperpigmented lesions in sun-exposed areas and occurs most commonly in women in the central facial area [55]. So far, the cause of melasma is not known but a large number of factors exist that can contribute to its development or aggravation (e.g. pregnancy and oral contraceptive/hormone replacement therapy, UV exposure, genetic influences, and cosmetics). Among those factors, UVR is regarded as the most important cause [56]. It was shown that lesional skin of melasma has higher amounts of melanin in the epidermis and dermis but no increase in melanocyte number, although the melanocytes were larger and more dendritic and produced higher amounts of eumelanin [57]. Elevated levels of estrogen and progesterone are associated with melasma [58] and several studies have shown a stimulating effect of estrogens on tyrosinase activity in cultured melanocytes [38,39].
In almost all skin disorders with an inflammatory component (e.g. acne, eczema, and psoriasis) there is a risk of postinflammatory hyperpigmentation (PIH) due to excessive cytokine secretion [59]. As with melasma, PIH occurs more often in patients with darker skin, although there is no gender predominance. PIH lesions, which may involve the epidermis and/or the dermis, are characteristically limited to sites of preceding inflammation and have hazy, feathered borders. In PIH, the number of melanocytes is typically normal, but the production of melanin is markedly increased. Often, melanin is abnormally transported into the dermis (termed pigmentary incontinence) where it accumulates within melanophages. During inflammatory processes and also after UVR, keratinocytes produce prostaglandins (PG), a group of inflammatory mediators, that are metabolites of arachidonic acid. PGE1 and PGE2 increase melanogenesis strongly while PGA1 and PGD2 represent strong inhibitors [60]. Leukotrienes such as LTC4 and LTD4, are metabolites of the lipoxygenase pathway and are able to induce the proliferation of melanocytes in vitro [61]. Histamine, another inflammatory agent, may activate melanogenesis via proteinase A activation [62].
Induction of hypopigmentation – specific mechanisms
Decreased levels of melanin in the epidermis is called hypomelanosis and results mainly from two different types of changes: a) decreased numbers or absence of melanocytes in the epidermis resulting in little or no melanin production (melanocytopenic hypomelanosis, e.g. vitiligo), and b) no decrease in the number of melanocytes but decreased levels of melanin production (melanopenic hypomelanosis). Hypomelanosis can also result from genetic (as in albinism), from autoimmune, toxic or inflammatory processes. During the senescence process, the density of melanocytes in the skin decreases physiologically ~10% per decade [63], but loss of pigmentation can occur at all ages after exposure to melanotoxic agents. Contact with certain chemicals can lead to cutaneous depigmentation [64–66] and a recent description of all agents that can induce depigmentation of the skin is available [67]. The majority of these agents are derivatives of phenol and catechols [68]. Generally, these agents induce chemical leucoderma (depigmentation at the contact sites), but in subjects with a genetic predisposition and chronic exposure, the initial depigmentation can extend and lead to progressive generalized vitiligo. Large differences in the appearance of toxic effects after exposure have been observed, and it seems that there is a genetic control of responses to melanotoxic agents [68].
Hypomelanosis can occur postinflammation (e.g. after resolving of psoriatic plaques or lesions of atopic dermatitis), possibly resulting from an increased keratinocyte turnover that interferes with melanosomal transfer as well as the activation of inhibitory cytokines. Decreased melanin content can be also related to infections (e.g. lepra and syphilis), although the mechanism(s) involved is currently unknown and it has been suggested to be postinflammatory [69]. Although there are some indications that properties of infectious agents might be responsible for the depigmentation: mycobacterium leprae contains an enzyme similar to TYR that potentially converts DOPA to a quinone, so that DOPA is unavailable for melanin production [70].
There are many different possible mechanisms for hypomelanocytosis, including the aforementioned melanotoxic effects of chemicals, or trauma. In vitiligo, there are several hypotheses to explain the loss of melanocytes, including the concept that vitiligo is an autoimmune response by anti-melanocyte antibodies [71] or is mediated by T-cells [72], or is an autocytotoxic response by melanin precursors [73], as well as being a genetic disease [74].
Modifying skin pigmentation
1. Stimulation of melanogenesis
Compared to fair skin, darkly pigmented skin has a up to 70-fold higher protection against skin cancer [75]. For decades, scientists have tried to enhance skin pigmentation without UV exposure to confer the protective properties of a tan without the associated DNA damage that is caused by UVR. Space doesn’t allow a full elaboration of all agents tested (many of them have been used in vitro only), so we will focus on some with different modes of actions that present interesting approaches to stimulate pigmentation in human skin. Interested readers are referred to a more detailed review that examined enhancers of skin pigmentation [76]. One possible approach lies in triggering MC1R function. In the 1960s, Lerner and McGuire discovered that injections with αMSH, one of the MC1R agonists (see Figure 2), increased human skin pigmentation [77]. More recently, several studies examined the effects of [Nle4-d-Phe7]-αMSH, a synthetic superpotent analogue of αMSH, on human skin in situ and reported increased pigmentation after a series of 10 injections [78–81]. In a larger study, injections of 65 subjects with a slow-release formulation of the same αMSH analogue over 3 months lead to an average 41% increase of melanin in subjects with high sun-sensitivity compared to a 12% increase in subjects with low sun-sensitivity, and there was no significant difference in pigmentation between sun-exposed and non-sun-exposed areas [82]. As the necessity to inject the drug on a regular basis to maintain a tan is a major drawback, there are currently attempts to develop αMSH analogs that are small enough to reach their target when administered topically. Abdel-Malek and colleagues developed potent tetrapeptide αMSH analogs (n-Pentadecanoyl- and 4-Phenylbutyryl-His-D-Phe-Arg-Trp-NH2) that are able to stimulate melanogenesis and enhance DNA repair after UVR of melanocytes in vitro [83]. However, αMSH analogs may not work in subjects with red hair who have an impaired MC1R function. A different approach was recently demonstrated by D’Orazio and colleagues in an animal model [84]. They were able to induce artificial tanning in red/blonde-haired mice that have an inactivating mutation of MC1R by treating the animals topically with forskolin. Forskolin, a cell permeable diterpenoid, is a natural product (root extract of Plectranthus barbatus, also known as Coleus forskohlii) that bypasses MC1R function by activating adenylate cyclase [85] and thus increasing cAMP levels. This chemically induced tan was able to protect UV-irradiated mice against sunburn, DNA damage and subsequent carcinogenesis. As virtually all cells contain adenylate cyclase, attempts are now being made to develop agents that affect the melanogenic pathway more specifically. UVR not only stimulates αMSH secretion but it also causes DNA damage, and it is widely believed that such damage can itself induce tanning and DNA repair responses [86]. Damage to telomere loops at the ends of chromosomes and overhang exposure is considered to be a DNA damage signal [87]. Oligonucleotides that imitate this telomere overhang (T-oligos) presumably induce protective DNA damage responses. Gilchrest and coworkers found that in human skin explants treated with T-oligos and irradiated with UVB, there was a strikingly reduced amount of DNA damage and a 3–5 fold increase in melanin content compared to untreated UV-irradiated samples [88]. Sunscreens containing T-oligos could be efficient in enhancing skin pigmentation and in protecting against photodamage and skin cancer. Bicyclic monoterpene (BMT) diols are small molecule compounds found in abundance in plants and food. In cultured cells and in guinea pig skin [89] and to some extent in human skin, BMT diols were able to increase melanin content when combined with α-hydroxy acids or retinoids [76]. BMT diols seem to have a good safety profile and might be interesting agents for further exploration as pigmentation enhancers.
2. Inhibition of pigmentation
The management of skin hyperpigmentation is still a challenging matter for dermatologists, as they are confronted with numerous different therapeutic options which often show unsatisfactory effects. As a further complication, relapses in hyperpigmentary disorders are common. Considering the numerous agents that have been suggested to have a hypopigmenting effect (and many of the available data are for in vitro results only), this review can only be selective in its coverage. For a more extensive description of hypopigmenting agents and their modes of action, readers are referred to recent reviews on this topic [90,91].
3. Biological effectors
One mechanism to induce hypopigmentation in the skin is to inhibit crucial factors such as tyrosinase and other melanogenic enzymes, for example by targeting MITF [92], a key regulator of melanocyte function. In this context, a number of biological compounds, including transforming growth factor (TGF-β1) [93], tumor necrosis factor (TNFα) interleukins 1 and 6 (IL-1, IL6) [94], dickkopf 1 (DKK1) [4], calpain inhibitors [95], lysophosphatidic acid [96] and C2 ceramides, are able to inhibit the function of tyrosinase and related enzymes (TYRP1 and DCT), mainly through down-regulation of MITF. Hypopigmenting properties of unsaturated fatty acids (e.g. linoleic acid) result from increased ubiquitination of tyrosinase that decreases its enzymatic function [97].
Another target is MC1R, as loss-of-function mutations in this receptor result in red hair and fair skin. As already mentioned, the physiological antagonist ASP blocks the binding of αMSH to MC1R and initiates a switch from eu- to pheo-melanogenesis. However, the specific mechanism regulating this switch has yet to be elucidated. The downstream target of MC1R, e.g. cAMP levels, can be decreased by androgens in combination with sex-hormone binding globulin, although drawbacks to this approach include a weak hypopigmenting effect and side effects of the agents.
Another possible way to inhibit skin pigmentation is by decreasing the transfer of melanosomes to keratinocytes. As mentioned above, the PAR2 receptor on keratinocytes plays an important role in regulating the uptake of melanosomes by keratinocytes. RWJ-50353, a serine protease inhibitor, leads to depigmentation in reconstructed skin and in dark-skinned Yucatan swine by affecting melanosome transfer and distribution [98]. In vitro, centaureidin [99], a flavone from Achillea millefolium (common yarrow), and methylophiopogonanone B [100] (MOPB), a homoisoflavonoid from Ophiopogon japonicus (mondo grass), block melanosome transfer by inducing the retraction of melanocyte dendrites through activation of GTPase Rho (the master regulator of dendrite formation in melanocytes) [101]). Niacinamide (the amide form of vitamin B3), inhibits melanosome transfer in vitro and has a significant effect in reducing hyperpigmentation in vivo, although its mode of action is not known [102]. Another in vitro study showed that plasma membrane lectins and their glycoconjugates interfere with melanocyte-keratinocyte interactions by binding to their specific plasma membrane receptors and inhibiting melanosome transfer [103].
Inducing melanocyte destruction is another approach to induce skin hypopigmentation. Tryptophan metabolites from malassezia yeast induce apoptosis in human melanocytes, which leads to depigmentation of the skin (pityriasis versicolor) [104]. Imiquimod, an immune response modifier that stimulates cytotoxic T-cell-mediated responses through activation of toll-like receptors, leads to tumor destruction and depigmentation in lentigo-maligna lesions [105,106].
Imatinib mesylate, a drug used to treat chronic myeloid leukemia, has been reported to induce vitiligo-like depigmentation of the skin and it was suggested that the impairment of pigmentation involved SCF and its receptor c-kit [107,108].
4. Chemical effectors
For many years, the complex mechanisms involved in the abnormal up-regulation of melanocyte function were not well understood. Therefore, therapeutic chemical hypopigmenting agents are restricted to mainly inhibitory or cytotoxic effects. Since good progress has been made in elucidating the paracrine and autocrine networks involved in pigmentary disorders, further development of new selective agonists and antagonists for specific targets is anticipated.
Many of the chemical agents used to induce hypopigmentation affect tyrosinase function. Phenolic compounds are widely used, such as hydroquinone (HQ), which is considered one of the most effective inhibitors of melanogenesis and is regarded as a reference standard when depigmenting agents are evaluated. HQ decreases tyrosinase activity by ~90% via the generation of quinones and reactive oxygen species (ROS) that induce damage in membrane lipids and proteins, by interacting with copper at the active site of the enzyme and by affecting RNA and DNA synthesis as well melanosome function [109]. In spite of its relatively high melanocyte-specific toxicity that improves hyperpigmentation in 14–70% of cases [110] and its general safety, there are common side effects of HQ such as skin irritation, contact dermatitis and (rarely) ochronosis, a blackish hyperpigmentation very resistant to treatment. The related phenol HQ monobenzyl ether (MBEH), which is used to eliminate residual spots of normal pigmentation in patients with generalized vitiligo, is metabolized to free radicals intracellularly which leads to selective melanocyte destruction and competitive tyrosinase inhibition, and results in an irreversible depigmentation even at sites that are distant from the application site.
Another common agent, arbutin, a natural β-glycoside of HQ isolated from the fruit of the California buckeye (Aesculus californica), as well as its more potent synthetic α-glycoside form, and synthetic deoxyarbutin are very efficient tyrosinase inhibitors. Compared to HQ, deoxyarbutin induces a more prolonged depigmentation and is not such an irritant.
Kojic acid, a fungal metabolic product from Aspergillus and Penicillum species, is commonly used in Asia as a whitening agent and a diet supplement, and it acts as a tyrosinase inhibitor by chelating copper at the active site of the enzyme [111]. Although it is effective as a hypopigmenting agent [112], especially in combination with other substances, it has a high sensitizing potential and can cause contact dermatitis [113]. Concerns have been raised about a possible carcinogenic effect since kojic acid was associated with hepatic tumors when fed to p53 deficient mice [114]. Aloesin, a natural derivative of aloe vera, acts as a competitive inhibitor of DOPA oxidation and a non-competitive inhibitor of tyrosine hydroxylase activity [115]. In a clinical trial, a combination of aloesin and arbutin inhibited UV-induced melanogenesis [116].
Azelaic acid, a dicarboxylic acid isolated from cultures of pityrosporum ovale, exerts antiproliferative and cytotoxic effects by inhibiting mitochondrial oxidoreductase and DNA synthesis [117] in highly active (or abnormal) melanocytes and acts as a weak inhibitor of tyrosinase activity. Its efficacy in treating hypermelanosis, which has been reported to be as good or even better than HQ [118], has been confirmed by several clinical studies [119].
Loss of melanin in the epidermis can also be obtained by stimulating the desquamation of stratum corneum cells. Retinoic acids are the most important agents in this context and act by disrupting pigment transfer, by inhibiting the dispersion of pigment granules in keratinocytes, by accelerating epidermal turnover and inducing desquamation [120]. Topical tretinoin has efficacy in treating hyperpigmentary disorders, although side effects such as erythema, peeling and PIH have been reported. To increase efficiency, various exfoliants (e.g. pretreatment with topical tretinoin followed by peels with trichloroacetic acid or α-hydroxy acids) can be combined. Another approach to increase efficacy is to combine different hypopigmenting agents that interfere with distinct steps of the melanogenic pathway (e.g. HQ and tretinoin).
Challenges for the future
As mentioned above, phenolic compounds are widely used as depigmenting agents, but it has been reported that instead of skin lightening, undesired counter-effects can occur. The flavone quercetin, originally described as a tyrosinase inhibitor [121], turned out to be a strong inducer of melanogenesis in vitro [122]. Although some hydroxylated derivatives of coumarins were reported to inhibit melanogenesis [123], several coumarins of seven umberiferae plants stimulated melanogenesis in murine melanoma cells [124]. The number of putative hypo- or hyper-pigmenting agents is vast, but promising effects detected in vitro are often not confirmed in clinical tests. Although topical application of compounds is most preferable, there are limitations to transdermal delivery as compounds applied to the surface of the skin are 100–1000 times diluted by the time they reach the lower layers of the epidermis [125]. Systemically applied substances carry the risk of greater side effects on other tissues. For pigmentation enhancing drugs, only MSH analogs have been tested extensively on humans, but there are concerns about their safety as injections with these synthetic compounds have been associated with nausea, facial flushing, fatigue and spontaneous erections [78,126].
Some critics have voiced their doubts whether enhancing pigmentation will significantly increase protection from skin cancers, as tanned skin has a sun protection factor (SPF) of only 2–4 and other physiological features might play a more important role in photoprotection. In addition, despite the fact that endogenous pigmentation is associated with a reduced cancer risk, it has been difficult to prove that facultative pigmentation has the same effect. Finally, there are some major concerns about the safety of artificially triggering the tanning response via the MC1R pathway since the stimulation of melanogenic activity also increases proliferation and might have undesired carcinogenic effects.
Conclusions
Facing the continuous rise in rates of skin cancer among the fair-skinned population and given the fact that in terms of skin cancer, highly pigmented skin is up to 70-fold more protected against the deleterious effects of UVR than is fair skin, artificially increasing pigmentation in the skin is more than ever a spotlight topic.
Consumer-driven demand for depigmenting drugs has risen in the past years due to the aging population’s interest in treating lesions resulting from photo-aging and due to demographic changes that have led to increases in non-Caucasian populations that are generally more prone to pigmentary disorders.
A better understanding of the complex interactions between regulators of the melanogenic pathway and the development of novel approaches to modulate pigmentation followed by strictly controlled clinical trials to assess safety and efficacy of these drugs would be desirable.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research,
Nonstandard Abbreviations Used
- ACTH
adrenocorticotropic hormone
- cAMP
cyclic adenosine monophosphate
- ASP
agouti signal protein
- bFGF
basic fibroblast growth factor
- DCT
DOPAchrome tautomerase
- DHI
5,6-dihydroxyindole
- DHICA
DHI-2-carboxylic acid
- DKK1
dickkopf 1
- DOPA
β-3,4-dihydroxyphenylalanine
- ET
endothelin
- HGF
hepatocyte growth factor
- HQ
hydroquinone
- IL
interleukin
- LS
lentigines solares
- MC1R
melanocortin 1 receptor
- MITF
microphthalmia-associated transcription factor
- αMSH
α-melanocyte stimulating hormone
- NGF
nerve growth factor
- OA1
ocular albinism type 1
- OCA
oculocutaneous albinism
- PG
prostaglandin
- PIH
postinflammatory hyperpigmentation
- PKA
protein kinase A
- POMC
proopiomelanocortin
- ROS
reactive oxygen species
- SCF
stem cell factor
- SPF
sun protection factor
- TYR
tyrosinase
- TYRP1
tyrosinase-related protein 1
- UVR
ultraviolet radiation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garland CF, et al. Epidemiologic evidence for different roles of ultraviolet A and B radiation in melanoma mortality rates. Ann Epidemiol. 2003;13(6):395–404. doi: 10.1016/s1047-2797(02)00461-1. [DOI] [PubMed] [Google Scholar]
- 2.Wolf P, et al. Effect of sunscreens on UV radiation-induced enhancement of melanoma growth in mice. J Natl Cancer Inst. 1994;86(2):99–105. doi: 10.1093/jnci/86.2.99. [DOI] [PubMed] [Google Scholar]
- 3.Tadokoro T, et al. UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin. Faseb J. 2003;17(9):1177–1179. doi: 10.1096/fj.02-0865fje. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi Y, et al. Mesenchymal-epithelial interactions in the skin: increased expression of dickkopf1 by palmoplantar fibroblasts inhibits melanocyte growth and differentiation. J Cell Biol. 2004;165(2):275–285. doi: 10.1083/jcb.200311122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzpatrick TB, Breathnach AS. the Epidermal Melanin Unit System. Dermatol Wochenschr. 1963;147:481–489. [PubMed] [Google Scholar]
- 6.Montagna W, Carlisle K. The architecture of black and white facial skin. J Am Acad Dermatol. 1991;24(6 Pt 1):929–937. doi: 10.1016/0190-9622(91)70148-u. [DOI] [PubMed] [Google Scholar]
- 7.Bennett DC, Lamoreux ML. The color loci of mice--a genetic century. Pigment Cell Res. 2003;16(4):333–344. doi: 10.1034/j.1600-0749.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 8.Chi A, et al. Proteomic and bioinformatic characterization of the biogenesis and function of melanosomes. J Proteome Res. 2006;5(11):3135–3144. doi: 10.1021/pr060363j. [DOI] [PubMed] [Google Scholar]
- 9.Hu ZZ, et al. Comparative Bioinformatics Analyses and Profiling of Lysosome-Related Organelle Proteomes. Int J Mass Spectrom. 2007;259(1–3):147–160. doi: 10.1016/j.ijms.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakamatsu K, et al. Diversity of pigmentation in cultured human melanocytes is due to differences in the type as well as quantity of melanin. Pigment Cell Res. 2006;19(2):154–162. doi: 10.1111/j.1600-0749.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- 11.Hennessy A, et al. Eumelanin and pheomelanin concentrations in human epidermis before and after UVB irradiation. Pigment Cell Res. 2005;18(3):220–223. doi: 10.1111/j.1600-0749.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, et al. Comparison of structural and chemical properties of black and red human hair melanosomes. Photochem Photobiol. 2005;81(1):135–144. doi: 10.1562/2004-08-03-RA-259.1. [DOI] [PubMed] [Google Scholar]
- 13.Ito S, Wakamatsu K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: a comparative review. Pigment Cell Res. 2003;16(5):523–531. doi: 10.1034/j.1600-0749.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 14.Toyofuku K, et al. Oculocutaneous albinism types 1 and 3 are ER retention diseases: mutation of tyrosinase or Tyrp1 can affect the processing of both mutant and wild-type proteins. Faseb J. 2001;15(12):2149–2161. doi: 10.1096/fj.01-0216com. [DOI] [PubMed] [Google Scholar]
- 15.Urabe K, et al. The inherent cytotoxicity of melanin precursors: a revision. Biochim Biophys Acta. 1994;1221(3):272–278. doi: 10.1016/0167-4889(94)90250-x. [DOI] [PubMed] [Google Scholar]
- 16.Nordlund JJ, Boissy RE, Hearing VJ, Oetting WS, King RA, Ortonne JP. The pigmentary system: Physiology and Pathophysiology. Blackwell Science; 2006. [Google Scholar]
- 17.Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445(7130):843–850. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- 18.Rees JL. The melanocortin 1 receptor (MC1R): more than just red hair. Pigment Cell Res. 2000;13(3):135–140. doi: 10.1034/j.1600-0749.2000.130303.x. [DOI] [PubMed] [Google Scholar]
- 19.Sturm RA, et al. Genetic association and cellular function of MC1R variant alleles in human pigmentation. Ann N Y Acad Sci. 2003;994:348–358. doi: 10.1111/j.1749-6632.2003.tb03199.x. [DOI] [PubMed] [Google Scholar]
- 20.Box NF, et al. Characterization of melanocyte stimulating hormone receptor variant alleles in twins with red hair. Hum Mol Genet. 1997;6(11):1891–1897. doi: 10.1093/hmg/6.11.1891. [DOI] [PubMed] [Google Scholar]
- 21.Palmer JS, et al. Melanocortin-1 receptor polymorphisms and risk of melanoma: is the association explained solely by pigmentation phenotype? Am J Hum Genet. 2000;66(1):176–186. doi: 10.1086/302711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaguchi Y, et al. The effects of dickkopf 1 on gene expression and Wnt signaling by melanocytes: mechanisms underlying its suppression of melanocyte function and proliferation. J Invest Dermatol. 2007;127(5):1217–1225. doi: 10.1038/sj.jid.5700629. [DOI] [PubMed] [Google Scholar]
- 23.Shriver MD, et al. Skin pigmentation, biogeographical ancestry and admixture mapping. Hum Genet. 2003;112(4):387–399. doi: 10.1007/s00439-002-0896-y. [DOI] [PubMed] [Google Scholar]
- 24.Graf J, et al. Single nucleotide polymorphisms in the MATP gene are associated with normal human pigmentation variation. Hum Mutat. 2005;25(3):278–284. doi: 10.1002/humu.20143. [DOI] [PubMed] [Google Scholar]
- 25.Lamason RL, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310(5755):1782–1786. doi: 10.1126/science.1116238. [DOI] [PubMed] [Google Scholar]
- 26.King RA, Hearing VJ, Creel DJ, Oetting WS. Albinism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. McGraw-Hill; 2001. [Google Scholar]
- 27.Incerti B, et al. Oa1 knock-out: new insights on the pathogenesis of ocular albinism type 1. Hum Mol Genet. 2000;9(19):2781–2788. doi: 10.1093/hmg/9.19.2781. [DOI] [PubMed] [Google Scholar]
- 28.Eller MS, Gilchrest BA. Tanning as part of the eukaryotic SOS response. Pigment Cell Res. 2000;13 Suppl 8:94–97. doi: 10.1034/j.1600-0749.13.s8.17.x. [DOI] [PubMed] [Google Scholar]
- 29.Rouzaud F, et al. Regulation of constitutive and UVR-induced skin pigmentation by melanocortin 1 receptor isoforms. Faseb J. 2006;20(11):1927–1929. doi: 10.1096/fj.06-5922fje. [DOI] [PubMed] [Google Scholar]
- 30.Busca R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000;13(2):60–69. doi: 10.1034/j.1600-0749.2000.130203.x. [DOI] [PubMed] [Google Scholar]
- 31.Tachibana M. MITF: a stream flowing for pigment cells. Pigment Cell Res. 2000;13(4):230–240. doi: 10.1034/j.1600-0749.2000.130404.x. [DOI] [PubMed] [Google Scholar]
- 32.Scott G, et al. Protease-activated receptor 2, a receptor involved in melanosome transfer, is upregulated in human skin by ultraviolet irradiation. J Invest Dermatol. 2001;117(6):1412–1420. doi: 10.1046/j.0022-202x.2001.01575.x. [DOI] [PubMed] [Google Scholar]
- 33.Tada A, et al. Endothelin-1 is a paracrine growth factor that modulates melanogenesis of human melanocytes and participates in their responses to ultraviolet radiation. Cell Growth Differ. 1998;9(7):575–584. [PubMed] [Google Scholar]
- 34.Yaar M, et al. Evidence for nerve growth factor-mediated paracrine effects in human epidermis. J Cell Biol. 1991;115(3):821–828. doi: 10.1083/jcb.115.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui R, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128(5):853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 36.Imokawa G. Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders. Pigment Cell Res. 2004;17(2):96–110. doi: 10.1111/j.1600-0749.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- 37.Abdel-Malek ZA, et al. Hormonal effects of vitamin D3 on epidermal melanocytes. J Cell Physiol. 1988;136(2):273–280. doi: 10.1002/jcp.1041360209. [DOI] [PubMed] [Google Scholar]
- 38.McLeod SD, et al. Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol. 1994;49(1):9–14. doi: 10.1016/0960-0760(94)90295-x. [DOI] [PubMed] [Google Scholar]
- 39.Ranson M, et al. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91(6):593–598. doi: 10.1111/1523-1747.ep12477126. [DOI] [PubMed] [Google Scholar]
- 40.Tadokoro T, et al. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J Invest Dermatol. 2005;124(6):1326–1332. doi: 10.1111/j.0022-202X.2005.23760.x. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi Y, et al. Human skin responses to UV radiation: pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. Faseb J. 2006;20(9):1486–1488. doi: 10.1096/fj.06-5725fje. [DOI] [PubMed] [Google Scholar]
- 42.Ortonne JP, et al. Treatment of solar lentigines. J Am Acad Dermatol. 2006;54(5 Suppl 2):S262–S271. doi: 10.1016/j.jaad.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 43.Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI. Fitzpatrick's dermatology in general medicine. McGraw-Hill; 1999. [Google Scholar]
- 44.Ortonne JP. Pigmentary changes of the ageing skin. Br J Dermatol. 1990;122 Suppl 35:21–28. doi: 10.1111/j.1365-2133.1990.tb16121.x. [DOI] [PubMed] [Google Scholar]
- 45.Kadono S, et al. The role of the epidermal endothelin cascade in the hyperpigmentation mechanism of lentigo senilis. J Invest Dermatol. 2001;116(4):571–577. doi: 10.1046/j.1523-1747.2001.01296.x. [DOI] [PubMed] [Google Scholar]
- 46.Hattori H, et al. The epidermal stem cell factor is over-expressed in lentigo senilis: implication for the mechanism of hyperpigmentation. J Invest Dermatol. 2004;122(5):1256–1265. doi: 10.1111/j.0022-202X.2004.22503.x. [DOI] [PubMed] [Google Scholar]
- 47.Imokawa G, et al. Intracellular signaling mechanisms leading to synergistic effects of endothelin-1 and stem cell factor on proliferation of cultured human melanocytes. Cross-talk via trans-activation of the tyrosine kinase c-kit receptor. J Biol Chem. 2000;275(43):33321–33328. doi: 10.1074/jbc.M004346200. [DOI] [PubMed] [Google Scholar]
- 48.Simons JJ, Morales A. Minocycline and generalized cutaneous pigmentation. J Am Acad Dermatol. 1980;3(3):244–247. doi: 10.1016/s0190-9622(80)80186-1. [DOI] [PubMed] [Google Scholar]
- 49.Eller MS, et al. DNA damage and melanogenesis. Nature. 1994;372(6505):413–414. doi: 10.1038/372413a0. [DOI] [PubMed] [Google Scholar]
- 50.Lio PA, Sober AJ. Drug-induced or -related pigmentation. In: Nordlund JJ, Boissy RE, Hearing VJ, Oetting WS, King RA, Ortonne JP, editors. The pigmentary system: Physiology and Pathophysiology. Blackwell Science; 2006. [Google Scholar]
- 51.Granstein RD, Sober AJ. Drug- and heavy metal--induced hyperpigmentation. J Am Acad Dermatol. 1981;5(1):1–18. doi: 10.1016/s0190-9622(81)70072-0. [DOI] [PubMed] [Google Scholar]
- 52.Namazi MR. Phenytoin as a novel anti-vitiligo weapon. J Autoimmune Dis. 2005;2:11. doi: 10.1186/1740-2557-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hashimoto K, et al. Imipramine hyperpigmentation: a slate-gray discoloration caused by long-term imipramine administration. J Am Acad Dermatol. 1991;25(2 Pt 2):357–361. doi: 10.1016/0190-9622(91)70204-f. [DOI] [PubMed] [Google Scholar]
- 54.Steele TE, Ashby J. Desipramine-related slate-gray skin pigmentation. J Clin Psychopharmacol. 1993;13(1):76–77. doi: 10.1097/00004714-199302000-00013. [DOI] [PubMed] [Google Scholar]
- 55.Resnik SS. Melasma and other skin manifestations or oral contraceptives. Trans N Engl Obstet Gynecol Soc. 1967;21:101–107. [PubMed] [Google Scholar]
- 56.Barankin B, et al. The skin in pregnancy. J Cutan Med Surg. 2002;6(3):236–240. doi: 10.1177/120347540200600308. [DOI] [PubMed] [Google Scholar]
- 57.Grimes PE, et al. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27(2):96–101. doi: 10.1097/01.dad.0000154419.18653.2e. [DOI] [PubMed] [Google Scholar]
- 58.Smith AG, et al. Chloasma, oral contraceptives, and plasma immunoreactive beta-melanocyte-stimulating hormone. J Invest Dermatol. 1977;68(4):169–170. doi: 10.1111/1523-1747.ep12492633. [DOI] [PubMed] [Google Scholar]
- 59.Lacz NL, et al. Postinflammatory hyperpigmentation: a common but troubling condition. Int J Dermatol. 2004;43(5):362–365. doi: 10.1111/j.1365-4632.2004.02267.x. [DOI] [PubMed] [Google Scholar]
- 60.Abdel-Malek ZA, et al. In vitro modulation of proliferation and melanization of S91 melanoma cells by prostaglandins. Cancer Res. 1987;47(12):3141–3146. [PubMed] [Google Scholar]
- 61.Medrano EE, et al. Chronic growth stimulation of human adult melanocytes by inflammatory mediators in vitro: implications for nevus formation and initial steps in melanocyte oncogenesis. Proc Natl Acad Sci U S A. 1993;90(5):1790–1794. doi: 10.1073/pnas.90.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshida M, et al. Histamine induces melanogenesis and morphologic changes by protein kinase A activation via H2 receptors in human normal melanocytes. J Invest Dermatol. 2000;114(2):334–342. doi: 10.1046/j.1523-1747.2000.00874.x. [DOI] [PubMed] [Google Scholar]
- 63.Gilchrest BA, et al. Effects of aging and chronic sun exposure on melanocytes in human skin. J Invest Dermatol. 1979;73(2):141–143. doi: 10.1111/1523-1747.ep12581580. [DOI] [PubMed] [Google Scholar]
- 64.Bleehen SS, et al. Depigmentation of skin with 4-isopropylcatechol,mercaptoamines, and other compounds. J Invest Dermatol. 1968;50(2):103–117. doi: 10.1038/jid.1968.13. [DOI] [PubMed] [Google Scholar]
- 65.Gellin GA, et al. Detection of environmental depigmenting substances. Contact Dermatitis. 1979;5(4):201–213. doi: 10.1111/j.1600-0536.1979.tb04853.x. [DOI] [PubMed] [Google Scholar]
- 66.O'Malley MA, et al. Occupational vitiligo due to unsuspected presence of phenolic antioxidant byproducts in commercial bulk rubber. J Occup Med. 1988;30(6):512–516. [PubMed] [Google Scholar]
- 67.Miyamoto L, Taylor JS. Chemical leukoderma. In: Hann SK, Nordlund JJ, editors. Vitiligo: A Comprehensive Monograph on Basic and Clinical Science. Blackwell Science; 2000. pp. 269–280. [Google Scholar]
- 68.Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17(3):208–214. doi: 10.1111/j.1600-0749.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- 69.Lacour J-P. Infectious hypomelanoses. In: Nordlund JJ, Boissy RE, Hearing VJ, Oetting WS, King RA, Ortonne JP, editors. The pigmentary system: Physiology and Pathophysiology. Blackwell Science; 2006. pp. 686–698. [Google Scholar]
- 70.Prabhakaran K. Unusual effects of reducing agents on 0-diphenoloxidase of Mycobacterium leprae. J Bacteriol. 1971;107(3):787–789. doi: 10.1128/jb.107.3.787-789.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cui J, et al. Identification of pigment cell antigens defined by vitiligo antibodies. J Invest Dermatol. 1992;98(2):162–165. doi: 10.1111/1523-1747.ep12555773. [DOI] [PubMed] [Google Scholar]
- 72.Steitz J, et al. Initiation and regulation of CD8+T cells recognizing melanocytic antigens in the epidermis: implications for the pathophysiology of vitiligo. Eur J Cell Biol. 2004;83(11–12):797–803. doi: 10.1078/0171-9335-00423. [DOI] [PubMed] [Google Scholar]
- 73.Mosher DB, et al. Monobenzylether of hydroquinone. A retrospective study of treatment of 18 vitiligo patients and a review of the literature. Br J Dermatol. 1977;97(6):669–679. doi: 10.1111/j.1365-2133.1977.tb14275.x. [DOI] [PubMed] [Google Scholar]
- 74.Fain PR, et al. A genomewide screen for generalized vitiligo: confirmation of AIS1 on chromosome 1p31 and evidence for additional susceptibility loci. Am J Hum Genet. 2003;72(6):1560–1564. doi: 10.1086/375451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kollias N, et al. Photoprotection by melanin. J Photochem Photobiol B. 1991;9(2):135–160. doi: 10.1016/1011-1344(91)80147-a. [DOI] [PubMed] [Google Scholar]
- 76.Brown DA. Skin pigmentation enhancers. In: Giacomoni PU, editor. Sun Protection in Man. Elsevier; 2001. pp. 637–675. [Google Scholar]
- 77.Lerner AB, McGuire JS. Effect of alpha- and betamelanocyte stimulating hormones on the skin colour of man. Nature. 1961;189:176–179. doi: 10.1038/189176a0. [DOI] [PubMed] [Google Scholar]
- 78.Dorr RT, et al. Increased eumelanin expression and tanning is induced by a superpotent melanotropin [Nle4-D-Phe7]-alpha-MSH in humans. Photochem Photobiol. 2000;72(4):526–532. doi: 10.1562/0031-8655(2000)072<0526:ieeati>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 79.Dorr RT, et al. Effects of a superpotent melanotropic peptide in combination with solar UV radiation on tanning of the skin in human volunteers. Arch Dermatol. 2004;140(7):827–835. doi: 10.1001/archderm.140.7.827. [DOI] [PubMed] [Google Scholar]
- 80.Levine N, et al. Induction of skin tanning by subcutaneous administration of a potent synthetic melanotropin. Jama. 1991;266(19):2730–2736. [PubMed] [Google Scholar]
- 81.Ugwu SO, et al. Skin pigmentation and pharmacokinetics of melanotan-I in humans. Biopharm Drug Dispos. 1997;18(3):259–269. doi: 10.1002/(sici)1099-081x(199704)18:3<259::aid-bdd20>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 82.Barnetson RS, et al. [Nle4-D-Phe7]-alpha-melanocyte-stimulating hormone significantly increased pigmentation and decreased UV damage in fair-skinned Caucasian volunteers. J Invest Dermatol. 2006;126(8):1869–1878. doi: 10.1038/sj.jid.5700317. [DOI] [PubMed] [Google Scholar]
- 83.Abdel-Malek ZA, et al. Melanoma prevention strategy based on using tetrapeptide alpha-MSH analogs that protect human melanocytes from UV-induced DNA damage and cytotoxicity. Faseb J. 2006;20(9):1561–1563. doi: 10.1096/fj.05-5655fje. [DOI] [PubMed] [Google Scholar]
- 84.D'Orazio JA, et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443(7109):340–344. doi: 10.1038/nature05098. [DOI] [PubMed] [Google Scholar]
- 85.Seamon KB, Daly JW. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- 86.Gilchrest BA, Eller MS. DNA photodamage stimulates melanogenesis and other photoprotective responses. J Investig Dermatol Symp Proc. 1999;4(1):35–40. doi: 10.1038/sj.jidsp.5640178. [DOI] [PubMed] [Google Scholar]
- 87.Karlseder J, et al. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science. 1999;283(5406):1321–1325. doi: 10.1126/science.283.5406.1321. [DOI] [PubMed] [Google Scholar]
- 88.Arad S, et al. T-oligos augment UV-induced protective responses in human skin. Faseb J. 2006;20(11):1895–1897. doi: 10.1096/fj.06-5964fje. [DOI] [PubMed] [Google Scholar]
- 89.Brown DA, et al. Aliphatic and alicyclic diols induce melanogenesis in cultured cells and guinea pig skin. J Invest Dermatol. 1998;110(4):428–437. doi: 10.1046/j.1523-1747.1998.00160.x. [DOI] [PubMed] [Google Scholar]
- 90.Ando H, et al. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J Invest Dermatol. 2007;127(4):751–761. doi: 10.1038/sj.jid.5700683. [DOI] [PubMed] [Google Scholar]
- 91.Solano F, et al. Hypopigmenting agents: an updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006;19(6):550–571. doi: 10.1111/j.1600-0749.2006.00334.x. [DOI] [PubMed] [Google Scholar]
- 92.Steingrimsson E, et al. Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet. 2004;38:365–411. doi: 10.1146/annurev.genet.38.072902.092717. [DOI] [PubMed] [Google Scholar]
- 93.Martinez-Esparza M, et al. Independent regulation of tyrosinase by the hypopigmenting cytokines TGF beta1 and TNF alpha and the melanogenic hormone alpha-MSH in B16 mouse melanocytes. Cell Mol Biol (Noisy-le-grand) 1999;45(7):991–1000. [PubMed] [Google Scholar]
- 94.Swope VB, et al. Interleukins 1 alpha and 6 and tumor necrosis factor-alpha are paracrine inhibitors of human melanocyte proliferation and melanogenesis. J Invest Dermatol. 1991;96(2):180–185. doi: 10.1111/1523-1747.ep12460991. [DOI] [PubMed] [Google Scholar]
- 95.Ohguchi K, et al. Involvement of calpain in melanogenesis of mouse B16 melanoma cells. Mol Cell Biochem. 2005;275(1–2):103–107. doi: 10.1007/s11010-005-1081-0. [DOI] [PubMed] [Google Scholar]
- 96.Kim DS, et al. Effects of lysophosphatidic acid on melanogenesis. Chem Phys Lipids. 2004;127(2):199–206. doi: 10.1016/j.chemphyslip.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 97.Ando H, et al. Intracellular composition of fatty acid affects the processing and function of tyrosinase through the ubiquitin-proteasome pathway. Biochem J. 2006;394(Pt 1):43–50. doi: 10.1042/BJ20051419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seiberg M, et al. Inhibition of melanosome transfer results in skin lightening. J Invest Dermatol. 2000;115(2):162–167. doi: 10.1046/j.1523-1747.2000.00035.x. [DOI] [PubMed] [Google Scholar]
- 99.Ito Y, et al. Centaureidin promotes dendrite retraction of melanocytes by activating Rho. Biochim Biophys Acta. 2006;1760(3):487–494. doi: 10.1016/j.bbagen.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 100.Ito Y, et al. Effects of methylophiopogonanone B on melanosome transfer and dendrite retraction. J Dermatol Sci. 2006;42(1):68–70. doi: 10.1016/j.jdermsci.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 101.Scott G. Rac and rho: the story behind melanocyte dendrite formation. Pigment Cell Res. 2002;15(5):322–330. doi: 10.1034/j.1600-0749.2002.02056.x. [DOI] [PubMed] [Google Scholar]
- 102.Hakozaki T, et al. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br J Dermatol. 2002;147(1):20–31. doi: 10.1046/j.1365-2133.2002.04834.x. [DOI] [PubMed] [Google Scholar]
- 103.Minwalla L, et al. Inhibition of melanosome transfer from melanocytes to keratinocytes by lectins and neoglycoproteins in an in vitro model system. Pigment Cell Res. 2001;14(3):185–194. doi: 10.1034/j.1600-0749.2001.140308.x. [DOI] [PubMed] [Google Scholar]
- 104.Kramer HJ, et al. Malassezin, a novel agonist of the aryl hydrocarbon receptor from the yeast Malassezia furfur, induces apoptosis in primary human melanocytes. Chembiochem. 2005;6(5):860–865. doi: 10.1002/cbic.200400247. [DOI] [PubMed] [Google Scholar]
- 105.Fleming CJ, et al. A pilot study of treatment of lentigo maligna with 5% imiquimod cream. Br J Dermatol. 2004;151(2):485–488. doi: 10.1111/j.1365-2133.2004.05983.x. [DOI] [PubMed] [Google Scholar]
- 106.Wolf IH, et al. Treatment of lentigo maligna (melanoma in situ) with the immune response modifier imiquimod. Arch Dermatol. 2005;141(4):510–514. doi: 10.1001/archderm.141.4.510. [DOI] [PubMed] [Google Scholar]
- 107.Legros L, et al. Imatinib mesilate (Glivec): a systemic depigmenting agent for extensive vitiligo? Br J Dermatol. 2005;153(3):691–692. doi: 10.1111/j.1365-2133.2005.06813.x. [DOI] [PubMed] [Google Scholar]
- 108.Tsao AS, et al. Imatinib mesylate causes hypopigmentation in the skin. Cancer. 2003;98(11):2483–2487. doi: 10.1002/cncr.11812. [DOI] [PubMed] [Google Scholar]
- 109.Briganti S, et al. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003;16(2):101–110. doi: 10.1034/j.1600-0749.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 110.Ortonne JP, Passeron T. Melanin pigmentary disorders: treatment update. Dermatol Clin. 2005;23(2):209–226. doi: 10.1016/j.det.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 111.Battaini G, et al. Inhibition of the catecholase activity of biomimetic dinuclear copper complexes by kojic acid. J Biol Inorg Chem. 2000;5(2):262–268. doi: 10.1007/s007750050370. [DOI] [PubMed] [Google Scholar]
- 112.Lim JT. Treatment of melasma using kojic acid in a gel containing hydroquinone and glycolic acid. Dermatol Surg. 1999;25(4):282–284. doi: 10.1046/j.1524-4725.1999.08236.x. [DOI] [PubMed] [Google Scholar]
- 113.Nakagawa M, et al. Contact allergy to kojic acid in skin care products. Contact Dermatitis. 1995;32(1):9–13. doi: 10.1111/j.1600-0536.1995.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 114.Takizawa T, et al. Hepatocellular tumor induction in heterozygous p53-deficient CBA mice by a 26-week dietary administration of kojic acid. Toxicol Sci. 2003;73(2):287–293. doi: 10.1093/toxsci/kfg094. [DOI] [PubMed] [Google Scholar]
- 115.Jones K, et al. Modulation of melanogenesis by aloesin: a competitive inhibitor of tyrosinase. Pigment Cell Res. 2002;15(5):335–340. doi: 10.1034/j.1600-0749.2002.02014.x. [DOI] [PubMed] [Google Scholar]
- 116.Choi S, et al. Aloesin inhibits hyperpigmentation induced by UV radiation. Clin Exp Dermatol. 2002;27(6):513–515. doi: 10.1046/j.1365-2230.2002.01120.x. [DOI] [PubMed] [Google Scholar]
- 117.Fitton A, Goa KL. Azelaic acid. A review of its pharmacological properties and therapeutic efficacy in acne and hyperpigmentary skin disorders. Drugs. 1991;41(5):780–798. doi: 10.2165/00003495-199141050-00007. [DOI] [PubMed] [Google Scholar]
- 118.Balina LM, Graupe K. The treatment of melasma. 20% azelaic acid versus 4% hydroquinone cream. Int J Dermatol. 1991;30(12):893–895. doi: 10.1111/j.1365-4362.1991.tb04362.x. [DOI] [PubMed] [Google Scholar]
- 119.Sarkar R, et al. A comparative study of 20% azelaic acid cream monotherapy versus a sequential therapy in the treatment of melasma in dark-skinned patients. Dermatology. 2002;205(3):249–254. doi: 10.1159/000065851. [DOI] [PubMed] [Google Scholar]
- 120.Nair X, et al. Combination of 4-hydroxyanisole and all-trans retinoic acid produces synergistic skin depigmentation in swine. J Invest Dermatol. 1993;101(2):145–149. doi: 10.1111/1523-1747.ep12363627. [DOI] [PubMed] [Google Scholar]
- 121.Kubo I, Kinst-Hori I. Flavonols from saffron flower: tyrosinase inhibitory activity and inhibition mechanism. J Agric Food Chem. 1999;47(10):4121–4125. doi: 10.1021/jf990201q. [DOI] [PubMed] [Google Scholar]
- 122.Nagata H, et al. Quercetin enhances melanogenesis by increasing the activity and synthesis of tyrosinase in human melanoma cells and in normal human melanocytes. Pigment Cell Res. 2004;17(1):66–73. doi: 10.1046/j.1600-0749.2003.00113.x. [DOI] [PubMed] [Google Scholar]
- 123.Yamamura T, et al. Antimelanogenic activity of hydrocoumarins in cultured normal human melanocytes by stimulating intracellular glutathione synthesis. Arch Dermatol Res. 2002;294(8):349–354. doi: 10.1007/s00403-002-0345-8. [DOI] [PubMed] [Google Scholar]
- 124.Matsuda H, et al. Melanogenesis stimulation in murine b16 melanoma cells by umberiferae plant extracts and their coumarin constituents. Biol Pharm Bull. 2005;28(7):1229–1233. doi: 10.1248/bpb.28.1229. [DOI] [PubMed] [Google Scholar]
- 125.Schalla W, Schaefer H. Localization of compounds in different skin layers and its use as an indicator of percutaneous absorption. In: Bronaugh RL, Maibach HI, editors. Percutaneous Absorption. Marcel Dekker; 1985. pp. 281–303. [Google Scholar]
- 126.Dorr RT, et al. Evaluation of melanotan-II, a superpotent cyclic melanotropic peptide in a pilot phase-I clinical study. Life Sci. 1996;58(20):1777–1784. doi: 10.1016/0024-3205(96)00160-9. [DOI] [PubMed] [Google Scholar]
- 127.Yamaguchi Y, Brenner M, Hearing VJ. The regulation of skin pigmentation. Biol Chem. 2007;282(38):27557–27561. doi: 10.1074/jbc.R700026200. [DOI] [PubMed] [Google Scholar]