STRUCTURED ABSTRACT
Objective
We tested a circumferential mechanical dyssynchrony index (circumferential uniformity ratio estimate, or CURE; 0–1, 1=synchrony) derived from magnetic resonance myocardial tagging (MR-MT) for predicting clinical function class improvement following cardiac resynchronization therapy (CRT).
Background
There remains a significant nonresponse rate to CRT, with recent data questioning the reproducibility of standard echocardiography-based dyssynchrony metrics. MR-MT provides high quality mechanical activation data throughout the heart, and delayed enhancement magnetic resonance imaging (DEMRI) offers precise characterization of myocardial scar and scar distribution.
Methods
MR-MT was performed in patients with cardiomyopathy, divided into: 1) a CRT-HF cohort (n=20) with mean (SD) LVEF 0.23 (0.057) in order to evaluate the clinical use of MR-MT and DEMRI prior to CRT; and 2) a multimodality cohort (n=27) with mean (SD) LVEF 0.20 (0.066) in order to compare MR-MT and tissue Doppler imaging (TDI) assessments of mechanical dyssynchrony. MR-MT was also performed in 9 healthy control subjects.
Results
MR-MT showed that control subjects had highly synchronous contraction (mean [SD] CURE 0.96 [0.01]) while TDI septal-lateral delay indicated dyssynchrony in 44% of normal controls. Using a cutoff of <0.75 for CURE based on ROC analysis (AUC 0.889), 56% of patients tested positive for mechanical dyssynchrony, and the MR-MT CURE predicted improved function class in CRT-HF patients with 90% accuracy (PPV 87%; NPV 100%). Adding DEMRI (% total scar<15%) data improved accuracy further to 95% (PPV 93%; NPV 100%). The correlation between CURE and QRSd was modest in all cardiomyopathy subjects (r=0.58, p<0.001), and somewhat less in the CRT-HF group (r=0.40, p=0.08). The multimodality cohort showed a 30% discordance rate between CURE and TDI septal-lateral delay.
Conclusions
MR-MT assessment of circumferential mechanical dyssynchrony predicts improvement in function class after CRT. The addition of scar imaging by DEMRI further improves this predictive value.
Keywords: magnetic resonance imaging, cardiac resynchronization therapy, biventricular, dyssynchrony, heart failure
INTRODUCTION
Cardiac resynchronization therapy (CRT) has been shown to improve heart failure symptoms and survival (1,2), yet 30% or more patients do not derive a clinical benefit from the therapy (3). To date, the standard criteria for receiving treatment include the presence of a QRS duration of >120 ms (slightly longer in some studies). Yet direct pre-CRT assessments of mechanical dyssynchrony using tissue Doppler imaging (TDI)/echocardiography (4) are common despite many limitations, including the poor intraobserver variability shown in the PROSPECT trial (3), dependence on longitudinal and radial motion rather than circumferential strain, limited acoustic windows, and ambiguities in the interpretation of these studies.
Magnetic resonance imaging with myocardial tissue tagging (MR-MT) provides quantitative and highly reproducible circumferential and longitudinal myocardial activation data along all three dimensions of the heart that are largely operator- and patient-independent (5), as well as characterization of myocardial scar and scar distribution (6). Myocardial fiber orientation is principally circumferential (7), and the circumferential strain data provide by MR-MT appears to have a much greater dynamic range for assessing mechanical dyssynchrony than longitudinal or radial strain (8). We have previously evaluated MR-MT for assessment of acute hemodynamic response to left ventricular pacing (9), but the role of MR-MT for clinical response after CRT implantation has not yet been defined. Accordingly, this study examined an MR-MT-based metric of global circumferential mechanical dyssynchrony in combination with MR scar imaging in patients with heart failure to test its predictive value for function class improvement in those receiving CRT, clarify its relation to TDI metrics, and determine whether scar imaging provides additional value for identifying responsive patients.
METHODS
Clinical Study Groups
The study protocol was approved by the Johns Hopkins Hospital Institutional Review Board, and all patients gave written informed consent. We studied 43 subjects with cardiomyopathy (divided into CRT-HF and multimodality cohorts) and 9 control subjects (total N=52). The CRT-HF cohort (n=20) included subjects referred for CRT, all NYHA function class III, in whom MR-MT was obtained prior to implantation. These patients all received an implantable cardioverter defibrillator (ICD) and CRT between August 2003 and May 2007. ICD selection and exclusion were based on MADIT II (10) or SCD-HeFT (11) criteria, and the decision to implant a CRT device was made based on approved clinical recommendations (12,13). As in prior studies (14), clinical improvement was defined as improvement to at least NYHA class II or better by 6 months as assessed by history, patient symptoms (e.g. dyspnea on exertion and/or fatigue), and functional capacity. Follow-up was assessed by staff different from those analyzing the MR-MT and echo imaging data.
The multimodality cohort (n=27) was used to determine discordance rates between MR-MT dyssynchrony and the most commonly used clinical measure of mechanical dyssynchrony, the TDI septal-lateral delay. Four (n=4) subjects in this cohort also received CRT and were included in the CRT-HF cohort. MR-MT studies and dyssynchrony echo studies with TDI were also obtained in a group of nine (n=9) normal volunteers.
Cardiac Magnetic Resonance Protocol
All patients in the CRT-HF cohort underwent CMR studies using a 1.5 Tesla clinical scanner (Signa CV/I, GE Medical Systems, Waukesha, WI), with a phased array receiver coil on the chest. After localization of the heart, 8 to 10 contiguous short-axis slices were prescribed to cover the entire left ventricle from base to apex. Cine images were acquired using a steady-state free precession pulse sequence. MR-MT was performed using 5 to 8 tagged short axis slices acquired at the left ventricular base, mid-level, and apex (repetition time 3.5–7.2 ms, echo time 2.0–4.2 ms, flip angle α=12°, 40 cm field of view, 8–10 mm slice thickness, matrix size 256×96 to 140, 4–9 phase encoding views per segment, bandwidth of 49 MHz with range 24.9–62.5, and tag spacing 7mm). Tagging was prescribed as a grid matrix in orthogonal orientations (0° and 90°) using an electrocardiogram-triggered spoiled gradient echo pulse sequence with spatial modulation of magnetization (15,16). The multimodality group studies were performed on a Phillips 3.0 Tesla clinical scanner with a nearly identical protocol.
Delayed-enhancement images in locations identical to the cine images were acquired 10 to 15 minutes after a bolus injection of 0.2 mmol/kg gadodiamide (Omniscan) in patients with glomerular filtration rates of at least 60 cc/minute, and with gadopentetate dimeglumine (Magnevist) in selected patients if the glomerular filtration rate was 45–60 cc/min. An inversion recovery fast gradient-echo pulse sequence was used for the acquisition. Imaging parameters for both 1.5 T and 3.0 T were: repetition time 5.4 ms, echo time 1.3 ms, 36–40 cm field of view, 8 mm slice thickness, matrix 256×192, inversion recovery time 150–250 ms (adjusted to null the signal of normal myocardium), and flip angle α=20°.
Image and Strain Analysis
Short axis tagged slices were analyzed in blinded fashion by the Harmonic Phase method (HARP, Diagnosoft, Palo-Alto, CA) to assess strain (17). Regional systolic circumferential strains and time from end-diastole to peak circumferential strain were determined in 24 left ventricular segments from the midwall layers using the HARP method and a custom Matlab program.
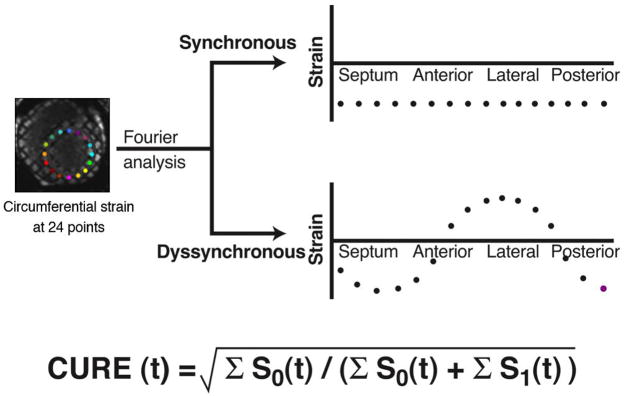
Mechanical dyssynchrony was indexed by the circumferential uniformity ratio estimate (CURE) (18,19) (Figure 1). CURE ranges from 0 (pure dyssynchrony) to 1 (perfectly synchronous). CURE was measured in 3 evenly spaced myocardial slices over the left ventricle, each containing approximately 15 cardiac phases. Slice-based CURE was measured by averaging CURE values for all systolic phases and several diastolic phases. The 3 slice-based CUREs were then averaged to yield a composite, patient-level CURE.
Figure 1. Quantification of circumferential mechanical dyssynchrony from MR-MT strain map.
Calculation of the CURE for mechanical dyssynchrony using Fourier analysis with extreme examples of spatial distribution of strain for synchrony (straight line) versus dyssynchrony (sine wave pattern). S0 is the zero-order or constant term of the Fourier transform, while S1 is the first-order term, representing low frequency changes at a given time. CURE for a given short-axis slice is generated first by determining instantaneous circumferential strains at 24 equally spaced segments in a short-axis slice at each time point, then subjecting this strain v. segment data to Fourier analysis with determination of the ratio of first to zero order power.
Delayed enhancement magnetic resonance imaging (DEMRI) was analyzed for percent total scar and posterolateral scar by standard methods (20). Abnormally enhanced myocardium (scar) was defined as high signal intensity regions with signal intensity at least two standard deviations or more relative to a remote region of interest, then percent left ventricular scar volume calculated using standard methods (20,21).
Echocardiography
Complete dyssynchrony echo studies equivalent to the clinical protocol at our hospital were performed in all multimodality cohort subjects. This included standard 2D views, tissue Doppler imaging in 3 views (apical 4-chamber, apical two-chamber, and apical 3-chamber), and M-mode left ventricular images. Timing delays by TDI were determined in a blinded fashion and reported as the septal-lateral delay, primarily based on peaks of tissue velocity in both basal and midwall planes in each view.
Statistical Analysis
Mean, standard deviation, and standard error of the mean were determined for continuous variables, and the Student t-test was used where appropriate. Fisher’s exact test (SAS 9.1.3, SAS Institute Inc., Cary, NC) was used for selected 2×2 comparisons. Correlation and linear regression analyses were used to compare CURE and TDI to QRSd (SAS). ROC analysis was performed using Johns Hopkins web-based ROC analysis software (22), and the optimal cutoffs for dyssynchrony determined by maximization of sensitivity with preservation of a reasonable specificity. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy for CRT response were calculated for CURE, DEMRI, and QRS duration. McNemar’s exact test (SAS) was used to compare MR-MT and TDI for dyssynchrony assessment in subjects with a narrow QRS in the multimodality cohort.
RESULTS
Baseline characteristics
Baseline demographic and left ventricular structural characteristics for the multimodality and CRT-HF cohorts are given in Table 1. All 9 normal subjects in the control group had normal left ventricular systolic function and normal QRS durations.
Table 1.
Baseline Characteristics
| CRT-HF Cohort (n=20) | Multimodality Cohort (n=27) | |
|---|---|---|
| Age, mean (SD), y | 58 ± 10 | 50 ± 15 |
| Gender, No. (%) | ||
| Male | 13 (65) | 16 (59) |
| Female | 7 (35) | 11 (41) |
| Cardiomyopathy, No. (%) | ||
| Ischemic | 8 (40) | 8 (30) |
| Nonischemic | 12 (60) | 19 (70) |
| LVEF, mean (SD) | 0.23 (0.057) | 0.20 (0.066) |
| QRSd, mean (SD), ms | 151 (26) | 138 (34) |
| NYHA Class, No. (%) | ||
| I | 0 (0) | 3 (11) |
| II | 0 (0) | 12 (44) |
| III | 20 (100) | 12 (44) |
| IV | 0 (0) | 0 (0) |
| Medication use, No. (%) | ||
| Beta Blocker | 19 (90) | 26 (96) |
| Amiodarone | 3 (15) | 1 (4) |
CRT-HF=Cardiac Resynchronization Therapy-Heart Failure
LVEF=left ventricular ejection fraction
QRSd = QRS duration
NYHA=New York Heart Association
Strain analysis
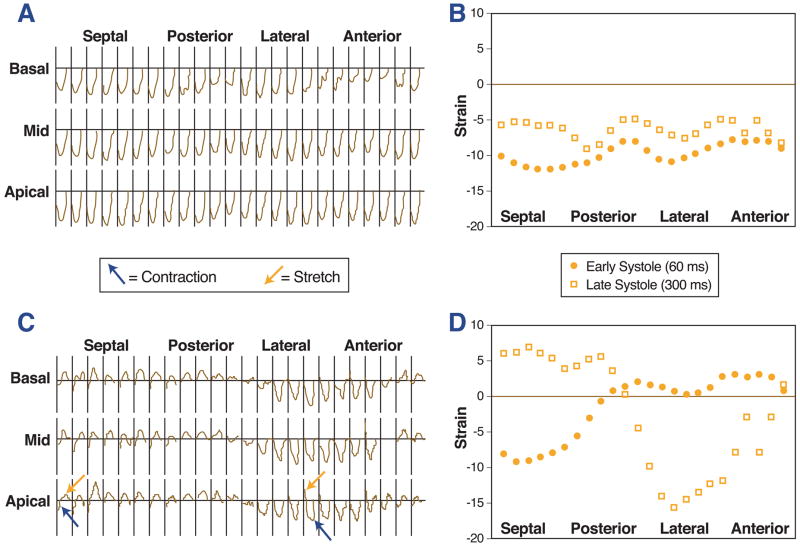
Fig. 2 displays strain plots for a normal control (Figs. 2A, 2B) and a patient with dyssynchronous cardiomyopathy (Figs. 2C, 2D). The important findings in these strain maps are described in the figure legends. The Online Movie Supplement shows the MR-MT images for the dyssynchronous subject, including color coding for varying stretch (positive strain) and contraction (negative strain).
Figure 2. MR-MT temporal and spatial circumferential strain maps for a normal subject and a subject with cardiomyopathy and dyssynchrony.
In the normal subject, the progression of the strain versus time (negative strain represents systole) is uniform in each of the 24 segments in each slice (A), and there is synchronous negative strain for each segment along the circumference of the left ventricle (B). In the subject with dyssynchrony and cardiomyopathy, the strain versus time maps show variable timing of contraction (blue arrows, negative strain) and stretch (red arrows, positive strain) in septal versus lateral segments (C). In this subject, some segments have positive strain (stretch) and others have negative strain (contraction) during systole (D).
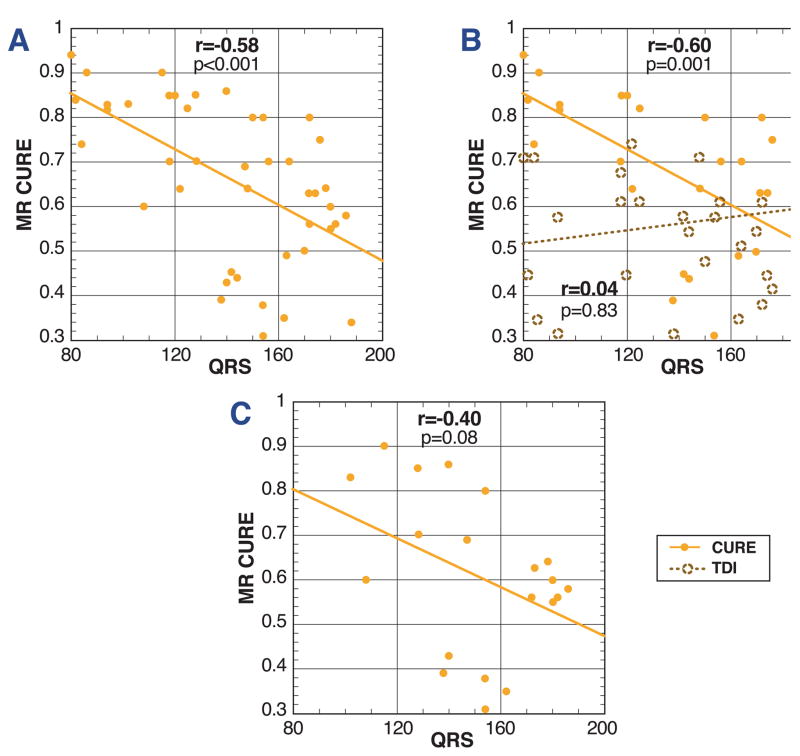
Relationship of MR-MT CURE to QRS duration
In cardiomyopathy subjects, there was a modest correlation between mechanical dyssynchrony by MR-MT (CURE) and the QRS duration (r =−0.58, p< 0.001; Fig. 3A). As anticipated, this correlation weakened when including only CRT-HF (wider QRS) patients (r=−0.40, p=0.08), as the variance range of QRS duration was more limited (Fig. 3C). Consistent with prior results, we found no correlation between the TDI septal-lateral delay and QRSd (r=0.04, p = 0.83) (Fig. 3B).
Figure 3. Correlations for MR-MT circumferential dyssynchrony (CURE), TDI septal-lateral delay, and QRSd.
The modest correlation between CURE and QRSd is shown (r=−0.58, p<0.001) for all 43 subjects in the CRT-HF and multimodality cohorts (A). For the multimodality cohort only (B), CURE and the TDI septal-lateral delay are shown versus QRS duration. As expected (based on prior published data), there is no correlation between TDI and QRSd (r=0.04, p=0.83), but there is a significant correlation between CURE and QRSd (r=−0.60, p=0.001) (B). The CURE-QRSd correlation is also shown (C) for the CRT-HF cohort only (r=−0.40, p=0.08).
MR-MT CURE in normal subjects
CMR studies from 9 normal volunteers (mean [SD] age 47 [13] years) with normal QRSd were also analyzed. In these 9 subjects, CURE approached unity with mean (SD) 0.96 (0.01), and the strain maps were similar to those shown in Figs. 2A and 2B. Despite synchronous cardiac function, 44% had a TDI septal-lateral delay of at least 65 ms, indicating dyssynchrony based on published data (23).
ROC of MR-MT for prediction of CRT response
Based on receiver operating characteristic (ROC) analysis, the AUC (SE) for CURE for prediction of improvement in function class was 0.889 (0.098) in the CRT-HF cohort. Based on this analysis, a cutoff of 0.75 for CURE was chosen because this was associated with maximal sensitivity and preserved specificity. For comparison purposes, QRS duration was associated with an AUC (SE) of 0.5077 (0.157).
Prediction of function class improvement after CRT using CURE
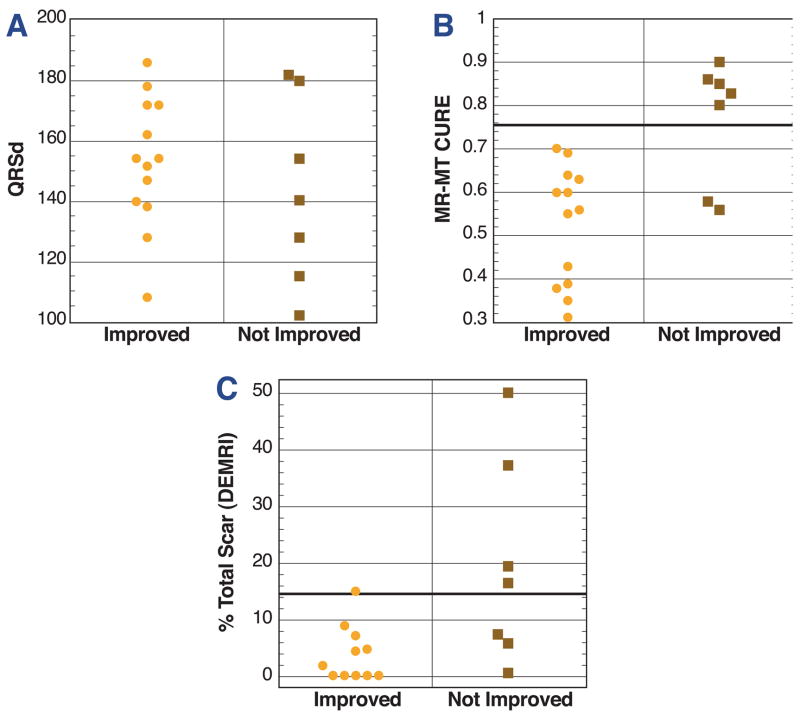
Improvement in function class was much more likely for subjects with dyssynchrony by CURE as compared to subjects without dyssynchrony by CURE (p<0.001; Fig. 4B). With a cutoff of < 0.75, CURE had 90% accuracy for predicting CRT response (PPV 87%; NPV 100%) (Table 2). As expected, QRSd did not predict function class improvement after CRT (Fig. 4A) in the CRT-HF cohort. One of the two subjects with CURE between 0.50 and 0.75 who did not improve had extensive scar by DEMRI (37% of total), as shown in Fig. 5.
Figure 4. Clinical response to CRT based on CMR findings.
These plots show data based on individual improvement in function class for patients in the CRT-HF cohort based on baseline characteristics such as QRSd (A), CURE (B), and percent left ventricular scar volume by DEMRI (C). Those with function class improvement are shown in the left column of each panel, while those without improvement are shown in the right panel. QRSd (A) has no association with improvement in function class. MR-MT (B) has better accuracy (90%) than DEMRI (78%) and (C) MR-MT and DEMRI combined have superior accuracy (95%). This highlights the superior accuracy of CURE for predicting function class improvement.
Table 2.
MR-MT and DEMRI for CRT response
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|
| % Total Scar < 15%* | 91 | 57 | 77 | 80 | 78 |
| MR-MT CURE < 0.75* | 100 | 71 | 87 | 100 | 90 |
| Both Present* | 100 | 86 | 93 | 100 | 95 |
All numbers reported are percentages.
Figure 5. CRT nonresponse due to extensive scar.
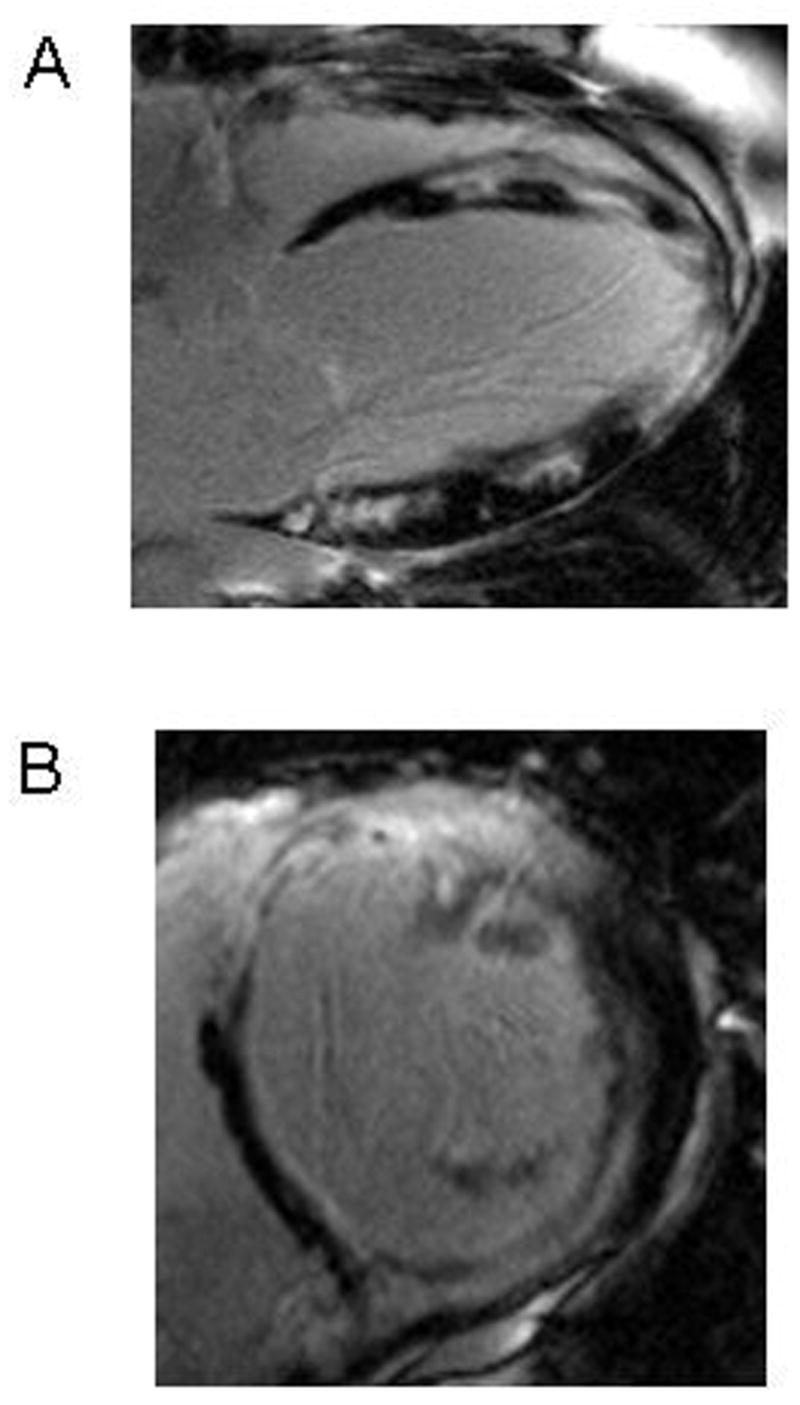
Scar imaging from a subject with significant circumferential dyssynchrony but CRT nonresponse shows extensive left ventricular scar on long axis (A) and short axis (B) images. This subject had a nonischemic cardiomyopathy (normal coronary angiography) despite the apparent regional transmural scar. This underscores the importance of CMR relative to echocardiography in the assessment of CRT candidates. Not only does MR-MT provide a very accurate measure of dyssynchrony, but the scar imaging data provides important information regarding the myocardial substrate such as extent and distribution of scar.
Scar imaging findings by DEMRI
With scar imaging of CRT-HF patients with an acceptable glomelular filtration rate (90%), 25% met the previously published criterion of percent total scar less than 15% (21) associated with CRT response. The CRT response rate in subjects with percent total scar less than 15% was significantly better that for subjects with percent total scar of 15% or greater (p=0.047; Fig. 4C). This criterion was associated with a PPV of 77%, NPV of 80%, and accuracy of 78% for CRT response. CURE < 0.75 without evidence of extensive (15% or more) left ventricular scar resulted in the most accurate prediction of CRT response, with a PPV of 93%, NPV of 100%, and accuracy of 95% (p < 0.001; Table 2).
Application of CURE cutoff to standard clinical dyssynchrony assessment
In the multimodality cohort (n=27), the mean (SE) CURE was 0.68 (0.03), and the mean (SE) TDI septal-lateral delay 74 (7.0) ms. Using the ROC-based CURE cutoff of 0.75, 56% of patients tested positive for mechanical dyssynchrony using either MR-MT or TDI, but the patients testing positive by MR-MT were not necessarily the same patients testing positive with TDI (discordance rate 30%).
In half of these discordant cases (15% of total), MR-MT indicated dyssynchrony and TDI did not, while in the other half of discordant cases (15% of total), TDI indicated dyssynchrony and MR-MT did not. TDI was more likely than MR-MT to indicate dyssynchrony in both cardiomyopathy subjects with a narrow QRS (63% v. 25%, TDI v. MR-MT, respectively) and control group subjects (44% v. 0%), who all had a narrow QRS (p =0.016 for all subjects with a narrow QRS). Of note, 77% of subjects with TDI/MR-MT discordance had two systolic peaks in the TDI tissue velocity versus time tracing from the septal or lateral walls, while only 33% of subjects with TDI/MR-MT concordance had double systolic velocity peaks in the septal or lateral walls.
DISCUSSION
Main findings
This is the first published series evaluating MR-MT assessment of circumferential mechanical dyssynchrony in patients referred for CRT with respect to prediction of clinical improvement after implantation. The most significant finding of this study is that MR-MT assessment of circumferential mechanical dyssynchrony had excellent predictive value for improved function class after CRT, and its accuracy could be further improved by combining MR-MT with scar imaging by DEMRI. This result is likely due to the precise circumferential strain map along all three dimensions of the heart provided by the MR-MT technique, integration of the entire strain map into a physiologic mechanical dyssynchrony index, and simultaneous evaluation of the underlying tissue substrate.
The CURE cutoff was based on ROC analysis. MR-TDI discordance was likely related to the use of circumferential strain by MR-MT (rather than longitudinal tissue velocity with TDI), as well as certain limitations of TDI for determining accurate time delays. Of note, our finding that TDI was more likely to indicate dyssynchrony in subjects with a narrow QRS (including normal subjects) is consistent with recent data from the RETHINQ study, in which TDI failed to identify narrow QRS patients who would benefit from CRT (24).
Present findings in the context of prior CMR dyssynchrony studies
This is the first study to support the use of MR-MT circumferential strain for CRT selection based on long-term function class improvement. Other MR protocols such as radial motion analysis (25) and CMR longitudinal phase velocity have essentially mimicked TDI by determining radial or longitudinal timing delays, and have not been shown to identify CRT responders. Of note, the usefulness of MR-MT circumferential strain for dyssynchrony had been suggested by prior small acute hemodynamic studies of left ventricular or biventricular pacing (9,26).
CRT selection
We have shown that MR-MT assessment of mechanical dyssynchrony has excellent predictive accuracy for improvement in function class after CRT and may be enhanced by DEMRI scar imaging. These findings highlight the unique value of MR-MT/DEMRI to characterize both mechanical function and scar extent/distribution in CRT candidates prior to implantation.
Limitations
There are several limitations to the study. Due to the retrospective nature of the assessment of clinical response, routine post-CRT echocardiographic studies and functional studies such as the 6-minute walk were not performed. Even so, the optimal CRT study endpoint is controversial, improvement in heart failure class is recognized as a valid post-CRT endpoint, and the overall agreement between clinical response (heart failure class improvement) and echocardiographic response was noted to be 76% in a recent series (14). Of note, the number of patients in the CRT-HF cohort was somewhat limited, and not all patients had pre-CRT TDI studies. Another limitation is that CURE was evaluated during the systolic and early diastolic frames, as the tags may fade somewhat after early diastole. The effect of not including a complete diastolic evaluation of dyssynchrony is likely minor, as dyssynchrony has been shown to peak in systole. Also, although the signal-to-noise ratio in MR-MT circumferential strain data is quite good, and the CURE filters out almost all of the noise based on exclusion of second order and higher Fourier terms, further refinement of the CMR protocol and analysis may be helpful.
CONCLUSIONS
MR-MT-based analysis of circumferential mechanical dyssynchrony with precise strain maps over all three dimensions of the heart is feasible, has excellent predictive accuracy for improvement in NYHA function class after CRT, and is complemented by substrate characterization using DEMRI scar imaging.
Supplementary Material
An online video has been provided for the MR-MT study for the subject with cardiomyopathy and dyssynchrony referenced in Figs. 2C and 2D. The images show color-coded strain superimposed upon a moving tagged circumferential short axis slice of the heart. Visual inspection of the tagging study shows early septal contraction and lateral wall stretch followed by late lateral wall contraction and septal stretch. The color coding confirms these observations, with red indicating stretch (positive strain) and green/blue indicating varying degrees of contraction (negative strain).
Acknowledgments
This work was supported by the Donald W. Reynolds Foundation, NIH Grant PO1 HL077180 (ACL, DAK, GFT), Guidant/Boston Scientific (ACL), and T32 HL07227 (RHM). Funding for this study was provided in part by Guidant/Boston Scientific. Drs. Lardo, Kass, Halperin, and Berger are paid consultants for Guidant/Boston Scientific. These relationships are managed by the Johns Hopkins University’s Committee on Conflict of Interest.
ABBREVIATIONS
- CURE
circumferential uniformity ratio estimate
- ICD
implantable cardioverter defibrillator
- DEMRI
delayed enhancement magnetic resonance imaging
- QRSd
QRS duration
- MR-MT
magnetic resonance imaging with myocardial tissue tagging
- NPV
negative predictive value
- NYHA
New York Heart Association
- PPV
positive predictive value
- ROC
receiver operating characteristic
- TDI
tissue Doppler imaging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 2.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 3.Chung ES, Leon AR, Tavazzi L, Nihoyannopoulos P, Merlino J, Abraham WT, Leclercq C. Predictors of Response to CRT (PROSPECT Study) Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.107.743120. In Press. [DOI] [PubMed] [Google Scholar]
- 4.Bleeker GB, Schalij MJ, Boersma E, et al. Relative merits of M-mode echocardiography and tissue Doppler imaging for prediction of response to cardiac resynchronization therapy in patients with heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2007;99:68–74. doi: 10.1016/j.amjcard.2006.07.068. [DOI] [PubMed] [Google Scholar]
- 5.Lardo AC, Abraham TP, Kass DA. Magnetic resonance imaging assessment of ventricular dyssynchrony: current and emerging concepts. J Am Coll Cardiol. 2005;46:2223–2228. doi: 10.1016/j.jacc.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Helm R, Wu K, Fernandes V, Schmidt A, Rosen B, Nazarian S, Byrne M, Osman N, Keel A, Weiss R, Berger R, Halperin H, Lima J, Lardo A. Quantitative MRI Assessment of Cardiac Dyssynchrony in Patients with Ischemic Cardiomyopathy: Effect of Infarct Location. Circulation. 2005;112:II-472. [Google Scholar]
- 7.Helm PA, Younes L, Beg MF, et al. Evidence of structural remodeling in the dyssynchronous failing heart. Circ Res. 2006;98:125–132. doi: 10.1161/01.RES.0000199396.30688.eb. [DOI] [PubMed] [Google Scholar]
- 8.Helm RH, Leclercq C, Faris OP, et al. Cardiac dyssynchrony analysis using circumferential versus longitudinal strain: implications for assessing cardiac resynchronization. Circulation. 2005;111:2760–2767. doi: 10.1161/CIRCULATIONAHA.104.508457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson GS, Curry CW, Wyman BT, et al. Predictors of systolic augmentation from left ventricular preexcitation in patients with dilated cardiomyopathy and intraventricular conduction delay. Circulation. 2000;101:2703–2709. doi: 10.1161/01.cir.101.23.2703. [DOI] [PubMed] [Google Scholar]
- 10.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 11.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 12.Gregoratos G, Abrams J, Epstein AE, et al. ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines) J Am Coll Cardiol. 2002;40:1703–1719. doi: 10.1016/s0735-1097(02)02528-7. [DOI] [PubMed] [Google Scholar]
- 13.Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) 2. J Am Coll Cardiol. 2005;46:e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Bleeker GB, Bax JJ, Fung JW, et al. Clinical versus echocardiographic parameters to assess response to cardiac resynchronization therapy. Am J Cardiol. 2006;97:260–263. doi: 10.1016/j.amjcard.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 15.Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology. 1989;171:841–845. doi: 10.1148/radiology.171.3.2717762. [DOI] [PubMed] [Google Scholar]
- 16.Zerhouni EA, Parish DM, Rogers WJ, et al. Human heart: tagging with MR imaging--a method for noninvasive assessment of myocardial motion. Radiology. 1988;169:59–63. doi: 10.1148/radiology.169.1.3420283. [DOI] [PubMed] [Google Scholar]
- 17.Kraitchman DL, Sampath S, Castillo E, et al. Quantitative ischemia detection during cardiac magnetic resonance stress testing by use of FastHARP. Circulation. 2003;107:2025–2030. doi: 10.1161/01.CIR.0000062684.47526.47. [DOI] [PubMed] [Google Scholar]
- 18.Helm RH, Leclercq C, Faris OP, et al. Cardiac dyssynchrony analysis using circumferential versus longitudinal strain: implications for assessing cardiac resynchronization. Circulation. 2005;111:2760–2767. doi: 10.1161/CIRCULATIONAHA.104.508457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leclercq C, Faris O, Tunin R, et al. Systolic improvement and mechanical resynchronization does not require electrical synchrony in the dilated failing heart with left bundle-branch block. Circulation. 2002;106:1760–1763. doi: 10.1161/01.cir.0000035037.11968.5c. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt A, Azevedo CF, Cheng A, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–2014. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White JA, Yee R, Yuan X, et al. Delayed enhancement magnetic resonance imaging predicts response to cardiac resynchronization therapy in patients with intraventricular dyssynchrony. J Am Coll Cardiol. 2006;48:1953–1960. doi: 10.1016/j.jacc.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 22.Eng J. ROC analysis: web-based calculator for ROC curves. 2006:5–17. [Google Scholar]
- 23.Bax JJ, Bleeker GB, Marwick TH, et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:1834–1840. doi: 10.1016/j.jacc.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Beshai JF, Grimm RA, Nagueh SF, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007;357:2461–2471. doi: 10.1056/NEJMoa0706695. [DOI] [PubMed] [Google Scholar]
- 25.Chalil S, Stegemann B, Muhyaldeen S, et al. Intraventricular dyssynchrony predicts mortality and morbidity after cardiac resynchronization therapy: a study using cardiovascular magnetic resonance tissue synchronization imaging. J Am Coll Cardiol. 2007;50:243–252. doi: 10.1016/j.jacc.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 26.Russel IK, Zwanenburg JJ, Germans T, et al. Mechanical dyssynchrony or myocardial shortening as MRI predictor of response to biventricular pacing? J Magn Reson Imaging. 2007;26:1452–1460. doi: 10.1002/jmri.21133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
An online video has been provided for the MR-MT study for the subject with cardiomyopathy and dyssynchrony referenced in Figs. 2C and 2D. The images show color-coded strain superimposed upon a moving tagged circumferential short axis slice of the heart. Visual inspection of the tagging study shows early septal contraction and lateral wall stretch followed by late lateral wall contraction and septal stretch. The color coding confirms these observations, with red indicating stretch (positive strain) and green/blue indicating varying degrees of contraction (negative strain).