Abstract
Objective
To assess risk factors for paediatric burn injuries in the Czech Republic and to suggest preventive measures.
Methods
This study included all children aged 0–16 years hospitalized during 1993–2000 at the Prague Burn Centre and data from the Czech Ministry of Health on national paediatric burn hospitalizations during 1996–2006. Personal, equipment and environmental risk factors were identified from hospital records.
Findings
The incidence of burn admissions among 0–14 year-olds increased from 85 to 96 per 100 000 between 1996 and 2006, mainly due to a 13% increase among 1–4 year-olds. Between 1993–2000 and 2006, the proportion of burn victims in the country hospitalized at the Prague Burn Centre increased from 9% to 21%. Detailed data were available on 1064 children (64% boys). Around 31% of all burn hospitalizations were in 1 year-olds. Some 79% of burns occurred at home: 70% in the kitchen, 14% in the living room or bedroom and 11% in the bathroom. Of the 18% occurring outdoors, 80% involved boys. Scalds from hot liquids accounted for 70% of all burns. The mean hospital stay was 22 days for boys and 18 days for girls.
Conclusion
Most burns involved scalds from hot liquids at home: beverages in kitchens and water in bathrooms. There is a need for passive preventive measures, such as redesigned domestic cooking and eating areas, safer electrical kettles and temperature control devices for bathrooms. Educational programmes should be developed for parents and caregivers. A national plan for child burn prevention with specific targets would be helpful.
Résumé
Objectif
Evaluer les facteurs de risque de brûlures pédiatriques dans la République tchèque et suggérer des mesures préventives.
Méthodes
Cette étude a porté sur l’ensemble des enfants de 0 à 16 ans hospitalisés entre 1993 et 2000 au Centre des grands brûlés de Prague et a utilisé les données du Ministère tchèque de la Santé sur les hospitalisations pour brûlure pédiatrique de 1996 à 2006. Les facteurs de risque humains, matériels et environnementaux ont été identifiés d’après les registres hospitaliers.
Résultats
L’incidence des admissions pour brûlure chez les enfants de 0 à 14 ans est passée de 85 à 96 pour 100 000 entre 1996 et 2006, en raison principalement de l’accroissement de 13 % de cette incidence dans la tranche d’âges 1-4 ans. Entre la période 1993-2000 et 2006, la proportion d’enfants brûlés dans le pays hospitalisées au Centre des grands brûlés de Prague a augmenté de 9 à 21 %. On disposait de données détaillées pour 1064 enfants (dont 64 % de garçons). Environ 31 % des hospitalisations pour brûlures concernaient des enfants d’un an. Près de 79 % des brûlures s’étaient produites au domicile des enfants : 70 % dans la cuisine, 14 % dans la salle de séjour ou une chambre et 11 % dans la salle de bain. Sur les 18 % de brûlures intervenant à l’extérieur, 80 % touchaient des garçons. Les enfants ébouillantés par des liquides chauds représentaient 70 % de l’ensemble des cas de brûlures. Le séjour moyen à l’hôpital était de 22 jours pour les garçons et de 18 jours pour les filles.
Conclusion
Dans la plupart des cas de brûlures, l’enfant avait été ébouillanté par un liquide chaud à son domicile : par une boisson à la cuisine et par de l’eau à la salle de bain. Des mesures de prévention passive s’imposent, comme le réaménagement des zones servant à la cuisine et à l’alimentation domestiques et comme l’utilisation de bouilloires électriques plus sûres et de dispositifs de contrôle de la température dans les salles de bains. Des programmes d’éducation doivent être développés à l’intention des parents et des personnes s’occupant des enfants. Un plan national pour la prévention des brûlures chez l’enfant, avec des objectifs précis, serait utile.
Resumen
Objetivo
Investigar los factores de riesgo de quemaduras en niños de la República Checa y proponer medidas preventivas.
Métodos
En el presente estudio se incluyeron todos los niños de 0 a 16 años hospitalizados entre 1993 y 2000 en el Centro de Quemados de Praga. Asimismo, se analizaron los datos del Ministerio de Salud de la República Checa sobre las hospitalizaciones por quemaduras registradas en el país durante el periodo 1996-2006. Los factores de riesgo personales, ambientales y relacionados con el equipo se identificaron a partir de los registros hospitalarios.
Resultados
La incidencia de ingresos por quemaduras en niños de 0 a 14 años aumentó de 85 a 96 por 100 000 entre 1996 y 2006, sobre todo debido a un aumento del 13% entre los niños de 1 a 4 años. Entre 1993-2000 y 2006, la proporción de quemados de todo el país ingresados en el Centro de Quemados de Praga aumentó del 9% al 21%. Se obtuvieron datos detallados sobre 1064 niños (el 64% de ellos del sexo masculino). Aproximadamente un 31% de todas las hospitalizaciones por quemaduras se registraron en niños de 1 año. Aproximadamente un 79% de las quemaduras se produjeron en casa: el 70% en la cocina, el 14% en el salón o la habitación, y el 11% en el baño. Del 18% de las quemaduras que se produjeron fuera de casa, el 80% afectó al sexo masculino. Las escaldaduras con líquidos calientes supusieron el 70% de la totalidad de las quemaduras. La duración media de la estancia en el hospital fue de 22 días en los niños y de 18 días en las niñas.
Conclusión
La mayoría de las quemaduras consisten en escaldaduras con líquidos calientes que se producen en casa: bebidas en la cocina y agua en el cuarto de baño. Son necesarias medidas preventivas pasivas, tales como la modificación del diseño de las zonas de la casa para cocinar y comer, una mayor seguridad de las teteras eléctricas y dispositivos de control de la temperatura del agua en los cuartos de baño. Asimismo, deben elaborarse programas de educación para padres y cuidadores. Sería útil disponer de un plan nacional de prevención de las quemaduras infantiles que tuviera metas concretas.
ملخص
الهدف
تقييم عوامل الاختطار للإصابة بالحروق لدى الأطفال في جمهورية الشيك ولاقتراح إجراءات وقائية.
الطريقة
ضمت الدراسة جميع الأطفال الذين تراوحت أعمارهم بين 0 و16 عاماً ممن أدخلوا المستشفى في الفتـرة 1993 – 2000 في مركز براغ للحروق إلى جانب المعطيات المستمدة من وزارة الصحة التشيكية حول الحالات التي أدخلت في المستشفيات الوطنية من الأطفال خلال الفترة 1996 – 2006. وقد تم التعرف من سجلات المستشفى على عوامل الاختطار التي تعود للأشخاص وللمعدات وللبيئة.
الموجودات
لقد زاد معدل وقوع الإدخالات إلى المستشفى لمن تتراوح أعمارهم بين 0 – 14 عاماً مما كان عليه عام 1996 والبالغ 85 لكل مئة ألف ليصبح 96 لكل مئة ألف عام 2006 ويعود ذلك بشكل رئيسي إلى زيادة مقدارها 13% في من تتراوح أعمارهم بين سنة وأربع سنوات. فيما ازدادت نسبة ضحايا الحروق الذين أدخلوا مركز براغ للحروق في الفترة بين 1993 و2000 من 9% إلى 21% وقد توافرت المعطيات حول 1064 طفلاً (64% منهم من الفتيان). وكان 31% من المصابين بالحروق الذين أدخلوا المستشفى ممن تتراوح أعمارهم بين 1 – 4 سنوات. وقد حدثت 79% من الحروق في المنزل؛ إذ حدث 70% منها في المطبخ و14% في غرفة المعيشة أو غرفة النوم و11% في الحمام. ومن بين 10% من الحالات التي حدثت خارج المنزل كان 80% لدى الفتيان. أما السمط الناجم عن المياه الساخنة فقد شكل 70% من مجمل الحروق. وكان وسطي المكوث في المستشفى 22 يوماً للفتيان و18 يوماً للفتيات.
الاستنتاج
تحدث معظم الحروق بما فيها السمط من المياه الحارة في المنزل؛ ومن المشروبات الساخنة في المطبخ والمياه الحارة في الحمام. وتمس الحاجة لاتخاذ إجراءات وقائية سلبية مثل إعادة تصميم أماكن الطبخ وتناول الطعام في المنزل، وتصميم أكثر أمناً لغلايات الشاي الكهربائية وأدوات التحكم بالحرارة في الحمامات. مع إعداد برامج تعليمية للآباء والقائمين على إيتاء الرعاية الصحية. وسيكون من المفيد إعداد خطة وطنية للوقاية من حروق الأطفال ويكون لها أهداف محددة.
Introduction
A severe nonfatal burn is one of the most devastating injuries a person can survive, and such injuries impose a substantial medical, social, economic and personal burden on society and the victims’ families. Patients often undergo numerous surgical procedures over a long period of hospitalization and some require readmission for reconstructive surgery. The emotional and physical scars can last a lifetime.1 The proportion of child injuries attributable to burns ranges from 2% to 6% in high-income developed countries.2–4
In the Czech Republic, about 1500 children aged 0–14 years were hospitalized annually for burns between 1996 and 2006.5 More than 60% were toddlers aged 1–4 years. Over one-third of the children were treated in a burn unit, while the remainder were treated in a surgical or paediatric department.
Clinical management of a severely burned child requires a multidisciplinary approach. Specialized burn centres with expert professionals can optimize therapy, survival and rehabilitation.6 The aims are to decrease mortality and achieve the best possible quality of life after treatment. In the Czech Republic, there are three burn centres: in Brno, Ostrava and Prague. The only criterion for admission to the Prague Burn Centre, the largest and most sophisticated, is burn severity, not geographical location.
The aim of this study was to investigate the epidemiological distribution and prevention of severe childhood burns in the Czech Republic by assessing the total number of hospitalizations for burns in the country and the proportion managed at the Prague Burn Centre, while taking into account burn severity and the risks associated with personal, equipment and environmental factors.
Methods
The study included all children aged 0–16 years who were admitted to the Prague Burn Centre during 1993–2000. Data on all hospitalizations for burns in the Czech Republic during 1996–2006 were also obtained from the Czech Ministry of Health, summarized and recorded.5 The Prague Burn Centre is part of the teaching hospital of the Third Faculty of Medicine Charles University. During the study period, the Centre provided complex treatment for 2278 (47%) of the most severely burned patients of all ages from the entire country and served as a national centre for undergraduate and postgraduate medical education. All admissions of 0–16 year-olds were included in the study, while readmissions were excluded. Detailed data on risk factors for burns were obtained by carrying out a retrospective analysis of hospital charts at the Prague Burn Centre for all 0–16 year-olds admitted between 1993 and 2000. In addition, the number and ages of all children aged 0–14 years hospitalized for burns throughout the Czech Republic, excluding those readmitted, were obtained, together with the proportion treated at the Prague Burn Centre. Census data were used to derive incidence rates.5
A structured questionnaire was developed to abstract data from hospital records, including data on personal risk factors such as age and sex, on burn hazards such as heat sources and on environmental factors such as the place and time of injury. Clinical data included the severity and anatomical site of the burn, the percentage of the total body surface area (TBSA) affected and the duration of hospitalization. Data were collected by the departmental head nurse. Data entry and analysis were carried out using Epi-Info version 6.04 (Centers for Disease Control and Prevention, Atlanta, GA, United States of America).
The study was approved by the ethics committee of the Third Faculty of Medicine of Charles University and conducted according to good clinical practice and the Helsinki Declaration, in agreement with institutional research review board requirements.
Results
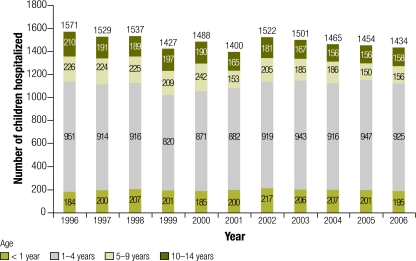
Nationally, annual paediatric burns admissions to all hospitals in the Czech Republic averaged 1510 during 1996–2000 and 1463 during 2001–2006 (Fig. 1). The incidence of burn hospitalizations among 0–14 year-olds increased by 13% between 1996 and 2006 due to a rise among toddlers aged 1–4 years. The increase in the proportion of all burn hospitalizations that occurred in toddlers was 17% (Table 1). The proportion of children hospitalized for burns in the Czech Republic who were treated at the Prague Burn Centre increased from 9% during 1993–2000 to 21% in 2006 (Table 2). The records of all 1064 children admitted to the Prague Burn Centre between 1993 and 2000 were available.
Fig. 1.
Hospitalizations for burns of children aged 0–14 years in the Czech Republic, by age group, 1996–2006 (n = 16 328 )
Table 1. Hospitalizations of children 0–14 years old in the Czech Republic due to all injuries and to burns, 1996 and 2006.
| Age group in years | Population in 1996 | Hospitalizations in 1996 |
Population in 2006 | Hospitalizations in 2006 |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All injuries | Burns | Burns as % of total injuries | Burns per 100 000 population | All injuries | Burns | Burns as % of total injuries | Burns per 100 000 population | |||||||||||||||
| < 1 | 90 014 | 1 697 | 184 | 10.8 | 204 | 101 134 | 2 090 | 195 | 9.3 | 193 | ||||||||||||
| 1–4 | 441 852 | 8 828 | 951 | 10.8 | 215 | 382 193 | 7 343 | 925 | 12.6 | 242 | ||||||||||||
| 5–14 | 1 310 813 | 23 413 | 436 | 1.9 | 33 | 1 003 871 | 17 190 | 314 | 1.8 | 31 | ||||||||||||
| 0–14 | 1 842 679 | 33 938 | 1571 | 4.6 | 85 | 1 487 198 | 26 623 | 1434 | 5.4 | 96 | ||||||||||||
Source: Ministry of Health of the Czech Republic.
Table 2. Number of hospitalizations for burns in children aged 0–14 years in the Czech Republic and at the Prague Burn Centre, 1993–2006.
| Year | All hospitals |
Prague Burn Centre |
||
|---|---|---|---|---|
| No. | No. | % of all hospitals | ||
| Average | 1510a | 132b | 9 | |
| 2001 | 1400 | 161 | 12 | |
| 2002 | 1522 | 200 | 13 | |
| 2003 | 1501 | 255 | 17 | |
| 2004 | 1465 | 234 | 16 | |
| 2005 | 1454 | 253 | 17 | |
| 2006 | 1434 | 303 | 21 | |
a The annual average for all hospitals was for the period 1996–2000. b The annual average for the Prague Burn Centre was for the period 1993–2000.
Personal risk factors
Of all children aged 0–16 years hospitalized at the Prague Burn Centre for more than 1 day, 64% were boys; the male:female ratio was 1.8:1 (Table 3). The mean age of the boys was 4.8 years (median: 2.9 years) and of the girls, 3.9 years (median: 2.2 years). Moreover, 54% (570) were < 3 years and 85% were < 10 years of age. One year-olds were at the highest risk and accounted for 31% of children with burns. Nearly all burns in infants occurred between 7 and 12 months of age.
Table 3. Number of children hospitalized for burns at the Prague Burn Centre, by age and sex, 1993–2000.
| Age | Male | Female | Total | %a | Average total per year of ageb | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| < 1 year | 79 | 47 | 126 | 12 | 126 | |||||
| < 3 months | 5 | 0 | 5 | 4 | NA | |||||
| 3–6 months | 4 | 3 | 7 | 6 | NA | |||||
| 7–8 months | 22 | 9 | 31 | 25 | NA | |||||
| 9–12 months | 48 | 35 | 83 | 66 | NA | |||||
| 1 year | 207 | 127 | 334 | 31 | 334 | |||||
| 2 years | 63 | 47 | 110 | 10 | 110 | |||||
| 3 years | 39 | 27 | 66 | 6 | 66 | |||||
| 4 years | 45 | 26 | 71 | 7 | 71 | |||||
| 5–9 years | 126 | 71 | 197 | 18 | 39 | |||||
| 10–14 years | 120 | 35 | 155 | 15 | 31 | |||||
| 15–16 years | 5 | 0 | 5 | 0.5 | 2.5 | |||||
| Total | 684 | 380 | 1064 | 100 | NA | |||||
NA, not applicable. a Percentages may not add up to 100% due to rounding. b To enable a comparison of groups covering 1 year and those covering more than 1 year of age.
Environmental risk factors and burn hazards
Some 79% (828) of child burns took place inside the home, while 18% (192) occurred outdoors, 2% (24) in kindergartens or schools and 2% (20) in another or unknown location. The kitchen was the location for 70% (577) of burns inside the home, the bathroom for 11% (90), the living room or child’s bedroom for 14% (115) and other locations for 5% (46). Boys comprised 60% of victims inside the home and 80% outdoors.
Gardens surrounding the home were the site for 17% (32) of outdoor burns, home courtyards for 13% (24), public locations such as streets for 13% (24), parks for 7% (13), train stations for 6% (12), and all other locations, including village greens, forests, electrical installations and unspecified locations, for 45% (87). The burn hazards involved in outdoor burns included: handling highly flammable and explosive liquids such as petrol, spirits or solvents, in 37% (71); activities connected with cooking on a campfire or grilling over charcoal, especially adding starter fluids to a fire, in 22% (43); electrical installations, mainly high-voltage, in 14% (26); explosives such as detonators, gunpowder and fireworks in 8% (16); and other hazards in 19% (36). Outdoor burns mainly involved older children (192).
Risk factors in the home
The most frequent cause of burns inside the home was scalding by hot water or another liquid, mainly in the kitchen or bathroom. Scalds accounted for 70% of all burns (740). Hot water accounted for 42% (308), tea for 21% (155), coffee for 20%, soup for 9%, oil for 5%, and other liquids for 3% of scalds. Around 65% (200) of hot water scalds occurred in the kitchen and 27% (82) in the bathroom. Scalds from tea were much more frequent in the kitchen (133) than in all other rooms (13). The situation was similar for coffee scalds, with 102 in the kitchen and 37 in other locations, mainly the living room.
Hot tap water during bathing or showering a child caused 62% (51) of scalds in the bathroom. Containers such as buckets and pots are mainly used for hot liquids in rural areas and are dangerous, with falls onto such containers accounting for nearly a quarter of thermal injuries in the bathroom. Another cause of burns was the use of a saucepan in lieu of a vaporizer for steam therapy of respiratory infections (24).
The most frequent causes of burns in other rooms, such as a child’s bedroom or the living room (115), were scalding by spilled hot coffee or tea in 43% of cases, household electrical burns in 18%, and contact burns from room heaters in 14% and from irons in 9% and others in 16%.
Electrical burns (56) affected children of all ages, from toddlers to adolescents, with boys comprising 80% of victims. Indoor and outdoor incidents were equally common. Around 46% (26) of electrical burns occurred in the living room or the child’s bedroom. Many victims had been playing unsupervised. High-voltage injuries made up 41% of electrical burns and mainly affected older boys. The most frequent (12) and dangerous outdoor location for such burns was the railway station. Grave injuries sustained by touching high-voltage railway cables resulted in the deaths of three boys and one girl.
Season, day of the week and time of day
Burn injuries were most frequent in the summer holiday months of July (105) and August (103) and in the winter months of November (100) and December (108). While the overall ratio of home-to-outdoor burns was 4.3:1, it varied by season: during the summer months, it was < 3:1 and in the winter months, > 10:1. Home burns took place mainly between November and January and in the summer, with > 12% of all kitchen burns occurring in July. Outdoor burns occurred predominantly between May and September.
Burns occurred most frequently on Saturdays, regardless of the victim’s gender or the environment. Most home burns took place on Saturday, including 16% (94) of kitchen burns and 9% (22) of burns in other rooms, as did 42% (17) of outdoor campfire incidents. As for scalds in bathrooms, 22% (19) took place on Fridays, as did 33% (4) of high-voltage electrical burns at railway stations.
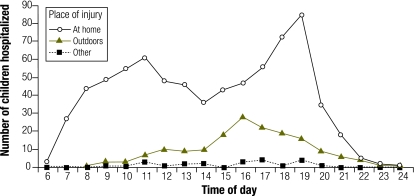
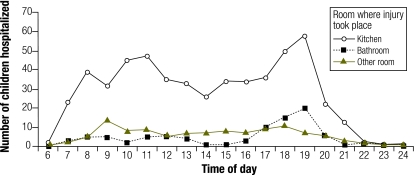
During the day, home burns peaked in frequency around 11:00 and 19:00. Outdoor burns increased during the afternoon, with the peak at around 16:00 (Fig. 2). For home burns, morning household activities were associated with burn injuries in the kitchen early in the day, and these increased up to 11:00 (Fig. 3). A decline in the afternoon was followed by a sharp increase culminating around 19:00, mainly due to dinner activities in the kitchen and, to a lesser extent, to bathing in the bathroom.
Fig. 2.
Children aged 0–16 years admitted to the Prague Burn Centre after injury at home, outdoors or elsewhere, by time of day of injury, 1993–2000a (n = 1064 )
a Data on 145 patients were missing.
Fig. 3.
Children aged 0–16 years admitted to the Prague Burn Centre, by type of room at home and time of day of injury, 1993–2000a (n = 1064 )
a Data on 331 patients were missing.
Burn severity
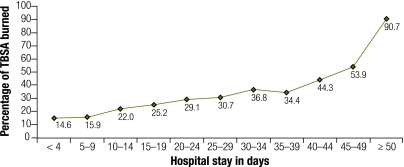
Factors affecting burn severity and the duration of the hospital stay included the TBSA affected by the burn and the depth, or degree, of the burn. Children with < 10% of TBSA affected were hospitalized for a mean of 16 days; those with ≥ 50% of TBSA affected had a mean stay of 91 days (Fig. 4).
Fig. 4.
Variation in hospital stay, by TBSA burned, among children aged 0–16 years admitted to the Prague Burn Centre, 1993–2000a (n = 1064 )
TBSA, total body surface area.
a Data on 14 patients were missing.
Breaking down the TBSA affected in terms of the depth of the burn was generally not possible since patients with third-degree burns often had lower-degree burns in other areas. Consequently, the TBSA of a burn was assessed as the sum of all areas burned, and the burn was categorized using the highest-degree burn sustained. Some 75% of children (799 in total, 495 boys) were classified as having a second-degree burn, while 24% (255 in total, 184 boys) had a third-degree burn and only 1% (9 in total, 4 boys), a first-degree burn. The male-to-female ratio for third-degree burns was 2.6:1, and a significantly higher number of boys was affected (P < 0.005).
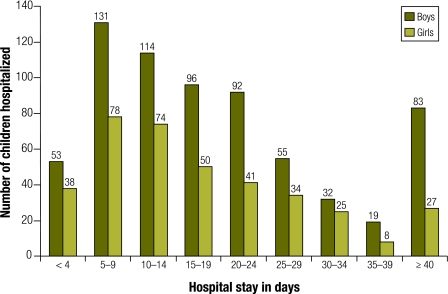
All admissions were for more than 1 day, although many hospitalizations were prolonged; the mean was 22 days in boys and 18 days in girls, and the mode was 10 days in both sexes (P < 0.005). The maximum duration was 154 days in boys and 95 days in girls. Boys had more severe burns requiring longer hospitalization (Table 4, Fig. 5) and comprised three of the four fatalities from high-voltage electrical burns.
Table 4. Duration of hospital stay among boys and girls aged 0–16 years admitted to the Prague Burn Centre, 1993–2000 (n = 1064 ).
| Hospital stay in days | Boys | Girls | Total | Male-to-female ratio | |||
|---|---|---|---|---|---|---|---|
| ≤ 4 | 54 | 38 | 92 | 1.4:1 | |||
| 5–19 | 343 | 204 | 547 | 1.7:1 | |||
| 20–39 | 202 | 108 | 310 | 1.9:1 | |||
| ≥ 40 | 88 | 27 | 115 | 3.3:1 | |||
Fig. 5.
Length of hospitalization of children aged 0–16 years admitted to the Prague Burn Centre, 1993–2000
Of those cases in which a relatively small TBSA was affected (≤ 15%), 78% occurred inside the home, while 68% of burns that involved a large TBSA (≥ 40%) took place outside. Frequent causes of severe injury were falling into a fire or hot embers from a partially extinguished fire, throwing flammable substances onto a grill or fire pit and contact with a high-voltage installation.
Discussion
The incidence of all paediatric hospitalizations for burns in the Czech Republic increased between 1996 and 2006 due to a rise in the rate among toddlers aged 1–4 years, who accounted for nearly two-thirds of 0–14-year-old patients. Similarly, about two-thirds of burn victims seen at the Prague Burn Centre between 1993 and 2000 were infants or toddlers aged 0–4 years and two-thirds were male. Overall, 1 year-olds accounted for nearly one-third of all hospitalizations. Most injuries were caused by hot liquids in the home. However, outdoor burns were responsible for the majority of the most severe cases, which mainly involved older children handling flammable and explosive liquids or cooking or barbecuing.
Low-voltage electrical burns mainly occurred in the home; nearly half took place in the living room or a child’s bedroom when young children chewed electrical cords or poked metal objects into power outlets. High-voltage electrical injuries caused devastating burns and occurred mainly in older boys at railway stations.
Burn hazards
Most burns were due to scalding by hot water, tea, coffee or soup, as has been reported elsewhere.7,8 There was an increasing trend in scalds from electric kettles, devices which were unknown in the 1980s. In 1993, no case was recorded, but in 2000, there were 22 cases.8,9 As the number of kettles increased, probably due to their low cost and ease of use, so did the frequency of scalds caused by kettles overturning. In Denmark, a comparison of scalds from electric kettles and those from other causes in children < 5 years old showed that kettle scalds involved younger children and resulted in larger and deeper burns.9
Home steam inhalation therapy for respiratory infections using a saucepan was hazardous, especially for infants, who were prone to scalding because of the difficulty of keeping them still. Overall, 24 scalds could have been averted by using vaporizers.10
Other burn hazards that were not addressed are hot milk and baby-walkers. In Turkey, children scalded by milk had more extensive burns and higher mortality than those scalded by water.11 In the United Kingdom, the increased use of baby-walkers was associated with a higher incidence of associated burns.12
Place of injury
Our finding that most burns occurred in the home was similar to that of several studies in which most child burns from scalds were reported to be linked to activities in the kitchen, living room, bedroom and bathroom.13–15 The most frequent indoor location for a thermal injury in our study was the kitchen, at 70% (577), a higher proportion than elsewhere.16,17 One possible explanation is that most of the one million or so prefabricated apartments in the Czech Republic were constructed without a dining room. Hence, cooking and eating both take place in the kitchen, often simultaneously. In contrast, in the Hong Kong Special Administrative Region, most domestic burns occurred in the living room because kitchens tend to be small and hot soup and kettles are brought into the living room to cool.17 Cultural differences should, therefore, be considered in planning effective prevention programmes.
Burns in the bathroom were most frequently due to tap water scalds. Many sinks, bathtubs and showers have low-level taps, easily reached by small children. The installation of thermostatic mixing valves at taps in bathtubs and showers could be useful.18,19 Lowering the temperature of the hot water heater is more controversial due to the potential risk of Legionella.20 While poor safety design is a contributing factor, caregivers, perhaps unaware that a child’s delicate skin burns faster than an adult’s, share responsibility if they do not take adequate precautions when bathing children.21
The increased use of barbecues and campfires, along with the dangerous practice of adding flammable liquids such as petrol, means that such sources accounted for about 22% of outdoor child burns. Windy weather adds to the risk.22
Time of injury
The late morning and evening peaks in burns were associated with cooking and serving food, as has been reported elsewhere.23–25 The early morning peak, which begins at 06:00, was associated with rushing breakfast and preparing for school and was followed by another peak at 11:00 that was linked to lunch preparation and to busy mothers being distracted. The evening peak was associated with the preparation and serving of dinner and with the bathing of children.
Personal risk factors
Severe burns were most frequent in children < 5 years old, similar to findings elsewhere.13,26 Infants, nearly all 7–12 months old, most frequently sustained scalds during bathing, while toddlers aged 1–4 years were scalded during other activities by hot liquids, food or tap water.15,27–31 Most admissions involved 1 year-olds. Toddlers and preschool children are mobile and at risk from spilled hot food and drink, hot tap water, hot surfaces (for example on irons and stoves), and electrical power outlets. The high risk of burns among infants and toddlers is attributable to their total dependence on caregivers,24 to rapid motor development preceding the understanding of risks, and to household hazards.
Scalds and contact burns become less common during later toddler and preschool years, perhaps reflecting the increased awareness of the consequences of personal actions that is associated with intellectual development.32 Older preschool children were burned during experimentation with matches, lighters and stoves, while typical burns in pre-adolescent and adolescent boys involved matches and petrol and, less frequently, high-voltage electricity.
As age increased, the sex difference in burn frequency widened. In 0–4 year-olds, the ratio of boys to girls was 1.6:1, compared with 3.3:1 among 10–14 year-olds. As Czech boys grew older, they undertook increasingly riskier activities.
Burn severity
Children with burns covering < 10% of the TBSA had a shorter mean hospitalization period of 16 days. As reported elsewhere, these minor burns resulted in low mortality.22,33–35 For severe burns affecting a TBSA ≥ 50%, the mean hospitalization period was 91 days. Elsewhere, high mortality was reported, especially for burns caused by flames.14,33,34,36–38 The longer duration of hospitalization in boys correlated with burn severity.
Adult supervision
Our study did not address adult supervision. In the United States, child burns were associated with the lack of parental supervision,39 whereas in Egypt and Kuwait most home scalds occurred in the presence of a parent or other caregiver.40,41 Hence, adult supervision may not be sufficient to prevent an injury at home when susceptibility is high because home hazards have not been eliminated or because cultural factors may be at play. The caregiver’s knowledge of passive measures for preventing child injury is often limited, with an over-reliance on vigilance.18
Limitations
The study of risk factors was based on data from the Prague Burn Centre. Although the Centre treats the majority of severe paediatric burn cases in the country, the results may not be representative of all three of the country’s burn centres. Furthermore, practice may have changed over the study period. Less severe burns may now be treated at other hospitals or clinics. Hence, these findings cannot be generalized to burns of all severities or to all regions of the country.
Conclusion
The susceptibility of children to burns increases with developmental achievements, such as independent mobility, exploratory behaviour and hand-to-mouth activity.15,42,43 Small children are less able to perceive danger, have less control over their environment and are slower to react in situations that can lead to burns.44 Thin skin is more susceptible at lower temperatures and sustains more severe injuries at higher temperatures.21 Hence, the introduction of effective passive interventions is of great importance.
Passive countermeasures that provide automatic protection include product safety legislation to mandate the safety of home heating devices such as electric kettles. Other measures include temperature-limiting valves on taps in bathtubs and showers, as well as placing taps and other sources of hot liquid high enough to be out of reach of small children. While a systematic review showed that home safety education was effective in increasing the proportion of families with “safe” hot tap water temperatures, there was a lack of evidence that such interventions reduced the burn rate.45 However, in Norway, a community programme targeting the prevention of burns in children < 5 years old was effective in reducing the most serious burns from stoves and taps.8
Over the long term, home design and building codes should preferably specify physically separate cooking and eating areas. Properly grounded electrical power outlets with switches could help. Childproof barriers around high-voltage installations in train stations should be universal.
Since most burns occurred in the home and involved infants or toddlers, effective educational programmes are needed to inform caregivers about the risks of scalds and fire-related injuries and about preventing access to hazardous areas and devices. This could help in the transition period before the development and introduction of passive preventive measures. Older children should be taught at school about volatile solvents, high-voltage electricity and hazards facing younger siblings.
Since few children were hospitalized because of fires in the home, the role of smoke detectors is unclear but might be revealed by mortality studies. National data on all burn hospitalizations would be helpful in many countries, not only the Czech Republic. Apart from the Australian National Injury Surveillance Unit,46 few centres produce publications detailing numbers, rates and risk factors for all hospitalizations for child burns. Field research could determine the hazard level for specific consumer products, for aspects of home design related to burns and for other risk factors amenable to passive protection. An assessment of the level of knowledge of parents and children of different ages about home safety, specifically burn prevention, and their attitudes towards it would be helpful for planning appropriate interventions. Finally, pilot projects on and evaluations of all proposed interventions are essential.
Since the study on risk factors in children admitted to the Prague Burn Centre was completed, the reported incidence of burn hospitalizations in the country has increased in the age group at the highest risk (i.e. toddlers). Although the Czech Republic is ahead of some other countries in the region in developing a national injury prevention programme and a child injury registry,15 policies on child burns prevention in 2006 did not score well on the European Child Safety Alliance’s Child Safety Report Card.47 One missing measure was “a government-approved national injury prevention strategy with specific targets and timelines related to child and adolescent burn/scald prevention”. Any such strategy should have a sound local research basis and should encompass, but not be limited to, the data in this paper. ■
Acknowledgements
We thank the nursing staff and management at the Prague Burn Centre for facilitating the study and helping with data collection, and the Institute of Health Information and Statistics of the Ministry of Health of the Czech Republic for supplying data on all burn hospitalizations. We are also grateful to Terri Everest, English Lecturer, for reviewing the manuscript.
Footnotes
Funding: None.
Competing interests: None declared.
References
- 1.Königová R. Factors influencing survival and quality of life in burns. Acta Chir Plast. 1996;38:116–8. [PubMed] [Google Scholar]
- 2.Vyrostek SB, Annest JL, Ryan GW. Surveillance for fatal and nonfatal injuries – United States 2001. MMWR Surveill Summ. 2004;53:1–57. [PubMed] [Google Scholar]
- 3.Scheidt PC, Harel Y, Trumble AC, Jones DH, Overpeck MD, Bijur PE. The epidemiology of nonfatal injuries among US children and youth. Am J Public Health. 1995;85:932–8. doi: 10.2105/AJPH.85.7.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kypri K, Chalmers DJ, Langley JD, Wright CS. Child injury morbidity in New Zealand, 1987-1996. J Paediatr Child Health. 2001;37:227–34. doi: 10.1046/j.1440-1754.2001.00668.x. [DOI] [PubMed] [Google Scholar]
- 5.Czech health statistics yearbook 2006 Prague: Institute of Health Information and Statistics of the Czech Republic; 2007. Available from: http://www.uzis.cz/download.php?ctg=10&mnu_id=5300 [accessed 13 February 2009].
- 6.Trop M. Burns in children and adolescents. First aid, treatment and aftercare. Tagliche Praxis. 2000;41:281–92. [Google Scholar]
- 7.Den Hertog PC, Blankendaal FA, Ten Hag SM. Burn injuries in the Netherlands. Accid Anal Prev. 2000;32:355–64. doi: 10.1016/S0001-4575(99)00056-1. [DOI] [PubMed] [Google Scholar]
- 8.Ytterstad B, Smith GS, Coggan CA. Harstad injury prevention study: prevention of burns in young children by community based intervention. Inj Prev. 1998;4:176–80. doi: 10.1136/ip.4.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheller JLP, Thuesen B. Scalds in children caused by water from electrical kettles: effect of prevention through information. Burns. 1998;24:420–4. doi: 10.1016/S0305-4179(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 10.Ebrahim MKH, Bang RL, Lari ARA. Scalds accidents during water aerosol inhalation in infants. Burns. 1990;16:291–3. doi: 10.1016/0305-4179(90)90142-J. [DOI] [PubMed] [Google Scholar]
- 11.Tarim A, Nursal TZ, Basaran O, Yildirim S, Türk E, Moray G, et al. Scalding in Turkish children: comparison of burns caused by hot water and hot milk. Burns. 2006;32:473–6. doi: 10.1016/j.burns.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Birchall MA, Henderson HP. Thermal injury associated with infant walking-aids. Burns. 1988;14:244–7. doi: 10.1016/0305-4179(88)90049-6. [DOI] [PubMed] [Google Scholar]
- 13.Al-Shehri M. The pattern of paediatric burn injuries in south-western Saudi Arabia. West Afr J Med. 2004;23:294–9. doi: 10.4314/wajm.v23i4.28144. [DOI] [PubMed] [Google Scholar]
- 14.Gupta M, Gupta OK, Goil P. Paediatric burns in Jaipur, India: an epidemiological study. Burns. 1992;18:63–7. doi: 10.1016/0305-4179(92)90125-E. [DOI] [PubMed] [Google Scholar]
- 15.World report on child injury prevention Geneva: World Health Organization; 2008. [PubMed] [Google Scholar]
- 16.Rossignol AM, Locke JA, Burke JF. Paediatric burn injuries in New England, USA. Burns. 1990;16:41–8. doi: 10.1016/0305-4179(90)90204-A. [DOI] [PubMed] [Google Scholar]
- 17.Tse T, Poon CH, Tse KH, Tsui TK, Ayyappan T, Burd A. Paediatric burn prevention: an epidemiological approach. Burns. 2006;32:229–34. doi: 10.1016/j.burns.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Eichelberger MR, Gotshall CS, Feely HB, Harstad P, Bowman LM. Parental attitudes and knowledge of child safety. A national survey. Am J Dis Child. 1990;144:714–20. doi: 10.1001/archpedi.1990.02150300112029. [DOI] [PubMed] [Google Scholar]
- 19.Collin T, Jeffery S, Reid C. Bath-water scalds in children and thermostatic mixer valves. Burns. 2006;32:909–12. doi: 10.1016/j.burns.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Barss P, Smith GS, Baker SP, Mohan D. Injury prevention: an international perspective. Epidemiology, surveillance & policy New York, NY: Oxford University Press; 1998. pp. 183-184. [Google Scholar]
- 21.McDonald EM, Girasek DC, Gielen AC. Home injuries. In: DeSafey Liller K, ed. Injury prevention for children and adolescents – research, practice and advocacy Washington, DC: American Public Health Association;2006:127. [Google Scholar]
- 22.Ryan CA, Shankowsky HA, Tredget EE. Profile of paediatric patient in a Canadian burn centre. Burns. 1992;18:267–72. doi: 10.1016/0305-4179(92)90146-L. [DOI] [PubMed] [Google Scholar]
- 23.Forjuoh SN, Guyer B, Smith GS. Childhood burns in Ghana: epidemiological characteristics and home-based treatment. Burns. 1995;21:24–8. doi: 10.1016/0305-4179(95)90776-V. [DOI] [PubMed] [Google Scholar]
- 24.Forjuoh SN. Burns in low- and middle-income countries: a review of available literature on descriptive epidemiology, risk factors, treatment, and prevention. Burns. 2006;32:529–37. doi: 10.1016/j.burns.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Chien WC, Pai L, Lin CC, Chen HC. Epidemiology of hospitalized burn patients in Taiwan. Burns. 2003;29:582–8. doi: 10.1016/S0305-4179(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 26.A WHO plan for burn prevention and care Geneva: World Health Organization; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharp RJ. Burns. In: Ashcraft KW, Holder TM, eds. Pediatric surgery Philadelphia, PA: WB Saunders Co; 1993. pp. 89-102. [Google Scholar]
- 28.Lindblad BE, Terkelsen CJ. Scalding accidents among children – an epidemiologic study. Ugeskr Laeger. 1990;152:1590–1. [PubMed] [Google Scholar]
- 29.Eadie PA, Williams R, Dickson WA. Thirty-five years of paediatric scalds: are lessons being learned? Br J Plast Surg. 1995;48:103–5. doi: 10.1016/0007-1226(95)90105-1. [DOI] [PubMed] [Google Scholar]
- 30.Fukunishi K, Takahashi H, Kitagishi H, Matushima T, Ohsawa H, Sakata I. Epidemiology of childhood burns in the critical care medical center of Kinki University Hospital in Osaka, Japan. Burns. 2000;26:465–9. doi: 10.1016/S0305-4179(99)00189-8. [DOI] [PubMed] [Google Scholar]
- 31.Cheng JCY, Leung KS, Lam ZCL, Leung PC. An analysis of 1704 burn injuries in Hong Kong children. Burns. 1990;16:182–4. doi: 10.1016/0305-4179(90)90035-U. [DOI] [PubMed] [Google Scholar]
- 32.Simon PA, Baron RC. Age as a risk factor for burn injury requiring hospitalization during early childhood. Arch Pediatr Adolesc Med. 1994;148:394–7. doi: 10.1001/archpedi.1994.02170040060010. [DOI] [PubMed] [Google Scholar]
- 33.Adamo C, Esposito G, Lissia M, Vonella M, Zagaria N, Scuderi N. Epidemiological data on burn injuries in Angola: a retrospective study of 7230 patients. Burns. 1995;21:536–8. doi: 10.1016/0305-4179(95)00038-D. [DOI] [PubMed] [Google Scholar]
- 34.Vilasco B, Bondurand A. Burns in Abidjan, Cote D’Ivoire. Burns. 1995;21:291–6. doi: 10.1016/0305-4179(94)00001-E. [DOI] [PubMed] [Google Scholar]
- 35.Ho WS, Ying SY. An epidemiological study of 1063 hospitalized burn patients in a tertiary burns centre in Hong Kong. Burns. 2001;27:119–23. doi: 10.1016/S0305-4179(00)00095-4. [DOI] [PubMed] [Google Scholar]
- 36.Bang RL, Saif JKH. Mortality for burns in Kuwait. Burns. 1989;15:315–21. doi: 10.1016/0305-4179(89)90009-0. [DOI] [PubMed] [Google Scholar]
- 37.Hemeda M, Maher A, Nasser S. Epidemiology of burns admitted to Ain Shams University Burns Unit, Cairo, Egypt. Burns. 2003;29:353–8. doi: 10.1016/s0305-4179(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 38.Boukind EH, Chafiki N, Terrab S, Alibou F, Bahechar N, Zerouali NO. Aetiology of burn injuries in childhood in Casablanca, Morocco: epidemiological data and preventive aspects. Burns. 1995;21:349–51. doi: 10.1016/0305-4179(95)00004-6. [DOI] [PubMed] [Google Scholar]
- 39.Joseph KE, Adams CD, Goldfarb IW, Slater H. Parental correlates of unintentional burn injuries in infancy and early childhood. Burns. 2002;28:455–63. doi: 10.1016/S0305-4179(02)00035-9. [DOI] [PubMed] [Google Scholar]
- 40.El-Badawy A, Mabrouk AR. Epidemiology of childhood burns in the burn unit of Ain Shams University in Cairo, Egypt. Burns. 1998;24:728–32. doi: 10.1016/S0305-4179(98)00097-7. [DOI] [PubMed] [Google Scholar]
- 41.Lari AR, Bang RL, Ebrahim MK, Dashti H. An analysis of childhood burns in Kuwait. Burns. 1992;18:224–7. doi: 10.1016/0305-4179(92)90074-5. [DOI] [PubMed] [Google Scholar]
- 42.Agran PF, Anderson C, Winn D, Trent R, Walton-Haynes L, Thayer S. Rates of pediatric injuries by 3-month intervals for children 0 to 3 years of age. Pediatrics. 2003;111:e683–92. doi: 10.1542/peds.111.6.e683. [DOI] [PubMed] [Google Scholar]
- 43.Flavin MP, Dostaler SM, Simpson K, Brison RJ, Pickett W. Stages of development and injury patterns in the early years: a population-based analysis. BMC Public Health. 2006;6:187. doi: 10.1186/1471-2458-6-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Widome MD, editor. Injury prevention and control for children and youth 3rd ed. Elk Grove Village: American Academy of Pediatrics;1997. pp. 55-59;242-4. [Google Scholar]
- 45.Kendrick D, Coupland C, Mulvaney C, Simpson J, Smith SJ, Sutton A, et al. Home safety education and provision of safety equipment for injury prevention. Cochrane Database Syst Rev. 2007;24:CD005014. doi: 10.1002/14651858.CD005014.pub2. [DOI] [PubMed] [Google Scholar]
- 46.Bradley C, Harrison J. Hospital separations due to injury and poisoning, Australia 2004–05. Injury research and statistics series number 47 [Cat. no. INJCAT 117]. Adelaide: Australian Institute of Health and Welfare; 2008. pp. 71-78. Available from: http://www.nisu.flinders.edu.au/pubs/reports/2008/injcat117.pdf [accessed 13 February 2009].
- 47.Child safety report card 2007: Czech Republic Amsterdam: European Child Safety Alliance; 2007.