Abstract
The bone diagnostic instrument (BDI) is being developed with the long-term goal of providing a way for researchers and clinicians to measure bone material properties of human bone in vivo. Such measurements could contribute to the overall assessment of bone fragility in the future. Here, we describe an improved BDI, the Osteoprobe II™. In the Osteoprobe II™, the probe assembly, which is designed to penetrate soft tissue, consists of a reference probe (a 22 gauge hypodermic needle) and a test probe (a small diameter, sharpened rod) which slides through the inside of the reference probe. The probe assembly is inserted through the skin to rest on the bone. The distance that the test probe is indented into the bone can be measured relative to the position of the reference probe. At this stage of development, the indentation distance increase (IDI) with repeated cycling to a fixed force appears to best distinguish bone that is more easily fractured from bone that is less easily fractured. Specifically, in three model systems, in which previous mechanical testing and∕or tests reported here found degraded mechanical properties such as toughness and postyield strain, the BDI found increased IDI. However, it must be emphasized that, at this time, neither the IDI nor any other mechanical measurement by any technique has been shown clinically to correlate with fracture risk. Further, we do not yet understand the mechanism responsible for determining IDI beyond noting that it is a measure of the continuing damage that results from repeated loading. As such, it is more a measure of plasticity than elasticity in the bone.
BACKGROUND
Recent measurements of material properties of bone have demonstrated that there is substantial deterioration of these properties with aging.1 For example, Nalla et al. have shown that the stress intensity necessary to initiate cracks in bone, the initiation toughness, decreases by 40% over six decades from 40 to 100 years in human bone even without diagnosed bone disease. Even more dramatically, the crack-growth toughness is effectively eliminated over the same age range.2 This recent research builds on earlier research by Wu and Vashishth3 that showed a reduction in crack-growth toughness with age. It is also consistent with research showing significant deterioration in another material property, fracture toughness, with age.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18
These measurements suggest that deteriorating material properties of bone, due to aging or disease, may play a role in bone fracture risk, in addition to the well-known factors of decreased areal bone mineral density and deterioration of microarchitecture in trabecular bone (i.e., loss of the structural network of the bone and∕or thinning of individual struts or trabeculae). Instruments already exist clinically to measure these two well-known factors. Dual energy x-ray absorptiometry and computed tomography are two examples of these instruments. There currently exists, to our knowledge, no instrument that can clinically measure the material properties of bone in vivo. Here, we report an improved design concept for the long-term goal of developing such an instrument. This design builds on our previous work and the work that has been done with indentation.19, 20, 21, 22, 23 Further, we present results from a prototype instrument based on this design concept and a few examples of the type of mechanical data that can be obtained on mineralized tissues harvested from humans and animals subjected to endogenous (aging) and exogenous perturbations (irradiation or cell transplantation to regenerate a bone defect). Further research, beyond the scope of this report, will be necessary to determine whether this instrument, or future instruments based on this design concept, will be useful for medical diagnosis of bone fragility, allowing the utility of this instrument to extend beyond that of a research tool to measure in vitro properties of tissues.
THE OSTEOPROBE II™
The Osteoprobe II™ bone diagnostic instrument (BDI) is an improved version of the Osteoprobe I™ BDI.1 The primary improvements are a new control system, which can collect and quantitatively analyze force versus distance curves (Fig. 1) and the substitution of a voice-coil-based force generator for the solenoid force generator used in the Osteoprobe I (Fig. 2). The control system, written in LABVIEW™, supplies a modified triangular wave to the force generator for the primary loading cycles used in measurements. The modified triangular wave consists of 1∕3 of a cycle of linear increase, followed by 1∕3 of a cycle hold at maximum current, and then 1∕3 of a cycle of linear decrease. A typical total cycle time is 500 ms. The purpose of the hold at maximum current is to monitor creep effects and to minimize the effect of the remaining creep during the linear decrease. This type of hold at the maximum load is used in instrumented indentation analysis, pioneered by Oliver and Pharr24 for getting valid retraction slopes for determining elastic modulus. Typically, for nanoindentation, the hold time and the unloading times are much longer than we use (and the indentations are much less deep). We have found that, for the BDI, what really counts in getting a valid retraction slope is the ratio of the unloading time to the hold time. We have found that the cycle described above gives valid retraction slopes comparable to those found with longer hold times (up to a minute) and correspondingly slower unloading.
Figure 1.
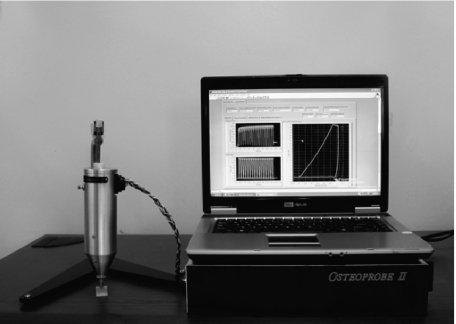
The Osteoprobe II™ bone diagnostic consists of a measurement head mounted on a stand, an electronics box, and a laptop computer. The electronics box sits under the laptop computer and contains drive electronics for the force generator in the head as well as readout electronics for the force and distance transducers. The laptop computer runs a custom LABVIEW™ program to cycle the current to the force generator and thus move the test probe relative to the reference probe and collect force vs distance data.
Figure 2.
The Osteoprobe II™ bone diagnostic instrument (BDI) uses a force generator to move a test probe relative to a reference probe. The force is monitored by a force transducer. The distance that the test probe moves relative to the reference probe is monitored with a distance transducer. The test probe, which is a ferromagnetic steel alloy, is attached to a magnet in the body of the instrument. The reference probe, which is a modified hypodermic needle, is attached to the body of the instrument with a Luer fitting.
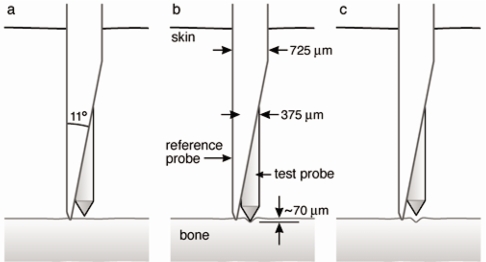
The basic idea for ultimately making in vivo diagnostic measurements is to do indentation measurements with a novel test probe∕reference probe geometry that allows measurements to be made on bone that is covered with skin and soft tissue (Fig. 3). In contrast to our previous work,1 we have now switched from beveled to conical test probes (Fig. 3). These new conical test probes are 375 μm in diameter and are beveled to 90° total included angles and then rounded to an approximate radius of 2.5 μm. The problem with the previous beveled test probes is that they were asymmetric and this asymmetry, together with the natural anisotropy of bone properties25 created an additional source of scatter in the data. Attempts to constrain the test probe to a fixed orientation relative to the bone asymmetry, for example, with the flat of the bevel parallel to the axis of a long bone versus perpendicular to the axis, proved inconvenient. Nevertheless, future studies of the bone indentation asymmetry with asymmetric indentation probes may be of interest, but are beyond the scope of this report.
Figure 3.
For measuring material properties of bone in vivo, the reference probe/test probe assembly is inserted through the skin down to the bone. The reference probe serves as a reference for measuring the distance that the test probe indented into the bone. The diameter of the test probe is 375 μm.
The substitution of a force generator, which operates with a coil in a nearly uniform magnetic field, together with flexures for nearly frictionless suspension, and a voltage to current converter to convert the voltage output of the digital to analog converter allow a well controlled force to be applied with the test probe to the sample. It is still, however, necessary to directly measure the force with a force transducer [a commercial 5 lb. (22.4 N) load cell] because some of the force generated by the force generator is used in deflecting the flexures. For our current prototypes, it takes approximately 1 N to deflect the flexures of 100 μm. The force transducer measurement is corrected to account for the weight of components in the mechanical path between the transducer and the indentation point. The weight of the rod that extends down from the force transducer through the distance transducer (a linear variable-differential transformer driven at 10 kHz) and to the magnet that holds the top of the test probe, as well as the weight of the test probe itself all must be added to the force sensed by the force transducer to get the actual force. Fortunately, this is a constant correction for standard test probes and just amounts to a need to zero the force transducer with these weights in place.
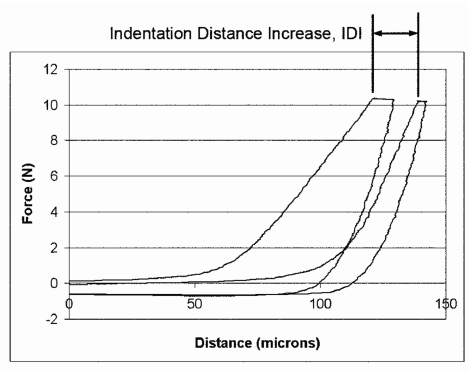
A more difficult problem is the friction between the test probe and the reference probe. This is typically between 0.05 and 0.1 N for bone samples and up to 1 N for bone samples covered with soft tissue (Fig. 4). For the case of Fig. 4, the friction, which causes the difference in force between the upper approach curves and the lower retraction curves, is of order 0.05 N. This friction causes the measured force to be 0.05 N greater as the test probe approaches the sample (upper curves) and 0.05 N less as the test probe retracts away from the sample (lower curves). Thus, the total separation between the approach and retract curves is of order 0.1 N at a distance of 0 μm (outside the bone) in this in figure. Note that another undesirable characteristic of these curves, from the standpoint of being able to use standard techniques for instrumented indentation analysis, is the very gradual onset of the force. This results in uncertainty as to where the soft tissue stops and the bone begins. Here, we report on a parameter that has been robust and does not depend on finding the precise position of the surface of the bone: the indentation distance increase (IDI).
Figure 4.
The indentation distance increase (IDI) is defined as the increase in the indentation distance in the last cycle relative to the indentation distance in the first cycle. In both cases, the indentation distance is measured just as the current to the force generator reaches its maximum value. (The current is then maintained at this maximum value for 1∕3 of the total cycle time to minimize the effects of creep on the measured slope of the retraction curve.) These curves, the first and last of 20 cycles, were obtained through the skin and soft tissue covering the tibia of a 68-year-old donor.
INDENTATION DISTANCE INCREASE
The IDI (Fig. 4) is defined as the increase in the indentation distance in the last cycle relative to the indentation distance in the first cycle. One of the key advantages of the IDI as a parameter is that it does not depend on finding the boundary between the soft tissue and the underlying bone precisely. That is, it does not depend on knowing the absolute values of the initial penetration distance and of the final penetration distance. It is just the increase in penetration distance.
The IDI is larger for bone in which the depth of the indentation at maximum load keeps increasing with successive indentation cycles. We do not yet understand the mechanism that determines IDI beyond noting that it is a measure of the damage that results from repeated loading. As such, it is more a measure of plasticity than elasticity. The IDI may reveal the capacity of the bone for resisting (or failing to resist) continuing fracture events under the indentation tip. There is growing evidence that the nanoscale processes that are involved in bone fracture include the relative motion of mineralized collagen fibrils and that the resistance of bone to fracture may depend on the ability of bone to dissipate energy in the interfibrillar matrix during this relative motion.26, 27, 28, 29, 30, 31
These same processes may be involved in resistance of bone to the continuing indentation that is measured by the IDI. So, perhaps, it is not surprising that, as shown below in model systems, bone that is more easily fractured has larger IDIs. Only clinical tests, however, can reveal whether the IDI is correlated with fracture risk in humans and only further basic research can reveal the nanoscale mechanisms that are involved in IDIs.
Since even the value of the IDI depends on many variables such as the magnitude of the maximum force, the number of cycles between the initial and final cycle (generally about 20) and the rate of cycling, it is generally also normalized. Most commonly, it is normalized to the value measured with the same probe assembly and same test protocol on a standardized sample of polished polymethyl methacrylate (PMMA), which has mechanical properties in the same general range as bone, but is more homogeneous and results in lower variance in properties
The automatic data collection protocol involves loading precycles before the primary loading cycles that were discussed above (the modified triangular waves). The purpose of the loading precycles is to establish a good zero reference for indentation distance measurements. When skin and soft tissue are present, there is no clear indication of the location of the bone surface. It has been impossible, so far, for us to come up with any algorithm that reproducibly locates a suitable reference position, much less the actual surface of the bone, from analysis of primary loading cycles like the ones in Fig. 4. So, instead, we have developed loading precycles. The precycles are modified triangular waves just as described for the primary cycles above. Unlike the primary cycles, which are of constant amplitude, the precycles have gradually increasing load amplitude. The maximum force during the precycles is monitored. When the maximum force reaches a preset threshold value, the reference position for indentation distance measurements is set at the distance where the preset threshold force was reached. For example, for the measurements of Fig. 4, the threshold force was 3.3 N. Note that this threshold force is a significant fraction of the maximum force in the primary cycles, on the order of 10 N. It has proved to be necessary to use a large threshold force to be sure that the reference position is safely inside the cortical bone, not still out in the soft tissue. This stable reference position has proved adequate for measurements, such as IDI, which are relative measurements and do not depend on accurate knowledge of the position of the surface of the bone. This works if force and indentation depth are large enough to ensure that the stable reference position is in bone, but will not work if the probe has not reached a depth sufficient to penetrate the bone, so we only want to err on the side of penetrating too far into bone. The situation is more complex, and beyond the scope of this paper, for measurements, such as elastic modulus E, and hardness H, which do conventionally depend on accurate knowledge of the position of the surface.
MODEL SYSTEMS
Irradiated versus control bovine femoral bone
Previously, we reported that the indentation distance increase in repetitive indentation cycles was related to resistance to fracture in a model system of bone subjected to temperatures of 250 °C for 2.5 h versus control bovine bone.1 This treatment is nonphysiological, but is used to dramatically degrade the organic component of the bone. This model system may simulate accelerated aging. The indentation distance increase was greater for the bone that had been heated under these conditions. Bone heated under these conditions had previously been shown to have reduced resistance to fracture.32, 33
Here, we report that this relationship, greater IDI for bone with degraded mechanical properties, is also seen in another model system: irradiated versus control bovine femur.
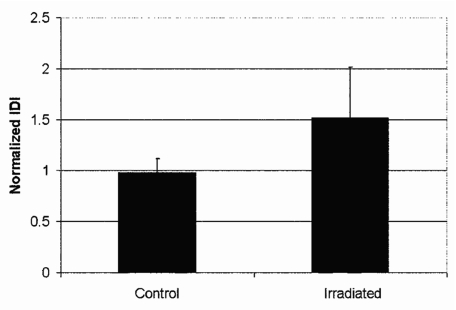
Fresh bovine femoral bones were purchased and quartered longitudinally. Two quartered sections were frozen as control specimens and two sections were shipped to Steris Isomedix Services (Ontario, CA) for gamma irradiation with high energy photons from the isotope, cobalt 60. Samples were subjected to 99.9–110 kGy of irradiation. Prior to testing, the bone was stripped of soft tissue, sectioned into pieces of order 15 mm on a side, held in a vice that was submerged in physiological buffer, and tested under the buffer state what physiological direction∕orientation was indented. The surface of the bone was not polished. Figure 5 shows that the IDI for irradiated bone is significantly larger than for control bone. Currey et al. previously shown that irradiated bone is more easily fractured than control bone.34 More specifically, irradiated bone has lower bending strength, work to fracture, and impact energy absorption.34
Figure 5.
The normalized IDI for control bovine femoral bone is smaller than that for irradiated bovine femoral bone. This difference is statistically significant at the level of P<0.001 for this sample of 100 tests of control and 99 tests of irradiated bone with the BDI. The normalization is with respect to a standardized PMMA sample measured, at the same time, with the same probe assembly and measurement protocol. The error bars show one standard deviation.
Elderly versus young human bone
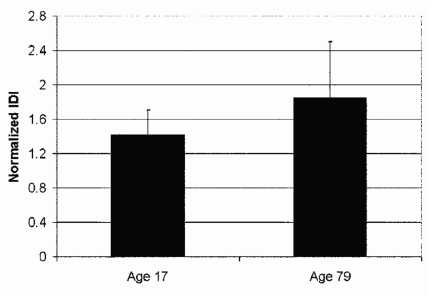
Human tibial bone from elderly females versus young females is another good model system in which to test the instrument (Fig. 6). Here, the bone was stripped of soft tissue, sectioned into pieces of order 15 mm on a side, held in a vice that was submerged in physiological buffer, and tested under the buffer. The indentations were normal to the outside surface of the cortical shell. The surface of the bone was not polished. As discussed above in the background section, there is a large body of work pointing toward the conclusion that, in general, bone from elderly donors is more easily fractured than bone from young donors. In this model system, the putatively more easily fractured bone from the elderly donor has a significantly larger IDI (Fig. 6), just as for the other model systems.
Figure 6.
The normalized IDI for the tibia of a 17-year-old female donor is smaller than that for the tibia of a 79-year-old female donor. This difference is statistically significant at the level of P<0.001 for this sample of 96 tests of the 17-year-old donor and 98 tests of the 79-year-old donor. The normalization is with respect to a standardized PMMA sample measured, at the same time, with the same probe assembly and measurement protocol. The error bars show one standard deviation.
The putative difference in fracture resistance of a 79-year-old donor relative to a 17-year-old donor was supported with four-point bending studies of small beams with lengths of order 20 mm in the longitudinal direction of the tibial diaphysis, widths of order 2 mm, and thickness of order 1 mm. They were cut from the same bone used for the BDI tests. There were five beams tested per subject. The beams were taken from the tibial diaphysis in the longitudinal direction. Mechanical properties were determined by monotonic loading to failure under four-point bending.35 Four-point bending develops a constant bending moment between the inner loading points. The advantage of this technique is that a local weakness in the testing region will be discovered by causing failure at that site. The loading fixture was custom designed to meet ASTM Standard D6272-02 for four-point bending and to ensure that a uniform moment could be applied to every specimen.36 The loading fixture was mounted on a pivot and had rollers as loading points to minimize testing errors.37 Tests were conducted with the medial side of the middiaphysis in tension and under position control with a crosshead displacement of 0.025 mm∕s tested until failure. Force and displacement data were recorded during the test and then converted to stress and strain using beam bending theory and caliper measurements from the fracture site. A custom designed MATLAB program (The MathWorks, Inc.) was used to perform this transformation and calculate all mechanical properties35). The result of the four-point bending tests was that the aged bone had, at the 0.01 level of significance, smaller yield stress, ultimate stress, failure stress, and toughness relative to the younger bones. (Table 1).
Table 1.
Mechanical properties determined by four-point bending from a young and old female subject. Mean (std. dev.)
| 17 year old | 79 year old | |
|---|---|---|
| Yield stress (MPa) | 164 (14) | 114 (25)a |
| Ultimate stress (MPa) | 257 (28) | 173 (24)a |
| Failure strain (millistrain) | 100 (20) | 52.7 (7.41)a |
| Toughness (MPa) | 19.2 (4.5) | 6.51 (0.65)a |
Significance of p<0.01.
Although, none of these parameters, or any measured mechanical parameters, have been shown to correlate with fracture risk in humans, they support the assumption that the bone from the older donor would be more easily fractured and thus the significance that the BDI measured a higher IDI for the bone from the older donor.
Differences between bones from humans of different ages
An important question is whether the BDI can measure a contribution to fracture risk that is not simply correlated to age. It is well known that bone material properties decline with age, as referenced above. For the BDI to be useful, it would have to give information about a risk factor that goes beyond what can simply be inferred from age. Only clinical tests can finally determine this, but our preliminary studies shed some light on the subject.
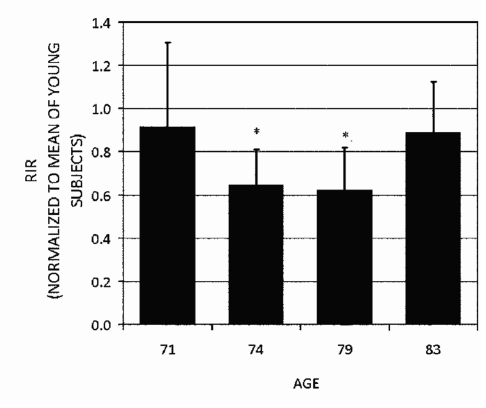
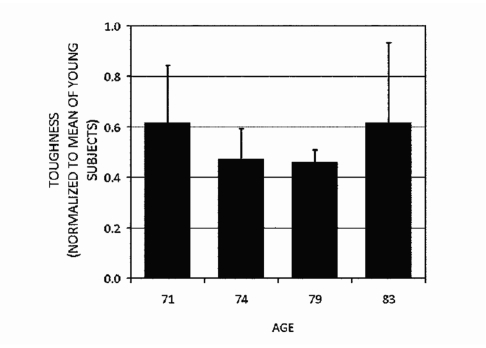
Figures 78 show that the normalized repetitive indentation resistance (RIR) which is defined as the inverse of the IDI, as measured by the Osteoprobe II™ has the same trends as measured by four-point bending. In particular, note that the RIR and toughness are not simply decreasing with age. The oldest donor had bone that had higher RIR and toughness than two out of three of the other donors in this group. The normalized RIR is used here rather than its inverse, IDI, so that bigger numbers correspond to putatively bigger fracture resistance as inferred from toughness. The normalization of the RIR in Fig. 7 and toughness in Fig. 8 is with respect to the averaged RIR from three young donors below 30 years of age. Thus, a value of 1 would indicate bone putatively as resistant to fracture as bone from young donors. That all the values for the older donors in Figs. 78 are below 1 is consistent with the results of the model system described in Sec. 4B: that older donors have less putative resistance to fracture than young donors. The four-point bending studies of small beams cut from the same bone used for the BDI tests were performed as described above. The figures show that, at least for these donors, these putative measures of fracture resistance are not a monotonic function of age. This is important. The BDI would not be necessary if its results could be simply predicted from the age of the patient.
Figure 7.
The repetitive indentation resistance (RIR) which is defined as the reciprocal of the IDI, of the tibia of four elderly donors normalized to the average RIR of tibia from three young donors (ages 17, 22, and 23 years). The stars here denote that the means are significantly less than both the 71 and 83 year old donors (p<0.05). Note that the normalized RIR is not a monotonic function of age. The most elderly donor actually had a larger normalized RIR than two younger donors. Thus, normalized RIR is not simply correlated with age. This graph is based on pooled data from ten tests on each of three different samples of bone from each of the four donors (a total of 120 tests).
Figure 8.
The toughness, measured with four-point bending to failure, of tibia from four elderly donors normalized to the average toughness of tibia from three young donors (ages 17, 22, and 23 years). Note that the normalized toughness shows the same trends as the normalized RIR shown in Fig. 7, but, in this case, the differences were not statistically significant because only one measurement could be made on each of the three samples of bone for each of the four donors.
We note, however, the downside of RIR compared to IDI. An issue stemming from abnormally low values of IDI may arise when, for example, the instrument is tipped during the primary cycling or the reference probe moves on the bone surface. The incidence of these low values was about 2% in a trial of 300 tests. These low values are not much of a problem in IDI measurements because even a value of zero is generally just a few standard deviations below the mean. However, when IDI is inverted to get RIR, these low values can become very large, over ten standard deviations above the mean. Even though they have a small incidence, they seriously affect the computed standard deviation of the whole set of measurements. This will be further elaborated and quantified in the next section.
Ten donor study and analysis: Bare bone
The data of Figs. 78 came from a study of a group of ten donors of age 17–83. The study had ten or more individual tests on each of three bone pieces cut from the tibia with dimensions of order 2 cm length and width and the full thickness of the cortical bone (of order 1 cm) cut from the tibia of each donor tested (a total of over 300 individual tests). Here, the bone pieces were stripped of soft tissue, sectioned into pieces of order 15 mm on a side, held in a vice that was submerged in physiological buffer, and tested under the buffer. The indentations were normal to the outside surface of the cortical shell. The surface of the bone was not polished. Each individual test was analyzed by software that was written to compute mechanical parameters such as IDI, elastic modulus, and hardness.
A measure is informative only when the subject-to-subject variability is greater than the bone specimen-to-bone specimen variability within one subject; otherwise, the variability between subjects may only be a reflection of the variability of the measurement process itself. Therefore, an analysis of variance was performed on each measure with replicates within bone nested within bone nested within subject. The hypothesis of interest was whether the subject-to-subject variance was greater than the bone specimen-to-bone specimen variance. Of the many parameters computed for each test, including E and H and total indentation distance, the IDI was the most significant; i.e., had the largest value of F(F=9.4) and consequently, a very significant, p<0.0001, difference between subjects. Although, a complete discussion of this analysis is beyond the scope of this paper, we note that there is a good reason for focusing on the IDI here. (The F values for other quantities are plotted in the graph in the Excel Worksheet under tab “F-sub-bone” in the supplementary documents for this paper. This Worksheet also contains all the raw data and computed variances for this study. The Worksheet also quantitates the downside of RIR, discussed above.) Even though RIR is simply the reciprocal of IDI, the F value for RIR is only F=4.7. This is due to the large contribution to the variance of the small number of tests with large values of RIR. Thus, we have only used RIR in comparison to toughness and prefer IDI.
One other note about this analysis is that elastic modulus and hardness were not useful in distinguishing one subject from another in this study and analysis. The F values were below one, corresponding to probabilities of order 50% that the null hypothesis was correct that the subject-to-subject variance was no greater than the bone-to-bone variance within one subject. In part, this is because values of E and H are highly tip dependent, much more than IDI, and different tips were used for different bone specimens. We now have a procedure to help correct for this variation. We use the tip to measure a standard sample of PMMA before and after a series of measurements on bone and then normalize the results on bone by the PMMA results. It is possible that E and H will prove less variable in future, normalized tests. Nevertheless, there is a good reason to normalize to PMMA, even though the results for IDI were highly significant even without this normalization.
Human cadaver tibiae: Bone versus bone covered with skin and soft tissue
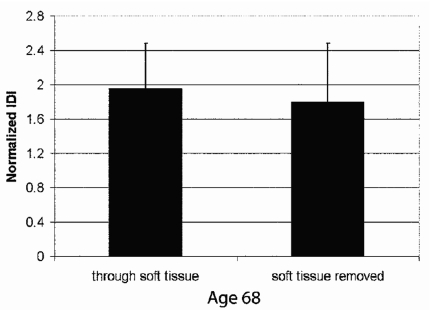
Another important question is whether or not the BDI can measure bone properties when the bone is covered with soft tissue that are comparable to the properties it would measure if the soft tissue were removed. The results of Figs. 5678 were obtained on bone from which the soft tissue had been removed. We have, however, been able to make measurements on an intact human cadaver tibia that still had the skin and all the soft tissue covering most of the tibia, but with some of the tibia exposed beyond the soft tissue. Figure 9 shows the IDI for tests done through the soft tissue compared to tests done on the part of the tibia that was exposed beyond the soft tissue, labeled “soft tissue removed.” The distance between the regions tested was approximately 10 cm. Although the small (and statistically insignificant) differences between the tests through soft tissue and with tissue removed may have had a contribution from differences in the bone between these locations, the more likely conclusion is that the material properties of bone measured subcutaneously are not significantly different from properties measured directly on bone.
Figure 9.
The normalized IDI measured through soft tissue, including the skin, on one part of the tibia from a 68-year-old donor was not significantly different from the normalized IDI measured on another part of the same tibia from which soft tissue had been removed, exposing the bone. This graph is based on the averages from ten tests each by two operators on each type of bone (a total of 40 tests).
The protocol for these tests involved inserting the probe assembly, by hand, through the skin and soft tissue. The BDI head was mounted on a stand with a slide in the vertical axis to hold the head in position after insertion. After insertion, the probe head was tapped by hand until the indentation distance remained constant. At this point, the indentation distance was usually negative, indicating that the reference probe, which is mounted directly on the head, had penetrated the periosteum, but the test probe had not. Next, the reference probe was scraped back and forth across the bone for a distance on the order of 1 mm to locally remove the periosteum. This was continued until the indentation distance was stable and near zero indicating that the test probe and reference probe were both on the surface of the bone. Then, the automatic data collection protocol was initiated. As mentioned above, loading precycles in the data collection protocol were used to provide a good reference distance for measuring the IDI. Since the IDI values were not significant different between measurements taken through soft tissue and with soft tissue removed, the BDI can work through skin and soft tissue to give measurements on the underlying bone. This is, of course, a key result for motivating and justifying human clinical tests.
Bone regeneration in a mouse model
Finally, as a last example of the potential application of the BDI, we turn to a mouse model of bone regeneration.38 The experiment was to surgically create defects (holes) in the skull of a mouse and then fill the defect with a synthetic gelatin scaffold infiltrated with murine stem cells. The bone that regenerated within the defect was then examined with microCT to evaluate the volume of regenerated bone.38
The skulls with regenerated bone were available from that experiment and probed with the BDI to see if there were differences in the material properties of the regenerated bone compared to native tissue surrounding the defect. Testing mouse calvarias required some modifications to make the BDI gentler for these thin bones: (1) the effective weight of the BDI on the sample, 12.2 N, was reduced to 2.2 N with a pulley and a 10 N counterweight, (2) the maximum force during the primary cycles was reduced from of order 10 N, as used above, to 1.5 N, (3) the reference probe was not a beveled hypodermic syringe, as in Fig. 3, but rather a blunted hypodermic syringe, cut off square and lightly sanded to better distribute the (reduced) weight of the BDI on the fragile mouse skulls, (4) the test probe was blunted to a 30 μm radius, rather than the radius of less than 5 μm that is more typically used.
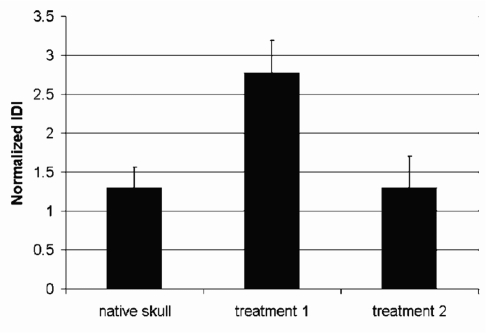
The skull fragments containing the regenerated bone in the defect were placed, with the convex (outer) surface of the skull on a piece of PMMA. The measurements were done on the exposed concave (inner, next to the brain) surface of the skull and regenerated bone. Figure 10 shows that there are significant differences in the IDI of the regenerated bone depending on the type of cells seeded in the scaffold. Thus, the BDI could distinguish the effectiveness of different treatments for bone regeneration. Further, the BDI results are consistent with the overall hypothesis that treatment 2 would more effectively regenerate bone.
Figure 10.
The normalized IDIs for bone regenerated under different tissue engineering strategies in a mouse model. Treatment 1 involved seeding Gelfoam in a cranial defect with bone marrow stromal cells. Treatment 2 involved seeding the Gelfoam with genetically modified bone marrow stromal cells. Note that though the normalized IDI was significantly greater than that of the native skull with treatment 1 (p<0.01), the genetic modification allowed regeneration of bone comparable to the native skull. This graph is based on ten tests on each of three samples of each type of bone (a total of 90 tests).
In this report, we stress that the BDI can measure the IDI in this system with only minor modifications. Clearly, much more research is needed for the development of methodology to adapt the BDI to other important systems.
PERSPECTIVES
The BDI has demonstrated, in model systems of human and animal tissue, an ability to distinguish putatively more easily fractured bone from putatively less easily fractured bone by measurement of the IDI, which is greater for putatively more easily fractured bone at least in the three model systems we have investigated. Specifically, in three model systems, in which previous mechanical testing and∕or tests reported here found degraded mechanical properties such as toughness and postyield strain, the BDI found increased IDI. However, it must be emphasized that, at this time, neither the IDI nor any other mechanical measurement by any technique has been shown clinically to correlate with fracture risk. Further, we do not yet understand the mechanism that determines IDI beyond noting that it is a measure of the damage that results from repeated loading. As such, it is more a measure of plasticity than elasticity. It may reveal the capacity of the bone for resisting (or failing to resist) continuing fracture events under the indentation tip. The IDI may also probe the same nanoscale mechanisms involving relative motion of mineralized collagen fibrils that are believed to be involved in bone fracture. Only further research can confirm or deny these possibilities.
The BDI may have demonstrated laboratory applications, but a significant open question is whether it can help in the assessment of fracture risk in living humans. Only clinical tests can answer this question.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institutes of Health (No. RO1 GM 065354). We thank Dr. Adolfo Diez Perez, Professor David Burr and Dr. Robert Recker for stimulating our interest in bone material properties and for useful suggestions about systems on which to test the BDI.
References
- Hansma P. K., Turner P. J., and Fantner G. E., Rev. Sci. Instrum. 10.1063/1.2221506 77, 075105 (2006). [DOI] [Google Scholar]
- Nalla R. K., Kruzic J. J., Kinney J. H., and Ritchie R. O., Bone (N.Y.) 10.1016/j.bone.2004.07.016 35, 1240 (2004). [DOI] [PubMed] [Google Scholar]
- Wu P. C. and Vashishth D., Proceedings of the Second Joint EMBS/BMES Conference, Houston, TX, USA2002.
- Currey J. D., Brear K., and Zioupos P., J. Biomech. 10.1016/S0021-9290(97)82890-4 30, 1001 (1997). [DOI] [Google Scholar]
- Rho J. Y., Kuhn-Spearing L., and Zioupos P., Med. Eng. Phys. 10.1016/S1350-4533(98)00007-1 20, 92 (1998). [DOI] [PubMed] [Google Scholar]
- Zioupos P., Currey J. D., and Hamer A. J., J. Biomed. Mater. Res. 45, 108 (1999). [DOI] [PubMed] [Google Scholar]
- Brown C. U., Yeni Y. N., and Norman T. L., J. Biomed. Mater. Res. 49, 380 (2000). [DOI] [PubMed] [Google Scholar]
- Phelps J. B., Hubbard G. B., Wang X., and Agrawal C. M., J. Biomed. Mater. Res. 51, 735 (2000). [DOI] [PubMed] [Google Scholar]
- Wang X., Shen X., Li X., and Agrawal C. M., Bone (N.Y.) 10.1016/S8756-3282(01)00697-4 31, 1 (2002). [DOI] [PubMed] [Google Scholar]
- Wang X., Li X., Shen X., and Agrawal C. M., Ann. Biomed. Eng. 10.1114/1.1623488 31, 1365 (2003). [DOI] [PubMed] [Google Scholar]
- Yeni Y. N. and Norman T. L., Bone (N.Y.) 10.1016/S8756-3282(00)00322-7 27, 327 (2000). [DOI] [Google Scholar]
- Wang X. D., Masilamani N. S., Mabrey J. D., Alder M. E., and Agrawal C. M., Bone (N.Y.) 10.1016/S8756-3282(98)00071-4 23, 67 (1998). [DOI] [PubMed] [Google Scholar]
- Currey J. D., Brear K., and Zioupos P., J. Biomech. 10.1016/0021-9290(95)00048-8 29, 257 (1996). [DOI] [PubMed] [Google Scholar]
- Diab T., Condon K. W., Burr D. B., and Vashishth D., Bone (N.Y.) 10.1016/j.bone.2005.09.002 38, 427 (2006). [DOI] [PubMed] [Google Scholar]
- Diab T. and Vashishth D., Bone (N.Y.) 10.1016/j.bone.2005.03.014 37, 96 (2005). [DOI] [PubMed] [Google Scholar]
- Schaffler M. B., Choi K., and Milgrom C., Bone (N.Y.) 10.1016/8756-3282(95)00370-3 17, 521 (1995). [DOI] [PubMed] [Google Scholar]
- Taylor D. and Lee T. C., J. Anat. 10.1046/j.1469-7580.2003.00194.x 203, 203 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zioupos P., J. Microsc. 10.1046/j.1365-2818.2001.00783.x 201, 270 (2001). [DOI] [PubMed] [Google Scholar]
- Rho J. Y., Tsui T. Y., and Pharr G. M., Biomaterials 10.1016/S0142-9612(97)00073-2 18, 1325 (1997). [DOI] [PubMed] [Google Scholar]
- Hoffler C. E., Moore K. E., Kozloff K., Zysset P. K., and Goldstein S. A., J. Orthop. Res. 10.1002/jor.1100180315 18, 432 (2000). [DOI] [PubMed] [Google Scholar]
- Hengsberger S., Kulik A., and Zysset P., Bone (N.Y.) 10.1016/S8756-3282(01)00624-X 30, 178 (2002). [DOI] [PubMed] [Google Scholar]
- Hengsberger S., Kulik A., and Zysset P., Eur. Cells Mater 1, 12 (2001). [DOI] [PubMed] [Google Scholar]
- Coats A. M., Zioupos P., and Aspden R. M., Calcif. Tissue Int. 10.1007/s00223-002-2080-8 73, 66 (2003). [DOI] [PubMed] [Google Scholar]
- Oliver W. C. and Pharr G. M., J. Mater. Res. 10.1557/jmr.2004.19.1.3 19, 3 (2004). [DOI] [Google Scholar]
- Peterlik H., Roschger P., Klaushofer K., and Fratzl P., Int. J. Fract. 10.1007/s10704-006-6634-z 139, 395 (2006). [DOI] [Google Scholar]
- Hansma P. K., Fantner G. E.Kindt J. H., Thurner P. J., Schitter G., Turner P. J., Udwin S. F., and Finch M. M., J. Musculoskeletal Neuronal Interactions 5, 313 (2005). [PubMed] [Google Scholar]
- Fantner G. E., Hassenkam T., Kindt J. H., Weaver J. C., Birkedal H., Pechenik L., Cutroni J. A., Cidade G. A. G., Stucky G. D., Morse D. E., and Hansma P. K., Nat. Mater. 10.1038/nmat1428 4, 612 (2005). [DOI] [PubMed] [Google Scholar]
- Gupta H. S., Seto J., Wagermaier W., Zaslansky P., Boesecke P., and Fratzl P., Proc. Natl. Acad. Sci. U.S.A. 10.1073/pnas.0604237103 103, 17741 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta H. S., Fratzl P., Kerschnitzki M., Benecke G., Wagermaier W., and Kirchner H. O. K., J. R. Soc., Interface 10.1098/rsif.2006.0172 4, 277 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta H. S., Wagermaier W., Zickler G. A., Aroush D. R. B., Funari S. S., Roschger P., Wagner H. D., and Fratzl P., Nano Lett. 10.1021/nl051584b 5, 2108 (2005). [DOI] [PubMed] [Google Scholar]
- Fantner G. E., Oroudjev E., Schitter G., Golde L. S., Thurner P., Finch M. M., Turner P., Gutsmann T., Morse D. E., Hansma H., and Hansma P. K., Biophys. J. 10.1529/biophysj.105.069344 90, 1411 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeni Y. N. and Norman T. L., Bone (N.Y.) 10.1016/S8756-3282(00)00322-7 27, 327 (2000). [DOI] [Google Scholar]
- Fantner G. E., Birkedal H., Kindt J. H., Hassenkam T., Weaver J. C., Cutroni J. A., Bosma B. L., Bawazer L., Finch M. M., Cidade G. A. G., Morse D. E., Stucky G. D., and Hansma P. K., Bone (N.Y.) 10.1016/j.bone.2004.05.027 35, 1013 (2004). [DOI] [PubMed] [Google Scholar]
- Currey J. D., Foreman J., Laketic I., Mitchell J., Pegg D. E., and Reilly G. C., J. Orthop. Res. 10.1002/jor.1100150116 15, 111 (1997). [DOI] [PubMed] [Google Scholar]
- Wallace J. M., Rajachar R. M., Allen M. R., Bloomfield S. A., Robey P. G., Young M. F., and Kohn D. H., Bone (N.Y.) 10.1016/j.bone.2006.12.002 40, 1120 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASTM Standard No. D6272-02, 2002.
- Hoagland R. G., Marschall C. W., and Duckworth W. H., J. Am. Ceram. Soc. 10.1111/j.1151-2916.1976.tb10929.x 59, 189 (1976). [DOI] [Google Scholar]
- Rossello R. A., Wang Z., Kizana E., Krebsbach P. H., and Kohn D. H., Transactions 32nd Annual Meeting of the Society for Biomaterials2007. (unpublished), Vol. 32, p. 714.