Abstract
Background
BK virus (BKV), which causes polyomavirus-associated nephropathy (PVN) in kidney transplant recipients (KTx), has 75% homology with JC virus (JCV), the etiologic agent of progressive multifocal leukoencephalopathy (PML). The large T-antigen (T-ag) is the main regulatory protein of polyomaviruses that is expressed early in the viral cycle.
Objectives
To characterize epitopes of BKV and JCV T-ag recognized by CD8+ T-cells and explore the role of these cells in containing polyomavirus infection.
Study design
We tested peripheral blood mononuclear cells of HLA A*0201+ BKV- and JCV-seropositive individuals, including patients with active BKV or JCV infection and healthy control subjects in a cross-sectional study.
Results
CD8+ T-cells that recognized the nonamer BKV Tp579, which is identical to JCV Tp578, were detected by tetramer staining in 10/13 (77%) healthy individuals, 3/10 (30%) KTx/PVN, and 4/9 (44%) patients with PML and/or HIV-infection. Conversely, BKV Tp398 and Tp410-specific CD8+ T cells were detected in 3/13(23%) and 1/13(8%) healthy individuals only.
Conclusion
These data suggests that, as it is the case for the VP1 protein, the same population of CD8+ T-cells may recognize epitopes located on the BKV and JCV T protein. The overall cellular immune response against polyomavirus T-ag, however, is lower than against the VP1 protein and is more frequently detected in healthy individuals than in patients with active BKV or JCV infection.
Keywords: BK virus, JC virus, large T antigen, cytotoxic T lymphocytes, tetramer, polyomavirus nephropathy, progressive multifocal leukoencephalopathy
Introduction
The cellular immune response to BK virus (BKV) VP1 is detectable in 80% of BKV-seropositive healthy individuals and plays an important role in the containment of BKV in kidney transplant recipients with polyomavirus nephropathy (KTx/PVN) (Binggeli et al., 2007, Chen et al., 2006, Krymskaya et al., 2005, Sharma et al., 2006). T-ag, which is the main viral regulatory protein, is the first viral protein to be expressed once BKV enters the host cell. Investigators have explored the immune response to BKV T-ag in healthy individuals (HI) or KTx recipients without PVN (Binggeli et al., 2007, Li et al., 2006, Provenzano et al., 2006, Randhawa et al., 2006). In this study, we investigated the specific cellular immune response against three BKV T-ag epitopes presented to CD8+ T cells cytotoxic T lymphocytes (CTL) by the A*0201 molecule. One of these BKV epitopes had a sequence identical with a corresponding epitope of JCV. We report for the first time testing patients with progressive multifocal leukoencephalopathy (PML) for the presence of CTL against T-ag, and comparing their responses to those of KTx/PVN patients.
Methods
We studied 32 HLA-A*0201+ individuals, including 13 healthy individuals (HI), 10 biopsy-proven KTx/PVN, and a group of 9 subjects (group PML/HIV), including 4 with PML (biopsy-proven or with CSF PCR positive for JCV: 3 HIV+ and 1 HIV−), and 5 HIV+-patients with other neurological diseases. All study subjects were shown to be BKV and JCV-seropositive except one HI whose serology could not be assessed for technical reasons. All KTx/PVN and 10/13 HI were the same as those reported in our study of the CD8+ T-cell response against BKV VP1 protein (Chen et al., 2006), and were tested in parallel using the same fresh blood samples. A total of four nonamer peptides of BKV T-ag, p410, p398, p579 and p570, predicted by the published algorithms (http://bimas.cit.nih.gov/molbio/hla_bind/, and http://www.syfpeithi.de) to bind the HLA-A*0201 molecule were synthesized, and the respective tetramers were constructed, as previously described(Du Pasquier et al., 2003, Koralnik et al., 2002). BKV Tp579 LLLIWFRPV and Tp570 ILQSGMTLL had complete homology with the corresponding epitopes of JCV Tp578 and Tp569, while BKV Tp398 CLLPKMDSV and Tp410 FLHCIVFNV had 2 and 3 amino acid (aa) difference with JCV Tp397 CLLPQMDTV and Tp409 FLKCIVLNI, respectively.
HLA typing, hemagglutination inhibition assay, 51Cr functional lysis and tetramer staining assays, and quantitation of BK viral load DNA by quantitative PCR were performed as previously described(Chen et al., 2006).
Results
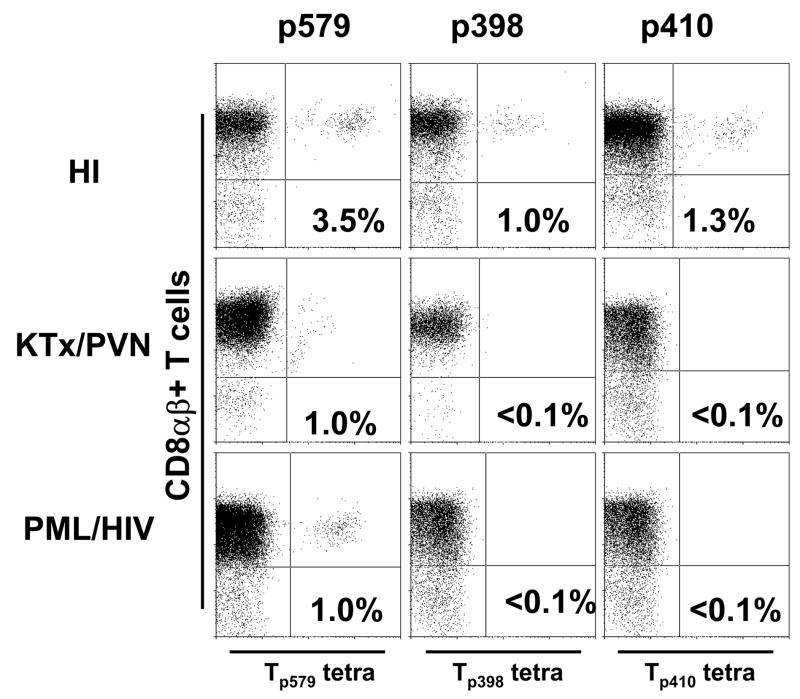
No tetramer staining of CD8αβ+ T-cells from any of the fresh blood samples was observed (data not shown). However, after 10–14 days of in vitro stimulation in the presence of peptide, CD8+ T-cells recognizing BKV Tp579, Tp398 and Tp410 were detected in 10, 3 and 1 of 13 healthy HI, respectively, while only CD8+ T-cells recognizing Tp579 were detected in 3/10 KTx/PVN and 4/9 of the PML/HIV group. No study subjects had a Tp570 response (Table 1). The percentage of CD8+ T-cells staining with the tetramers was low in all groups, between 0.2 and 3.5 % (Figure 1).
Table 1.
Immunological, virological and serological data in 32 study subjects
| Subjects | T-aga |
VL (copies/ml) |
HAI titer |
Weeks after diagnosis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p579 | p410 | p398 | p570 | Urine | Plasma | BKV | JCV | |||
| (BKV) | ||||||||||
| HI | #1 | − | − | − | − | − | NA | 1/512 | 1/32 | |
| #2 | 0.8% | − | − | − | − | NA | 1/1024 | 1/32 | ||
| #3 | 3.5% | 1.3% | 0.4% | − | − | NA | 1/512 | 1/32 | ||
| #4 | 0.2% | − | − | − | − | NA | 1/256 | 1/32 | ||
| #5 | 0.5% | − | − | − | + | NA | 1/512 | 1/32 | ||
| #6 | 0.3% | − | − | − | − | NA | 1/256 | 1/32 | ||
| #7 | 0.5% | − | − | − | − | NA | 1/2048 | 1/16 | ||
| #8 | 1.2% | − | − | − | − | NA | 1/512 | 1/64 | ||
| #9 | − | − | 1.0% | − | + | NA | 1/256 | 1/64 | ||
| #10b | 0.8% | − | − | − | NA | NA | NA | NA | ||
| #11 | 0.2% | − | − | − | − | NA | 1/1024 | 1/64 | ||
| #12 | 0.4% | − | − | − | NA | NA | 1/2048 | 1/128 | ||
| #13 | − | − | 0.2% | − | NA | NA | 1/256 | 1/64 | ||
| Total | 77% | 8% | 23% | 0% | ||||||
| (BKV) | ||||||||||
| KTx/PVN | #1 | − | − | − | − | − | − | 1/16384 | 1/1024 | 6 |
| #2 | 0.7% | − | − | − | − | − | 1/16384 | 1/64 | 90 | |
| #3 | 1.3% | − | − | − | 1.23×105 | − | 1/1024 | 1/256 | 172 | |
| #4 | − | − | − | − | 4.51×105 | 1.21×105 | 1/131072 | 1/256 | 11 | |
| #5 | − | − | − | − | 5.29×109 | 5.83×105 | 1/16384 | 1/64 | 5 | |
| #6 | − | − | − | − | 3.60×105 | 5.69×103 | 1/262144 | 1/256 | 7 | |
| #7 | − | − | − | − | 5.08×105 | − | 1/131072 | 1/256 | 16 | |
| #8 | − | − | − | − | 2.22×109 | 1.31×103 | 1/65536 | 1/32 | 4 | |
| #9 | − | − | − | − | 4.67×107 | 7.94×103 | 1/32768 | 1/128 | 70 | |
| #10 | 1.0% | − | − | − | 1.95×105 | − | 1/131072 | 1/128 | 170 | |
| Total | 30% | 0% | 0% | 0% | ||||||
| (JCV) | ||||||||||
| HIV+/PML | #1c | 0.3% | − | − | − | − | − | 1/512 | 1/2048 | 40 |
| #2d | − | − | − | − | − | − | 1/1024 | 1/2048 | 728 | |
| #3c | 0.3% | − | − | − | 7.5×103 | − | 1/512 | 1/512 | 12 | |
| HIV−/PML | #1d | − | − | − | − | − | − | 1/64 | 1/256 | 780 |
| HIV+ | #1 | 1.0% | − | − | − | 2.7×107 | − | 1/64 | 1/128 | |
| #2 | 0.2% | − | − | − | − | − | 1/512 | 1/32 | ||
| #3 | − | − | − | − | 2.27×108 | − | 1/128 | 1/64 | ||
| #4 | − | − | − | − | 1.08×107 | − | 1/256 | 1/64 | ||
| #5 | − | − | − | − | − | − | 1/256 | 1/16 | ||
| Total | 44% | 0% | 0% | 0% | ||||||
T-ag: BKV large T antigen, VL: BKV or JCV viral load, NA: not available, +: positive using qualitative PCR, −: undetectable.
: percentage of tetramer positive CD8+ T-cells,
: serology couldn’t be performed for technical reason.
: JCV CSF PCR-proven PML.
: biopsy-proven PML
Figure 1.
Staining of PBMC from an HLA A*0201+ healthy individual, a PML patient and a KTx/PVN patient with tetrameric HLA-A*0201/BKV VP1p579, VP1p410 and VP1p398 complexes after in vitro stimulation with the respective peptides for 10–14 days. The percentages of CD8αβ+ T cells that bind the tetramers (dots in right upper quadrant of each panel) are indicated. Results were considered positive if the percentage of tetramer staining cells was equal or greater to 0.1% of CD8αβ+ T cells and formed a distinct population of cells on the dot plot.
In KTx/PVN, the percentage of cells staining with Tp579 tetramer was comparable to the results obtained in the same subjects with BKV VP1p44, but lower than for VP1p108 (patients 2,3 and 10, Fig 4 (Chen et al., 2006)). In HI the T-ag-specific response was globally lower than the VP1-specific response (Chen et al., 2006) in 7/13, comparable in 5/13, and in one subject only a T-ag, but no VP1-specific response, was detected. Finally, in the PML/HIV group the T-ag response was always at least one log below that of the VP1 response (data not shown).
As shown in table 1, 12/13 HI (92%) had CD8+ T cells recognizing either one or more of the T epitopes tested, which was significantly higher than 3/10 (30%) KTx/PVN (p<0.01), and 4/9 (44%) HIV+/PML group (p=0.02) (Fisher’s exact test, 2 tail). These data suggest that in immunocompromised patients, the cellular immune response against T-ag is lower than in HI regardless of the antigenic stimulation associated with an elevated BKV viral load in KTx/PVN and JCV viral load in the HIV+/PML group.
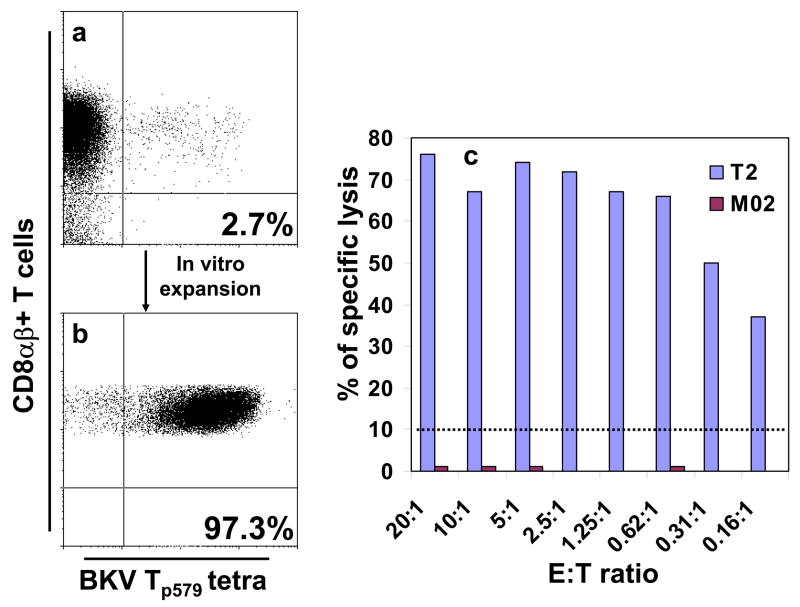
We then performed 51Cr lysis assays using the same peptide-stimulated peripheral blood mononuclear cells (PBMC) from HI and KTx/PVN. The results were negative in all cases, commensurate with the low percentages of tetramer staining cells after in vitro stimulation. However, an enriched Tp579-specific T-cell line from a HI (Fig 2, panel b), used as effectors, could lyse a Tp579-pulsed T2 cell line, which expresses the A*0201 molecule only, but not the M02 BLCL that is totally mismatched for MHC class-I alleles(Figure 2, panel c). These results demonstrate that Tp579-specific CD8+ T-cells are functionally active CTL that are A*0201-restricted in their target cell recognition.
Figure 2.
Effector cell recognition of the BKV Tp579 peptide is HLA-A*0201-restricted. BKV Tp579- stimulated PBMC from an HLA A*0201+ healthy individual were stained with the respective tetramer, gating on CD8αβ+ T cells. A small population comprising 2.7% of tetramer-binding CD8+ T cells (panel a, right upper quadrant) was sorted by flow cytometry and propagated in vitro for 2 months. A 97.3% enriched BKV Tp579-specific CTL cell line (panel b, right upper quadrant) could lyse the p579-pulsed T2 cell line, expressing the A*0201 class I molecule only, but not the p579-pulsed M02 BLCL (totally mismatched for HLA class I alleles with the p579 CTL line) (panel c)
The three KTx/PVN with positive BKV Tp579 tetramer staining had BK DNA viral load that was undetectable in serum and their viral load in urine was undetectable, 1.23 × 105/ml and 1.5 × 105/ml, respectively, as measured by quantitative PCR(Chen et al., 2006). These patients were tested at 90, 170 and 172 weeks after PVN diagnosis. Conversely, there was a trend for the viral load of eight patients with a negative BKV Tp579 tetramer staining to be higher and ranged from undetectable to 5.83 × 105 copies/ml serum (mean:1.43 × 105) and from undetectable to 5.29 × 109 copies/ml urine (mean:1.25×109)(p=0.1462, one way Anova). These patients were tested between 4 and 70 weeks after KTx/PVN diagnosis (mean: 17 weeks).
To explore further potential cross-reactivities between BKV and JCV CTL epitopes, we constructed A*0201 tetramers with the JCV Tp397 and Tp409 which have 2 and 3 amino acids different from the corresponding BKV Tp398 and Tp410, respectively. No detectable tetramer staining was found in PBMC of 9 PML/HIV patients and 8 HI tested which suggest that these JCV peptides are not CTL epitopes.
Discussion
Our results confirm that T-ag is a target of the cellular immune response (Binggeli et al., 2007, Comoli et al., 2004, Koralnik et al., 2001, Li et al., 2006, Provenzano et al., 2006, Randhawa et al., 2006, Tong, 2006). Provenzano, et al. have also identified the BKV Tp579 epitope in A*0201+ healthy BKV-seropositive individuals and shown that a Tp579-specific cell line could lyse a human melanoma cell line (HBL) transfected with BKV Tag despite low CTL precursor frequencies, estimated to be around 1/20,000 by limiting dilution analysis. This result suggests that BKV Tp579/JCV Tp578 can be naturally processed and presented to the cell surface, where it can be recognized by p579/p578-specific CTL. That low frequency of CTL is consistent with our present data showing that Tp579-specific CD8+ T-cells were undetectable by tetramer staining ex vivo in fresh blood samples of all our study subjects, including KTx/PVN. Furthermore, a CTL response against this epitope could only be detected by Provenzano and colleagues after 3 rounds of in vitro stimulation of PBMC with peptide-loaded autologous dendritic cells over 21 days, and using a Tp579-specific CD8+ T-cell line in our study (Fig 2C).
Our data suggest that while A*0201-restricted CTL epitopes are present in BKV and JCV T-ag, they do not elicit a stronger CTL response compared to previously identified epitopes of the major capsid protein, VP1 (Chen et al., 2006, Du Pasquier et al., 2003, Koralnik et al., 2002, Koralnik et al., 2001). This finding has also been reported by a recent study by Binggeli (Binggeli et al., 2007) who examined the global T cell response to BKV T and VP1 proteins using libraries of overlapping peptides, and found lower responses against Tag compared to VP1. Interestingly, the most frequently recognized epitope, BKV Tp579, is identical to the corresponding epitope of JCV Tp578. This cross-recognition of BKV and JCV epitopes by CTL was already demonstrated in the VP1 protein (Chen et al., 2006, Krymskaya et al., 2005, Sharma et al., 2006). Since most adults are seropositive for both viruses, a correct interpretation of the CTL response depends on the baseline condition of the patients. In PML, the importance of the CTL response is indicated by the correlation of detectable cellular immunity and outcome of PML, indicating that the functional significance of this response is directed against JCV (Du Pasquier et al., 2004). In KTx/PVN, a strong anti-polyomavirus CTL response is associated with a lower BK viral load and antibody titers, suggesting that these cells are directed against BKV (Chen et al., 2006). Finally, it is safe to assume that dually JC and BK-infected individuals have responses against antigens present in both viruses.
In KTx/PVN, the Tp579–specific CTL could only be detected more than a year after PVN diagnosis. A longitudinal study of KTx/PVN, which is outside of the scope of the present study, will be needed to determine whether BKV Tp579-specific CTL may be part of a chronic, rather than an acute, response against BKV, and to understand how they participate in the control BKV replication and disease outcome.
Acknowledgments
We are grateful to Michele Lifton and Darci Gorgone for technical assistance. This work was supported in part by Public Health Service grant and NS/AI 041198 to IJK, and the Paul Teschan Research Fund from Dialysis Clinics to JT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Binggeli S, Egli A, Schaub S, Binet I, Mayr M, Steiger J, Hirsch HH. Polyomavirus BK-specific cellular immune response to VP1 and large T-antigen in kidney transplant recipients. Am J Transplant. 2007;7:1131–9. doi: 10.1111/j.1600-6143.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Trofe J, Gordon J, Du Pasquier RA, Roy-Chaudhury P, Kuroda MJ, Woodle ES, Khalili K, Koralnik IJ. Interplay of cellular and humoral immune responses against BK virus in kidney transplant recipients with polyomavirus nephropathy. J Virol. 2006;80:3495–505. doi: 10.1128/JVI.80.7.3495-3505.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoli P, Azzi A, Maccario R, Basso S, Botti G, Basile G, Fontana I, Labirio M, Cometa A, Poli F, Perfumo F, Locatelli F, Ginevri F. Polyomavirus BK-specific immunity after kidney transplantation. Transplantation. 2004;78:1229–32. doi: 10.1097/01.tp.0000137932.44791.d3. [DOI] [PubMed] [Google Scholar]
- Du Pasquier RA, Kuroda MJ, Schmitz JE, Zheng Y, Martin K, Peyerl FW, Lifton M, Gorgone D, Autissier P, Letvin NL, Koralnik IJ. Low frequency of cytotoxic T lymphocytes against the novel HLA-A*0201-restricted JC virus epitope VP1(p36) in patients with proven or possible progressive multifocal leukoencephalopathy. J Virol. 2003;77:11918–26. doi: 10.1128/JVI.77.22.11918-11926.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Pasquier RA, Kuroda MJ, Zheng Y, Jean-Jacques J, Letvin NL, Koralnik IJ. A prospective study demonstrates an association between JC virus-specific cytotoxic T lymphocytes and the early control of progressive multifocal leukoencephalopathy. Brain. 2004;127:1970–8. doi: 10.1093/brain/awh215. [DOI] [PubMed] [Google Scholar]
- Koralnik IJ, Du Pasquier RA, Kuroda MJ, Schmitz JE, Dang X, Zheng Y, Lifton M, Letvin NL. Association of prolonged survival in HLA- A2+ progressive multifocal leukoencephalopathy patients with a CTL response specific for a commonly recognized JC virus epitope. J Immunol. 2002;168:499–504. doi: 10.4049/jimmunol.168.1.499. [DOI] [PubMed] [Google Scholar]
- Koralnik IJ, Du Pasquier RA, Letvin NL. JC virus-specific cytotoxic T lymphocytes in individuals with progressive multifocal leukoencephalopathy. J Virol. 2001;75:3483–7. doi: 10.1128/JVI.75.7.3483-3487.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krymskaya L, Sharma MC, Martinez J, Haq W, Huang EC, Limaye AP, Diamond DJ, Lacey SF. Cross-reactivity of T lymphocytes recognizing a human cytotoxic T-lymphocyte epitope within BK and JC virus VP1 polypeptides. J Virol. 2005;79:11170–8. doi: 10.1128/JVI.79.17.11170-11178.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Melenhorst J, Hensel N, Rezvani K, Sconocchia G, Kilical Y, Hou J, Curfman B, Major E, Barrett AJ. T-cell responses to peptide fragments of the BK virus T antigen: implications for cross-reactivity of immune response to JC virus. J Gen Virol. 2006;87:2951–60. doi: 10.1099/vir.0.82094-0. [DOI] [PubMed] [Google Scholar]
- Provenzano M, Bracci L, Wyler S, Hudolin T, Sais G, Gosert R, Zajac P, Palu G, Heberer M, Hirsch HH, Spagnoli GC. Characterization of highly frequent epitope-specific CD45RA+/CCR7+/− T lymphocyte responses against p53-binding domains of the human polyomavirus BK large tumor antigen in HLA-A*0201+ BKV-seropositive donors. J Transl Med. 2006;4:47. doi: 10.1186/1479-5876-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randhawa PS, Popescu I, Macedo C, Zeevi A, Shapiro R, Vats AN, Metes D. Detection of CD8+ T cells sensitized to BK virus large T antigen in healthy volunteers and kidney transplant recipients. Hum Immunol. 2006;67:298–302. doi: 10.1016/j.humimm.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Sharma MC, Zhou W, Martinez J, Krymskaya L, Srivastava T, Haq W, Diamond DJ, Lacey SF. Cross-reactive CTL recognizing two HLA-A*02-restricted epitopes within the BK virus and JC virus VP1 polypeptides are frequent in immunocompetent individuals. Virology. 2006;350:128–36. doi: 10.1016/j.virol.2006.02.040. [DOI] [PubMed] [Google Scholar]
- Tong D, Miller JD, Kokko KE, McLaughlin S, Lukacher AE, Gangappa S, Larsen CP. Healthy individuals generate predominantly CD4 response against polyoma virus BK. Am J Transplant. 2006;5(S11):452. [Google Scholar]