Abstract
CD4+CD25+FOXP3+ regulatory T cells (Treg) successfully control graft-versus-host-disease (GVHD) in animal models. In humans, incomplete reconstitution of Treg after allogeneic hematopoietic stem cell transplantation (HSCT) has been associated with chronic GVHD. Recent studies have demonstrated that IL-2 infusions expand Treg in vivo. However, the effectiveness of this therapy depends on the number of cells capable of responding to IL-2. We examined the effect of low-dose IL-2 infusions on Treg populations after HSCT in patients who also received infusions of donor CD4+ lymphocytes. Utilizing FOXP3 as a Treg marker, we found that patients who received CD4+DLI concomitantly with IL-2 had greater expansion of Treg compared to patients who received IL-2 (p=0.03) or CD4+DLI alone (p=0.001). FOXP3 expression correlated with absolute CD4+CD25+ cell counts. Moreover, expanded CD4+CD25+ T cells displayed normal suppressive function and treatment with CD4+DLI and IL-2 was not associated with GVHD. This study suggests that administration of low-dose IL-2 combined with adoptive CD4+ cellular therapy may provide a mechanism to expand Treg in vivo.
Introduction
CD4+CD25+FOXP3+ regulatory T cells (Treg) play a critical role in controlling immune reactions following allogeneic hematopoietic stem cell transplantation (HSCT) (1). In mice, infusion of purified Treg at the time of transplant can prevent the development of lethal graft versus host disease (GVHD) while depletion of these cells worsens GVHD (2–5). In most experimental models, prevention of GVHD is achieved while preserving a beneficial graft versus leukemia effect (GVL) (3–5). In humans, poor reconstitution of Treg after HSCT correlates with the subsequent development of chronic GVHD (6, 7). Taken together, these findings suggest that methods to increase the number of Treg in vivo may provide a new approach for either prevention or treatment of GVHD following allogeneic HSCT (1, 8, 9). Based on murine models, several investigators have proposed that adoptive therapy with purified Treg that have previously been expanded in vitro may be able to prevent or treat GVHD (10, 11). Recent clinical studies have also shown that IL-2 therapy induces the selective expansion of Treg following HSCT as well as in patients with solid tumors (12–14). The effect of IL-2 however, depends on the presence of sufficient numbers of Treg capable of responding to cytokine stimulation. Combining adoptive cellular therapy together with IL-2 administration could therefore represent a strategy to effectively expand Treg in patients where these cells are severely reduced. In the present study, we examined the modulation of Treg in patients who received concomitant CD8-depleted donor lymphocyte infusion (CD4+DLI) together with prolonged low-dose IL-2 following HSCT. We provide evidence that this combined therapy substantially expands Treg in vivo to a level greater than CD4+DLI or low-dose IL-2 alone.
Materials and Methods
Patients and samples
Patients included in this study were enrolled in clinical protocols approved by the Dana-Farber Cancer Institute Investigational review board (IRB). All patients received myeloablative conditioning and peripheral blood mononuclear cells (PBMC) were isolated from blood before and after treatment and cryopreserved. Patient and treatment characteristics are summarized in Table 1.
Quantitative PCR
Quantitative PCR for FOXP3 has been described previously (7, 14). Briefly, experiments were performed using universal PCR Master Mix (Applied Biosystems) and FOXP3 specific primers and probe. Results were normalized based on the amplification of TFRC (CD71, transferrin receptor).
Immunofluorescence analysis
Intracellular staining of FOXP3, was performed using a commercially available kit (eBioscience, San Diego, CA). Absolute numbers of CD4+ and CD4+CD25+ lymphocytes were calculated based on total lymphocyte counts and phenotypic data.
Suppression of T cell proliferation by Treg
Standard suppression assays were performed as described previously (7). Briefly, CD4+CD25− and CD4+CD25+ cells were purified from patient samples by cell sorting and incubated either alone or together (ratio 1:1) in the presence of soluble anti CD3 (OKT3; 1μg/ml) and irradiated allogeneic PBMC (1×104 cells/well). Responder cell proliferation was measured after 6 days.
Statistical analysis
FOXP3 expression level was log-transformed (base 10). Student t-test was used for two-sample comparisons. Linear regression models were fit to examine the group effect after adjusting for age, sample collection days, time from HSCT/DLI to therapy, IL-2 dose, duration of IL-2, and DLI dose.
Results
Patient groups
In the present study we investigated whether a combination of IL-2 and adoptive cellular therapy could enhance the expansion of Treg in vivo. We examined changes in circulating Treg in 3 groups of patients who had previously undergone allogeneic HSCT following a myeloablative conditioning regimen consisting of cyclophosphamide and total body irradiation. Patient characteristics are summarized in Table 1. The first group includes 5 patients with relapsed hematologic malignancy who received CD8-T cell depleted DLI (CD4+ DLI) and low-dose IL-2. These patients received defined doses of CD4+ DLI (3 × 107 to 1 × 108 CD4+ cells/kg) followed by daily administration of 6 × 105 IU/m2 IL-2 for 12 continuous weeks. One patient had to discontinue treatment after 5 weeks for accelerated disease (pt. 1). This Phase I trial was designed to evaluate safety of the combined therapy as a primary endpoint and anti-tumor effect of the combined therapy as a secondary endpoint. The second group of 5 patients received lower doses of IL-2 (2 × 105 IU/m2/day) for 12–13 continuous weeks. For these patients, IL-2 was administered relatively early after CD6 depleted allogeneic transplant when leukemia was in remission with the intent to increase the GVL effect and prevent relapse(15, 16). Patients were monitored for safety and the effects of IL-2 therapy on reconstitution of NK cells (CD56+) and T cells (CD3+CD4+ and CD3+CD8+) in peripheral blood. Group 3 was comprised of 4 patients who received CD4+ DLI alone without IL-2 for relapsed leukemia. This Phase I trial was undertaken to evaluate the safety of escalating doses of CD4+ DLI as well as efficacy of DLI. None of the patients included in the 3 groups had prior episodes of acute or chronic GVHD. As shown in Table 1, times from HSCT to therapy varied for the different groups. Patients who received IL-2 alone were treated prophylactically beginning at 6–12 weeks post-transplant. In contrast, patients who received DLI with or without IL-2 were treated for relapsed leukemia at later times post-transplant. Toxicity specifically associated with IL-2 administration has been reported previously (17, 18). IL-2 toxicity in these patients was minimal including transient fatigue, fever, nausea, myalgia and rash and was consistent with previous findings (17, 18). CD4+ DLI was also well tolerated and did not lead to any severe side effects as previously reported in similar trials (19, 20). None of the patients examined in this study developed GVHD following IL-2 infusion or CD4+ DLI. One patient who received CD4+ DLI alone developed GVHD after treatment.
Increase in FOXP3 gene expression following CD4+ DLI and IL-2 therapy
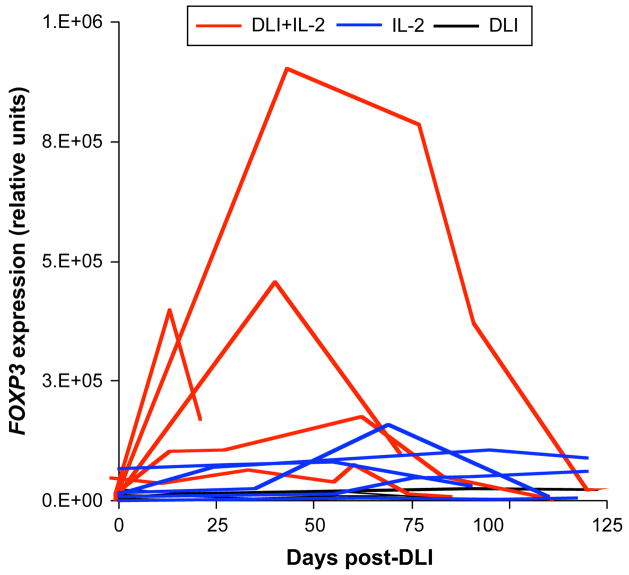
To measure changes in Treg during therapy we assessed FOXP3 gene expression by quantitative PCR in PBMC collected before and after treatment. Results are reported in Figure 1 for the 3 patient groups. As depicted in this figure, concomitant administration of low-dose IL-2 and CD4+DLI (red lines) induced a significant increase in FOXP3 gene expression levels when compared to CD4+DLI only (black lines; Student t-test for largest value, p=0.001) or low-dose IL-2 only (blue lines; Student t-test for largest value, p=0.03). Increase in FOXP3 gene expression following CD4+DLI + IL-2 was also confirmed in a linear regression model for which age, sampling time, time from HSCT to therapy, DLI and IL-2 doses as well as duration of IL-2 therapy were adjusted (p<0.001). Increased FOXP3 gene expression was not seen in any of the patients who only received CD4+DLI. In addition, compared to previously published reports, IL-2 had a relatively weak effect on FOXP3 gene expression in the 5 patients who only received this treatment (12–14). This was likely due to low numbers of CD4+CD25+ T cells in these patients prior to therapy early after stem cell transplantation. The median absolute CD4+CD25+ T cell counts in these patients before treatment was only 7.5 +/−3.8 cells/μl compared to approximately 200 CD4+CD25+ cells/μl in the peripheral blood of healthy donors. Only one patient (Pt. 4) did not experience an increase in FOXP3 gene expression following CD4+DLI + IL-2. This patient had relapsed AML at the time therapy was started.
Figure 1. Increased FOXP3 gene expression following CD4+ DLI plus low dose IL-2.
FOXP3 expression was assessed by real time PCR in PBMC from patients who received CD4+ DLI (black lines), low-dose IL-2 (blue lines) or combined CD4+ DLI and low-dose IL-2 (red lines). FOXP3 expression levels were normalized based on the expression of TFRC (transferrin receptor).
CD4+ DLI + IL-2 induces peripheral Treg expansion and increased FOXP3 protein expression in peripheral Treg
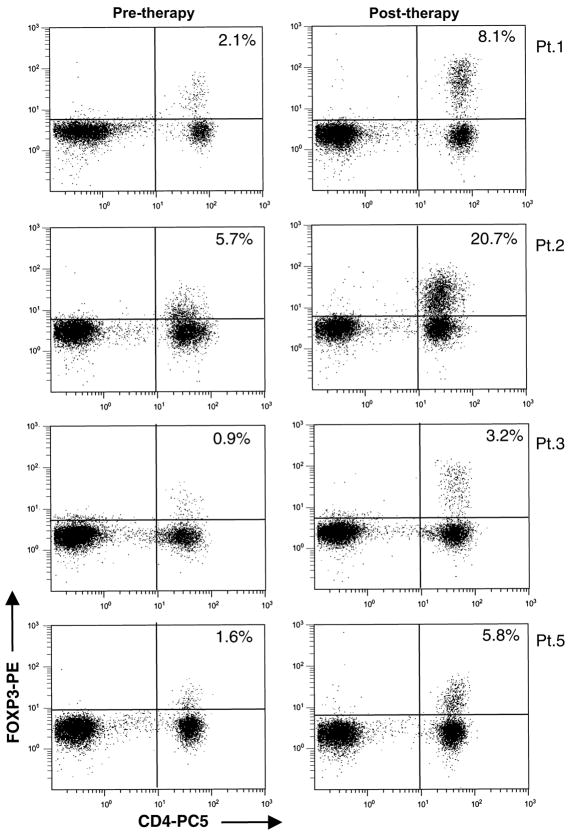
FOXP3 protein expression in peripheral blood lymphocytes was also examined by flow cytometry before and after CD4+ DLI and IL-2. As shown in Figure 2 for the 4 patients in Group 1 who had increased FOXP3 gene expression by quantitative RT-PCR, this was confirmed by an expansion of CD4+ T cells expressing intracellular FOXP3 protein. Staining intensity for FOXP3 was increased for all CD4+CD25+ T cells indicating that IL-2 also induced up-regulation of FOXP3 gene expression at the single cell level.
Figure 2. Intracellular staining for FOXP3 protein in patients who received CD4+ DLI and IL-2.
Patient PBMC were tested for expression of cell membrane CD4 and intracellular FOXP3. Samples were collected before patients received CD4+DLI and IL-2 and between 13 to 77 days after starting therapy. Dot-plots are shown after gating on lymphocyte populations.
FOXP3 gene expression levels correlate with absolute CD4+CD25+ T cell counts
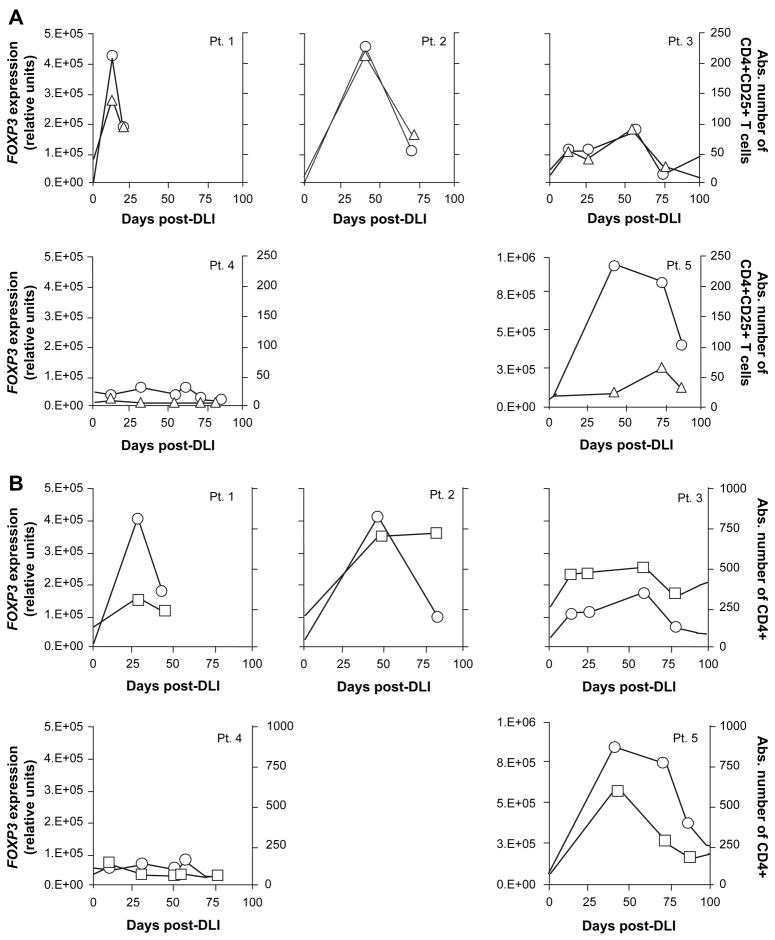
Consistent with the IL-2 effect on Treg expansion, we observed a highly significant correlation between FOXP3 gene expression levels and absolute CD4+CD25+ counts (Spearman correlation coefficient r=0.79, p=0.0003; Figure 3A). These results provided additional support for assessing FOXP3 gene expression as a surrogate marker for Treg populations. Correlation between FOXP3 gene expression levels and CD4+ counts was also significant in these patients (r=0.76, p=0.0006: Figure 3B).
Figure 3. Correlation of FOXP3 gene expression and CD4+CD25+ Treg and total CD4 T cells in peripheral blood after CD4+ DLI and IL-2.
A. FOXP3 gene expression (circles) was assessed by quantitative PCR in PBMC collected from patients who received CD4+DLI + IL-2 and is shown together with absolute blood CD4+CD25+ T cell counts (triangles; cells/μl). Spearman correlation coefficient r=0.79, p=0.0003. B. FOXP3 gene expression is depicted (circles) together with absolute blood CD4+ T cell counts (squares; cells/μl) for all patients treated with CD4+DLI + IL-2. Spearman correlation coefficient r=0.76, p=0.0006.
CD4+ DLI + IL-2 expanded Treg exhibit normal immune suppressive function in vivo
To determine whether Treg that expanded in vivo after CD4+DLI and IL-2 maintained their suppressive function, we tested purified CD4+CD25+ cells from these patients for their ability to inhibit proliferation of autologous CD4+CD25− T cells in vitro. As shown in Figure 4, Treg purified from patient samples collected during combined therapy at a time when FOXP3 gene expression was increased, displayed similar levels of suppressive function as Treg isolated from healthy donors.
Figure 4. Suppressive activity of CD4+CD25+ Treg following IL-2 and CD4+ DLI combined therapy.
Suppressive activity of purified CD4+CD25+ T cells was assessed for patients 1, 2, 3 and 5 using samples collected after DLI and during IL-2 treatment. Suppression by Treg purified from 4 healthy donors was also measured. Solid lines represent median values for the 2 groups.
Discussion
The present studies demonstrate that the addition of low dose IL-2 to CD4+ lymphocyte infusions results in a marked expansion of CD4+CD25+FOXP3+ regulatory T cells. The increase was significantly greater than that observed in patients receiving rIL-2 alone. As this latter group of patients received a lower dose of IL-2 that patients who received both DLI and IL-2, it is possible that the dose of IL-2 contributed to the different effects in these 2 groups of patients. In contrast, patients who received CD8 depleted DLI alone had no increase in circulating CD4+CD25+FOXP3+ regulatory T cells whatsoever. These results provide a framework in which allogeneic Tregs can be expanded in vivo without the need for long term ex vivo culture.
It is noteworthy that previous studies from our institution have documented that low dose IL-2 can be safely administered to patients early after T cell depleted allogeneic transplantation without inducing acute or chronic GVHD. We previously demonstrated that such treatment dramatically expands CD56+CD3− NK cells, but also CD4+CD25+FOXP3+ regulatory T cells (14, 17, 18). The patients receiving CD8+ depleted DLI plus IL-2 also did not develop any GVHD. Nor, however, was any GVL activity noted. None of the patients receiving CD8 depleted DLI plus IL-2 achieved significant tumor responses. It is possible that the anti-tumor effect of DLI was diminished by the expansion of Treg though all of these patients had advanced hematologic malignancies prior to treatment. While the original intent of this clinical trial was to augment cytotoxic effector activity, the combination of CD8 depleted DLI and low dose IL-2 appears to have served to instead expand cells with potent immune suppressive properties. We have reported on the expansion of effector T cells with potent anti-tumoral activity following CD4+ DLI (21). It is possible that expanded Treg in patients who received the combined therapy may have diminished the efficacy of such GVL effector cells.
Other studies published by Ahmadzadeh et al. and Zhang et al. have also demonstrated the capacity of IL-2 therapy to expand human Treg in vivo using higher doses and intermittent therapy with IL-2 (12–14). We have also reported similar expansion of Treg in vivo following administration of low doses of IL-2 comparable to the doses given to DLI recipients in this study (14). IL-2 directly induces the up-regulation of FOXP3 gene expression through the phosphorylation of STAT proteins downstream of the IL-2 receptor that subsequently translocate to the nucleus and bind to regulatory motifs located in the first intron of the FOXP3 gene (14). This signaling pathway appears active in CD4+CD25+ Treg that already express FOXP3 but not in CD4+CD25− cells. Consistent with this mechanism, the intensity of FOXP3 intra-cellular staining in Treg increased following IL-2 treatment, reflecting an up-regulation of gene expression at the single cell level. The effect of IL-2 therapy is therefore dependent on preexisting populations of Treg in treated patients. Following HSCT, T cell populations are often reduced due to the preparative regimen and concomitant administration of calcineurin inhibitors. Our studies suggest that a combination of both CD8-T cell depleted DLI to replenish CD4+ T cell pools together with administration of low dose IL-2 can more effectively expand Treg in vivo.
Recent investigations have revealed a crucial role for Treg in controlling immune responses post-HSCT and especially the development of GVHD. In animal models, infusion of Treg at the time of stem cell infusion or immediately following transplantation successfully prevented GVHD (2, 3, 5). In most studies, this was achieved while maintaining the beneficial GVL effect. In the autologous setting however, Treg have been shown on numerous occasions to impair anti-tumor immunity (22). In humans, we and others have shown that poor Treg reconstitution correlates with chronic GVHD (6, 7, 23, 24). Treg cellular therapy could thus be utilized to replenish Treg pools in vivo and prevent or even correct GVHD. A number of studies have addressed the feasibility of purifying and expanding human Treg ex vivo in order to use these cells for adoptive therapy (10, 25, 26). Overall, our retrospective study suggests that combined therapy with CD4+ donor lymphocytes and low-dose IL-2 may be an alternative strategy for expanding Treg in vivo. This strategy does not rely on the purification and selective expansion of Treg in vitro. Furthermore, previous findings that combined CD4+DLI and low-dose IL-2 have not been associated with GVHD suggest that this approach could be safely tested in clinical trials with careful monitoring of immunological effects and toxicity.
Acknowledgments
EZ and MM designed and performed the research, analyzed the data and wrote the paper. HK performed the statistical analysis of the data. FP analyzed the data. AL performed part of the research. RB analyzed the data. CC provided patient samples and clinical data. EPA and RJS contributed to the design of the study and analyzed the data. JR contributed to the design of the study, analyzed the data and edited the manuscript.
Financial Support: This study was supported by the Jock and Bunny Adams Research and Education Endowment, the Ted and Eileen Pasquarello Research Fund and NIH grants AI29530 and HL70149.
Abbreviations
- Treg
T Regulatory Cell
- HSCT
Hematopoietic Stem Cell Transplantation
- GVHD
Graft-Versus-Host Disease
- DLI
Donor Lymphocyte Infusion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoffmann P, Edinger M. CD4+CD25+ regulatory T cells and graft-versus-host disease. Semin Hematol. 2006;43(1):62–9. doi: 10.1053/j.seminhematol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4(+)CD25(+) immunoregulatory T cells: new therapeutics for graft-versus-host disease. J Exp Med. 2002;196(3):401–6. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9(9):1144–50. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 4.Jones SC, Murphy GF, Korngold R. Post-hematopoietic cell transplantation control of graft-versus-host disease by donor CD425 T cells to allow an effective graft-versus-leukemia response. Biol Blood Marrow Transplant. 2003;9(4):243–56. doi: 10.1053/bbmt.2003.50027. [DOI] [PubMed] [Google Scholar]
- 5.Trenado A, Charlotte F, Fisson S, et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest. 2003;112(11):1688–96. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miura Y, Thoburn CJ, Bright EC, et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004;104(7):2187–93. doi: 10.1182/blood-2004-03-1040. [DOI] [PubMed] [Google Scholar]
- 7.Zorn E, Kim HT, Lee SJ, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106(8):2903–11. doi: 10.1182/blood-2005-03-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hess AD. Modulation of graft-versus-host disease: role of regulatory T lymphocytes. Biol Blood Marrow Transplant. 2006;12(1 Suppl 2):13–21. doi: 10.1016/j.bbmt.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Zorn E. CD4+CD25+ regulatory T cells in human hematopoietic cell transplantation. Semin Cancer Biol. 2006;16(2):150–9. doi: 10.1016/j.semcancer.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann P, Boeld TJ, Eder R, et al. Isolation of CD4+CD25+ regulatory T cells for clinical trials. Biol Blood Marrow Transplant. 2006;12(3):267–74. doi: 10.1016/j.bbmt.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Karakhanova S, Munder M, Schneider M, Bonyhadi M, Ho AD, Goerner M. Highly efficient expansion of human CD4+CD25+ regulatory T cells for cellular immunotherapy in patients with graft-versus-host disease. J Immunother. 2006;29(3):336–49. doi: 10.1097/01.cji.0000203080.43235.9e. [DOI] [PubMed] [Google Scholar]
- 12.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107(6):2409–14. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Chua KS, Guimond M, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4(+)CD25(+) regulatory T cells. Nat Med. 2005 doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 14.Zorn E, Nelson EA, Mohseni M, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108(5):1571–9. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soiffer RJ, Fairclough D, Robertson M, et al. CD6-depleted allogeneic bone marrow transplantation for acute leukemia in first complete remission. Blood. 1997;89(8):3039–47. [PubMed] [Google Scholar]
- 16.Soiffer RJ, Murray C, Mauch P, et al. Prevention of graft-versus-host disease by selective depletion of CD6-positive T lymphocytes from donor bone marrow. J Clin Oncol. 1992;10(7):1191–200. doi: 10.1200/JCO.1992.10.7.1191. [DOI] [PubMed] [Google Scholar]
- 17.Soiffer RJ, Murray C, Cochran K, et al. Clinical and immunologic effects of prolonged infusion of low-dose recombinant interleukin-2 after autologous and T-cell-depleted allogeneic bone marrow transplantation. Blood. 1992;79(2):517–26. [PubMed] [Google Scholar]
- 18.Soiffer RJ, Murray C, Gonin R, Ritz J. Effect of low-dose interleukin-2 on disease relapse after T-cell-depleted allogeneic bone marrow transplantation. Blood. 1994;84(3):964–71. [PubMed] [Google Scholar]
- 19.Alyea EP, Soiffer RJ, Canning C, et al. Toxicity and efficacy of defined doses of CD4(+) donor lymphocytes for treatment of relapse after allogeneic bone marrow transplant. Blood. 1998;91(10):3671–80. [PubMed] [Google Scholar]
- 20.Soiffer RJ, Alyea EP, Hochberg E, et al. Randomized trial of CD8+ T-cell depletion in the prevention of graft-versus-host disease associated with donor lymphocyte infusion. Biol Blood Marrow Transplant. 2002;8(11):625–32. doi: 10.1053/bbmt.2002.v8.abbmt080625. [DOI] [PubMed] [Google Scholar]
- 21.Zorn E, Wang KS, Hochberg EP, et al. Infusion of CD4+ donor lymphocytes induces the expansion of CD8+ donor T cells with cytolytic activity directed against recipient hematopoietic cells. Clin Cancer Res. 2002;8(7):2052–60. [PubMed] [Google Scholar]
- 22.Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Semin Cancer Biol. 2006;16(2):115–23. doi: 10.1016/j.semcancer.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Rieger K, Loddenkemper C, Maul J, et al. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107(4):1717–23. doi: 10.1182/blood-2005-06-2529. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez J, Casano J, Alvarez MA, et al. Kinetic of regulatory CD25high and activated CD134+ (OX40) T lymphocytes during acute and chronic graft-versus-host disease after allogeneic bone marrow transplantation. Br J Haematol. 2004;126(5):697–703. doi: 10.1111/j.1365-2141.2004.05108.x. [DOI] [PubMed] [Google Scholar]
- 25.Godfrey WR, Ge YG, Spoden DJ, et al. In vitro-expanded human CD4(+)CD25(+) T-regulatory cells can markedly inhibit allogeneic dendritic cell-stimulated MLR cultures. Blood. 2004;104(2):453–61. doi: 10.1182/blood-2004-01-0151. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood. 2004;104(3):895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]