Abstract
Background
New markers to distinguish benign reactive glands from infiltrating ductal adenocarcinoma of the pancreas are needed.
Design
The gene expression patterns of 24 surgically resected primary infiltrating ductal adenocarcinomas of the pancreas were compared with 18 non-neoplastic samples using the Affymetrix U133 Plus 2.0 Arrays and the Gene Logic GeneExpress Software System. Gene fragments from 4 genes (annexin A8, claudin 18, CXCL5, and S100 A2) were selected from the fragments found to be highly expressed in infiltrating adenocarcinomas when compared with normal tissues. The protein expression of these genes was examined using immunohistochemical labeling of tissue microarrays.
Results
Claudin 18 labeled infiltrating carcinomas in a membranous pattern. When compared with normal and reactive ducts, claudin 18 was overexpressed, at least focally, in 159 of 166 evaluable carcinomas (96%). Strong and diffuse claudin 18 overexpression was most often seen in well-differentiated carcinomas (P=0.02). Claudin 18 was overexpressed in 51 of 52 cases (98%) of pancreatic intraepithelial neoplasia. Annexin A8 was at least focally overexpressed in 149 of 154 evaluable infiltrating carcinomas (97%). S100 A2 was at least focally overexpressed in 118 of 154 evaluable infiltrating carcinomas (77%). Non-neoplastic glands also frequently expressed S100 A2 diminishing its potential diagnostic utility. Immunolabeling with antibodies directed against CXCL5 did not reveal any significant differences in protein expression between infiltrating adenocarcinomas and normal pancreatic ducts.
Conclusions
Claudin 18 and annexin A8 are frequently highly overexpressed in infiltrating ductal adenocarcinomas when compared with normal reactive ducts, suggesting a role for these molecules in pancreatic ductal adenocarcinomas. Furthermore, these may serve as diagnostic markers, as screening tests and as therapeutic targets.
Keywords: pancreas, pancreatic cancer, claudin, annexin, markers, pancreatic intraepithelial neoplasia
It is estimated that this year 37,170 Americans will be diagnosed with pancreatic cancer and that 33,370 will die from it.3 Much of the high mortality rate from pancreatic cancer can be attributed to the advanced incurable stage at which most patients present. Yet, a growing body of evidence has made it clear that most pancreatic cancers, like other epithelial neoplasms, start as a curable noninvasive lesion such as pancreatic intraepithelial neoplasia (PanIN) and intraductal papillary mucinous neoplasm.26–29 The origin of pancreatic cancer from noninvasive precursor lesions suggests a window of opportunity for a cure, if pancreatic neoplasia can be detected before it invades. A great example of the potential of such approach can be seen with breast neoplasia. Invasive breast cancers arise from morphologically defined noninvasive neoplasms (intraductal carcinoma and in situ lobular carcinoma), and early detection through mammography accounts for half of the reduction in breast cancer mortality over the last 25 years in this country.7,12 It is reasonable to hypothesize that new markers of pancreatic cancer could form the basis for an early detection test for pancreatic cancer and its precursors, and that such a test could be used to diagnose presymptomatic disease.
New markers are also needed to aid in the morphologic diagnosis of pancreatic cancer.29 This is one of the great paradoxes in pathology. One of the deadliest of all solid malignancies, pancreatic cancer, can be one of the most difficult to diagnose. Well-differentiated infiltrating ductal adenocarcinoma can appear better differentiated than the reactive glands of chronic pancreatitis.29 New markers to aid in the interpretation of pancreatic biopsies are urgently needed.
Global analyses of gene expression in pancreatic cancer have led to the development of a number of potential new markers of pancreatic cancer.4,5,9,10,18,19,31–33,36,40,49,50,60 For example, mesothelin was identified by serial analysis of gene expression as overexpressed in pancreatic cancer and mesothelin has proven to be a useful diagnostic aid and a therapeutic target.4,38,53
The Affymetrix Company has recently developed a new gene expression array, the Human Genome U133 Plus 2.0 Array, which provides the same coverage as the Human Genome U133 Set, plus it contains 6500 additional genes allowing for an analysis of over 47,000 transcripts (http://www.a.ymetrix.com/index.a.x). This platform has been successfully used to identify a number of disease markers.6,44,57 For example, Osman et al44 profiled circulating blood RNA from patients with bladder cancer and they identified a set of 7 gene transcripts that could be used to distinguish between patients with bladder cancer and controls.
We applied the new Affymetrix Human Genome U133 Plus 2.0 Array to a series of pancreatic cancers and control tissue and identified 4 gene fragments that were overexpressed in pancreatic cancer compared with the controls. We then evaluated the protein expression patterns of the genes corresponding to these gene fragments in well-characterized tissue microarrays and compared the results of immunolabeling with clinical features including patient outcome.
METHODS
This study was approved by the institutional review board of The Johns Hopkins Medical Institutions (JHMI).
Tissues
Samples (0.5 g) of chronic pancreatitis (n=4), normal duodenal mucosa (n=14), and primary infiltrating pancreatic adenocarcinoma (n=24) were collected from surgical resection specimens from patients at The Johns Hopkins Hospital.31 The samples were harvested and snap frozen in liquid nitrogen before storage at −80°C. Hematoxylin and eosin-stained sections were examined to confirm the presence of appropriate tissue for the gene expression experiments. The sections of chronic pancreatitis were specifically examined for the presence of neoplastic tissue, including PanINs or invasive carcinomas. For the chronic pancreatitis and duodenum samples, only non-neoplastic tissues were submitted for the analyses. The normal duodenum and chronic pancreatitis samples were included in the analyses to facilitate the identification of markers of pancreatic cancer that would be useful in screening secondary sources, such as in duodenal fluid or stool samples.14,17 The neoplastic cellularity varied between 5% and 55%. The resected cancers were not microdissected to foster identification of genes expressed due to neoplastic cellstromal interaction.
RNA Extraction, Quality Control, and Expression Profiling
RNA was extracted from samples by homogenization in Trizol Reagent (Invitrogen, Carlsbad, CA) followed by isolation with a RNAeasy kit (Qiagen, Valencia, CA) as recommended by the manufacturer. RNA was evaluated for quality and integrity (Agilent 2100 Bioanalyzer derived 28S/18S ratio and RNA integrity number), purity (via absorbance ratio at A260/A280), and quantity (via absorbance at A260 or alternative assay). Gene expression levels were assessed using Affymetrix GeneChip Human Genome U133 Plus 2.0 Array (greater than 54,000 probe sets representing more than 47,000 transcripts and variants derived from approximately 39,000 well-substantiated human genes). Two micrograms of total RNA was used to prepare cDNA using Superscript II (Invitrogen) with a T7 oligo-dT primer for cDNA synthesis and an Affymetrix GeneChip IVT Labeling Kit (Affymetrix, Santa Clara, CA). Quantity and purity of cDNA synthesis product was assessed using UV absorbance. Quality of cDNA synthesis was assessed using either the Agilent Bioanalyzer or a 3-(N-morpholino) propane sulfonic acid agarose gel. The labeled cDNA was subsequently fragmented, and 10 μg was hybridized to each array at 45°C over 16 to 24 hours. Arrays were washed and stained according to manufacturer recommendations and scanned on Affymetrix GeneChip Scanner 3000 7G. Array data quality was evaluated using a proprietary high throughput application, which assesses the data against multiple objective standards including 5′/3′ GAPDH ratio, signal/noise ratio, and background as well as other additional metrics (eg, outlier, vertical variance), which must be passed before inclusion for analysis. GeneChip analysis was performed with Microarray Analysis Suite version 5.0, Data Mining Tool 2.0, and Microarray database software (available at: http://www.a.ymetrix.com). All of the genes represented on the GeneChip were globally normalized and scaled to a signal intensity of 100.
Data Analysis
The gene expression patterns of the 24 surgically resected primary infiltrating adenocarcinomas of the pancreas were compared with the 18 non-neoplastic samples (14 duodenal mucosa and 4 chronic pancreatitis) using the GeneExpress Software System Fold Change Analysis tool.31 For each gene fragment, the ratio of the geometric means of the expression intensities in the normal control tissues and the pancreatic cancer samples was calculated, and the fold change then calculated on a per fragment basis.31 Confidence limits were calculated using a 2-sided Welch modified t test on the difference of the means of the logs of the intensities. Fragments that were highly overexpressed in the cancer samples as compared with normal (defined as ≥5-fold change) and which were expressed in a significant proportion of the cancer samples (defined as expressed in >70% of the cancers) were selected and the genes corresponding to these fragments were identified. From these overexpressed genes, we selected those for which antibodies were commercially available to the corresponding protein product. These were annexin A8, claudin 18, CXCL5, and S100 A2.
Immunohistochemical Labeling
The protein expression of these genes was examined using immunohistochemical labeling of tissue microarrays and whole tissue sections. Tissue microarrays containing a total of 168 different surgically resected infiltrating ductal pancreatic adenocarcinomas of the pancreas and a variety of normal tissues were constructed as previously described.54 The microarray cores measured 1.5mm in diameter. Each carcinoma was represented twice in each tissue microarray as was normal pancreas to account for potential tumor heterogeneity. Unstained 4-μm sections of each tissue microarray were deparaffnized by routine techniques before placing in 200mL DIVA Antigen Retrieval Solution, pH 6.0 (BioCare Medical, Concord, CA) for 40 minutes at 100°C. After cooling for 20 minutes, slides were quenched with 3% H2O2 for 5 minutes, before incubating with the appropriate dilution of each primary antibody [a 1:1000 dilution of rabbit monoclonal antihuman claudin 18 (Invitrogen, clone ZMD 395), a 1:400 dilution of goat polyclonal antihuman annexin A8 antibody (BioVision Research Products), a 1:150 dilution of mouse monoclonal antihuman CXCL5 (R&D Research Products, clone 33160), or a 1:150 dilution of mouse monoclonal antihuman S100 A2 (Sigma, clone SH-L1]. Incubation was overnight at room temperature. Labeling was detected with the BioCare MACH 4 Universal Polymer Detection System for mouse and rabbit antibodies. Dako LSAB+ Detection system (Dako, Carpinteria, CA) was used for goat antibodies following the manufacturers’ protocols. Labeling was detected by adding biotinylated secondary antibodies, avidin-biotin complex, and 3,3′-diaminobenzidine. All sections were counterstained with hematoxylin.
Only microarray cores involved by at least 30% invasive carcinoma were scored. The percentage of neoplastic cells that labeled with each antibody was scored, as was the relative intensity of labeling (from 0 to 3+) by a single observer unaware of the patients clinical characteristics. A second observer independently scored a subset of the data. The scores of the 2 observers unaware of the clinical characteristics of the patients were compared and then reconciled at a multiheaded microscope, if different. In the statistical analyses, labeling was considered strong and diffuse if >80% of the neoplastic cells labeled at an intensity of 2 or 3+.
Statistical Data Analysis
Survival analysis included the 168 patients whose cancers were represented in the tissue microarrays. These were all patients with resectable infiltrating adenocarcinoma of the pancreas and they all underwent pancreaticoduodenectomy at The Johns Hopkins Hospital, Baltimore, MD, between 2000 and 2003. For analyses of follow-up we excluded patients with disease left beyond the Whipple margins, either grossly or microscopically, and those with distant metastasis. Time to event analysis was performed measuring overall survival from the date of surgery to the time of last follow-up or death. Patients were censored if they were still alive at last follow-up, with a maximum follow-up of 60 months. Contact with patients, their family or their primary physician to confirm patient status occurred at least annually. All clinical and pathologic patient information is maintained in a regularly updated clinical database with the last observation recorded in April 2007. Two-sided Fisher exact tests for r-by-c tables were used for comparison of categorical characteristics across groups. Cox proportional hazards regression models were used to control the following prognostic features: tumor size 3 cm versus <3 cm, positive versus negative margins, positive versus negative nodes, poor versus well to moderate differentiation, age (analyzed as 3 categories <60, 60 to 70, and >70). Final multivariable model contained covariates with known biologic associations with pancreas cancer and mortality. Statistical analysis completed with Stata 8.2 (StataCorp 2003. Stata Statistical Software: Release 8.0. College Station, TX).
RESULTS
Patients
The demographics of the 168 patients represented in the tissue microarrays are provided in Table 1. These patient demographics are representative of all patients treated surgically at The Johns Hopkins Hospital for pancreatic cancer.47,59
TABLE 1.
Baseline Characteristics of Cases in 168 Patients Undergoing a Whipple Procedure Whose Carcinomas Were Represented in the Tissue Microarrays
| Node positive | 137 (82%) |
| Margin positive | 68 (41%) |
| Poor vs. well-moderately differentiation | 74 (44%) |
| Known to have received adjuvant therapy | 71 (43%) |
| Size >3 cm | 89 (53%) |
| Mean age in years (SD) | 67 (11) |
| Mean tumor size in centimeters (SD) | 3.1 (1.5) |
| Strong and diffuse claudin 18 labeling (n = 166) | 83 (50%) |
Gene Expression
Four genes were selected from the gene fragments found to be highly expressed in Affymetrix analyses. These 4 genes were selected on the basis of the commercial availability of antibodies to the corresponding proteins and included annexin A8, claudin 18, S100 A2, and CXCL5. The Affymetrix analyses revealed that a fragment corresponding to the annexin A8 gene was expressed (“present call” using the GeneExpress Software System Fold Change Analysis tool) in 92% of cancers, and only 11% of normals. The mean intensity (in relative units) of expression for this fragment was 393 in cancers, and 79 in normals. A fragment corresponding to the claudin 18 gene was expressed in 100% of the cancers, and only 6% of normals. The mean intensity was 743 in cancers, and 19 in normals. A fragment corresponding to the CXCL5 gene was expressed in 100% of the cancers, and 39% of the normals. The mean intensity of expression of this fragment was 1096 in the cancers, and 52 in normals. A fragment corresponding to the S100 A2 gene was expressed in 71% of the cancers and 11% of normals. The mean intensity was 829 in the cancers, and only 27 in the normals.
Claudin 18
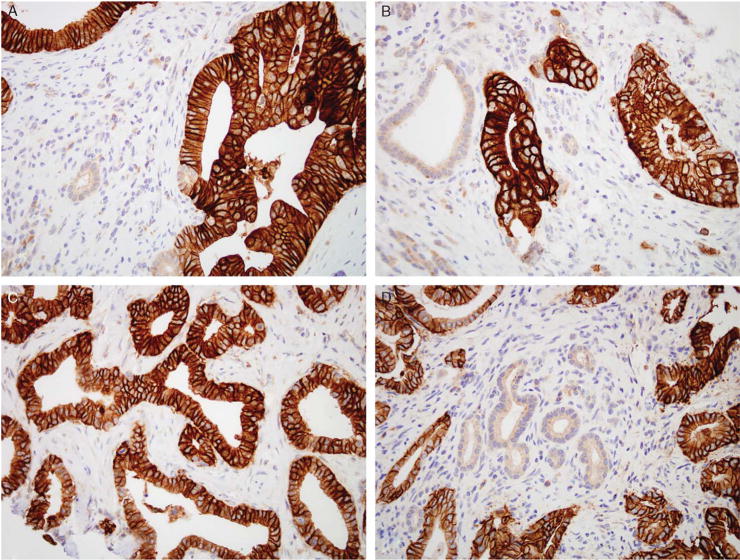
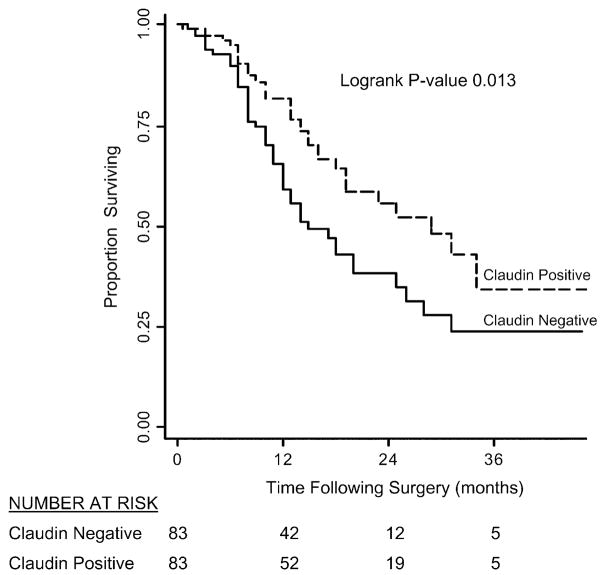
Only 4 of 105 (4%) matched control cases containing normal and reactive ducts labeled with claudin 18 with a weak intensity (1+), cytoplasmic distribution of labeling. Antibodies to claudin 18 labeled the infiltrating carcinomas in a membranous pattern, consistent with its reported role as a tight junction protein (Fig. 1). When compared with normal and reactive ducts, claudin 18 was overexpressed, at least focally, in 159 of 166 evaluable infiltrating carcinomas (96%) (Table 2). Of these 159 cancers, 50% expressed claudin 18 strongly (2 or 3+) and diffusely (>80% of the neoplastic cells labeling). Strong and diffuse claudin 18 overexpression was most often seen in well-differentiated carcinomas, whereas more poorly differentiated carcinomas often labeled focally or did not label. Only 34% of the cases with strong and diffuse (positive) labeling were poorly differentiated, whereas 53% of the cases with weak or no labeling were poorly differentiated (P=0.02). The intense membranous pattern of labeling found in the carcinomas (Fig. 1) was useful in making the distinction between carcinoma and reactive ducts in diagnostically challenging cases. No statistically significant differences were seen with the other clinical parameters listed in Table 3 when the strong and diffuse claudin 18 expressing cases were compared with claudin 18 weak or negative labeling cases. To control for tumor heterogeneity, the microarrays consisted of 2 separately sampled areas of a patient’s tumor for each case. When compared with the tissue microarrays, immunohistochemistry performed on the corresponding whole tumor sections demonstrated similar patterns of expression. By Kaplan-Meier analysis, patients whose carcinomas strongly and diffusely labeled with the antibody to claudin 18 had a significantly better survival than did patients whose carcinomas weakly labeled or did not label (log-rank P=0.013; Fig. 2). In the multivariable regression model based on 166 patients, the adjusted Cox proportional hazards regression for patients whose carcinomas expressed claudin 18 strongly and diffusely was 0.52 (95% confidence interval, 0.32–0.84) compared with patients whose carcinomas only weakly expressed or did not express claudin 18. This difference was independent of tumor grade and other known factors associated with pancreatic cancer mortality including tumor size, positive lymph nodes, positive margins, and older age. Additionally, claudin 18 was highly overexpressed in PanIN lesions (Table 4). Overall, 98% (51/52) PanINs overexpressed claudin 18 with a similar membranous pattern of expression to that observed in infiltrating carcinomas (Fig. 3). Strong and diffuse overexpression was identified in both low-grade and high-grade PanIN lesions (PanIN grades 1 to 3).
FIGURE 1.
Immunohistochemical labeling for claudin 18 demonstrates strong, membranous labeling of the infiltrating carcinoma and absence of labeling of the non-neoplastic glands and stroma (A–D).
TABLE 2.
Immunohistochemical Labeling of Infiltrating Pancreatic Adenocarcinomas With Antibodies to Claudin 18 and Annexin A8
| At Least Focal Labeling (%) | Strong and Diffuse Labeling (%) | Focal or Weak Labeling (%) | No Labeling (%) | |
|---|---|---|---|---|
| Claudin 18 (n = 166) | 96 | 50 | 46 | 4 |
| Annexin A8 (n = 154) | 97 | 67 | 30 | 3 |
TABLE 3.
Baseline Characteristics of Cases as Stratified by Claudin 18 Expression Status in 166 Patients Undergoing Pancreaticoduodenectomy for Adenocarcinoma of the Pancreas Between 2000 and 2003 at Johns Hopkins Hospital
| Weak or Negative Labeling, N = 83 | Strong and Diffuse Labeling, N = 83 | P* | |
|---|---|---|---|
| Node positive | 66 (80%) | 70 (84%) | 0.55 |
| Margin positive | 29 (35%) | 38 (46%) | 0.21 |
| Poorly differentiated | 44 (53%) | 28 (34%) | 0.02 |
| Tumor size >3 cm | 43 (52%) | 44 (53%) | 1.00 |
| Age | 0.96 | ||
| <60 | 23 (28%) | 22 (27%) | |
| 60-70 | 21 (25%) | 23 (28%) | |
| >70 | 39 (47%) | 38 (46%) |
All P values using Fisher exact test with rows and columns.
FIGURE 2.
Kaplan-Meier survival curves for patients with pancreatic ductal adenocarcinoma after pancreaticoduodenectomy based on claudin 18 expression. Patients whose carcinomas strongly and diffusely labeled with the antibody to claudin 18 had a significantly better survival than did patients whose carcinomas weakly labeled or did not label, even when controlling for tumor grade (log-rank P = 0.013).
TABLE 4.
Immunohistochemical Labeling of PanIN Lesions With Antibodies to Claudin 18
| n | At Least Focal Labeling (%) | Strong and Diffuse Labeling (%) | Focal or Weak Labeling (%) | No Labeling (%) | |
|---|---|---|---|---|---|
| PanIN | |||||
| 1A | 12 | 92 | 75 | 17 | 8 |
| 1B | 10 | 100 | 80 | 20 | 0 |
| 2 | 20 | 100 | 65 | 35 | 0 |
| 3 | 10 | 100 | 60 | 40 | 0 |
| All PanIN lesions | 52 | 98 | 69 | 31 | 2 |
FIGURE 3.
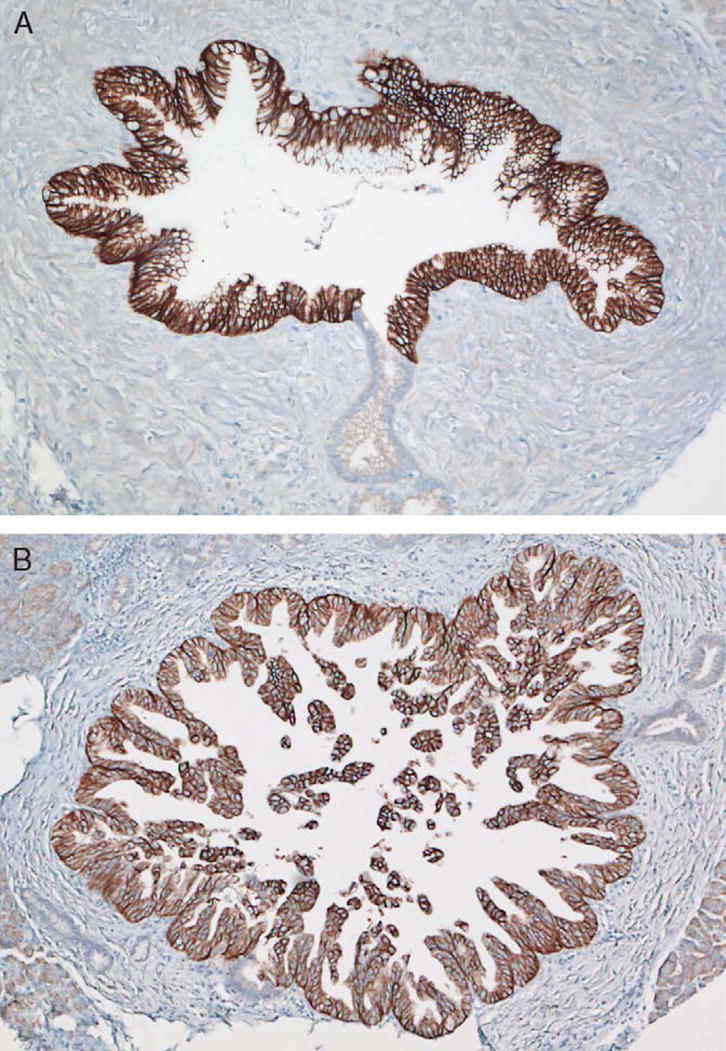
Immunohistochemical labeling for claudin 18 labels (A) PanIN-2 and (B) PanIN-3 precursor lesions. The pattern of labeling is membranous and is similar to the labeling seen in PanIN-1 lesions (not shown).
Annexin A8
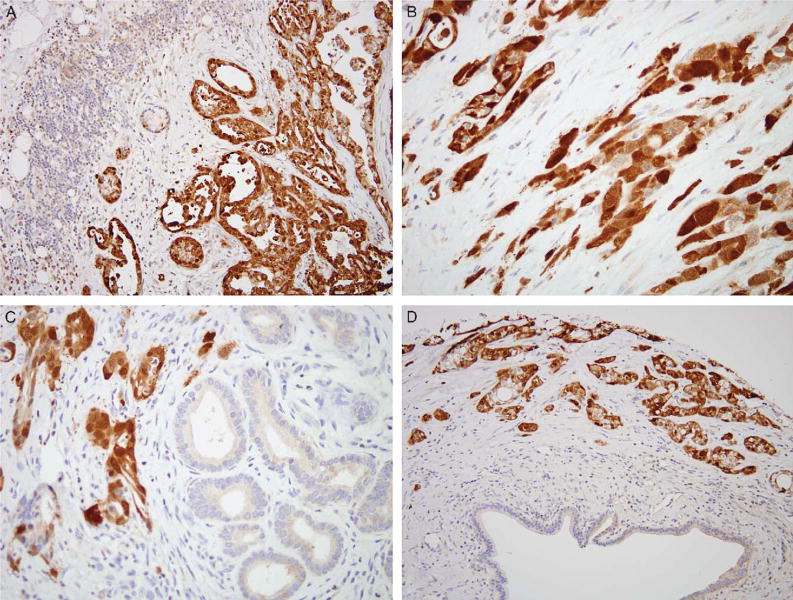
Annexin A8 is a member of a family of calcium-regulated membrane-binding proteins that have proposed roles in mediating calcium signaling, and in membrane structure and transport. Weak (1+), punctuate cytoplasmic labeling was observed in the acinar cells of normal pancreatic tissues. Additionally, weak (1+) cytoplasmic labeling of reactive ducts was observed in 13 of 110 (12%) of cases. By contrast, annexin A8 was at least focally overexpressed in 149 of 154 evaluable infiltrating carcinomas (97%). Of these 149 cancers, 69% expressed annexin A8 strongly and diffusely (Fig. 4). Unlike reactive ducts, the pattern of annexin A8 labeling observed in infiltrating carcinomas was both nuclear and cytoplasmic. No statistically significant differences were seen with the clinical parameters when the strong and diffuse annexin A8 expressing cases were compared with annexin A8 weak or negative labeling cases. Unlike claudin 18, annexin A8 was not overexpressed in low-grade and high-grade PanIN lesions (0/40).
FIGURE 4.
Immunohistochemical labeling for annexin A8 demonstrates strong and diffuse labeling of the neoplastic glands (A–D). The adjacent lymphocytes, reactive glands, and stroma do not label.
S100 A2 and CXCL5
S100 A2, a calcium-binding protein, was at least focally overexpressed in 118 of 154 evaluable infiltrating carcinomas (77%). Of these 118 cancers, 34% demonstrated strong and diffuse overexpression of S100 A2. Non-neoplastic glands also frequently expressed S100 A2 diminishing its potential diagnostic utility.
Immunolabeling with antibodies directed against CXCL5 did not reveal any significant differences in protein expression between infiltrating adenocarcinomas and normal pancreatic ducts. Both well and poorly differentiated carcinomas and benign ducts showed a diffuse, cytoplasmic reactivity.
DISCUSSION
Claudin 18 and annexin A8 are frequently highly overexpressed in infiltrating ductal adenocarcinomas when compared with normal reactive ducts, suggesting a biologic role for these molecules in pancreatic ductal adenocarcinomas. Additionally, claudin 18 overexpression is detected in PanIN precursor lesions. Furthermore, claudin 18 and annexin A8 may serve as diagnostic markers, as screening tests and as therapeutic targets.
The claudins are a large family of tight junction proteins with 4 transmembrane domains.41 Over 20 claudins have been described and are expressed in epithelial cells from a variety of tissues. Claudins interact with one another to form a branching network that helps create the tight junctions of epithelial cells.55 Claudins function to produce a seal between cells, and they play a critical role in maintaining cell polarity.41,52,55
Although the biologic role of claudins in cancer is not entirely understood, the abnormal expression of a number of claudins has been reported in a variety of cancer types.41,51 Early reports identified the down-regulation of selected claudin members in some cancer types. For example, claudin 7 levels are decreased in some breast carcinomas,35 and in squamous cell carcinomas of the head and neck.2 In these cases, the loss of cell polarity normally maintained by the claudins likely contributed to tumorigenesis. Other claudins are increased in cancers, including claudin 10 in hepatocellular and papillary thyroid carcinomas, claudins 3 and 4 in ovarian carcinoma, and claudin 4 in pancreatic cancer.41 Here we demonstrate that claudin 18 protein is also overexpressed in pancreatic cancer, and PanIN lesions, a finding supported by recent reports from Iacobuzio-Donahue et al32 and Hewitt et al24 that claudin 18 mRNA is overexpressed in pancreatic cancer as measured by oligonucleotide arrays and real time reverse transcriptionpolymerase chain reaction, respectively.
It has been suggested that the overexpression of claudins in cancers may be related to a tight junction independent function.24 Recent studies, using human ovarian epithelial cells, have shown that claudin 3 and claudin 4 overexpression may enhance tumorigenesis and metastasis.24
The overexpression of selected claudins by pancreatic cancer may have therapeutic implications. Our group has previously demonstrated that most of pancreatic cancers overexpress claudin 4.42 Claudin 4 is also a receptor for the Clostridium perfringens cytolytic enterotoxin, and recent studies in pancreatic cancer xenografts have demonstrated that the C. perfringens enterotoxin causes tumor cell necrosis in vivo.39,42 Similarly, the demonstration here that claudin 18 is also strongly and diffusely overexpressed in the majority of pancreatic cancers and their precursors suggests that claudin 18 may also be a useful therapeutic target.45 Additionally, the detection of PanINs, a precursor to invasive carcinoma, offers a window of opportunity for early surgical resection and treatment of neoplasia before the development of an incurable invasive adenocarcinoma.
It is interesting to note that we found that claudin 18 protein expression was most pronounced in well-differentiated cancers, because it is the well-differentiated carcinomas that present the greatest challenge diagnostically. Immunolabeling for claudin 18 may be useful in distinguishing the reactive glands of chronic pancreatitis from well-differentiated adenocarcinoma.
Claudin 18 expression levels may also have prognostic implications for patients with pancreatic carcinoma. We found that patients whose carcinomas diffusely and strongly labeled with antibodies to the claudin 18 protein had a significantly better survival when compared with those cancers that had weak or no claudin 18 protein expression (Fig. 2). The multivariable Cox proportional hazards model suggests a 48% reduction in mortality associated with this labeling pattern, independent of other known prognostic factors including tumor grade, tumor size, positive lymph nodes, positive margins, and older age.
The annexins are a family of calcium-regulated proteins that bind the cell membrane and provide a link between calcium signaling and cell membrane functions. 15,46 The overexpression of annexin A8 in pancreatic cancer is biologically interesting for several reasons. Annexins have calcium regulatory functions and a number of other calcium-regulated proteins, particularly the S100 family of proteins, are also overexpressed in pancreatic cancers suggesting a critical role for calcium homeostasis in pancreatic cancer.11,37,48,56 In addition, one of the other annexins, annexin A2, binds S100 A10, and S100 A10 is also overexpressed in pancreatic cancer.11,33,37,48,50,56 Our finding that annexin A8 is overexpressed in pancreatic cancers is supported by other reports that have also identified annexin A8 overexpression by cDNA representational difference analysis and oligonucleotide-directed gene array experiments.16,36
The strong and diffuse overexpression of annexin A8 and claudin 18 in the majority of pancreatic cancers suggests that annexin A8 and claudin 18 may be useful markers for pancreatic cancer. For example, we have previously identified a number of proteins that are overexpressed in pancreatic cancer and subsequently some of these proteins have been used to create new diagnostic markers, imaging and therapeutic targets for pancreatic cancer.1,13,42 Using serial analysis of gene expression, we discovered that mesothelin is overexpressed in most of pancreatic cancers, and several clinical trials are now underway treating pancreatic cancer by targeting mesothelin.4,20,22,30,51,53 Similarly, mesothelin has also proven useful in evaluating difficult pancreas biopsies and cytology samples, and mesothelin may be useful as an early detection marker for pancreatic neoplasia.8,21,23,25,34,38,43,58
In summary, we report the overexpression of 2 new proteins in pancreatic cancer. Claudin 18 and annexin A8 may serve as diagnostic markers, as screening tests and as therapeutic targets.
Acknowledgments
Supported by The Sol Goldman Pancreatic Cancer Research Center, the Michael Rolfe Pancreatic Cancer Foundation, and the NIH SPORE (Specialized Programs of Research Excellence) in Gastrointestinal Cancer Grant CA62924.
Footnotes
Disclosure: This work was supported by a collaborative agreement between Johns Hopkins University and Gene Logic, Gaithersburg, MD.
References
- 1.Agarwal R, D’Souza T, Morin PJ. Claudin-3 and claudin-4 expression in ovarian epithelial cells enhances invasion and is associated with increased matrix metalloproteinase-2 activity. Cancer Res. 2005;65:7378–7385. doi: 10.1158/0008-5472.CAN-05-1036. [DOI] [PubMed] [Google Scholar]
- 2.Al Moustafa AE, Alaoui-Jamali MA, Batist G, et al. Identification of genes associated with head and neck carcinogenesis by cDNA microarray comparison between matched primary normal epithelial and squamous carcinoma cells. Oncogene. 2002;21:2634–2640. doi: 10.1038/sj.onc.1205351. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer Facts & Figures 2007. Cancer, 1-52. New York, NY: American Cancer Society; 2007. [Google Scholar]
- 4.Argani P, Iacobuzio-Donahue CA, Ryu B, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 5.Argani P, Rosty C, Reiter RE, et al. Discovery of new markers of cancer through serial analysis of gene expression: prostate stem cell antigen is overexpressed in pancreatic adenocarcinoma. Cancer Res. 2001;61:4320–4324. [PubMed] [Google Scholar]
- 6.Bachtiary B, Boutros PC, Pintilie M, et al. Gene expression profiling in cervical cancer: an exploration of intratumor heterogeneity. Clin Cancer Res. 2006;12:5632–5640. doi: 10.1158/1078-0432.CCR-06-0357. [DOI] [PubMed] [Google Scholar]
- 7.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 8.Corso CD, Stubbs DD, Lee SH, et al. Real-time detection of mesothelin in pancreatic cancer cell line supernatant using an acoustic wave immunosensor. Cancer Detect Prev. 2006;30:180–187. doi: 10.1016/j.cdp.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Crnogorac-Jurcevic T, Efthimiou E, Capelli P, et al. Gene expression profiles of pancreatic cancer and stromal desmoplasia. Oncogene. 2001;20:7437–7446. doi: 10.1038/sj.onc.1204935. [DOI] [PubMed] [Google Scholar]
- 10.Crnogorac-Jurcevic T, Efthimiou E, Nielsen T, et al. Expression profiling of microdissected pancreatic adenocarcinomas. Oncogene. 2002;21:4587–4594. doi: 10.1038/sj.onc.1205570. [DOI] [PubMed] [Google Scholar]
- 11.Crnogorac-Jurcevic T, Missiaglia E, Blaveri E, et al. Molecular alterations in pancreatic carcinoma: expression profiling shows that dysregulated expression of S100 genes is highly prevalent. J Pathol. 2003;201:63–74. doi: 10.1002/path.1418. [DOI] [PubMed] [Google Scholar]
- 12.Elkin EB, Hudis C, Begg CB, et al. The effect of changes in tumor size on breast carcinoma survival in the US: 1975-1999. Cancer. 2005;104:1149–1157. doi: 10.1002/cncr.21285. [DOI] [PubMed] [Google Scholar]
- 13.Foss CA, Fox JJ, Feldmann G, et al. Radiolabeled anti-claudin 4 and anti-prostate stem cell antigen: initial imaging in experimental models of pancreatic cancer. Mol Imaging. 2007;6:131–139. [PubMed] [Google Scholar]
- 14.Fukushima N, Walter KM, Uek T, et al. Diagnosing pancreatic cancer using methylation specific PCR analysis of pancreatic juice. Cancer Biol Ther. 2003;2:78–83. doi: 10.4161/cbt.183. [DOI] [PubMed] [Google Scholar]
- 15.Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- 16.Gress TM, Wallrapp C, Frohme M, et al. Identification of genes with specific expression in pancreatic cancer by cDNA representational difference analysis. Genes Chromosomes Cancer. 1997;19:97–103. doi: 10.1002/(sici)1098-2264(199706)19:2<97::aid-gcc5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Grönborg M, Bunkenborg J, Kristiansen TZ, et al. Comprehensive proteomic analysis of human pancreatic juice. J Proteome Res. 2004;3:1042–1055. doi: 10.1021/pr0499085. [DOI] [PubMed] [Google Scholar]
- 18.Grutzmann R, Foerder M, Alldinger I, et al. Gene expression profiles of microdissected pancreatic ductal adenocarcinoma. Virchows Arch. 2003;443:508–517. doi: 10.1007/s00428-003-0884-1. [DOI] [PubMed] [Google Scholar]
- 19.Han H, Bearss DJ, Browne LW, et al. Identification of differentially expressed genes in pancreatic cancer cells using cDNA microarray. Cancer Res. 2002;62:2890–2896. [PubMed] [Google Scholar]
- 20.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–3942. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 21.Hassan R, Laszik ZG, Lerner M, et al. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol. 2005;124:838–845. [PubMed] [Google Scholar]
- 22.Hassan R, Williams-Gould J, Steinberg SM, et al. Tumor-directed radiation and the immunotoxin SS1P in the treatment of mesothelin-expressing tumor xenografts. Clin Cancer Res. 2006;12:4983–4988. doi: 10.1158/1078-0432.CCR-06-0441. [DOI] [PubMed] [Google Scholar]
- 23.Hellstrom I, Raycraft J, Kanan S, et al. Mesothelin variant 1 is released from tumor cells as a diagnostic marker. Cancer Epidemiol Biomarkers Prev. 2006;15:1014–1020. doi: 10.1158/1055-9965.EPI-05-0334. [DOI] [PubMed] [Google Scholar]
- 24.Hewitt KJ, Agarwal R, Morin PJ. The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer. 2006;6:186. doi: 10.1186/1471-2407-6-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornick JL, Lauwers GY, Odze RD. Immunohistochemistry can help distinguish metastatic pancreatic adenocarcinomas from bile duct adenomas and hamartomas of the liver. Am J Surg Pathol. 2005;29:381–389. doi: 10.1097/01.pas.0000149710.01559.fe. [DOI] [PubMed] [Google Scholar]
- 26.Hruban RH, Goggins M, Parsons JL, et al. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–2972. [PubMed] [Google Scholar]
- 27.Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 29.Hruban RH, Pitman MB, Klimstra DS. Tumors of the Pancreas. Atlas of Tumor Pathology. Washington, DC: American Registry of Pathology and Armed Forces Institute of Pathology; 2007. [Google Scholar]
- 30.Hung CF, Calizo R, Tsai YC, et al. A DNA vaccine encoding a single-chain trimer of HLA-A2 linked to human mesothelin peptide generates anti-tumor effects against human mesothelin-expressing tumors. Vaccine. 2007;25:127–135. doi: 10.1016/j.vaccine.2006.06.087. [DOI] [PubMed] [Google Scholar]
- 31.Iacobuzio-Donahue CA, Maitra A, Shen-Ong GL, et al. Discovery of novel tumor markers of pancreatic cancer using global gene expression technology. Am J Pathol. 2002;160:1239–1249. doi: 10.1016/S0002-9440(10)62551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iacobuzio-Donahue CA, Ashfaq R, Maitra A, et al. Highly expressed genes in pancreatic ductal adenocarcinomas: a comprehensive characterization and comparison of the transcription profiles obtained from three major technologies. Cancer Res. 2003;63 [PubMed] [Google Scholar]
- 33.Iacobuzio-Donahue CA, Maitra A, Olsen M, et al. Exploration of global gene expression patterns in pancreatic adenocarcinoma using cDNA microarrays. Am J Pathol. 2003;162:1151–1162. doi: 10.1016/S0002-9440(10)63911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jhala N, Jhala D, Vickers SM, et al. Biomarkers in diagnosis of pancreatic carcinoma in fine-needle aspirates. Am J Clin Pathol. 2006;126:572–579. doi: 10.1309/cev30be088cbdqd9. [DOI] [PubMed] [Google Scholar]
- 35.Kominsky SL, Argani P, Korz D, et al. Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene. 2003;22:2021–2033. doi: 10.1038/sj.onc.1206199. [DOI] [PubMed] [Google Scholar]
- 36.Logsdon CD, Simeone DM, Binkley C, et al. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 2003;63:2649–2657. [PubMed] [Google Scholar]
- 37.Mazzucchelli L. Protein S100A4: too long overlooked by pathologists? Am J Pathol. 2002;160:7–13. doi: 10.1016/S0002-9440(10)64342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarthy DM, Maitra A, Argani P, et al. Novel markers of pancreatic adenocarcinoma in fine-needle aspiration: mesothelin and prostate stem cell antigen labeling increases accuracy in cytologically borderline cases. Anticancer Res. 2003;11:238–243. doi: 10.1097/00129039-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Michl P, Barth C, Buchholz M, et al. Claudin-4 expression decreases invasiveness and metastatic potential of pancreatic cancer. Cancer Res. 2003;63:6265–6271. [PubMed] [Google Scholar]
- 40.Missiaglia E, Blaveri E, Terris B, et al. Analysis of gene expression in cancer cell lines identifies candidate markers for pancreatic tumorigenesis and metastasis. Int J Cancer. 2004;112:100–112. doi: 10.1002/ijc.20376. [DOI] [PubMed] [Google Scholar]
- 41.Morin PJ. Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res. 2005;65:9603–9606. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]
- 42.Nichols LS, Ashfaq R, Iacobuzio-Donahue CA. Claudin 4 protein expression in primary and metastatic pancreatic cancer: support for use as a therapeutic target. Am J Clin Pathol. 2004;121:226–230. doi: 10.1309/K144-PHVD-DUPD-D401. [DOI] [PubMed] [Google Scholar]
- 43.Ordonez NG. Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol. 2003;27:1418–1428. doi: 10.1097/00000478-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Osman I, Bajorin DF, Sun TT, et al. Novel blood biomarkers of human urinary bladder cancer. Clin Cancer Res. 2006;12:3374–3380. doi: 10.1158/1078-0432.CCR-05-2081. [DOI] [PubMed] [Google Scholar]
- 45.Pomper MG. Translational molecular imaging for cancer. Cancer Imaging. 2005;5:S16–S26. doi: 10.1102/1470-7330.2005.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rescher U, Gerke V. Annexins—unique membrane binding proteins with diverse functions. J Cell Sci. 2004;117:2631–2639. doi: 10.1242/jcs.01245. [DOI] [PubMed] [Google Scholar]
- 47.Riall TS, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma—part 3: update on 5-year survival. J Gastrointest Surg. 2005;9:1191–1206. doi: 10.1016/j.gassur.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 48.Rosty C, Ueki T, Argani P, et al. Overexpression of S100A4 in pancreatic ductal adenocarcinomas is associated with poor differentiation and DNA hypomethylation. Am J Pathol. 2002;160:45–50. doi: 10.1016/S0002-9440(10)64347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryu B, Jones JB, Hollingsworth MA, et al. Invasion-specific genes in malignancy: serial analysis of gene expression comparisons of primary and passaged cancers. Cancer Res. 2001;61:1833–1838. [PubMed] [Google Scholar]
- 50.Ryu B, Jones JB, Blades NJ, et al. Relationships and differentially expressed genes among pancreatic cancers examined by large-scale serial analysis of gene expression. Cancer Res. 2002;62:819–826. [PubMed] [Google Scholar]
- 51.Sato N, Hassan R, Axworthy DB, et al. Pretargeted radio-immunotherapy of mesothelin-expressing cancer using a tetravalent single-chain Fv-streptavidin fusion protein. J Nucl Med. 2005;46:1201–1209. [PubMed] [Google Scholar]
- 52.Swisshelm K, Macek R, Kubbies M. Role of claudins in tumorigenesis. Adv Drug Deliv Rev. 2005;57:919–928. doi: 10.1016/j.addr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 53.Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Heek NT, Meeker AK, Kern SE, et al. Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am J Pathol. 2002;161:1541–1547. doi: 10.1016/S0002-9440(10)64432-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 56.Vimalachandran D, Greenhalf W, Thompson C, et al. High nuclear S100A6 (calcyclin) is significantly associated with poor survival in pancreatic cancer patients. Cancer Res. 2005;65:3218–3225. doi: 10.1158/0008-5472.CAN-04-4311. [DOI] [PubMed] [Google Scholar]
- 57.Wamunyokoli FW, Bonome T, Lee JY, et al. Expression profiling of mucinous tumors of the ovary identifies genes of clinicopathologic importance. Clin Cancer Res. 2006;12:690–700. doi: 10.1158/1078-0432.CCR-05-1110. [DOI] [PubMed] [Google Scholar]
- 58.Watanabe H, Okada G, Ohtsubo K, et al. Expression of mesothelin mRNA in pure pancreatic juice from patients with pancreatic carcinoma, intraductal papillary mucinous neoplasm of the pancreas, and chronic pancreatitis. Pancreas. 2005;30:349–354. doi: 10.1097/01.mpa.0000160281.56828.76. [DOI] [PubMed] [Google Scholar]
- 59.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single institution experience. J Gastrointest Surg. 2006;10:1199–1211. doi: 10.1016/j.gassur.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 60.Zhou W, Sokoll LJ, Bruzek DJ, et al. Identifying markers for pancreatic cancer by gene expression analysis. Cancer Epidemiol Biomarkers Prev. 1998;7:109–112. [PubMed] [Google Scholar]