Abstract
Psychostimulant abuse is a serious social and health problem, for which no effective treatments currently exist. A number of review articles have described predominantly ‘clinic’-based pharmacotherapies for the treatment of psychostimulant addiction, but none have yet been shown to be definitively effective for use in humans. In the present article, we review various ‘hypothesis’- or ‘mechanism’-based pharmacological agents that have been studied at the preclinical level and evaluate their potential use in the treatment of psychostimulant addiction in humans. These compounds target brain neurotransmitter or neuromodulator systems, including dopamine (DA), γ-aminobutyric acid (GABA), endocannabinoid, glutamate, opioid and serotonin, which have been shown to be critically involved in drug reward and addiction. For drugs in each category, we first briefly review the role of each neurotransmitter system in psychostimulant actions, and then discuss the mechanistic rationale for each drug’s potential anti-addiction efficacy, major findings with each drug in animal models of psychostimulant addiction, abuse liability and potential problems, and future research directions. We conclude that hypothesis-based medication development strategies could significantly promote medication discovery for the effective treatment of psychostimulant addiction.
Keywords: Psychostimulant, addiction, reward, reinstatement, dopamine, glutamate, GABA, endocannabinoids
1. INTRODUCTION
Psychostimulant abuse is a major medical and social problem. Cocaine, amphetamine, methamphetamine and N-methyl-3,4-methylenedioxymethamphetamine (MDMA, ecstasy) are the most commonly abused psychostimulants in humans. The acute rewarding effects produced by psychostimulants and craving and relapse after abstinence are the core features of addiction to these drugs [1, 2]. Currently, there are no effective medications available for the treatment of psychostimulant addiction [3–5].
The lack of effective medications has spurred increased research attention. Recently, several review articles have described the progress of a number of pharmacological agents that are believed to be possibly effective in treatment of psychostimulant addiction [3–10]. However, most of these have focused on preliminary clinical findings (or ‘clinical’-based medication discovery), and none of the potentially effective agents described has yet been clearly shown to be effective in humans [4–9]. We have previously argued that knowledge of the underlying brain mechanisms of addiction, garnered from diligent and astute use of preclinical animal models relevant to addiction, provides an alternative – and arguably more rational – approach to the development of effective anti-addiction treatment medications [11, 12]. On this rationale, we focus in the present review on ‘mechanism’-based medication development strategies and we evaluate the pharmacological efficacy in animal models of drug addiction of various novel pharmacological agents possibly effective for the treatment of psychostimulant addiction. Animal models of disease are, by definition, approximations of the corresponding human clinical conditions. Since no single model can fully emulate all aspects of human drug abuse, multiple animal models or paradigms have been developed to reflect different aspects of the human addictive process. Among them, drug self-administration, brain stimulation reward (BSR), conditioned place preference (CPP) and drug discrimination are the most commonly used animal paradigms for studying the acute rewarding effects of drugs of abuse. Reinstatement models of drug-seeking behavior -triggered by the addictive drug itself, drug-paired environmental cues, or stress - are the most commonly used animal paradigms to emulate drug craving and relapse to drug use in humans. Detailed descriptions of each of these animal paradigms have been reported elsewhere [1, 3, 11, 12].
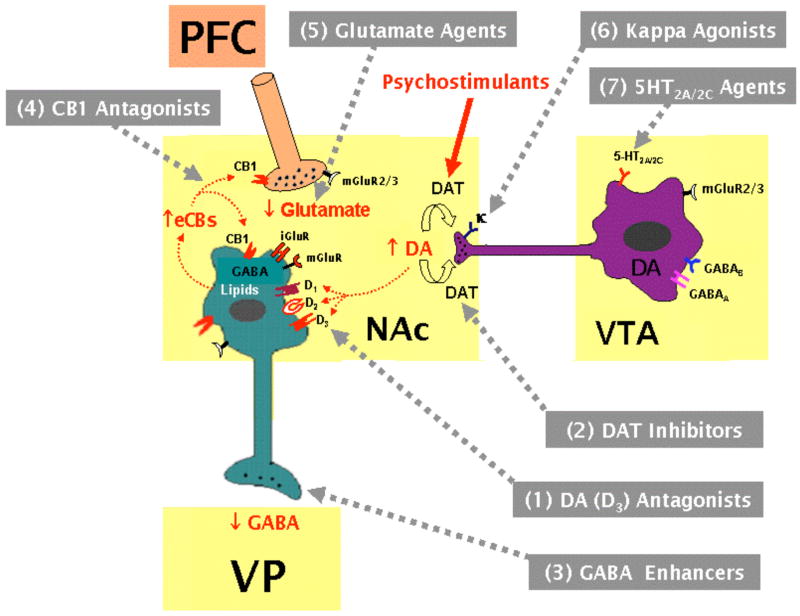
Extensive studies during several decades indicate that the neurotransmitter or neuromodulator systems most critically involved in drug reward and addiction are those involving dopamine (DA), γ-aminobutyric acid (GABA), endocannabinoids, glutamate, endogenous opioids, and serotonin [13–19]. Therefore, in the present review article, we first briefly review the functional role of each neurotransmitter in psychostimulant reward and relapse, and then summarize major findings in animal models of psychostimulant addiction produced by 27 pharmacological agents that target these neurotransmitter systems. We then discuss the potential use of these compounds in the clinical treatment of psychostimulant addiction. Recent studies also suggest that acetylcholine, several neuropeptides, and other neuro-signaling systems are involved in psychostimulant addiction [20, 21]. We do not review these systems in the present article because many findings are controversial or inconclusive, or reported elsewhere.
2. DOPAMINE-BASED MEDICATION DISCOVERY – ANTAGONIST STRATEGIES
Mesolimbic DA hypothesis of drug reward
It is well documented that the mesolimbic DA system is critically involved in drug reward and addiction [13–14, 17]. This system originates from the DA neurons in the ventral tegmental area (VTA) in the midbrain and predominantly projects to the NAc, prefrontal cortex (PFC) and amygdala in the forebrain. A great deal of evidence supports the importance of this DA system in drug addiction. First, almost all addictive drugs, including cocaine, amphetamine, opiates, nicotine, marijuana or ethanol, increase extracellular DA in the NAc [13–14, 17, 22]. Second, almost all addictive drugs are self-administered by animals either intravenously or locally into brain DA loci, an effect that can be blocked by either selective lesions of DA terminals or by pharmacological blockade of DA receptors [23, 24]. Third, electrical stimulation of brain DA loci maintains BSR, which is enhanced by drugs of abuse and attenuated by DA receptor antagonists [25, 26]. Cocaine-induced increases in extracellular DA are mediated by blockade of DA reuptake, while amphetamine- or methamphetamine-induced increases in extracellular DA are mediated predominantly by reversal of DA transporters [14, 17, 27]. These increases in synaptic DA levels in forebrain reward loci — especially in the NAc — are thought to underlie the euphoria associated with psychostimulant use [13–14, 17, 28]. Based on these findings, much development of new medications for treatment of psychostimulant addiction has focused on manipulation of DA transmission in the reward circuitry of the brain. Two major pharmacological strategies for manipulating brain DA transmission have emerged as the basis of anti-psychostimulant medication development: one being to modulate brain DA receptors, and the other being to modulate DA transporters. The former strategy has – to date – primarily utilized DA receptor antagonists, while the latter strategy has – to date – primarily utilized indirect DA receptor agonists that can be viewed as pharmacologically substituting for the addictive psychostimulants in a manner analogous to methadone substitution for heroin. Recently, extensive studies have demonstrated that the DA agents summarized in Table 1 appear to be promising for the treatment of psychostimulant addiction.
Table 1.
Pharmacological Actions of DA Receptor Antagonists in Animal Models or Paradigms of Psychostimulant Addiction
| l-THP | BP-897 | SB-277011A | NGB2904 | S33138 | |
|---|---|---|---|---|---|
| Pharmacological Actions | DA (D1, D2, and maybe D3) antagonist | D3 partial agonist | D3 antagonist | D3 antagonist | D3-preferring antagonist |
| Self-Administration (SA) | ↓ Cocaine SA (FR, PR) | ↓ Cocaine SA (PR, 2nd-order, not FR) | ↓ Cocaine SA (PR, 2nd-order, not FR)
↓ METH SA (PR, not FR) |
↓ Cocaine SA (PR, not FR) | ↓ Cocaine SA (PR, not FR) |
| Reinstatement of Drug-Seeking (Relapse) | ↓ Relapse by cocaine | ↓ Relapse by cocaine, by cues | ↓ Relapse by cocaine, cues or stress | ↓ Relapse by cocaine or cues | ↓ Relapse by cocaine |
| Conditioned Place Preference (CPP) | ↓ CPP by cocaine or METH | ↓ CPP by cocaine | ↓ CPP by cocaine | ||
| Enhanced Brain-Stimulation Reward (eBSR) | ↓ eBSR by cocaine | ↓ eBSR by cocaine or METH | ↓ eBSR by cocaine or METH | ↓ eBSR by cocaine or METH | ↓ eBSR by cocaine or METH |
| Psychostimulant-like Discriminative Stimulus Effects (DS) | ↓ DS by cocaine or Amphetamine | ||||
| Behavioral Sensitization (BS) | ↓ BS by cocaine Cue | ||||
| Abuse Liability | No SA by itself | No SA by itself | No SA by itself | No SA by itself | |
| Natural Reward | ↓ Sucrose-taking
↓ Food-taking |
No effect on sucrose-seeking | No effect on food-taking | No effect on sucrose-seeking | ↓ Sucrose-taking |
| Clinical Use or Trials | For pain and insomnia for 40 years | Phase II | No | No | No |
l-THP: Levo-tetrahydropalmantine
FR: Pixed-ratio reinforcement
PR: Progressive-ratio reinforcement
2nd-order: Self-administration under second-order reinforcement
METH: Methamphetamine.
2.1. Levo-tetrahydropalmatine (l-THP)
It is well documented that both D1 and D2 receptors are critically involved in drug reward and addiction [3, 29, 30]. However, clinical trials with D1- or D2-like receptor antagonists have failed because of lack of therapeutic effect with D1-like antagonists or severe side-effects with D2-like antagonists - such as dysphoria, supression of natural reward or abnormal movements [3, 29–32]. In contrast to D1- or D2-like receptor antagonists, l-THP is a non-selective D1 and D2 (and possibly D3) receptor antagonist [33–36], purified from the Chinese herb Stephanie [33]. l-THP has been used in China as a traditional sedative-analgesic agent for more than 40 years for the treatment of chronic pain and anxious insomnia. Recent studies have shown that l-THP significantly inhibits: 1) cocaine- or methamphetamine-induced CPP [37, 38]; 2) cocaine self-administration under fixed-ratio (FR) reinforcement and progressive-ratio (PR) reinforcement schedules [36, 39]; 3) cocaine-enhanced electrical BSR [39]; and 4) cocaine-triggered reinstatement of drug-seeking behavior in rats [36]. Such data support the potential use of l-THP in the treatment of psychostimulant dependence. The attenuation of cocaine’s actions in the above cited animal models of drug addiction are unlikely due to l-THP-induced sedation or locomotor inhibition, because the effective doses for attenuation of cocaine’s actions are much lower (3–10 fold) than those for inhibition of locomotion or sucrose self-administration [39]. In addition, l-THP also inhibits opioid tolerance and withdrawal syndromes in rats [40], as well as locomotor sensitization to oxycodone in mice [41], suggesting an anti-addictive utility broader than just for psychostimulants. A recent clinical trial demonstrates that l-THP significantly attenuates drug craving and relapse in heroin addicts [42].
In vivo microdialysis demonstrates that l-THP modestly elevates extracellular NAc DA by itself, and also dose-dependently potentiates cocaine-augmented DA [39], suggesting an action on presynaptic DA D2/3 receptors. However, such presynaptic action is unlikely to mediate the antagonism by l-THP of cocaine’s actions, suggesting a post-synaptic mechanism underlying the pharmacotherapeutic effects of l-THP. Although l-THP modestly elevates NAc DA, it can not maintain intravenous self-administration behavior in rats previously self-administered cocaine (Xi et al., unpublished data), suggesting that l-THP has no abuse potential by itself. Given that chronic cocaine appears to produce a reduction in basal mesolimbic DA transmision in both humans and experimental animals after withdrawal or abstinence [23, 43–46], the l-THP-induced modest augmentation of extracellular DA may also contribute to its therapeutic anti-cocaine actions by correcting the hypofunctional DA state. Since l-THP is a natural and cost-effective substance purified from Chinese herbs, and is well-tolerated in humans with few side-effects [33], the above pre-clinical findings support the potential use of l-THP for the treatment of cocaine or other pyschostimulant addiction.
2.2. BP-897
Recent research interest in DA D3 receptor-selective compounds in medication discovery for the treatment of psychostimulant addiction is derived from the unique anatomical distribution of D3 receptors in the brain. D3 receptors are preferentially localized in the mesolimbic DA system with the highest receptor densities in the NAc, islands of Calleja and olfactory tubercle [47, 48]. This restricted neuroanatomic localization suggests an important role for D3 receptors in drug reward and addiction [49, 50]. In addition, D3 receptors have the highest affinity for endogenous DA of all known receptors [49, 51], suggesting a crucial role for D3 receptors in the normal functioning of the mesolimbic reward system. Based on these and other considerations, it was hypothesized that pharmacological agents that block brain D3 receptors might be effective in the treatment of psychostimulant dependence [49, 50, 52].
BP-897 [1-(4-(2-naphthoylamino)butyl)-4-(2-methoxyphenyl)-1A-piperazine] is the first developed D3-selective pharmacological agent. It has been claimed to act as a D3 partial agonist [53] or antagonist [54, 55]. It has modest (60–70 fold) selectivity for human D3 versus D2 receptors, and similar (60–70 fold) selectivity over other receptors such as α1-, α2-adrenergic, and 5-HT1A receptors [53]. Several studies have assessed the efficacy of BP-897 in animal models of drug addiction [56, 57]. Briefly, it has been reported that BP-897 produces a significant dose-dependent reduction in: 1) cocaine self-administration under second-order reinforcement [53], but not under FR reinforcement [58]; 2) cocaine- or cocaine-cue-triggered reinstatement of cocaine-seeking behavior [59–61]; 3) cocaine-induced CPP [62–64]; 4) cocaine’s or amphetamine’s discriminative stimulus properties [65]; and 5) cocaine cue-induced increases in locomotion and behavioral sensitization [66]. These data support the potential use of BP-897 in the treatment of cocaine or other psychostimulant addiction [56, 57, 67, 68].
However, enthusiasm for BP-897 has waned due to recent findings that BP-897 also displays full antagonist properties at both DA D2 and D3 receptors [54, 55, 57]. Given that D2 receptor antagonism usually produces severe unwanted side-effects, such as dysphoria, inhibition of natural reward, and abnormal extrapyramidal movements [3, 29, 30], BP-897’s D2 antagonist properties raise the possibility of unwanted side-effects at the human level. BP-897 has recently entered Phase II clinical studies, but detailed pharmacokinetic and toxicological data have not yet been reported.
2.3. SB-277011A
SB-277011A [trans-N-[4-[2-(6-cyano-1,2,3,4-tetrahydroisoquinolin-2-yl)ethyl]cyclohexyl]-4-quinolinecarboxamide] is the most well characterized DA D3 receptor in antagonist to date. SB-277011A has high affinity for the human cloned DA D3 receptor, and the ratio of in vitro D3/D2 affinity of SB-277011A for human and rat is 120 and 80, respectively [69]. SB-277011A has a 100-fold selectivity or better over 180 other receptors, enzymes and ion channels [69]. Recently, we and others have assessed the pharmacological efficacy of SB-277011A in animal models of drug addiction. We found that SB-277011A attenuates: 1) cocaine- or methamphetamine-enhanced BSR [70, 71]; 2) cocaine-induced CPP [70]; 3) cocaine or methamphetamine self-administration under PR or high FR (FR10) reinforcement schedules [72, 73]; 4) cocaine-seeking behavior under second-order reinforcement conditions [74]; 5) cocaine-, cocaine cue- or stress-triggered relapse to cocaine-seeking behavior [59–60, 70, 75]; and 6) incubation of cocaine craving in rats [76]. These data suggest that SB-277011A significantly inhibits the acute rewarding effects of psychostimulants, incubation of cocaine craving, and reinstatement of cocaine-seeking behavior [57]. However, further development of SB-277011A has been halted by Glaxo-SmithKline Pharmaceuticals, due to unexpectedly poor bioavailability (~2%) and a very short half-life (<20 min) in primates [77, 78]. Therefore, development of other D3-selective antagonists with higher bioavailability and more promising pharmacotherapeutic profiles is required [79].
2.4. NGB 2904
NGB 2904 [N-(4-[4-{2,3-dichlorophenyl}-1-piperazinyl]butyl)-2-fluorenylcarboxamide] is another highly selective D3 receptor antagonist which demonstrates high binding affinities at primate and rat D3 receptors [79–81]. NGB 2904 has been reported to have >150-fold selectivity for primate D3 over D2 receptors, and >800-fold selectivity for rat D3 versus D2 receptors [79, 80]. In addition, it was found to have >5000-fold selectivity over D1, D4, and D5 receptors, 200- to 600-fold selectivity over α1, 5HT2 receptors, and >1000-fold selectivity versus other CNS targets in a 60-receptor Panlabs screen [80]. These in vitro profiles of NGB 2904 suggest it to be a promising D3 antagonist.
Based on this, we recently examined the pharmacological efficacy of NGB 2904 in animal models of psychostimulant addiction. We found that systemic administration of NGB 2904 inhibits: 1) intravenous cocaine self-administration maintained under PR reinforcement [82]; 2) cocaine- or cocaine cue-triggered reinstatement of cocaine-seeking behavior [60, 82]; and 3) cocaine- or methamphetamine-enhanced BSR [71, 82]. In addition, NGB 2904 inhibits nicotine- and heroin-enhanced BSR [83]. Further, NGB 2904 neither produces a dysphorigenic shift in BSR functions nor substitutes for cocaine in maintenance of self-administration behavior, suggesting that NGB 2904 itself has no addictive liability [83].
The effectiveness of NGB 2904 on PR cocaine self-administration and reinstatement of drug-seeking triggered by cocaine or cocaine-associated cues suggests a clinical therapeutic potential, particularly for relapse to drug-seeking behavior. NGB 2904 is currently not under clinical trial and detailed data regarding bioavailability and pharmacokinetic properties must still be gathered.
2.5. S33138
As described above, the selective D3 receptor antagonists SB-277011A and NGB 2904 are ineffective against intravenous cocaine and methamphetamine (and also nicotine or alcohol) self-administration under low fixed-ratio (FR1, FR2) reinforcement conditions. In addition, NGB 2904’s antagonism of cocaine- and methamphetamine-enhanced BSR displays an obvious “dose-window effect”. That is, only lower doses of NGB 2904 were effective in attenuating cocaine- or methamphetamine-enhanced BSR, and the anti-reward effects could be overcome by increasing the doses of cocaine or methamphetamine [71, 83]. These data suggest that selective D3 receptor antagonists may have limited potential against the direct rewarding effects produced by psychostimulants. Consequently, it was proposed that preferential D3 versus D2 receptor antagonists may be an effective strategy for treating psychostimulant addiction [39, 83–84], based upon the finding that blockade of both D3 and D2 receptors produces additive or synergistic effects in antagonizing drug reward, but produced fewer unwanted side-effects than selective D2 antagonists due to opposite locomotor effects produced by blockade of D3 (facilitation) versus D2 (suppression) receptors [84, 85].
S33138 (N-[4-[2-[(3aS,9bR)-8-cyano-1,3a,4,9b-tetrahydro [1]benzopyrano[3,4-c]pyrrol-2(3H)-yl)-ethyl]phenyl acetamide) is such a preferential D3 versus D2 receptor antagonist [86], displaying 25-fold selectivity for human (h) D3 over hD2 receptors (pKi, 8.7 vs. 7.1 and 7.3). Recently, we carried out a series of experiments to evaluate the efficacy of S33138 in animal models relating to cocaine addiction [87]. We found that systemic administration of S33138 dose-dependently attenuated cocaine-enhanced BSR, but at the highest dose S33138 produced a significant aversive-like shift in BSR reward functions. S33138 produced biphasic effects on cocaine self-administration, i.e., a moderate dose increased, while a high dose inhibited, cocaine self-administration under FR2 reinforcement. We suggest that the increase in cocaine self-administration produced by the moderate dose of S33138 could be a compensatory response to a partial reduction in cocaine reward. In addition, S33138 also dose-dependently inhibited oral sucrose self-administration at high doses. In the reinstatement model of relapse to drug-seeking behavior, S33138 dose-dependently inhibited cocaine-triggered reinstatement of drug-seeking behavior. This reduction in reward-taking/seeking behaviors is unlikely due to impaired locomotor performance, as S33138 decreased neither Ymax levels in the BSR paradigm nor locomotor activity. Finally, S33138, at doses of 0.04–0.36 mg/kg/infusion, is clained to not be self-administered intravenously in rhesus monkeys (France et al., unpublished data). These findings suggest that the preferential D3 versus D2 receptor antagonist S33138 may be useful for the treatment of cocaine addiction, and may even show advantages relative to selective D2 antagonists, although the aversive-like BSR effects and inhibition of natural reward at high doses is a caution. Currently, S33138 is under clinical trial as an anti-psychotic agent [84, 88].
3. DOPAMINE-BASED MEDICATION DISCOVERY – AGONIST SUBSTITUTION STRATEGIES
Agonist Substitution Hypothesis
Agonist substitution as a pharmacotherapy for psychostimulant addiction is based on, first, the success of methadone in the treatment of opiate dependence and the nicotine patch for smoking cessation; second, the finding that the speed with which addictive drugs enter the brain and elevate NAc DA is positively correlated with addictive potential [89, 90], with slow-onset long-acting compounds having lower addictive potential; third, the hypothesis that a compound with low addictive potential may substitute for one with high addictive potential, thereby decreasing the use of the highly addictive drug; and finally, the hypothesis that long-term increases in NAc DA may ameliorate the hypodopaminergic dysfunction believed to exist in human cocaine addicts, thereby minimizing drug craving and relapse [23, 43, 45, 46]. To achieve such DA agonist effects, medication development for anti-addiction purposes has concentrated on the development of DA reuptake or DA transporter (DAT) inhibitors rather than direct DA receptor agonists. Indeed, this strategy has been at the forefront of medication development for the treatment of cocaine addiction for more than a full decade [11, 91]. To date, a wide variety of structural classes have served as chemical templates for the development of slow-onset long-acting DAT inhibitors, including benztropines, mazindol, substituted piperazines, tropanes, indanamines and trans-aminotetralins (Table 2).
Table 2.
Pharmacological Actions of DA or Monoamine Transporter Inhibitors in Animal Models or Paradigms of Psychostimulant Addiction
| GBR12909 | RTI-336 | CTDP30,640 | CTDP31,345 | |
|---|---|---|---|---|
| Pharmacological Actions | DA transporter inhibitor | DA transporter inhibitor | Monoamine transporter inhibitor | Monoamine transporter inhibitor |
| Self-Administration (SA) | ↓ Cocaine SA (FR, PR) | ↓ Cocaine SA (FR, 2nd-order) | ↓ Cocaine SA (FR) | ↓ Cocaine SA (FR) |
| Reinstatement of Drug-Seeking (Relapse) | ↓ Relapse by cocaine or cues | No effect on relapse by cocaine | ||
| Enhanced Brain-Stimulation Reward (eBSR) | ↓ eBSR by cocaine | ↑ eBSR by cocaine | ↑ eBSR by cocaine | |
| Psychostimulant-like Discriminative Stimulus Effects (DS) | ↑ DS by cocaine, by METH | |||
| Abuse Liability | SA by itself; Sensitization by itself | SA by itself; DS effect by itself | Sensitization by itself; DS effect by itself | SA by itself |
| Natural Reward | No effect on food-taking | ↓ Food-taking | ↓ Sucrose-taking | |
| Clinical Trials | Phase I/IIa | No | No | No |
3.1. GBR-12909 (Vanoxerine)
GBR-12909, a phenyl-substituted piperazine derivative, is a relatively slow-onset long-acting DAT inhibitor [92]. To date, it is the most extensively studied DAT inhibitor proposed to be beneficial in the treatment of cocaine addiction [93–96]. GBR-12909 binds to the DAT site with high affinity, and selectively inhibits DA re-uptake [97]. GBR-12909 can also compete with psychostimulants at the DAT site, thus blocking cocaine- or amphetamine-induced increases in NAc extracellular DA [98, 99]. Compared with the same doses of cocaine, GBR-12909-induced increases in striatal DA and locomotion are relatively slow-onset (<10 min vs. 10–20 min) and long-lasting (1 hr vs. 3–4 hr) [99, 100]. Pretreatment with GBR-12909 significantly inhibits cocaine self-administration in rats and nonhuman primates at doses that have little or no effect on food self-administration [101–104]. Repeated treatment with low doses of GBR-12909 also sustains the selective suppression of cocaine self-administration versus food self-administration [105]. Further, a single injection of a decanoate ester slow-release formulation of GBR-12909 produced a prolonged (up to one month) suppression of cocaine self-administration in nonhuman primates [92, 106]. These findings support GBR-12909 as a potential candidate for the treatment of cocaine addiction [96]. However, there are significant cautions associated with it. GBR-12909 itself produces cocaine-like augmentment of BSR [107] and cocaine-like discriminative stimulus effects [108]. It is also self-administered and triggers reinstatement of extinguished drug-seeking behavior in both rats and primates [109, 110]. These data suggest that GBR-12909 itself may have abuse potential. Finally, GBR-12909 produced rate-dependent cardiac QTc interval prolongation in five out of five subjects studied in Phase I human trials, prompting discontinuation of the trials [112].
3.2. RTI-336
RTI-336 [3beta-(4-chlorophenyl)-2beta-[3-(4′-methylphenyl)isoxazol-5-yl]tropane] is a novel slow-onset (30 min) long-acting (4 hrs) DAT inhibitor [90, 111]. It has >1000- and >400-fold selectivity for the DAT relative to the serotonin transporter (SERT) and norepinepherine transporter (NET), respectively [113]. Pretreatment with RTI-336 produced dose-dependent reduction in cocaine self-administration in both rats and nonhuman primates [114, 115]. The ED50 dose of RTI-336 for reducing cocaine self-administration resulted in approximately 90% DAT occupancy, suggesting that high levels of DAT occupancy by RTI-336 are required to reduce cocaine self-administration. However, co-administration of the ED50 dose of RTI-336 with the SERT inhibitor fluoxetine or citalopram produced robust reductions in cocaine self-administration in nonhuman primates [115], suggesting that blockade of both DAT and SERT may be more effective in attenuating cocaine’s reinforcement than selective blockade of DAT alone [116]. However, RTI-336, at the doses that effectively suppressed cocaine self-administration, also inhibited food-taking behavior under multiple second-order reinforcement schedules [115]. This is different from the relative selectivity of GBR-12909 in suppressing cocaine self-administration over food-taking behavior, as noted above. As with many other DAT inhibitors, RTI-336 itself reliably maintained self-administration behavior in all non-human primates tested [115], suggesting abuse liability by itself. In addition, RTI-336 itself also produced locomotor stimulating and cocaine-like drug-discrimination effects in mice and rats. However, when compared with cocaine, RTI-336 maintained lower rates of self-administration, produced weaker locomotion and cocaine-like drug discriminative stimulus effects across a broad range of doses, and showed very low locomotor sensitization [113, 117]. These data suggest that RTI-336 may have lower abuse potential than cocaine. So far, there are no human trials with RTI-336 reported.
3.3. CTDP30,640
CTDP30,640 is an indanamine analog with slow-onset and long-acting monoamine re-uptake inhibitor properties [118, 119]. The “CTDP” terminology derives from the “Cocaine Treatment Discovery Program” of the Extramural Program of the U.S. National Institute on Drug Abuse. Our interest in such a non-selective monoamine transporter inhibitor as a potential anti-cocaine medication originally stemmed from the fact that cocaine is also a non-selective monoamine transporter inhibitor. Thus, a monoamine transporter inhibitor with a slow-onset and long-acting profile might constitute a therapeutically useful substitute for cocaine. This possiblity is supported by a recent finding that combined DAT and SERT inhibition appears more effective in suppressing cocaine self-administration than a selective DAT inhibitor (RTI-336) alone, as mentioned above.
CTDP30,640 is a prodrug, which is metabolized to active metabolites by rate-limited N-demethylation to achieve the desired slow-onset long-lasting pharmacological profile. CTDP30,640 itself has little or no activity [118]. We have evaluated the efficacy of CTDP30,640 in preclinical animal paradigms of drug addiction. We found that: 1) CTDP30,640 produced a cocaine like enhancement of locomotor activity, but with a slow-onset (<10 min vs. 20 min) and striking long duration (2h vs 8h) compared with cocaine [118]; 2) CTDP30,640 produced cocaine-like drug-discrimination effects in rats [118]; 3) CTDP30640 produced a cocaine-like enhancement of BSR, but with a marked slow onset and prolonged duration (up to 25–48 hrs after 3–5 mg/kg) relative to cocaine [119]; 4) CTDP30,640 dose-dependently lowered cocaine self-administration, an effect that lasted for up to 28, 50, and 90 hrs after 2.5, 5.0, 10 mg/kg, respectively [119]; and finally, 5) in vivo brain microdialysis demonstrated that CTDP30,640 produced a marked cocaine-like enhancement of NAc DA with a very pronounced slow onset and long duration profile relative to cocaine [119]. These findings suggest that CTDP30,640 has cocaine-like rewarding, psychomotor stimulating and neurochemical properties with slow onset and extralong duration. The prolonged suppression of cocaine self-administration after a single dose of CTDP30,640 suggests the potential use of CTDP30,640 in the treatment of cocaine addiction. However, its strong psychomotor stimulant and stereotypy-inducing effects decrease enthusiasm for its further development [118].
3.4. CTDP31,345
Based on our work with CTDP30,640, we designed, synthesized and tested additional slow-onset long-acting monoamine transporter inhibitors as candidate compounds for the treatment of psychostimulant addiction. CTDP31,345 is a trans-aminotetralin derivative [118, 120]. Like CTDP 30,640, CTDP31,345 is also a prodrug, which is metabolized (N-demethylated) to CTDP31,346, a slow-onset long-acting non-selective monoamine transporter inhibitor. In vitro transporter binding assays demonstrate similar binding affinities of CTDP31,345 to cloned human DAT (Ki = 18 ± 5 nM) and SERT (Ki = 23 ± 7 nM), but a lower affinity to cloned human NET (Ki = 81 ± 10 mM). Functional reuptake assays with cell lines expressing each transporter revealed the IC50 of CTDP31,345 for inhibition of DA, serotonin (5-HT) and NE reuptake to be 330 ± 120, 3.6 ± 0.2 and 120 ± 60 nM, respectively (Froimowitz, unpublished data).
To determine whether CTDP31,345 is superior to CTDP30,640, Froimowitz et al. observed the effects of CTDP31,345 on locomotion and stereotypic behaviors in rats. They found that the locomotor stimulation effects of CTDP31,345 (3–56 mg/kg i.p.) appeared to be mild and not dose-dependent. CTDP31,345, at 10 mg/kg, produced the most robust increase among all the doses (3–56 mg/kg i.p.) tested, with peak effects occurring at 40 min and lasting for about 8 hr. At higher doses, CTDP31,345 lost locomotor stimulating effects in mice. In contrast to CTDP30,640, CTDP31,345 failed to produce stereotypic behaviors in the majority (~80%) of mice tested (Froimowitz and Forster, unpublished data). These data suggest that CTDP31,345 appears superior to CTDP30,640, at least with respect to possible motoric side effects.
Therefore, we went on to assess the actions of CTDP31,345 in animal models of drug addiction. We found that: 1) CTDP31,345 itself has cocaine-like properties in rats. Systemic administration of CTDP31,345 produced slow-onset (20–50 min) and long-lasting (at least 5–7 hrs) increases in locomotion and NAc extracellular DA, and a sustained (>24 hrs) enhancement of BSR in rats [121, 122]; 2) pretreatment with CTDP31,345 dose-dependently inhibited cocaine self-administration in rats, an effect that lasted for at least 24 hrs after a single injection of 20 mg/kg of CTDP31,345; 3) this reduction in cocaine self-administration appeared to be due to CTDP31,345’s substitution effects, because pretreatment with CTDP31,345 produced an enhancement of cocaine’s effects on locomotion, electrical BSR and NAc DA; 4) such enhancing effects of CTDP31,345 on cocaine’s actions are strikingly different from those of methadone on heroin’s actions, in which methadone pretreatment produces a dose-dependent reduction in heroin self-administration, heroin-enhanced BSR and heroin-enhanced NAc DA; and finally 5) CTDP31,345 itself also maintained self-administration at lower rates than cocaine when tested at comparable doses in rats [121, 122].
The potential use of CTDP31,345 in the treatment of cocaine addiction remains to be determined. Although its cocaine-like rewarding effects and potential abuse liability may limit its use, the long-term inhibition of cocaine self-administration after a single dose of CTDP31,345 administration does support its potential utility. In addition, CTDP31,345-induced long-term increases in NAc DA may also be helpful in relieving drug craving and relapse to drug use by restoring reduced synaptic DA function in brain reward circuits [43, 45]. Clearly, more studies are required to determine whether CTDP31,345 or other slow-onset long-acting monoamine transporter inhibitors with different chemical structures will have similar or different pharmacological profiles in animal paradigms of drug addiction, as compared to methadone, a ‘gold standard’ in the treatment of opiate addiction.
4. GABA-BASED MEDICATION DISCOVERY
GABAergic hypothesis of drug reward
As noted above, an increase in mesolimbic DA transmission has been thought to underlie the drug reward produced by addictive drugs. However, how increased NAc DA mediates drug reward remains unclear. It is well documented that the neurotransmitter GABA plays an important role in the mesolimbic DA reward circuit [25, 123]. Anatomically, the majority of neurons in the striatum are medium-spiny GABAergic output neurons, which project predominantly to the dorsal globus pallidus (from the dorsal striatum) and the ventral pallidum (from the ventral striatum, i.e. the NAc) [124–126]. In addition, striatal GABAergic neurons also receive DA projections from the VTA and glutamatergic projections predominantly from the prefrontal cortex (PFC), and regulate striatal DA and glutamate release [28, 127]. Overall, DA produces an inhibitory effect on striatal medium-spiny GABAergic neurons [128–130], predominantly by activation of D2-like DA receptors [131, 132]. Similarly, psychostimulants, such as cocaine or amphetamine, produce an inhibitory effect on VTA GABAergic neurons [133], striatal GABAergic neurons [131, 132, 134–136], and on GABA release in the ventral pallidum (VP) [137–139]. Based on this, the VTA-NAc-VP pathway, particularly the downstream NAc-VP GABAergic projection, has been proposed as a common final pathway underlying psychostimulant reward [13–14, 17]. Thus, it has been hypothesized that any pharmacological strategy that increases GABAergic transmission by either elevating extracellular GABA levels or directly activating GABA receptors within brain reward circuits might directly counteract psychostimulant-induced inhibition of GABAergic neurons, thereby antagonizing psychostimulant abuse [123, 140, 141]. Based on this hypothesis, several GABAergic compounds, which are summarized in Table 3, are being explored as candidate medications for the treatment of psychostimulant addiction.
Table 3.
Pharmacological Actions of GABAergic Agents in Animal Models or Paradigms of Psychostimulant Addiction
| GVG | Gabapentin | Tiagabine | Topiramate | Baclofen | |
|---|---|---|---|---|---|
| Pharmacological Actions | GABA transaminase inhibitor | GABAmimetic | GABA transporter inhibitor | Positive GABAA receptor modulator | GABAB receptor agonist |
| Self-Administration (SA) | ↓ Cocaine SA (FR, PR) | No effect on cocaine SA | ↓ SA of low dose cocaine | ↓ Cocaine (amphetamine, METH) SA (FR, PR, 2nd-order) | |
| Reinstatement of Drug-Seeking | ↓ Relapse by cocaine | No effect on relapse by cocaine | No effect on relapse by cocaine | ↓ Relapse by cocaine, by amphetamine | |
| Conditioned Place Preference (CPP) | ↓ CPP by Cocaine or amphetamine | ↓ CPP by METH | |||
| Enhanced Brain-Stimulation Reward (eBSR) | ↓ eBSR by cocaine | ↓ eBSR by cocaine | |||
| Psychostimulant-like Discriminative Stimulus Effects (DS) | No effect on DS by cocaine | No effect on DS by cocaine | No effect on DS by cocaine | No effect on DS by cocaine | No effect on DS by cocaine |
| Behavioral Sensitization (BS) | ↓ BS by Cocaine | Conflicting | No effect on cocaine BS | ↓ BS by cocaine, by amphetamine | |
| Abuse Liability | No SA by itself | Cases reported | No cocain-like DS by itself | No cocain-like DS by itself | No SA by itself |
| Natural Reward | ↓ Food-taking
↓ Sucrose-seeking |
↓ Food-taking | ↓ Food-taking | ||
| Clinical Trials | Phase II | Phase I/II | Phase II | Phase II | Phase II |
4.1. Gamma-vinyl GABA (GVG)
GVG is an irreversible GABA transaminase inhibitor [142]. GABA transaminase is the primary enzyme involved in GABA’s metabolic breakdown, and therefore, and its inhibition elevates brain GABA levels. Since many anti-epilepsy and anticonvulsant medications are either direct or indirect GABA agonists, GVG (as an indirect agonist) was initially developed as an anticonvulsant, and has been used for that purpose in many countries for many years. About 10 years ago, Dewey and colleagues first proposed that GVG might be useful in the treatment of psychostimulant addiction by elevating brain GABA levels [140, 143]. So far, findings in their preclinical studies appear to be highly promising [12]. Systemic administration of GVG has been shown to inhibit: 1) cocaine-enhanced electrical BSR, but without significant effects on motor performance [144]; 2) cocaine self-administration under both FR5 and PR reinforcement [145, 146]; 3) cocaine-induced CPP [140]; 4) cocaine-induced behavioral sensitization [147]; and 5) cocaine-triggered reinstatement of drug-seeking behavior [146, 148]. In addition, at higher doses (~300 mg/kg), GVG also inhibits food-taking and sucrose-triggered reinstatement [145, 148, 149], but fails to inhibit the discriminative stimulus effects of cocaine [149].
Regarding the effects of GVG on NAc DA, previous studies by Dewey’s group have shown that GVG significantly inhibits cocaine- or cocaine-associated cue-induced increases in NAc DA in rats [140, 150–152]. However, our recent studies demonstrate that GVG, when given systemically or locally into the NAc, dose-dependently elevates NAc extracellular GABA levels, but fails to alter either basal levels of, or cocaine-enhanced, NAc DA in drug naïve or cocaine-treated rats [148]. The same doses of GVG also fail to inhibit, but rather increase, NAc extracellular glutamate levels (Xi et al., unpublished data). Clearly, these findings conflict with those from Dewey’s group noted above. The reasons for this discrepancy are unclear. It does not appear to be related to GVG dose, GVG pretreatment time or animals’ cocaine experience, but likely to be related to differences in rat strains (Long-Evans vs Sprague-Dawley) and/or brain regions of microdialysis sampling (medial NAc core/shell vs unspecified NAc subdomains) (see more discussion in [148]). Whatever the reasons for this discrepancy, our recent studies have further demonstrated that GVG-induced increases in NAc GABA are derived predominantly from non-neuronal (presumably glial) sources (Xi et al., unpublished data). This new finding provides a reasonable explanation for the ineffectiveness of GVG on NAc DA, i.e., that GVG-enhanced extrasynaptic GABA may not diffuse sufficiently into synaptic clefts to inhibit DA release. This is consistent with previous findings that local NAc perfusion of GABAA or GABAB receptor agonists, but not GABA itself, inhibits NAc DA and glutamate [127, 153, 154], and similarly inhibits cocaine or heroin self-administration and reinstatement of drug-seeking behaviors [154, 155]. GVG’s ineffectiveness on NAc DA suggests that non-DA mechanisms may underlie GVG-induced inhibition of drug-seeking behavior.
We have proposed an alternative mechanistic hypothesis - that GVG-elevated extracellular GABA levels in GABAergic projection loci such as the VP or VTA, may underlie GVG’s effects. That is, GVG-induced GABA elevations may directly counteract cocaine-induced reductions in GABA release, and therefore, may underlie the potential therapeutic effect of GVG on cocaine addiction [148]. This is consistent with our previous studies demonstrating that microinjection of GVG into the NAc failed to inhibit heroin self-administration, while microinjections of GVG into the VTA or VP significantly inhibited heroin self-administration [123].
Whatever the underlying mechanisms, the above-cited evidence strongly supports the potential use of GVG in the treatment of psychostimulant abuse. GVG is currently under double-blind placebo-controlled clinical trials for treatment of cocaine addiction. Preliminary open-label clinical trials demonstrated that GVG appears to manifest clinically-relevant anti-addiction, anti-craving, and anti-relapse properties in humans [156, 157]. The major concern for its use in humans, however, is the potential for visual field defects, which may limit its use.
4.2. Gabapentin
Gabapentin is structurally analogous to GABA but, unlike the latter, it crosses the blood–brain barrier and can be administered systemically [158]. Pharmacologically, gabapentin is a GABAmimetic drug that increases extracellular GABA levels, possibly by increasing the synthesis and nonvesicular release of GABA as well as by preventing GABA catabolism (reviewed in [159]). In addition, gabapentin also inhibits alpha2delta subunit-composed voltage-dependent Ca++ channels [160]. Early studies and small-scale, open-label outpatient trials suggested that gabapentin reduced cocaine craving and use [161–165]. However, this finding was later challenged by larger-scale, double-blind, placebo-controlled clinical trials demonstrating that gabapentin, at doses up to 2400–3200 mg/day for 6–12 weeks, had no effect on abstinence rates, craving or subjective effects of cocaine [166–169].
Recently, we assessed its efficacy in animal models of drug addiction. We found that gabapentin (25–200 mg/kg i.p.) failed to inhibit i.v. cocaine self-administration under FR2 reinforcement or cocaine-triggered reinstatement of cocaine-seeking behavior in rats [170]. Congruent with our findings, Filip et al. [146] recently reported that lower doses of gabapentin (10–30 mg/kg, i.p.) neither alter cocaine self-administration nor cocaine-induced reinstatement of drug-seeking behavior in rats. In addition, 1–30 mg/kg gabapentin was reported to have no effect on cocaine-induced hyperactivity or cocaine-induced behavioral sensitization [171, 172].
To study possible mechanisms underlying such ineffectiveness of gabapentin on cocaine’s addictive behaviors, we further observed the effects of gabapentin on NAc extracellular GABA, DA and cocaine-enhanced NAc DA in rats. We found that gabapentin, at 100–200 mg/kg, produced a modest increase (maximally ~50 %) in extracellular NAc GABA levels, but failed to alter either basal or cocaine-enhanced NAc DA [170]. This is in striking contrast to our findings with GVG in which we observed a robust and dose-dependent increase (200–400% at 100–300 mg/kg) in NAc extracellular GABA and an inhibition of both cocaine self-administration and cocaine-triggered reinstatement [148]. These data suggest that gabapentin is a weak GABA enhancer and has limited potential in the treatment of psychostimulant addiction.
4.3. Tiagabine
Tiagabine is a selective type 1 GABA transporter (GAT1) inhibitor, approved as an antiepileptic medication that increases synaptic levels of GABA. There are four types of GABA transporter identified, GAT1 being the most abundant, with predominant localization on GABAergic neurons and glial cells [173, 174]. Given the effectiveness of GVG in preclinical animal models of drug addiction, it was proposed that tiagabine may also be effective in attenuating cocaine use in humans [175]. However, the results of clinical trials with tiagabine are conflicting. Two small-scale (45 and 76 subjects, respectively) placebo-controlled clinical trials indicated that tiagabine produced a moderate reduction (~30%) in self-reported and urine-measured cocaine use in methadone-treated cocaine addicts [168, 175]. In contrast to this finding, other studies demonstrated that the same doses of tiagabine neither altered the acute effects of oral cocaine [176], nor significantly lowered cocaine use in human cocaine addicts [177, 178].
Similar conflicting findings are also reported in preclinical animal studies. It was reported that tiagabine produced a dose-dependent reduction in self-administration maintained by a very low dose (0.032 mg/kg) of cocaine under a FR10 schedule of reinforcement in baboons [179]. In contrast, Filip et al. [146] reported that tiagabine significantly inhibited lever responding for cocaine (0.5 mg/kg/infusion) self-administration under FR5 reinforcement, but had no effect on either total cocaine intake during self-administration, cocaine-induced reinstatement of drug-seeking behavior, or cocaine’s discriminative stimulus effects. The reasons for such conflicting findings are unclear. Possibly, tiagabine’s binding properties could play a role. [3H]Tiagabine has highest binding density in frontal cortex and parietal cortex, and lowest binding in NAc and putamen [180]. Overall, present data suggest that tiagabine may have limited potential for the treatment of psychostimulant addiction.
4.4. Topiramate
GABAergic neurotransmission can be increased by drugs that elevate endogenous GABA levels (such as GVG, gabapentin or tiagabine) or by positive modulators at GABAA or GABAB receptors. Topiramate is a positive modulator of GABAA receptors, licensed as an antiepileptic drug. In addition to its enhancement of GABAA receptor-mediated currents at non-benzodiazepine sites on the GABAA receptor, topiramate also has other pharmacological actions in the brain, including antagonism of AMPA/kainate glutamate receptors, inhibition of voltage-gated sodium and calcium channels and inhibition of carbonic anhydrase [181]. Based on this, topiramate was proposed to be able to inhibit cocaine-enhanced NAc DA and cocaine abuse by enhancing GABAA-mediated inhibition and blocking excitatory glutamate activity [181, 182]. In a small preliminary clinical study (6 subjects), topiramate appeared to be effective at producing cessation of cocaine use, and alcohol consumption as well [183]. In another bigger double-blind, placebo-controlled clinical trial (40 subjects), topiramate significantly increased abstinance rates in patients with low cocaine withdrawal scores when compaired with placebo [184]. In contrast, a recent clinical report indicates that acute dosing with topiramate appears to enhance, rather than attenuate, the positive subjective effects of methamphetamine [185]. These data suggest that more large-scale clinical trials and preclinical mechanistic studies are required to determine whether or not topiramate is truly effective in antagonizing the addictive actions of psychostimulants.
4.5. Baclofen
Baclofen is a selective GABAB receptor agonist, licensed as an antispasmodic for patients with spinal cord injuries or multiple sclerosis. The efficacy of baclofen against cocaine use and relapse has been studied in both humans and animals extensively for many years, and appears promising for the treatment of cocaine addiction [186]. In rodents, pretreatment with baclofen dose-dependently attenuated cocaine-induced increases in NAc DA [187], cocaine-enhanced BSR [188], and cocaine self-administration under FR and PR reinforcement [189–191]. Baclofen also reduced cocaine-taking and cocaine-seeking behavior under second-order reinforcement in rats [192] and cocaine-triggered reinstatement of drug-seeking behavior [193, 194].
In an initial open-label clinical trial, baclofen (20 mg × 3) reduced self-reports of craving and cocaine use in 10 cocaine abusers [195]. In a subsequent 16-week double-blind study in 35 cocaine-dependent subjects, the same doses of baclofen reduced cocaine use and increased the number of cocaine-free urines [196], but did not significantly alter cocaine craving. In a recent placebo-controlled, double-blind study, baclofen (20 mg × 3/day, for 7 days) decreased self-administration of a low dose of smoked cocaine in 10 cocaine addicts, decreased cocaine craving in 7 opioid addicts, attenuated cocaine’s cardiovascular effects, but not cocaine’s subjective effects (‘high,’ ‘stimulated’) in both cocaine- and opioid-dependent subjects [197]. Another similar double-blind controlled study (7 subjects) demonstrated that a single dose of baclofen (10–30 mg/kg, p.o.) had no effect on either subject-rated or cardiovascular effects of intranasal cocaine [198]. Overall, baclofen seems to be well tolerated with no major side effects. The major limitation is its short-duration (3–4 hrs) of action, but slow-release preparations are likely to circumvent this limitation.
5. CANNABINOID-BASED MEDICATION DISCOVERY
Cannabinoid hypothesis of drug reward
Marijuana is the most widely used illicit drug in the United States and many other countries. As with other addictive drugs, cannabinoids produce significant rewarding and psychostimulating effects in both humans and experimental animals [199]. However, the underlying mechanisms are still unclear. The main psychoactive ingredient in marijuana is Δ9-tetrahydrocannabinol (Δ9-THC). Two major types of cannabinoid receptors, CB1 and CB2, have been cloned [200, 201]. CB1 receptors are found primarily in the brain, whereas CB2 receptors are located mainly in peripheral tissues associated with the immune system [202, 203].
As discussed above, a GABAergic mechanism may underlie the rewarding effects of addictive drugs. Here, we propose that a similar cannabinoid-induced inhibition of GABAergic neurons in the mesolimbic system may also underlie the actions of cannabinoids. This is supported by the following evidence: first, previous studies show that Δ9-THC enhances NAc extracellular DA [204, 205], which may inhibit postsynaptic medium-spiny GABAergic neurons; and second, high densities of CB1 receptors are found on medium-spiny GABAergic neurons and glutamatergic terminals in the striatum [203, 206]. Since CB1 receptors are Gi-coupled, activation of CB1 receptors located on GABAergic neurons produces an inhibitory effect on GABAergic neurons. Similarly, activation of CB1 receptors located on glutamatergic terminals decreases glutamate release, thereby decreasing excitatory impact on striatal GABAergic neurons. Growing evidence demonstrates that cocaine, opioids, alcohol and DA significantly increase endocannabinoid release in the striatum [207–210], suggesting that such an increase in endocannabinoids release may contribute to the acute rewarding effects of psychostimulants by increasing endocannabinoid tone on CB1 receptors located on both medium-spiny GABAergic neurons and glutamate terminals. This endocannabinoid hypothesis of drug reward may explain why deletion or pharmacological blockade of CB1 receptors broadly affects almost all addictive drug actions in experimental animals [18, 211]. Based on this hypothesis, the selective CB1 receptor antagonists summarized in Table 4 could be effective in attenuating the rewarding effects and relapse properties of drugs of abuse, including psychostimulants.
Table 4.
Pharmacological Actions of CB1 Receptor Antagonists in Animal Models or Paradigms of Psychostimulant Addiction
| SR141716 | AM251 | |
|---|---|---|
| Pharmacological Actions | Prototypic CB1 receptor antagonist | Novel CB1 receptor antagonist |
| Self-Administration (SA) | Conflicting | ↓ Cocaine SA (PR, not FR)
↓ METH SA (FR) |
| Reinstatement of Drug-Seeking (Relapse) | ↓ Relapse by cocaine, cues, not stress | ↓ Relapse by cocaine |
| Conditioned Place Preference (CPP) | No effect on cocaine CPP | |
| Enhanced Brain-Stimulation Reward (eBSR) | ↓ eBSR by cocaine | ↓ eBSR by cocaine |
| Behavioral Sensitization (BS) | Conflicting | ↓ BS by cocaine |
| Natural Reward | ↓ Food-taking | ↓ Food-taking |
| Clinical Trials | Phase III for nicotine and alcohol, but not psychostimulant, dependence | No |
5.1. SR141716A
SR141716A [N-(piperidin-1-yl-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-carboxamide] is the first developed selective CB1 receptor antagonist [212], and has become a considerable focus for research on medication discovery for the treatment of drug abuse. It is well documented that SR141716A appears to be widely effective in attenuating self-administration of heroin, nicotine, or alcohol, and in attenuating reinstatement of drug-seeking behavior produced by these drugs [213–215]. However, the effects of SR141716A on cocaine’s actions have been controversial [216]. The positive findings include: 1) SR141716A (0.3–3.0 mg/kg i.p.) significantly inhibited cocaine- or cocaine-associated environmental cue-, but not footshock stress-, induced reinstatement of drug-seeking behavior [217, 218]; 2) SR141716A (0.3–3.0 mg/kg) dose-dependently lowered the break-point for cocaine self-administration under PR reinforcement conditions in mice [219]; and 3) SR141716 significantly inhibited cocaine-induced phasic increase in NAc DA in rats as assessed by in vivo voltammetry [220]. In contrast to these positive findings, most other studies demonstrate that SR141716A, within the dose range of 0.3–10 mg/kg, has no significant effect on: 1) cocaine self-administration under FR or PR reinforcement conditions in mice, rats or nonhuman primates [139, 217, 221–224]; 2) cocaine-induced CPP [225]; 3) cocaine’s discriminative stimulus effects [223]; 4) cocaine-enhanced BSR in rats [224]; 5) cocaine-induced behavioral sensitization [223, 225]; 6) cocaine-induced increases in NAc DA [139, 219]; and 7) cocaine-induced decreases in extracellular VP GABA levels [139]. These data suggest that SR141716A may have certain therapeutic potential in attenuating drug craving or relapse to drug-seeking behavior, but has limited potential in attenating cocaine’s acute rewarding and neurochemical effects [211, 216]).
5.2. AM251
AM251 [N-(piperidin-1-yl)-5-(4-iodophenyl)-l-(2,4-dichlorophenyl)-4-methyl-l-H-pyrazole-3-carboxamide] is a another CB1 receptor antagonist. Compared with SR141716A, AM 251 is roughly 2-fold more potent (Ki=7.49 vs. 11.5 nM) and more selective (1: 306 vs. 1: 143) than SR141716 for CB1 over CB2 receptors in vitro [226, 227]. The efficacy of AM251 on psychostimulant action has been assessed recently in preclinical animal models. In summary, it was reported that AM 251, within the dose range of 1–10 mg/kg i.p., dose-dependently inhibited: 1) methamphetamine (0.08 mg/kg/infusion) self-administration under FR3 reinforcement in rats [228]; 2) cocaine self-administration under PR, but not under FR2 reinforcement conditions in rats [224]; 3) cocaine-triggered reinstatement of drug-seeking behavior in rats [229]; 4) cocaine-enhanced electrical BSR in rats [224]; 5) cocaine- or amphetamine-induced behavioral sensitization in mice [230]; 6) cocaine-induced increases in NAc glutamate, but not in DA in rats [39]; and 7) cocaine-induced increases in phosphorylated extracellular signal-regulated kinase (p-ERK) [230]. In contrast to these positive findings, it was recently reported that, at lower doses (0.032–0.32 mg/kg i.v.), AM251 had no effect on methamphetamine-induced reinstatement of drug-seeking behavior in rats [231].
Together, these data suggest that AM251 appears to be more potent and more effective in attenuating cocaine’s acute rewarding effects than SR141716. This is consistent with their in vitro binding properties noted above. In addition, the non-selective binding of SR141716A to other non-CB1 receptor proteins may also contribute to its relative ineffectiveness in attenuating cocaine’s rewarding efficacy (for more comprehensive review, see [211]).
Our most recent studies further demonstrate that a GABAergic mechanism within the NAc-VP GABAergic pathway appears to underlie AM251’s anti-reward action [211]. This is largely based on our recent finding that systemic cocaine produced a significant and dose-dependent increase in extracellular DA in both the NAc and VP, but a dose-dependent reduction in extracellular GABA levels in the VP. Pretreatment with AM251, when administered systemically or locally into the VP, dose-dependently blocked cocaine-induced reductions in VP GABA levels, but had no effect on cocaine-induced increases in NAc or VP DA levels. In contrast to AM251, SR141716 significantly inhibited cocaine-induced reductions in VP GABA levels when administered locally, but not systemically [211]. In addition to this GABAergic mechanism, a glutamate-mGluR2/3-dependent mechanism may also underlie the antagonism of AM251 or SR141716 on cocaine-triggered reinstatement of drug-seeking behavior [229]. Briefly, CB1 receptor blockade-induced increases in NAc glutamate leads to an increase in glutamate tone on mGluR2/3 auto-receptors on glutamatergic terminals, which subsequently inhibits cocaine-induced increases in NAc glutamate release and cocaine-induced reinstatement of drug-seeking behaviors [211, 229].
Taken together, these data support an important role for CB1 receptors in cocaine reward and relapse. AM251 or other more potent and more selective CB1 receptor antagonists appear promising for the treatment of psychostimulant addiction. The effectiveness of AM251 in attenuating cocaine’s rewarding effects also supports the endocannabinoid hypothesis of drug reward proposed above.
6. GLUTAMATE-BASED MEDICATION DISCOVERY
Glutamate hypothesis of drug reward
L-glutamate is the major excitatory neurotransmitter in the brain and acts through two heterogeneous families of glutamate receptors: ionotropic (iGluR) and metabotropic (mGluR). While iGluRs (i.e. NMDA, AMPA, kainate) are ligand-gated ion channels and responsible for fast excitatory neurotransmission, mGluRs (mGluR1–8) are G-protein coupled receptors linked to intracellular second messenger pathways. The eight subtypes of mGluRs have been classified into three groups on the basis of sequence similarities and pharmacological properties. Group I (mGluR1,5) receptors activate phospholipase C via Gαq/11 proteins, whereas group II (mGluR2,3) and group III (mGluR4,6,7,8) receptors inhibit adenylate cyclase via Gαi/o proteins [232].
The role of NAc glutamate in mediating cocaine’s rewarding effects is controversial. Growing evidence suggests that NAc glutamate transmission is negatively correlated to brain reward function and/or psychostimulant’s rewarding effects. This view is supported by the following evidence: 1) repeated cocaine injections or cocaine self-administration produces long-term depression in basal glutamate transmission in the mouse NAc [233, 234]; 2) chronic cocaine self-administration produces a significant reduction in basal levels of extracellular glutamate in the rat NAc, an effect that persists for 5 days after the extinction of cocaine self-administration [235]; 3) repeated cocaine administration results in a decrease in glutamate immunoreactivity in presynaptic NAc terminals [236, 237]; 4) microinjections of NMDA receptor antagonists (phencyclidine, MK-801) produce an enhancement of electrical BSR in rats [238, 239]; further, NMDA receptor antagonists are self-administered locally into the NAc [240]; 5) systemic administration of MK-801 increases cocaine’s rewarding efficacy, as assessed by an increase in break-point levels for cocaine self-administration under PR reinforcement conditions [241]; 6) activation of presynaptic mGluR2/3 receptors, which inhibits glutamate release, enhances amphetamine reinforcement in amphetamine-sensitized rats [242], while mGluR2/3 antagonists, which facilitate glutamate release, discrupt intra-NAc amphetamine-induced CPP [243]; 7) genetic deletion or pharmacological blockade of mGluR5, which results in an increase in extracellular NAc glutamate, inhibits cocaine self-administration and cocaine-induced reinstatement of drug-seeking behavior [244] (see more discussion in section 6.2. below); and 8) electrical stimulation of PFC, which increases NAc glutamate release, or increased GluR1 expression in the NAc, inhibits cocaine self-administration and cocaine-triggered reinstatement of drug-seeking behavior [245, 246]. These findings suggest that an increase in NAc glutamate transmission may attenuate, while a reduction in glutamate transmission may significantly contribute to, the rewarding effects produced by cocaine or amphetamine.
In contrast to these findings, other studies suggest an important role of enhanced glutamate transmission in mediating cocaine reward (for review, see [247]). The major evidence includes: 1) high doses of cocaine (30 mg/kg) or amphetamine (2 mg/kg) produce an increase in extracellular glutamate in the NAc in drug naïve rats [248, 249]; and 2) activation of NAc AMPA or NMDA receptors appears to enhance cocaine’s rewarding effect as suggested by a compensatory reduction in cocaine self-administration behavior [250], while blockade of AMPA or NMDA receptors attenuate cocaine self-administration and cocaine- or amphetamine-induced CPP [250–253].
Glutamate hypothesis of relapse
It is well documented that drug craving and reinstatement (relapse) of cocaine-seeking behavior are related to repeated cocaine-induced reduction in basal levels of glutamate transmission in the NAc during cocaine withdrawal or abstinence and to enhanced glutamate responses to acute cocaine priming (largely due to a reduction in basal levels of glutamate) [16, 127, 155, 233, 235, 254, 255]. In addition, such glutamate responses also can be seen in the presence of cocaine-associated environmental cues or footshock stress [256–258]. These data suggest that both a reduction in basal glutamate transmission and enhanced glutamate responding may constitute a neurobiological substrate of cocaine-, cocaine-associated cue-, or stress-triggered relapse. Such preclinical findings suggest a number of potential pharmacotherapeutic strategies (summarized in Table 5): first, normalization (increase) of reduced basal glutamate neurotransmission might be helpful in attenuating cocaine reward and relapse; and 2) blockade of enhanced glutamate responses to cocaine priming, cocaine-associated cues, or stress might attenuate reinstatement of drug-seeking behavior evoked by these relapse-triggers.
Table 5.
Pharmacological Actions of Glutamate Agents in Animal Models or Paradigms of Psychostimulant Addiction
| N-Acetylcysteine | MPEP | LY379268 | 2-PMPA | AMN082 | |
|---|---|---|---|---|---|
| Pharmacological Actions | Cystine prodrug, glutathione prodrug | mGluR5 antagonist | mGluR2/3 agonist | NAALADase inhibitor | mGluR7 agonist |
| Self-Administration | No effect on cocaine SA | ↓ Cocaine SA (FR, PR) | ↓ Cocaine SA (FR) | ↓ Cocaine SA (PR, notFR) | ↓ Cocaine SA (FR, PR) |
| Reinstatement of Drug-Seeking | ↓ Relapse by cocaine | ↓ Relapse by cocaine, by cues, but not by stress | ↓ Relapse by cocaine or cues | ↓ Relapse by cocaine | ↓ Relapse by cocaine |
| Conditioned Place Preference (CPP) | ↓ CPP by cocaine, amphetamine or MDMA | ↓ CPP by cocaine | |||
| Enhanced Brain-Stimulation Reward (eBSR) | Conflicting | ↓ eBSR by cocaine | ↓ eBSR by cocaine | ||
| Psychostimulant-like Discriminative Stimulus Effects (DS) | ↓ DS by cocaine | ||||
| Behavioral Sensitization (BS) | ↓ BS by cocaine | ↓ BS by cocaine | ↓ BS by amphetamine | ↓ BS by cocaine | |
| Natural Reward | ↓ Food-taking | ↓ Food-seeking | No effect on CPP for food | No effect on sucrose-taking | |
| Clinical Trials | Phase I | No | No | No | No |
6.1. N-acetylcysteine
N-acetylcysteine is a cystine prodrug. Recently, it was reported that systemic administration of N-acetylcysteine or local perfusion of cystine itself into the NAc restored decreased NAc glutamate levels in chronic cocaine-treated rats by stimulating cystine-glutamate exchange (i.e. exchange of extracellular cystine for intracellular glutamate), and dose-dependently inhibited cocaine-triggered reinstatement of drug-seeking behavior [255, 259]. Although N-acetylcysteine did not inhibit cocaine self-administration or acute cocaine-induced hyperactivity, it inhibited escalation of drug intake and behavioral sensitization seen after repeated administration [259]. In addition, pretreatment with N-acetylcysteine inhibited cocaine-induced increases in NAc glutamate by a mechanism that increased glutamate tone on presynaptic mGluR2/3 receptors [229, 255, 260, 261]. Also of interest is the fact that N-acetylcysteine is also a prodrug for the synthesis of the endogenous antioxidant glutathione, and that N-acetylcysteine pretreatment protected animals from high dose methamphetamine- or amphetamine-induced DA neurotoxicity and behavioral changes by lowering oxidative stress levels [262–265].
N-acetylcysteine is currently under Phase I trial for the treatment of cocaine addiction. So far, N-acetylcysteine appears to be safe and well tolerated as observed in 13 cocaine addicts [266]. An open-label clinical trial demonstrated that N-acetylcysteine, at 2400–3600 mg/day for 4 weeks, significantly reduced cocaine use in 16 of 23 human cocaine-addicted subjects [267]. Similarly, another double-blind, placebo-controlled trial demonstrated that N-acetylcysteine (600 mg/12 h × 4) significantly inhibited desire to use cocaine, interest in cocaine, or cue viewing time triggered by exposure to cocaine-related cues in 15 cocaine-addicted participants during a 3-day hospitalization study [266].
6.2. MPEP
MPEP [2-methyl-6-(phenylethynyl)-pyridine] is a selective group I mGluR5 antagonist. The mGluR5 receptor has become an important target in medication discovery for treatment of psychostimulant addiction, largely because of its relatively selective regional distribution in the NAc [268]. A large body of literature indicates that mGluR5 plays an important role in behavioral responses to psychostimulants [269]. Systemic administration of MPEP significantly attenuated the rewarding effects of psychostimulants, as assessed by: 1) cocaine self-administration under FR or PR reinforcement in mice, rats and nonhuman primates [24, 270–276]; and 2) cocaine-, amphetamine- or MDMA-induced CPP in rats [277, 278]; MPEP also attenuated cocaine- or amphetamine-induced hyperactivity in rats or mice [279–281]. In addition, MPEP pre-treatment inhibited reinstatement of drug-seeking behavior triggered by cocaine in rats and nonhuman primates [271,272,276] or by cocaine-associated cues in rats [282,283], but not by footshock stress [272].
To further determine the site(s) of action of MPEP and the neurochemical mechanisms underlying the antagonism by MPEP of cocaine’s actions described above, we microinjected MPEP into the NAc in conjunction with cocaine-triggered relapse. We found that intra-NAc MPEP (3–10 μg/side) significantly inhibited cocaine-triggered reinstatement of drug-seeking behavior (Xi et al., unpublished data). These data suggest that the NAc is an important site underlying MPEP’s anti-relapse action. Further, in vivo microdialysis studies demonstrated that MPEP itself selectively elevated NAc extracellular glutamate, but not DA levels. Further, pretreatment with MPEP selectively prevented cocaine-induced increases in NAc glutamate, but not in NAc DA [271]. Such increased NAc glutamate produced by MPEP is tetrodotoxin (TTX)-insensitive, suggesting non-vesicular glutamate origins. We believe that this increase in NAc glutamate by MPEP may play an important role in attenuating cocaine reward and relapse, likely by attenuating cocaine-induced reduction in VP GABA release, renormalizing reduced basal levels of glutamate transmission, and potentiating mGluR2/3-mediated inhibition of cocaine-enhanced glutamate responding as discussed above. Taken together, these preclinical data suggest that the mGluR5 antagonist MPEP could be promising in the treatment of psychostimulant addiction. So far, however, no clinical trials with MPEP have been undertaken.
6.3. LY379268
LY379268 [(−)-2-oxa-4-aminobicylco [3.1.0] hexane-4,6-dicarboxylic acid] is a selective and systemically effective group II receptor (mGluR2/3) agonist [284]. Recently, mGluR2/3 receptors have become attractive targets in medication development for the treatment of drug addiction because group II mGluR2/3 receptors function as glutamate autoreceptors, and mGluR2/3 activation inhibits glutamate release from neuronal and/or glial cells [285,286]. In addition, mGluR2/3 activation inhibits the release of other striatal neuro-transmitters, including DA [232,287]. Also, in comparison to other mGluRs, mGluR2/3 are highly expressed in the NAc, hippocampus and PFC [268], suggesting that the mGluR2/3 could be a target on which cocaine acts. Given that cocaine-induced increases in NAc DA and glutamate have been thought to play an important role in drug reward and relapse, it has been proposed that mGluR2/3 agonists could be effective in the treatment of cocaine addiction [285,286,288].
With the recent development and widespread use of LY379268, its efficacy in animal models of drug addiction has been recently studied. It has been reported that: 1) systemic administration of LY379268 significantly inhibits both acute and sensitized locomotor behaviors induced by amphetamine [289,290]; 2) systemic administration of LY379268 inhibits cocaine self-administration and cocaine-associated cue-triggered reinstatement of drug-seeking behavior [291]; 3) systemic administration or microinjection of LY369268 into the NAc core significantly inhibits cocaine- or food-triggered reinstatement of reward-seeking behavior [292]; 4) systemic or local administration of LY379268 into the central amydala inhibits incubation of cocaine craving in rats [293]; and 5) systemic or intra-NAc administration of LY379268 inhibits heroin-associated contextual cue-triggered reinstatement of drug-seeking behavior [294,295]. Together, these data suggest that mGluR2/3 agonists may be promising in the development of anti-cocaine medications.
6.4. 2-PMPA
2-PMPA [2-(Phosphono-methyl)-pentanedioic acid] is a highly potent and selective inhibitor of the enzyme N-acetylated-α-linked acidic dipeptidase (NAALADase), which hydrolyzes N-acetylaspartate-glutamate (NAAG) to N-acetylaspartate (NAA) and glutamate [296]. NAAG is an endogenous mGluR3 agonist, which negatively modulates the release of glutamate and other neurotransmitters [297]. At high concentrations, NAAG may also act as a mixed agonist/antagonist of NMDA receptors [298]. In addition to functioning as an mGluR3 agonist, NAAG may also serve as a glutamate precursor, because it can be hydrolyzed by the enzyme NAALADase to NAA and glutamate. Immunohistochemical studies have shown that NAAG is located in neurons [299,300] and released in a Ca++-dependent manner [301], while NAAL-ADase and its mRNA are located primarily in astrocytes [302, 303]. This suggests that synaptically released NAAG is degraded by NAALADase predominantly located on astrocytic membranes. Thus, NAALADase appears to be a key enzyme regulating extracellular glutamate and neuronal glutamate availability [297]. Given the important role of NAc glutamate in drug reward and relapse, it has been hypothesized that a NAALADase inhibitor, such as 2-PMPA, may be a promising anti-cocaine medication - acting by inhibiting cocaine-induced increases in NAc DA and glutamate via mGluR3 receptors, secondary to an increase in extracellular NAAG levels [304, 305].
Previous studies have shown that systemic administration of 2-PMPA significantly inhibits cocaine-induced CPP [306] and cocaine-induced behavioral sensitization [307]. Recently, we have shown that systemic administration of 2-PMPA, within a low dose window, significantly inhibited: 1) cocaine self-administration under a PR, but not FR2, reinforcement schedule; 2) cocaine-enhanced BSR; and 3) cocaine-triggered reinstatement of drug-seeking behavior [304, 305]. The same doses of 2-PMPA alone had no effect on BSR. These data suggest that inhibition of NAALADase by 2-PMPA produces an inhibitory effect on cocaine’s rewarding effects and cocaine-triggered reinstatement of drug-seeking behavior.
To further determine the neurochemical mechanisms underlying these behavioral effects, we observed the effects of 2-PMPA on NAc DA, glutamate and GABA using in vivo brain microdialysis. We found that systemic administration of 2-PMPA (10–100 mg/kg, i.p.) dose-dependently decreased NAc extracellular DA, glutamate and GABA levels, an effect that was blocked by intra-NAc perfusion of the mGluR2/3 antagonist LY341495 (Xi et al., unpublished data). These data suggest mediation by activation of NAc mGluR3 receptors secondary to an increase in NAAG levels. This is consistent with previous findings that mGluR2/3 activation inhibits the release of DA, glutamate, GABA and many other neurotransmitters in the brain [232]. This nonselective inhibition of neurotransimitter release may explain the “dose-window” effectiveness of 2-PMPA in animal models of drug addiction noted above, and also suggests a potential limitation for the use of 2-PMPA or other mGluR2/3 agonists in the treatment of addictive diseases. So far, no clinical trials with drugs from this receptor-active category have been undertaken.
6.5. AMN082
AMN082 [N,N′-Bis(diphenylmethyl)-1,2-ethanediamine dihydrochloride] is a selective systemically effective mGluR7 agonist [308]. The mGluR7 receptor subtype is of particular interest, because: 1) mGluR7 is the most abundant subtype among group III mGluR receptors in reward-related brain regions such as striatum, hippocampus and olfactory tubercle [268]; 2) mGluR7 is the most highly conserved mGluR subtype across different mammalian species [309], suggesting that selective mGluR7 ligands that are effective in laboratory animals are more likely to be effective in humans; and 3) microinjections of L-AP4, a non-selective brain-impermeable group III mGluR agonist, into the NAc or dorsal striatum inhibit NAc DA and glutamate [287, 310], and cocaine-induced increases in striatal DA and locomotion [311, 312]. These data may imply a potential role for mGluR7 in the modulation of drug-taking and drug-seeking behaviors. However, the role of group III receptors in drug reward and relapse is comparatively unstudied because of lack of selective pharmacological tools.
With the recent availability of the systemically effective mGluR7-selective agonist AMN082, we have assessed AMN082’s actions in animal models of drug addiction. We found that systemic administration of AMN082 (1–20 mg/kg i.p.) dose-dependently inhibits: 1) cocaine self-administration behavior under both FR2 and PR reinforcement schedules; 2) cocaine-enhanced BSR; and 3) cocaine-triggered reinstatement of drug-seeking behavior. In contrast, the same doses of AMN082 had no effect on locomotion or sucrose self-administration behavior. AMN082 alone, at lower doses (1–10 mg/kg), did not alter BSR itself, while a higher dose (20 mg/kg) decreased BSR in an aversive-like manner [313].
To further determine the mechanisms underlying these effects, we observed the action of AMN082 alone or in combination with cocaine on NAc DA, glutamate and GABA using in vivo brain microdialysis. We found that AMN082 significantly lowered NAc extracellular GABA, increased extracellular glutamate, but had no effect on NAc DA levels [314]. Further, pretreatment with AMN082 did not inhibit cocaine-enhanced NAc DA [313], suggesting that a DA-independent mechanism underlies the observed behavioral effects. Consistent with the GABAergic hypothesis of drug reward discussed above, a decrease in NAc GABA and an increase in glutamate would increase NAc medium-spiny GABAergic neuronal activity, thereby counteracting cocaine-induced GABAergic inhibition and cocaine reward. In addition, AMN082-induced increases in NAc glutamate may also restore decreased basal levels of extracellular glutamate, and increase glutamate tone on presynaptic mGluR2/3 receptors, thereby inhibiting cocaine priming-induced increases in NAc glutamate and cocaine-triggered reinstatement of drug-seeking behavior. Together, these preclinical experimental data suggest a potential utility for AMN082, a selective mGluR7 agonist, in the treatment of cocaine or other psychostimulant addiction.
6.6. Modafinil
Modafinil is a stimulant drug used in clinical medicine to treat narcolepsy and idiopathic hypersomnia [315]. The neurochemical mechanisms underlying modafinil’s actions are not fully understood. One of its most important actions appears to be to elevate extracellular levels of glutamate in such brain regions as striatum, thalamus, hippocampus, and hypothalamus [315, 316]. In addition, it may also inhibits GABA release [316], and elevate extracellular DA levels by inhibiting DA reuptake and/or potentiating DA release in the striatum [317, 318]. Consistent with these actions, modafinil has been shown to have weak cocaine-like discriminative stimulus and rewarding effects in both rats and nonhuman primates [319, 320], and weak stimulant-like subjective effects in humans [321, 322]. This being the case, modafinil has been suggested as a ‘substitution treatment’ for cocaine addiction, similar to methadone for the treatment of opioid addiction. Recent clinical trials have shown that modafinil significantly relieves cocaine’s withdrawal symptoms, including hypersomnia, lethargy, dysphoric mood, cognitive impairment, and increased appetite [323]. It also significantly blunts the euphorigenic effects of cocaine, reduces daily cocaine use, and prolongs abstinence in human cocaine addicts [324–326]. Modafinil itself does not appear to produce euphoria or evoke cocaine craving in humans [316,327], suggesting that it has low abuse potential [320,328]. Overall, these findings support the above-noted glutamate hypothesis that a drug that increases extracellular glutamate levels may produce a reduction in cocaine reward and intake, likely by ‘normalization’ of reduced extracellular glutamate and/or ‘restoration’ of glutamate tone on presynaptic mGluR2/3 receptors that attenuates the ability of cocaine to further increase glutamate release [261]. Additional studies are needed to study the efficacy of modafinil in attenuating cocaine self-administration and preventing relapse to cocaine use in both humans and experimental animals.
7. OPIOID-BASED MEDICATION DISCOVERY
Kappa Opioid Receptor–DA Hypothesis
It is well documented that opioid μ and κ receptors differentially modulate DA release in the mesolimbic DA system. In general, opioid μ receptor agonists stimulate, while κ receptor agonists inhibit DA release [19,329]. Based on this, κ receptor agonists have been considered as functional psychostimulant antagonists that may have possible utility as treatments for psychostimulant addiction. Dynorphin A (Dyn A) is an endogenous κ receptor agonist. Dyn A has been shown to significantly decrease basal levels of extracellular NAc DA, and also to block cocaine-induced increases in NAc DA, cocaine-induced CPP, and cocaine-induced hyperactivity [19]. Since Dyn A is a peptide that cannot penetrate the blood-brain barrier after systemic administration, it has no potential for clinical use. However, since the early 1980s, a series of nonpeptide κ receptor selective agonists have been developed, including the benzomorphans (cyclazocine, bremazocine), arylacetamides (U50, 488, U59,693, spiradoline), epoxymorphinans (nalfurafine), and several natural products from plants or herbs (ibogaine, salvinorin A) [329, 330]. The actions of a number of κ receptor agonists (Table 6) in animal models of psychostimulant abuse are reviewed below.
Table 6.
Pharmacological Actions of Kappa Opioid Receptor Agonists in Animal Models or Paradigms of Psychostimulant Addiction
| U50488 | U69593 | Nalfurafine | |
|---|---|---|---|
| Pharmacological Actions | Kappa receptor agonist | Kappa receptor agonist | Kappa receptor agonist |
| Self-Administration (SA) | ↓ Cocaine SA (FR) | ↓ Cocaine SA (FR) | |
| Reinstatement of Drug-Seeking (Relapse) | ↓ Relapse by cocaine or amphetamine | ||
| Conditioned Place Preference (CPP) | ↓ CPP by cocaine | ↓ CPP by cocaine, amphetamine | ↓ CPP by cocaine |
| Psychostimulant-like Discriminative Stimulus Effects (DS) | ↓ DS by cocaine | ↓ DS by cocaine, amphetamine | ↓ DS by cocaine |
| Behavioral Sensitization (BS) | ↓ BS by cocaine | ↓ BS by cocaine, amphetamine | |
| Abuse Liability | Cocaine-like DS by itself; Rewarding or aversive by itself; | No cocaine-like DS effects; No rewarding effects by itself | |
| Natural Reward | Conflicting | ↓ Food-taking | |
| Clinical Trials | No | No | No |
7.1. U50,488
U50,488 is a prototypic κ receptor selective agonist. The first suggestions that κ agonists might be useful as functional cocaine antagonists were based on the actions of U50,488 in animal models of drug addiction. Maisonneuve et al. [331] first showed that pretreatment with U50,488 attenuated cocaine-induced increases in extracellular NAc DA, an effect that was reversed by the κ opioid antagonist norbinaltorphimine (nor-BNI). Later work showed that acute U50,488 produced a dose-related decrease in cocaine self-administration in rats [332, 333]. Perhaps even more importantly, chronic administration of U50,488 dose-dependently inhibited cocaine self-administration in nonhuman primates [334]. However, at doses that inhibited cocaine self-administration, U50488 also produced emesis, sedation, and decreased food-maintained responding. In the drug discriminative paradigm, U50,488 was found to attenuate low-dose (3.0 mg/kg), but not high-dose (10.0 mg/kg), cocaine-induced discriminative stimulus effects [335]. However, U50,488 itself also produced weak cocaine-like discriminative stimulus effects [336] and potentiated the reinforcing effects of cocaine in nonhuman primates [337]. These data suggest that U50,488 may have therapeutic potential but also may have significant side-effects by itself.
7.2. U69,593
U69,593 is another prototypic κ receptor agonist (an analog of U50,488) [329]. Various studies have shown that U69,593 modulates the neurochemical and behavioral effects of cocaine. Treatment with U69,593 was reported to attenuate cocaine’s discriminative stimulus properties, its conditioned reinforcing effects, its self-administration, and reinstatement of extinguished drug-taking behavior in rats [338–340]. In addition, U69,593 attenuates the psychomotor stimulant effects of amphetamine and cocaine and modulates neurotoxic effects of methamphetamine [341–343]. Furthermore, U69,593 attenuates the discriminative stimulus effects of amphetamine in squirrel monkeys [344], albeit with large inter-individual differences.
7.3. Nalfurafine (TRK-820)
Nalfurafine is a novel potent and selective κ receptor agonist [345, 346]). The antinociceptive effects produced by nalfurafine are approximately 350-fold greater that that of U50,488 [347, 348]. Behavioral studies have shown that nalfurafine significantly attenuated the discriminative and rewarding effects of cocaine in rats, an effect that was blocked by nor-BNI [349]. Nalfurafine, at low doses, did not produce CPP or conditioned place aversion. However, at high doses, it significantly induced place aversion [349]. Nalfurafine produced neither cocaine-like discriminative stimulus effects in rats [350], nor psychotomimetic effects or significant tolerance in healthy human volunteers [351]. These findings suggest that nalfurafine may have potential for the treatment of psychostimulant addiction without severe side-effects such as dysphoria and psychotomimesis.
In addition to these 3 compounds, the pharmacological efficacy of several other selective κ receptor agonists or mixed κ agonist/μ antagonist agents has also been reported [19,329,330]. Overall, both selective and nonselective κ agonists have been shown to attenuate stimulant self-administration in a variety of animal models. While highly selective κ agonists attenuate psychostimulant self-administration in non-human primates, they are associated with behavioral side effects such as sedation, dysphoria and emesis. Mixed-action κ agonists appear to be more effective in attenuating cocaine’s actions and intake with a lower incidence of undesirable effects [352].
8. SEROTONIN-BASED MEDICATION DISCOVERY
5-HT-DA Interaction hypothesis
Serotonin (5-HT) neurons originate in the raphe nuclei in the midbrain and project to numerous regions throughout the brain, interacting with many other neurotransmitter systems [353]. The actions of 5-HT are mediated through at least 14 subtypes of 5-HT receptors, which are grouped into seven families according to their structural and functional characteristics [353]. In general, an increase in 5-HT levels produces an inhibitory effect on cocaine’s rewarding actions [15]. This effect is mediated indirectly by actions on 5-HT receptors located within the mesolimbic DA system [15, 354]. Of the 14 5-HT receptors, the 5-HT2A and 5-HT2C receptors are the two most important modulators of DA output. Briefly, 5-HT2C receptors tonically inhibit, while 5-HT2A receptors potentiate mesolimbic DA output [15, 353, 354]. Thus, 5-HT2A receptor antagonists and 5-HT2C receptor agonists have been proposed as possibly effective in alternating the neuro-chemical and behavioral effects of psychostimulants. Preclinical studies appear to support this suggestion - 5-HT2A receptor antagonists and 5-HT2C receptor agonists appear to reduce cocaine intake and craving and/or relapse [15, 354]. Although selective 5-HT2A receptor antagonists or 5-HT2C agonists are presently unavailable for use in human cocaine abusers, a number of selective 5-HT2 ligands are currently under development at preclinical levels. Two representive 5-HT2 ligands are reviewed below (Table 7).
Table 7.
Pharmacological Actions of Serotonine (5-HT2A, 5-HT2C) Agents in Animal Models or Paradigms of Psychostimulant Addiction
| M100907 | Ro 60-0175 | |
|---|---|---|
| Pharmacological Actions | 5-HT2A antagonist | 5-HT2C agonist |
| Self-Administration (SA) | No effect on cocaine SA | ↓ Cocaine SA (FR, PR) |
| Reinstatement of Drug-Seeking (Relapse) | ↓ Relapse by cocaine or cues | ↓ Relapse by cocaine or cues |
| Conditioned Place Preference (CPP) | ↓ CPP by cocaine | |
| Psychostimulant-like Discriminative Stimulus Effects (DS) | ↓ DS by cocaine | ↑ DS by cocaine |
| Behavioral Sensitization (BS) | ↓ BS by cocaine or amphetamine | ↓ Cocaine-induced hyperactivity |
| Abuse Liability | cocaine-like DS by itself | cocaine-like DS by itself |
| Natural Reward | ↓ food-taking | |
| Clinical Trials | No | No |
8.1. M100907
M100907 is a potent and selective 5-HT2A receptor antagonist [355]. In vitro, M100907 binds with subnanomolar affinity to 5-HT2A receptors, with at least 100-fold lower affinity for other receptors [356]). It has been reported that M100907 does not alter basal DA neuron function [357]. However, pretreatment with M100907 markedly diminished the effects of amphetamine on the firing rate of VTA DA neurons, and attenuated MDMA-induced DA release [358, 359]. In behavioral studies, M100907 attenuated cocaine- or amphetamine-induced hyperactivity [360–362], cocaine-induced CPP [354], and cocaine- or cocaine cue-induced reinstatement of extinguished drug-seeking behavior [354, 363]. However, M100907 was also reported to alter neither cocaine self-administration [363], nor amphetamine-enhanced BSR [360]. It also did not alter amphetamine’s discriminative stimulus properties [363]. These data suggest that the selective 5-HT2A receptor antagonist M100907 may lack efficacy for reducing cocaine or amphetamine intake, but may be effective at reducing relapse to drug use and enhancing abstinence. Currently, it is under Phase II/III trials for the treatment of depression and/or sleep disorders, but not for psychostimulant addiction [354].
8.2. Ro 60-0175
Ro 60-0175 is a non-selective 5-HT2C receptor agonist with similar high affinity for human 5-HT2C (pKi=9.0) and 5-HT2B receptors (pKi=9.3) [364,365]. In vivo microdialysis studies demonstrate that systemic administration of Ro 60-0175 (1 mg/kg, i.p.) did not alter cocaine, but attenuated haloperidol (a D2-like antagonist)-induced increases in extracellular DA in the NAc [366]. However, intra-VTA injection of Ro 60-0175 dose-dependently attenuated cocaine-induced increases in NAc DA [367]. Behavioral studies indicate that systemic administration of Ro 60-0175 (0.1–3 mg/kg, i.p. or s.c.) dose-dependently attenuated cocaine-induced hyperactivity, intravenous cocaine self-administration and cocaine- or cocaine-associated cue-induced reinstatement of drug-seeking behavior [363, 368, 369]. Also, intra-VTA injection of Ro 60-0175 produced a dose-dependent inhibition of cocaine-induced hyperactivity and of intravenous cocaine self-administration under both FR5 and PR reinforcement conditions [370]. Importantly, this antagonism of cocain’s effects by Ro 60-0175, after systemic administration or microinjection, in all these animal models was completely blocked by SB-242084, a highly selective 5-HT2C receptor antagonist [371]. These data suggest that the antagonism by Ro 60-0175 of cocaine’s actions is mediated by activation of VTA 5-HT2C receptors. In addition, Ro 60-0175, at the doses that inhibit cocaine self-administration, also inhibited food-maintained behavior, an effect that was also blocked by SB-242084 [363, 368, 370]. Taken together, these findings support a potential use of Ro 60-0175 in the treatment of cocaine or other psychostimulant addiction, although with possible adverse effects on natural reward-driven behavior.
In addition to M100907 and Ro 60-0175, several other selective or nonselective 5-HT2A antagonists (SR 46349B, risperidone) or 5-HT2C agonists (m-chlorophenylpiperazine, MK 212) have been proposed as treatments for psychostimulant addiction [354, 372]. Taken together such work suggests that 5-HT2A antagonists and/or 5-HT2C agonists may be effective in reducing craving and enhancing abstinence in treatment-seeking psychostimulant addicts, while 5-HT2C agonists may also be effective for reducing cocaine use. Currently, no selective ligands are available for use in human cocaine abusers. In addition to compounds that target 5-HT2A and 5-HT2C receptors, several compounds that target other 5-HT receptor subtypes (5-HT1A, 5-HT1B, 5-HT3) have been also reported to affect animal drug-taking and drug-seeking behavior [15]. Given that the findings are controversial or inconclusive, we do not discuss them in this review article.
9. CONCLUSION
In this review article, we reviewed the role of NAc DA, GABA, endocannabinoids, glutamate, opioids, and 5-HT in drug reward and relapse. We also evaluated the pharmacological actions of 27 pharmacological agents that target these brain substrates and mechanisms in multiple animal models of drug addiction. Briefly, the VTA-NAc-VP pathway and, in particular, functional inhibition of NAc-VP GABAergic projection neurons appears to play a critical role in mediating psychostimulant reward and relapse. The same pathway may also mediate endocannabinoid reward by activating CB1 receptors located on both GABAergic and glutamatergic neurons and/or terminals that results in an inhibition of medium-spiny GABAergic neurons. This GABAergic hypothesis may also explain why NMDA receptor antagonists have rewarding effects. Further, decreased NAc glutamate transmission during cocaine self-administration or early withdrawal/abstinence appears to play a critical role in drug craving and relapse to drug use. Decreased glutamate tone on mGluR2/3 autoreceptors located on presynaptic glutamate terminals may also provide an explanation for the increased glutamate response upon re-exposure to cocaine or cocaine-associated environmental cues. Thus, pharmacological strategies that either block NAc DA receptors or increase GABAergic transmission in brain reward circuits could antagonize a given drug’s rewarding effects (“antagonist” treatment), while restoration of reduced basal glutamate or DA transmission could be helpful in relieving drug craving or relapse to drug use (“agonist” treatment).
Based upon these mechanistic hypotheses, we summarize DA-based medication discovery strategies predominantly from our research group at the U.S. National Institute on Drug Abuse. We found that a non-selective DA receptor antagonist (l-THP), D3-selective or D3-preferring receptor antagonists (SB-277011A, NGB 2904, S33138), or slow-onset long-acting DA or monoamine transporter inhibitors (GBR 12909, RTI-336, CTDP31,345) appear to be promising approaches for the development of anti-psychostimulant medications. Next, we summarized recent studies with various GABAmimetic drugs, and found that the GABA enhancer GVG and GABAB receptor agonist baclofen appear to be promising for the treatment of psychostimulant addiction. Then, we reviewed recent research progress on the potential use of selective CB1 receptor antagonists for the treatment of psychostimulant dependence. We found that the novel CB1 receptor antagonist AM251 appears to be more promising than the prototypic CB1 receptor antagonist SR141716 for the treatment of drug addiction. For glutamate-based medication discovery, we reviewed recent progress on the mGluR2/3 agonists LY379268 and 2-PMPA, the mGluR5 antagonist MPEP, and the mGluR7 agonist AMN082 in pre-clinical models. Finally, we briefly reviewed current studies of opioid- and serotonin-based pharmacological strategies for the treatment of psychostimulant abuse. Although it is difficult to predict from where promising new compounds for the treatment of addictive disease may arise, we believe that the probability is high that potentially useful compounds may arise from mechanistic considerations of these 6 neurotransmitter systems and from hypothesis-driven medication development strategies based upon such mechanistic considerations. If any of the discussed compounds reach human trials and demonstrate anti-reward, anti-craving, and/or anti-relapse efficacy, the beneficial impact on addiction medicine will be considerable.
Learning Objectives
Psychostimulant abuse represents a significant global public health problem. Although a number of preliminary ‘clinic’-based pharmacotherapies have been reported, none has yet been proven to be effective for use in humans.
Given that a better understanding of psychostimulant actions in the brain may better guide development of effective treatment medications, in this review, we first discuss the role of brain dopamine, GABA, endocannabinoids, glutamate, opioids and serotonin in psychostimulant addiction, and then evaluate the pharmacological efficacy, in animal models of drug addition, of various novel pharmacological agents that target these neurotransmitter systems.
Such “mechanism”-based medication development strategies may significantly promote medication discovery for effective treatment of psychostimulant addiction in humans.
Future Research Directions
For the DA receptor antagonist strategy, more highly selective D3 receptor antagonists and more D3-preferring mixed D3/D2 receptor antagonists should be developed and pharmacologically evaluated. This is based on recognition that highly selective D3 receptor antagonists have proven highly promising in preclinical animal models of addiction and blockade of both D3 and D2 receptors may produce an additive therapeutic effect, with fewer unwanted side-effects (which seem to be produced predominantly by blockade of D2 receptors).
For the DA agonist substitution strategy, any compound that “competitively” inhibits psychostimulant binding to DAT or monoamine transporters should be pharmacologically evaluated. This is based on evidence that the therapeutic effects of methadone on heroin addiction appear to be mediated by functional antagonism of heroin’s actions, not a simple “agonist substitution” effect as previously thought.
For the GABA-based medication strategy, development of GVG-like GABA transaminase inhibitors with GABAC receptor antagonist properties may represent a new research direction in medication development for the treatment of psychostimulant addiction. This is based on recognition that the visual field defects reported with long-term GVG use in humans are probably mediated by activation of GABAC receptors located in retina secondary to the increase in extracellular GABA levels.
For the cannabinoid-based medication strategy, development of more potent and selective neutral CB1 receptor antagonists may be more promising than the existing CB1 receptor antagonists SR141716A and AM251, which have significant inverse agonist properties.
For the glutamate-based medication strategy, any compound that modestly elevates extracellular NAc glutamate, but with fewer unwanted side-effects, should be tested in animal models related to drug craving and relapse. In addition, more studies are required to clarify the functional role of brain glutamate in drug reward and relapse.
For the opioid-based medication strategy, mixed kappa-preferring opioid receptor agonists with partial mu receptor agonist properties may be more promising in attenuating psychostimulant addiction and also lowering the unwanted side-effects such as dysphoria and emesis produced by activation of kappa opioid receptors.
For the serotonin-based medication strategy, development of more effective and systemically active 5-HT2C receptor agonists may represent another medication development direction.
Fig. (1).
Schematic diagram of the VTA-NAc-VP reward pathway, illustrating the actions of psychostimulants (including cocaine, amphetamine, methamphetamine) on extracellular DA, endocannabinoids (eCBs), glutamate and GABA in both the NAc and VP, and the sites of action of 6 classes of mechanism-based pharmacological agents in development for the treatment of psychostimulant addiction, as reviewed in the present article. The mesolimbic DA system originates in the midbrain ventral tegmental area (VTA) and projects predominantly to the forebrain nucleus accumbens (NAc). DA afferents from the VTA and glutamatergic afferents originating predominantly from the prefrontal cortex (PFC) synapse on NAc medium-spiny GABAergic neurons, which predominantly project to the ventral pallidum (VP). In general, activation of ionotropic glutamate receptors (iGluRs) activates, while activation of DA (D2-like) receptors inhibits NAc medium-spiny GABAergic neurons. Functional inhibition of the NAc-VP GABAergic pathway is believed to play a critical role in drug reward and addiction. Psychostimulants elevate extracellular NAc DA by blocking and/or reversing DA transporters (DAT) on NAc DA axon terminals, which stimulates the release of eCBs from medium-spiny GABAergic neurons, leading to a reduction in release of NAc glutamate and VP GABA. Based on this hypothesis and these underlying neural substrates of addiction, it is proposed that: (1) blockade of DA receptors, in particular D3 receptors, may directly block psychostimulant-enhanced NAc DA transmission and psychostimulant-induced reward and relapse; (2) slow-onset long-acting DAT or monoamine transporter inhibitors may substitute for psychostimulants by blocking DA or monoamine transport, but with pharmacokinetic properties that militate against addiction; (3) GABAmimetic compounds (at least that subset with therapeutic potential for psychostimulant addiction) may act at GABAergic terminals within the (a) VP (shown) or (b) VTA on GABAergic afferents synapsing upon DA neurons (not shown); (4) cannabinoid CB1 receptor antagonists may modulate NAc-VP GABAergic neuronal function by acting on CB1 receptors located on both NAc GABAergic neurons and glutamatergic terminals; (5) compounds acting on mGluRs may alter psychostimulant action by modulating release of presynaptic glutamate and/or the neuronal activity of post-synaptic NAc GABAergic neurons; (6) kappa opioid receptor agonists may attenuate psychostimulant actions by inhibiting DA release in the NAc; and (7) compounds acting on 5-HT2A and/or 5HT2C receptors may attenuate psychostimulant action by modulating DA neuron activity and DA release in the NAc. More details are discussed in the text of this review.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health. The authors thank Dr. Xiao-Qing Peng, Xia Li and Krista Spiller for their assistance with the figure, references and manuscript editing.
References
- 1.Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 2.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 3.Gorelick DA, Gardner EL, Xi Z-X. Agents in development for the management of cocaine abuse. Drugs. 2004;64:1547–73. doi: 10.2165/00003495-200464140-00004. [DOI] [PubMed] [Google Scholar]
- 4.Vocci F, Ling W. Medications development: successes and challenges. Pharmacol Ther. 2005;108:94–108. doi: 10.1016/j.pharmthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Karila L, Gorelick D, Weinstein A, et al. New treatments for cocaine dependence: a focused review. Int J Neuropsychopharmacol. 2008;11:425–38. doi: 10.1017/S1461145707008097. [DOI] [PubMed] [Google Scholar]
- 6.Ling W, Rawson R, Shoptaw S, Ling W. Management of methamphetamine abuse and dependence. Curr Psychiatr Rep. 2006;8:345–54. doi: 10.1007/s11920-006-0035-x. [DOI] [PubMed] [Google Scholar]
- 7.Castells X, Casas M, Vidal X, Bosch R, Roncero C, Ramos-Quiroga JA, Capellà D. Efficacy of central nervous system stimulant treatment for cocaine dependence: a systematic review and meta-analysis of randomized controlled clinical trials. Addiction. 2007;102:1871–87. doi: 10.1111/j.1360-0443.2007.01943.x. [DOI] [PubMed] [Google Scholar]
- 8.Kalivas PW. Neurobiology of cocaine addiction: implications for new pharmacotherapy. Am J Addict. 2007;16:71–78. doi: 10.1080/10550490601184142. [DOI] [PubMed] [Google Scholar]
- 9.Preti A. New developments in the pharmacotherapy of cocaine abuse. Addict Biol. 2007;12:133–151. doi: 10.1111/j.1369-1600.2007.00061.x. [DOI] [PubMed] [Google Scholar]
- 10.Vocci FJ, Appel NM. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction. 2007;102(Suppl 1):96–106. doi: 10.1111/j.1360-0443.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- 11.Wise RA, Gardner EL. Animal models of addiction. In: Charney DS, Nestler EJ, editors. Neurobiology of Mental Illness. 2. London: Oxyford University Press; 2004. pp. 683–697. [Google Scholar]
- 12.O’Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 14.Self DW, Nestler EJ. Molecular mechanisms of drug reinforcement and addiction. Annu Rev Neurosci. 1995;18:463–495. doi: 10.1146/annurev.ne.18.030195.002335. [DOI] [PubMed] [Google Scholar]
- 15.Higgins GA, Fletcher PJ. Serotonin and drug reward: focus on 5-HT2C receptors. Eur J Pharmacol. 2003;480:151–162. doi: 10.1016/j.ejphar.2003.08.102. [DOI] [PubMed] [Google Scholar]
- 16.Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005;493:115–121. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–232. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams MJ, Adinoff B. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology. 2008;33:1779–97. doi: 10.1038/sj.npp.1301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman A. Neurobiology of addiction. An integrative review Biochem Pharmacol. 2008;75:266–322. doi: 10.1016/j.bcp.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 22.Bardo MT. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- 23.Gardner EL. What we have learned about addiction from animal models of drug self-administration. Am J Addict. 2000;9:285–313. doi: 10.1080/105504900750047355. [DOI] [PubMed] [Google Scholar]
- 24.Bressan RA, Crippa JA. The role of dopamine in reward and pleasure behaviour - review of data from preclinical research. Acta Psychiatr Scand Suppl. 2005;111(427):14–21. doi: 10.1111/j.1600-0447.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 25.Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- 26.Kornetsky C. Brain-stimulation reward, morphine-induced oral stereotypy, and sensitization: implications for abuse. Neurosci Biobehav Rev. 2004;27:777–786. doi: 10.1016/j.neubiorev.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- 28.Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Rev. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- 29.Rothman RB, Glowa JR. A review of the effects of dopaminergic agents on humans, animals, and drug-seeking behavior, and its implications for medication development: focus on GBR 12909. Mol Neurobiol. 1995;11:1–19. doi: 10.1007/BF02740680. [DOI] [PubMed] [Google Scholar]
- 30.Platt DM, Rowlett JK, Spealman RD. Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology. 2002;163:265–282. doi: 10.1007/s00213-002-1137-8. [DOI] [PubMed] [Google Scholar]
- 31.Haney M, Ward AS, Foltin RW, Fischman MW. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology. 2001;155:330–337. doi: 10.1007/s002130100725. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien CP. Research advances in the understanding and treatment of addiction. Am J Addict. 2003;12:S36–S47. [PubMed] [Google Scholar]
- 33.Jin G-Z. (−)-Tetrahydropalmatine and its analogues as new dopamine receptor antagonists. Trends Pharmacol Sci. 1987;8:81–82. [Google Scholar]
- 34.Xu S-X, Yu L-P, Han Y-P, Chen Y, Jin G-Z. Effects of tetrahydroprotoberberines on dopamine receptor subtypes in brain. Acta Pharmacol Sin. 1989;10:104–110. [PubMed] [Google Scholar]
- 35.Guo X, Wang L-M, Liu J, Jin G-Z. Characteristics of tetrahydroprotoberberines on dopamine D1 and D2 receptors in calf striatum. Acta Pharmacol Sin. 1997;18:225–230. [PubMed] [Google Scholar]
- 36.Mantsch JR, Li SJ, Risinger R, et al. Levo-tetrahydropalmatine attenuates cocaine self-administration and cocaine-induced reinstatement in rats. Psychopharmacology. 2007;192:581–591. doi: 10.1007/s00213-007-0754-7. [DOI] [PubMed] [Google Scholar]
- 37.Ren Y-H, Zhu Y, Jin G-Z, Zheng J-W. Levo-tetrahydropalmatine inhibits the expression of methamphetamine-induced conditioned place preference in rats. Chin J Drug Depend. 2000;9:182–186. [Google Scholar]
- 38.Luo J-Y, Ren Y-H, Zhu R, Lin D-Q, Zheng J-W. The effect of l-tetrahydropalmatine on cocaine induced conditioned place preference. Chin J Drug Depend. 2003;12:177–179. [Google Scholar]
- 39.Xi Z-X, Yang Z, Li SJ, Li X, Dillon C, Peng X-Q, Spiller K, Gardner EL. Levo-tetrahydropalmatine inhibits cocaine’s rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropharmacology. 2007;53:771–782. doi: 10.1016/j.neuropharm.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ge X-Q, Zhang H-Q, Zhou H-Z, Xu Z-X, Bian C-P. Experimental studies with tetrahydropalmatine analogs in relieving morphine withdrawal syndromes. Chin J Drug Depend. 1999;8:108–112. [Google Scholar]
- 41.Liu Y-L, Liang J-H, Yan L-D, Su R-B, Wu C-F, Gong Z-H. Effects of l-tetrahydropalmatine on locomotor sensitization to oxycodone in mice. Acta Pharmacol Sin. 2005;26:533–538. doi: 10.1111/j.1745-7254.2005.00101.x. [DOI] [PubMed] [Google Scholar]
- 42.Yang Z, Chen H, Hao W, Jin G-Z, Li S-J. Medication of l-tetrahydropalmatine significantly increased the abstinence rate in heroin addicts. Acta Pharamcol Sin; Abstract at the 68th Annual Meeting of the College on Problems of Drug Dependence; Scottsdale, AR. 2006; 2008. Abstract #109. in press. [Google Scholar]
- 43.Gardner EL. Neurobiology and genetics of addiction: implications of “reward deficiency syndrome” for therapeutic strategies in chemical dependency. In: Elster J, editor. Addiction: Entries and Exits. New York: Russell Sage Foundation; 1999. pp. 57–119. [Google Scholar]
- 44.Kuhar MJ, Pilotte NS. Neurochemical changes in cocaine withdrawal. Trends Pharmacol Sci. 1996;17:260–264. doi: 10.1016/0165-6147(96)10024-9. [DOI] [PubMed] [Google Scholar]
- 45.Volkow ND, Fowler JS, Wang G-J. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J Psychopharmacol. 1999;13:337–345. doi: 10.1177/026988119901300406. [DOI] [PubMed] [Google Scholar]
- 46.Volkow ND, Wang G-J, Telang F, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stanwood GD, Artymyshyn RP, Kung M-P, Kung HF, Lucki I, McGonigle P. Quantitative autoradiographic mapping of rat brain dopamine D3 binding with [125I]7-OH-PIPAT: evidence for the presence of D3 receptors on dopaminergic and nondopaminergic cell bodies and terminals. J Pharmacol Exp Ther. 2000;295:1223–1231. [PubMed] [Google Scholar]
- 48.Diaz J, Pilon C, Le Foll B, et al. Dopamine D3 receptors expressed by all mesencephalic dopamine neurons. J Neurosci. 2000;20:8677–8684. doi: 10.1523/JNEUROSCI.20-23-08677.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sokoloff P, Le Foll B, Perachon S, Bordet R, Ridray S, Schwartz J-C. The dopamine D3 receptor and drug addiction. Neurotox Res. 2001;3:433–441. doi: 10.1007/BF03033202. [DOI] [PubMed] [Google Scholar]
- 50.Sokoloff P, Diaz J, Le Foll B, et al. The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets. 2006;5:25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- 51.Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev. 1997;49:231–252. [PubMed] [Google Scholar]
- 52.Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260:1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- 53.Pilla M, Perachon S, Sautel F, et al. Selective inhibition of cocaine- seeking behaviour by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- 54.Wood MD, Boyfield I, Nash DJ, Jewitt FR, Avenell KY, Riley G. Evidence for antagonist activity of the human dopamine D3 receptor partial agonist, BP 897, at human dopamine D3 receptor. Eur J Pharmacol. 2000;407:47–51. doi: 10.1016/s0014-2999(00)00732-9. [DOI] [PubMed] [Google Scholar]
- 55.Wicke K, Garcia-Ladona J. The dopamine D3 receptor partial agonist, BP-897, is an antagonist at human dopamine D3 receptors and at rat somatodendritic dopamine D3 receptors. Eur J Pharmacol. 2001;424:85–90. doi: 10.1016/s0014-2999(01)01054-8. [DOI] [PubMed] [Google Scholar]
- 56.Garcia-Ladona FJ, Cox BF. BP 897, a selective dopamine D3 receptor ligand with therapeutic potential for the treatment of cocaine-addiction. CNS Drug Rev. 2003;9:141–158. doi: 10.1111/j.1527-3458.2003.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heidbreder CA, Gardner EL, Xi Z-X, et al. The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaál K, Gyertyán I. Targeting the dopamine D3 receptor cannot influence continuous reinforcement cocaine self-administration in rats. Brain Res Bull. 2003;61:595–601. doi: 10.1016/s0361-9230(03)00217-x. [DOI] [PubMed] [Google Scholar]
- 59.Cervo L, Carnovali F, Stark JA, Mennini T. Cocaine-seeking behavior in response to drug-associated stimuli in rats: involvement of D3 and D2 dopamine receptors. Neuropsychopharmacology. 2003;28:1150–1159. doi: 10.1038/sj.npp.1300169. [DOI] [PubMed] [Google Scholar]
- 60.Gilbert JG, Newman AH, Gardner EL, et al. Acute administration of SB-277011A, NGB 2904, or BP 897 inhibits cocaine cue-induced reinstatement of drug-seeking behavior in rats: role of dopamine D3 receptors. Synapse. 2005;57:17–128. doi: 10.1002/syn.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gaál K, Gyertyán I. Dopamine D3 as well as D2 receptor ligands attenuate the cue-induced cocaine-seeking in a relapse model in rats. Drug Alcohol Depend. 2006;81:63–70. doi: 10.1016/j.drugalcdep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 62.Duarte C, Lefebvre C, Chaperon F, Hamon M, Thiébot MH. Effects of a dopamine D3 receptor ligand, BP 897, on acquisition and expression of food-, morphine-, and cocaine-induced conditioned place preference, and food-seeking behavior in rats. Neuropsychopharmacology. 2003;28:1903–1915. doi: 10.1038/sj.npp.1300276. [DOI] [PubMed] [Google Scholar]
- 63.Gyertán I, Gál K. Dopamine D3 receptor ligands show place conditioning effect but do not influence cocaine-induced place preference. NeuroReport. 2003;14:93–98. doi: 10.1097/00001756-200301200-00018. [DOI] [PubMed] [Google Scholar]
- 64.Cervo L, Burbassi S, Colovic M, Caccia S. Selective antagonist at D3 receptors, but not non-selective partial agonists, influences the expression of cocaine-induced conditioned place preference in free-feeding rats. Pharmacol Biochem Behav. 2005;82:727–734. doi: 10.1016/j.pbb.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 65.Beardsley PM, Sokoloff P, Balster RL, Schwartz J-C. The D3R partial agonist, BP 897, attenuates the discriminative stimulus effects of cocaine and D-amphetamine and is not self-administered. Behav Pharmacol. 2001;12:1–11. doi: 10.1097/00008877-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 66.Le Foll B, Francès H, Diaz J, Schwartz J-C, Sokoloff P. Role of the dopamine D3 receptor in reactivity to cocaine-associated cues in mice. Eur J Neurosci. 2002;15:2016–2026. doi: 10.1046/j.1460-9568.2002.02049.x. [DOI] [PubMed] [Google Scholar]
- 67.Preti A. BP-897 Bioprojet. Curr Opin Investig Drugs. 2000a;1:110–115. [PubMed] [Google Scholar]
- 68.Le Foll B, Goldberg SR, Sokoloff P. The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology. 2005;49:525–541. doi: 10.1016/j.neuropharm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 69.Reavill C, Taylor SG, Wood MD, et al. Pharmacological actions of a novel, high-affinity, and selective human dopamine D3 receptor antagonist, SB-277011-A. J Pharmacol Exp Ther. 2001;294:1154–1165. [PubMed] [Google Scholar]
- 70.Vorel SR, Ashby CR, Jr, Paul M, et al. Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci. 2002;22:9595–9603. doi: 10.1523/JNEUROSCI.22-21-09595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spiller K, Xi Z-X, Peng X-Q, et al. The putative dopamine D3 receptor antagonists SB-277011A, NGB 2904 or BP 897 inhibit methamphetamine-enhanced brain stimulation reward in rats. Psychopharmacology. 2008;196:533–542. doi: 10.1007/s00213-007-0986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xi Z-X, Gilbert JG, Pak AC, Ashby CR, Jr, Heidbreder CA, Gardner EL. Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost--variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci. 2005;21:3427–3438. doi: 10.1111/j.1460-9568.2005.04159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Higley A, Li X, Dillon C, et al. The dopamine D3 receptor antagonist SB277011A inhibits methamphetamine self-administration under a progressive-ratio reinforcement schedule. Abstract at the 37th Annual Meeting of the Society for Neuroscience; San Diego, California. November 3–7, 2007; p. Abstract 814.1. [Google Scholar]
- 74.Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ. Attenuation of cue-controlled cocaine-seeking by a selective D3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology. 2003;28:329–338. doi: 10.1038/sj.npp.1300148. [DOI] [PubMed] [Google Scholar]
- 75.Xi Z-X, Gilbert J, Campos AC, et al. Blockade of mesolimbic dopamine D3 receptors inhibits stress-induced reinstatement of cocaine-seeking in rats. Psychopharmacology. 2004;176:57–65. doi: 10.1007/s00213-004-1858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xi Z-X, Li X, Gilbert J, et al. Blockade of dopamine D3 receptors by SB-277011A inhibits incubation of craving for cocaine in rats. Abstract at the 69th Annual Meeting of the College on Problems of Drug Dependence; Quebec, Canada. June, 2007. [Google Scholar]
- 77.Austin NE, Baldwin SJ, Cutler L, et al. Pharmacokinetics of the novel, high-affinity and selective dopamine D3 receptor antagonist SB-277011 in rat, dog and monkey: in vitro/in vivo correlation and the role of aldehyde oxidase. Xenobiotica. 2001;31:677–686. doi: 10.1080/00498250110056531. [DOI] [PubMed] [Google Scholar]
- 78.Remington G, Kapur S. SB-277011 GlaxoSmithKline. Curr Opin Investig Drugs. 2001;2:946–949. [PubMed] [Google Scholar]
- 79.Newman AH, Grundt P, Nader MA. Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. J Med Chem. 2005;48:3663–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]
- 80.Yuan J, Chen X, Brodbeck R, et al. NGB 2904 and NGB 2849: highly selective dopamine D3 receptor antagonists. Bioorg Med Chem Lett. 1998;8:2715–2718. doi: 10.1016/s0960-894x(98)00469-7. [DOI] [PubMed] [Google Scholar]
- 81.Robarge MJ, Husbands SM, Kieltyka A, Brodbeck R, Thurkauf A, Newman AH. Design and synthesis of [(2,3-dichlorophenyl)piper-azin-1-yl]alkylfluorenylcarboxamides as novel ligands selective for the dopamine D3 receptor subtype. J Med Chem. 2001;44:3175–3186. doi: 10.1021/jm010146o. [DOI] [PubMed] [Google Scholar]
- 82.Xi Z-X, Newman AH, Gilbert JG, et al. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine’s rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology. 2006;31:1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- 83.Xi Z-X, Gardner EL. Pharmacological actions of NGB 2904, a selective dopamine D3 receptor antagonist, in animal models of drug addiction. CNS Drug Rev. 2007;13:240–259. doi: 10.1111/j.1527-3458.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Joyce JN, Millan MJ. Dopamine D3 receptor antagonists as therapeutic agents. Drug Discov Today. 2005;10:917–925. doi: 10.1016/S1359-6446(05)03491-4. [DOI] [PubMed] [Google Scholar]
- 85.Millan MJ, Girardon S, Monneyron S, Dekeyne A. Discriminative stimulus properties of the dopamine D3 receptor agonists, PD128,907 and 7-OH-DPAT: a comparative characterization with novel ligands at D3 versus D2 receptors. Neuropharmacology. 2000;39:586–598. doi: 10.1016/s0028-3908(99)00180-x. [DOI] [PubMed] [Google Scholar]
- 86.Millan MJ, Mannoury la Cour C, Novi F, et al. S33138 [N[4-[2-[(3aS,9bR)-8-cyano-1,3a,4,9b-tetrahydro[1]-benzopyrano[3,4-c]pyrrol- 2(3H)-yl)-ethyl]phenylacetamide], a preferential dopamine D3versus D2 receptor antagonist and potential antipsychotic agent: I. Receptor-binding profile and functional actions at G-protein-coupled receptors J Pharmacol Exp Ther. 2008;324:587–99. doi: 10.1124/jpet.107.126706. [DOI] [PubMed] [Google Scholar]
- 87.Peng X-Q, Ashby CR, Spiller K, et al. The preferential dopamine D3versus D2 receptor antagonist S33138 inhibits cocaine reward and cocaine-triggered relapse to drug-seeking in rats. Neuropsychopharmacol. doi: 10.1016/j.neuropharm.2008.12.007. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Millan MJ, Loiseau F, Dekeyne A, et al. S33138, (N-[4-[2-[3aS,9bR)-8-cyano-1,3a,4,9b-tetrahydro[1]benzopyrano[3,4-c]pyrrol-2(3H)-yl- ethyl]phenyl-acetamide preferential dopamine D3versus D2receptor an- tagonist and potential antipsychotic agent. III Actions in models of therapeutic activity and induction of side-effects J Pharmacol Exp Ther. 2008;324(3):1212–26. doi: 10.1124/jpet.107.134536. [DOI] [PubMed] [Google Scholar]
- 89.Volkow ND, Ding Y-S, Fowler JS, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain Arch Gen Psychiatry. 1995;52:456–463. doi: 10.1001/archpsyc.1995.03950180042006. [DOI] [PubMed] [Google Scholar]
- 90.Kimmel HL, O’Connor JA, Carroll FI, Howell LL. Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys. Pharmacol Biochem Behav. 2007;86:45–54. doi: 10.1016/j.pbb.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochem Pharmacol. 2008;75:196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 92.Howell LL, Wilcox KM. The dopamine transporter and cocaine medication development: drug self-administration in nonhuman primates. J Pharmacol Exp Ther. 2001;298:1–6. [PubMed] [Google Scholar]
- 93.Wong DF, Cantilena L, Elkashef A, et al. In vivo human dopamine transporter occupancy of a potential cocaine treatment agent, GBR 12909. Soc Neurosci Abstr. 1999;25:1298. Abstract 522.7. [Google Scholar]
- 94.Preti A. Vanoxerine National Institute on Drug Abuse. Curr Opin Investig Drugs. 2000b;1:241–251. [PubMed] [Google Scholar]
- 95.Stafford D, Rice KC, Lewis DB, Glowa JR. Response requirements and unit dose modify the effects of GBR 12909 on cocaine-maintained behavior. Exp Clin Psychopharmacol. 2000;8:539–548. doi: 10.1037//1064-1297.8.4.539. [DOI] [PubMed] [Google Scholar]
- 96.Rothman RB, Baumann MH, Prisinzano TE, Newman AH. Dopamine transport inhibitors based on GBR12909 and benztropine as potential medications to treat cocaine addiction. Biochem Pharmacol. 2008;75:2–16. doi: 10.1016/j.bcp.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andersen PH. The dopamine uptake inhibitor GBR 12909: selectivity and molecular mechanism of action. Eur J Pharmacol. 1989;166:493–504. doi: 10.1016/0014-2999(89)90363-4. [DOI] [PubMed] [Google Scholar]
- 98.Rothman RB, Mele A, Reid AA, et al. GBR 12909 antagonizes the ability of cocaine to elevate extracellular levels of dopamine. Pharmacol Biochem Behav. 1991;40:387–397. doi: 10.1016/0091-3057(91)90570-r. [DOI] [PubMed] [Google Scholar]
- 99.Baumann MH, Char GU, de Costa BR, Rice KC, Rothman RB. GBR 12909 attenuates cocaine-induced activation of mesolimbic dopamine neurons in the rat. J Pharmacol Exp Ther. 1994;271:1216–1222. [PubMed] [Google Scholar]
- 100.Kelley AE, Lang CG. Effects of GBR 12909, a selective dopamine uptake inhibitor, on motor activity and operant behavior in the rat. Eur J Pharmacol. 1989;167:385–395. doi: 10.1016/0014-2999(89)90447-0. [DOI] [PubMed] [Google Scholar]
- 101.Tella SR. Effects of monoamine reuptake inhibitors on cocaine self-administration in rats. Pharmacol Biochem Behav. 1995;51:687–692. doi: 10.1016/0091-3057(94)00438-o. [DOI] [PubMed] [Google Scholar]
- 102.Glowa JR, Wojnicki FHE, Matecka D, et al. Effects of dopamine reuptake inhibitors on food-and cocaine-maintained responding: I. Dependence on unit dose of cocaine. Exp Clin Psychopharmacol. 1995;3:219–231. [Google Scholar]
- 103.Villemagne VL, Rothman RB, Yokoi F, et al. Doses of GBR12909 that suppress cocaine self-administration in non-human primates substantially occupy dopamine transporters as measured by [11C] WIN35,428 PET scans. Synapse. 1999;32:44–50. doi: 10.1002/(SICI)1098-2396(199904)32:1<44::AID-SYN6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 104.Schenk S. Effects of GBR 12909, WIN 35,428 and indatraline on cocaine self-administration and cocaine seeking in rats. Psychopharmacology. 2002;160:263–270. doi: 10.1007/s00213-001-0972-3. [DOI] [PubMed] [Google Scholar]
- 105.Glowa JR, Wojnicki FHE, Matecka D, et al. Effects of dopamine reuptake inhibitors on food-and cocaine-maintained responding: II. Comparisons with other drugs and repeated administrations. Exp Clin Psychopharmacol. 1995;3:232–239. [Google Scholar]
- 106.Glowa JR, Fantegrossi WF, Lewis DB, et al. Sustained decrease in cocaine-maintained responding in rhesus monkeys with 1-[2-[bis(4-fluorophenyl)methyoxy]ethyl]-4-(3-hydroxy-3-phenylpropyl)piperazinyl decanoate, a long-acting ester derivative of GBR 12909. J Med Chem. 1996;39:4689–4691. doi: 10.1021/jm960551t. [DOI] [PubMed] [Google Scholar]
- 107.Maldonado-Irizarry CS, Stellar JR, Kelley AE. Effects of cocaine and GBR-12909 on brain stimulation reward. Pharmacol Biochem Behav. 1994;48:915–920. doi: 10.1016/0091-3057(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 108.Holtzman SG. Differential interaction of GBR 12909, a dopamine uptake inhibitor, with cocaine and methamphetamine in rats discriminating cocaine. Psychopharmacology. 2001;155:180–186. doi: 10.1007/s002130100684. [DOI] [PubMed] [Google Scholar]
- 109.Roberts DCS. Self-administration of GBR 12909 on a fixed ratio and progressive ratio schedule in rats. Psychopharmacology. 1993;111:202–206. doi: 10.1007/BF02245524. [DOI] [PubMed] [Google Scholar]
- 110.De Vries TJ, Schoffelmeer AN, Binnekade R, Vanderschuren LJ. Dopaminergic mechanisms mediating the incentive to seek cocaine and heroin following long-term withdrawal of IV drug self-administration. Psychopharmacology. 1999;143:254–260. doi: 10.1007/s002130050944. [DOI] [PubMed] [Google Scholar]
- 111.Carroll FI, Fox BS, Kuhar MJ, Howard JL, Pollard GT, Schenk S. Effects of dopamine transporter selective 3-phenyltropane analogs on locomotor activity, drug discrimination, and cocaine self-administration after oral administration. Eur J Pharmacol. 2006;553:149–156. doi: 10.1016/j.ejphar.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 112.Vocci FJ, Elkashef A. Pharmacotherapy and other treatments for cocaine abuse and dependence. Curr Opin Psychiatry. 2005;18:265–270. doi: 10.1097/01.yco.0000165596.98552.02. [DOI] [PubMed] [Google Scholar]
- 113.Carroll FI, Howard JL, Howell LL, Fox BS, Kuhar MJ. Development of the dopamine transporter selective RTI-336 as a pharmacotherapy for cocaine abuse. AAPS J. 2006;8:E196–203. doi: 10.1208/aapsj080124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Haile CN, Zhang XY, Carroll FI, Kosten TA. Cocaine self-administration and locomotor activity are altered in Lewis and F344 inbred rats by RTI 336, a 3-phenyltropane analog that binds to the dopamine transporter. Brain Res. 2005;1055:186–195. doi: 10.1016/j.brainres.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 115.Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:757–765. doi: 10.1124/jpet.106.108324. [DOI] [PubMed] [Google Scholar]
- 116.Rothman RB, Blough BE, Baumann MH. Dual dopamine/serotonin releasers as potential medications for stimulant and alcohol addictions. AAPS J. 2007;9:E1–10. doi: 10.1208/aapsj0901001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Carroll FI, Pawlush N, Kuhar MJ, Pollard GT, Howard JL. Synthesis, monoamine transporter binding properties, and behavioral pharmacology of a series of 3β-(substituted phenyl)-2β-(3′-substituted isoxazol-5-yl)tropanes. J Med Chem. 2004;47:296–302. doi: 10.1021/jm030453p. [DOI] [PubMed] [Google Scholar]
- 118.Froimowitz M, Wu KM, Moussa A, et al. Slow-onset, long-duration 3-(3′,4′-dichlorophenyl)-1-indanamine monoamine reuptake blockers as potential medications to treat cocaine abuse. J Med Chem. 2000;43:4981–4992. doi: 10.1021/jm000201d. [DOI] [PubMed] [Google Scholar]
- 119.Gardner EL, Liu X, Paredes W, et al. A slow-onset, long-duration indanamine monoamine reuptake imnhibitor as a potential maintenance pharmacotherapy for psychostimulant abuse: effects in laboratory rat models relating to addiction. Neuropharmacology. 2006;51:993–1003. doi: 10.1016/j.neuropharm.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 120.Welch WM, Kraska AR, Sarges R, Koe BK. Nontricyclic antidepressant agents derived from cis-and trans-1-Amino-4-aryltetralins. J Med Chem. 1984;27:1508–1515. doi: 10.1021/jm00377a021. [DOI] [PubMed] [Google Scholar]
- 121.Peng X-Q, Xi Z-X, Gilbert J, et al. Behavioral and neurochemical effects of CTDP-31,345, a novel slow-onset and long-duration dopamine transporter inhibitor: alone and in combination with cocaine. Abstract at the 35th Annual Meeting of the Society for Neuroscience; Washington, DC. November 12–16, 2005; p. Abstract 227.10. [Google Scholar]
- 122.Peng X-Q, Xi Z-X, Li X, et al. Methadone pretreatment attenuates heroin’s rewarding effects and heroin-induced dopamine release in the nucleus accumbens: comparison to the effects of CTDP-31,345, a long-acting dopamine transporter inhibitor. Abstract at the 36th Annual Meetings of the Society for Neuroscience; Atlanta, Georgia. October 14–18, 2006; p. Abstract 591.8. [Google Scholar]
- 123.Xi Z-X, Stein EA. Increased mesolimbic GABA concentration blocks heroin self-administration in the rat. J Pharmacol Exp Ther. 2000;294:613–619. [PubMed] [Google Scholar]
- 124.Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci. 1990;13:259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- 125.Bennett BD, Bolam JP. Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum of the rat. Neuroscience. 1994;62:707–719. doi: 10.1016/0306-4522(94)90471-5. [DOI] [PubMed] [Google Scholar]
- 126.Groenewegen HJ, Wright CI, Beijer AV. The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- 127.Xi Z-X, Ramamoorthy S, Shen H, Lake R, Samuvel DJ, Kalivas PW. GABA transmission in the nucleus accumbens is altered after withdrawal from repeated cocaine. J Neurosci. 2003;23:3498–505. doi: 10.1523/JNEUROSCI.23-08-03498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lindefors N. Dopaminergic regulation of glutamic acid decarboxylase mRNA expression and GABA release in the striatum: a review. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:887–903. doi: 10.1016/0278-5846(93)90018-n. [DOI] [PubMed] [Google Scholar]
- 129.Nicola SM, Malenka RC. Dopamine depresses excitatory and inhibitory synaptic transmission by distinct mechanisms in the nucleus accumbens. J Neurosci. 1997;17:5697–710. doi: 10.1523/JNEUROSCI.17-15-05697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Umemiya M, Raymond LA. Dopaminergic modulation of excitatory postsynaptic currents in rat neostriatal neurons. J Neurophysiol. 1997;78:1248–55. doi: 10.1152/jn.1997.78.3.1248. [DOI] [PubMed] [Google Scholar]
- 131.Qiao J-T, Dougherty PM, Wiggins RC, Dafny N. Effects of microiontophoretic application of cocaine, alone and with receptor antagonists, upon the neurons of the medial prefrontal cortex, nucleus accumbens and caudate nucleus of rats. Neuropharmacology. 1990;29:379–85. doi: 10.1016/0028-3908(90)90098-c. [DOI] [PubMed] [Google Scholar]
- 132.Centonze D, Picconi B, Baunez C, et al. Cocaine and amphetamine depress striatal GABAergic synaptic transmission through D2 dopamine receptors. Neuropsychopharmacology. 2002;26:164–175. doi: 10.1016/S0893-133X(01)00299-8. [DOI] [PubMed] [Google Scholar]
- 133.Cameron DL, Williams JT. Cocaine inhibits GABA release in the VTA through endogenous 5-HT. J Neurosci. 1994;14:6763–6767. doi: 10.1523/JNEUROSCI.14-11-06763.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Uchimura N, North RA. Actions of cocaine on rat nucleus accumbens neurones in vitro. Br J Pharmacol. 1990;99:736–740. doi: 10.1111/j.1476-5381.1990.tb12999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.White FJ, Hu X-T, Henry DJ. Electrophysiological effects of cocaine in the rat nucleus accumbens: microiontophoretic studies. J Pharmacol Exp Ther. 1993;266:1075–1084. [PubMed] [Google Scholar]
- 136.Kiyatkin EA, Rebec GV. Dopamine-independent action of cocaine on striatal and accumbal neurons. Eur J Neurosci. 2000;12:1789–1800. doi: 10.1046/j.1460-9568.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- 137.Bourdelais A, Kalivas PW. Amphetamine lowers extracellular GABA concentration in the ventral pallidum. Brain Res. 1990;516:132–136. doi: 10.1016/0006-8993(90)90907-s. [DOI] [PubMed] [Google Scholar]
- 138.Tang X-C, McFarland K, Cagle S, Kalivas PW. Cocaine-induced reinstatement requires endogenous stimulation of μ-opioid receptors in the ventral pallidum. J Neurosci. 2005;25:4512–4520. doi: 10.1523/JNEUROSCI.0685-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Caille S, Parsons LH. Cannabinoid modulation of opiate reinforcement through the ventral striatopallidal pathway. Neuropsychopharmacology. 2006;31:804–13. doi: 10.1038/sj.npp.1300848. [DOI] [PubMed] [Google Scholar]
- 140.Dewey SL, Morgan AE, Ashby CR, Jr, et al. A novel strategy for the treatment of cocaine addiction. Synapse. 1998;30:119–29. doi: 10.1002/(SICI)1098-2396(199810)30:2<119::AID-SYN1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 141.Xi Z-X, Stein EA. GABAergic mechanisms of opiate reinforcement. Alcohol Alcohol. 2002;37:485–94. doi: 10.1093/alcalc/37.5.485. [DOI] [PubMed] [Google Scholar]
- 142.Cubells JF, Blanchard JS, Smith DM, Makman MH. In vivo action of enzyme-activated irreversible inhibitors of glutamic acid decarboxylase and γ-aminobutyric acid transaminase in retina vs. brain. J Pharmacol Exp Ther. 1986;238:508–514. [PubMed] [Google Scholar]
- 143.Gerasimov MR, Ashby CR, Jr, Gardner EL, et al. Gamma-vinyl GABA inhibits methamphetamine, heroin, or ethanol-induced increases in nucleus accumbens dopamine. Synapse. 1999;34(1):11–9. doi: 10.1002/(SICI)1098-2396(199910)34:1<11::AID-SYN2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 144.Kushner SA, Dewey SL, Kornetsky C. Gamma-vinyl-GABA attenuates cocaine-induced lowering of brain-stimulation reward thresholds. Psychopharmacology. 1997;133:383–88. doi: 10.1007/s002130050418. [DOI] [PubMed] [Google Scholar]
- 145.Kushner SA, Dewey SL, Kornetsky C. The irreversible γ-aminobutyric acid (GABA) transaminase inhibitor γ-vinyl-GABA blockscocaineself-administration in rats. J Pharmacol Exp Ther. 1999;290:797–802. [PubMed] [Google Scholar]
- 146.Filip M, Frankowska M, Zaniewska M, Golda A, Przegalinski E, Vetulani J. Diverse effects of GABA-mimetic drugs on cocaine-evoked self-administration and discriminative stimulus effects in rats. Psychopharmacology. 2007;192:17–26. doi: 10.1007/s00213-006-0694-7. [DOI] [PubMed] [Google Scholar]
- 147.Gardner EL, Schiffer WK, Horan BA, et al. Gamma-vinyl GABA, an irreversible inhibitor of GABA transaminase, alters the acquisition and expression of cocaine-induced sensitization in male rats. Synapse. 2002;46:240–250. doi: 10.1002/syn.10138. [DOI] [PubMed] [Google Scholar]
- 148.Peng X-Q, Li X, Gilbert JG, et al. Gammavinyl GABA inhibits cocaine-triggered reinstatement of drug-seeking behavior in rats by a non-dopaminergic mechanism. Drug Alcohol Depend. 4 Dec 2007; doi: 10.1016/j.drugalcdep.2007.10.004. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Barrett AC, Negus SS, Mello NK, Caine SB. Effect of GABA agonists and GABA-A receptor modulators on cocaine-and food-maintained responding and cocaine discrimination in rats. J Pharmacol Exp Ther. 2005;315:858–71. doi: 10.1124/jpet.105.086033. [DOI] [PubMed] [Google Scholar]
- 150.Morgan AE, Dewey SL. Effects of pharmacologic increases in brain GABA levels on cocaine-induced changes in extracellular dopamine. Synapse. 1998;28:60–65. doi: 10.1002/(SICI)1098-2396(199801)28:1<60::AID-SYN7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 151.Schiffer WK, Gerasimov MR, Bermel RA, Brodie JD, Dewey SL. Stereoselective inhibition of dopaminergic activity by gamma vinyl-GABA following a nicotine or cocaine challenge: a PET/microdialysis study. Life Sci. 2000;66:PL169–173. doi: 10.1016/s0024-3205(00)00432-x. [DOI] [PubMed] [Google Scholar]
- 152.Gerasimov MR, Schiffer WK, Brodie JD, Lennon IC, Taylor SJC, Dewey SL. γ-Aminobutyric acid mimetic drugs differentially inhibit the dopaminergic response to cocaine. Eur J Pharmacol. 2000;395:129–35. doi: 10.1016/s0014-2999(00)00267-3. [DOI] [PubMed] [Google Scholar]
- 153.Xi Z-X, Stein EA. Nucleus accumbens dopamine release modulation by mesolimbic GABAA receptors - an in vivo electrochemical study. Brain Res. 1998;798:156–65. doi: 10.1016/s0006-8993(98)00406-5. [DOI] [PubMed] [Google Scholar]
- 154.Xi Z-X, Stein EA. Baclofen inhibits heroin self-administration behavior and mesolimbic dopamine release. J Pharmacol Exp Ther. 1999;290:1369–74. [PubMed] [Google Scholar]
- 155.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–37. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brodie JD, Figueroa E, Dewey SL. Treating cocaine addiction: from preclinical to clinical trial experience with γ-vinyl GABA. Synapse. 2003a;50:261–65. doi: 10.1002/syn.10278. [DOI] [PubMed] [Google Scholar]
- 157.Brodie JD, Figueroa E, Laska EM, Dewey SL. Safety and efficacy of γ-vinyl GABA (GVG) for the treatment of methamphetamine and/or cocaine addiction. Synapse. 2003;55:122–25. doi: 10.1002/syn.20097. [DOI] [PubMed] [Google Scholar]
- 158.Goa KL, Sorkin EM. Gabapentin: a review of its pharmacological properties and clinical potential in epilepsy. Drugs. 1993;46:409–27. doi: 10.2165/00003495-199346030-00007. [DOI] [PubMed] [Google Scholar]
- 159.Taylor CP, Gee NS, Su T-Z, et al. Summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res. 1998;29:233–49. doi: 10.1016/s0920-1211(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 160.Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271:5768–76. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- 161.Raby WN. Gabapentin therapy for cocaine cravings. Am J Psychiatry. 2000;157:2058–59. doi: 10.1176/appi.ajp.157.12.2058-a. [DOI] [PubMed] [Google Scholar]
- 162.Raby WN, Coomaraswamy S. Gabapentin reduces cocaine use among addicts from a community clinic sample. J Clin Psychiatry. 2004;65:84–86. doi: 10.4088/jcp.v65n0114. [DOI] [PubMed] [Google Scholar]
- 163.Myrick H, Henderson S, Brady KT, Malcolm R. Gabapentin in the treatment of cocaine dependence: a case series. J Clin Psychiatry. 2001;62:19–23. doi: 10.4088/jcp.v62n0105. [DOI] [PubMed] [Google Scholar]
- 164.Hart CL, Ward AS, Collins ED, Haney M, Foltin RW. Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug Alcohol Depend. 2004;73:279–87. doi: 10.1016/j.drugalcdep.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 165.Haney M, Hart C, Collins ED, Foltin RW. Smoked cocaine discrimination in humans: effects of gabapentin. Drug Alcohol Depend. 2005;80:53–61. doi: 10.1016/j.drugalcdep.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 166.Bisaga A, Aharonovich E, Garawi F, et al. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alcohol Depend. 2006;81:267–74. doi: 10.1016/j.drugalcdep.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 167.Berger SP, Winhusen TM, Somoza EC, et al. A medication screening trial evaluation of reserpine, gabapentin and lamotrigine pharmacotherapy of cocaine dependence. Addiction. 2005;100(Suppl 1):58–67. doi: 10.1111/j.1360-0443.2005.00983.x. [DOI] [PubMed] [Google Scholar]
- 168.González G, Desai R, Sofuoglu M, et al. Clinical efficacy of gabapentin versus tiagabine for reducing cocaine use among cocaine dependent methadone-treated patients. Drug Alcohol Depend. 2007;87:1–9. doi: 10.1016/j.drugalcdep.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 169.Hart CL, Haney M, Collins ED, Rubin E, Foltin RW. Smoked cocaine self-administration by humans is not reduced by large gabapentin maintenance doses. Drug Alcohol Depend. 2007;86:274–77. doi: 10.1016/j.drugalcdep.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 170.Peng X-Q, Li X, Li J, et al. Effects of gabapentin on cocaine self-administration, cocaine-triggered relapse and cocaine-enhanced nucleus accumbens dopamine in rats. Drug Alcohol Depend. 2007 doi: 10.1016/j.drugalcdep.2007.09.019. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Itzhak Y, Martin JL. Effect of riluzole and gabapentin on cocaine- and methamphetamine-induced behavioral sensitization in mice. Psychopharmacology. 2000;151:226–33. doi: 10.1007/s002130000394. [DOI] [PubMed] [Google Scholar]
- 172.Filip M, Frankowska M, Golda A, Zaniewska M, Vetulani J, Przegalinski E. Various GABA-mimetic drugs differently affect cocaine-evoked hyperlocomotion and sensitization. Eur J Pharmacol. 2006;541:163–70. doi: 10.1016/j.ejphar.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 173.Adkins JC, Noble S. Tiagabine: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in the management of epilepsy. Drugs. 1998;55:437–60. doi: 10.2165/00003495-199855030-00013. [DOI] [PubMed] [Google Scholar]
- 174.Soudijn W, van Wijngaarden I. The GABA transporter and its inhibitors. Curr Med Chem. 2000;7:1063–79. doi: 10.2174/0929867003374363. [DOI] [PubMed] [Google Scholar]
- 175.González G, Sevarino K, Sofuoglu M, et al. Tiagabine increases cocaine-free urines in cocaine-dependent methadone-treated patients: results of a randomized pilot study. Addiction. 2003;98:1625–32. doi: 10.1046/j.1360-0443.2003.00544.x. [DOI] [PubMed] [Google Scholar]
- 176.Lile JA, Stoops WW, Glaser PE, Hays LR, Rush CR. Acute administration of the GABA reuptake inhibitor tiagabine does not alter the effects of oral cocaine in humans. Drug Alcohol Depend. 2004b;76:81–91. doi: 10.1016/j.drugalcdep.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 177.Winhusen TM, Somoza EC, Harrer JM, et al. A placebo-controlled screening trial of tiagabine, sertraline and donepezil as cocaine dependence treatments. Addiction. 2005;100:68–77. doi: 10.1111/j.1360-0443.2005.00992.x. [DOI] [PubMed] [Google Scholar]
- 178.Winhusen T, Somoza E, Ciraulo DA, et al. A double-blind, placebo-controlled trial of tiagabine for the treatment of cocaine dependence. Drug Alcohol Depend. 2007 doi: 10.1016/j.drugalcdep.2007.05.028. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 179.Weerts EM, Froestl W, Griffiths RR. Effects of GABAergic modulators on food and cocaine self-administration in baboons. Drug Alcohol Depend. 2005;80:369–76. doi: 10.1016/j.drugalcdep.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 180.Eriksson IS, Allard P, Marcusson J. [3H]tiagabine binding to GABA uptake sites in human brain. Brain Res. 1999;851:183–88. doi: 10.1016/s0006-8993(99)02183-6. [DOI] [PubMed] [Google Scholar]
- 181.Johnson BA. Recent advances in the development of treatments for alcohol and cocaine dependence: focus on topiramate and other modulators of GABA or glutamate function. CNS Drugs. 2005;19:873–96. doi: 10.2165/00023210-200519100-00005. [DOI] [PubMed] [Google Scholar]
- 182.Cubells JF. Topiramate for cocaine dependence. Curr Psychiatry Rep. 2006;8:130–31. doi: 10.1007/s11920-006-0011-5. [DOI] [PubMed] [Google Scholar]
- 183.Ait-Daoud N, Johnson BA. Open-label trial of topiramate for treating cocaine dependence. Abstract presented at the College on Problems of Drug Dependence Annual Meeting; 2004, June 13; San Juan. [Google Scholar]
- 184.Kampman KM, Pettinati H, Lynch KG, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75:233–40. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 185.Johnson BA, Roache JD, Ait-Daoud N, et al. Effects of acute topiramate dosing on methamphetamine-induced subjective mood. Int J Neuropsychopharmacol. 2007;10:85–98. doi: 10.1017/S1461145705006401. [DOI] [PubMed] [Google Scholar]
- 186.Roberts DCS. Preclinical evidence for GABAB agonists as a pharma- cotherapy for cocaine addiction. Physiol Behav. 2005;86:18–20. doi: 10.1016/j.physbeh.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 187.Fadda P, Scherma M, Fresu A, Collu M, Fratta W. Baclofen antagonizes nicotine-, cocaine-, and morphine-induced dopamine release in the nucleus accumbens of rat. Synapse. 2003;50:1–6. doi: 10.1002/syn.10238. [DOI] [PubMed] [Google Scholar]
- 188.Slattery DA, Markou A, Froestl W, Cryan JF. The GABAB receptor-positive modulator GS39783 and the GABAB receptor agonist baclofen attenuate the reward-facilitating effects of cocaine: intracranial self-stimulation studies in the rat. Neuropsychopharmacology. 2005;30:2065–72. doi: 10.1038/sj.npp.1300734. [DOI] [PubMed] [Google Scholar]
- 189.Roberts DC, Andrews MM, Vickers GJ. Baclofen attenuates the reinforcing effects of cocaine in rats. Neuropsychopharmacology. 1996;15:417–23. doi: 10.1016/0893-133X(96)00002-4. [DOI] [PubMed] [Google Scholar]
- 190.Roberts DC, Andrews MM. Baclofen suppression of cocaine self-administration: demonstration using a discrete trials procedure. Psychopharmacology. 1997;131:271–77. doi: 10.1007/s002130050293. [DOI] [PubMed] [Google Scholar]
- 191.Brebner K, Phelan R, Roberts DC. Effect of baclofen on cocaine self-administration in rats reinforced under fixed-ratio 1 and progressive-ratio schedules. Psychopharmacology. 2000;148:314–21. doi: 10.1007/s002130050056. [DOI] [PubMed] [Google Scholar]
- 192.Di Ciano P, Everitt BJ. The GABA(B) receptor agonist baclofen attenuates cocaine-and heroin-seeking behavior by rats. Neuropsychopharmacology. 2003;28:510–18. doi: 10.1038/sj.npp.1300088. [DOI] [PubMed] [Google Scholar]
- 193.Campbell UC, Lac ST, Carroll ME. Effects of baclofen on maintenance and reinstatement of intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1999;143:209–14. doi: 10.1007/s002130050937. [DOI] [PubMed] [Google Scholar]
- 194.Weerts EM, Froestl W, Kaminski BJ, Griffiths RR. Attenuation of cocaine-seeking by GABAB receptor agonists baclofen and CGP44532 but not the GABA reuptake inhibitor tiagabine in baboons. Drug Alcohol Depend. 2007;89:206–13. doi: 10.1016/j.drugalcdep.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ling W, Shoptaw S, Majewska D. Baclofen as a cocaine anti-craving medication: a preliminary clinical study. Neuropsychopharmacology. 1998;18:403–04. doi: 10.1016/S0893-133X(97)00128-0. [DOI] [PubMed] [Google Scholar]
- 196.Shoptaw S, Yang X, Rotheram-Fuller EJ, et al. Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. J Clin Psychiatry. 2003;64:1440–8. doi: 10.4088/jcp.v64n1207. [DOI] [PubMed] [Google Scholar]
- 197.Haney M, Hart CL, Foltin RW. Effects of baclofen on cocaine self-administration: opioid-and nonopioid-dependent volunteers. Neuropsychopharmacology. 2006;31:1814–21. doi: 10.1038/sj.npp.1300999. [DOI] [PubMed] [Google Scholar]
- 198.Lile JA, Stoops WW, Allen TS, Glaser PE, Hays LR, Rush CR. Baclofen does not alter the reinforcing, subject-rated or cardiovascular effects of intranasal cocaine in humans. Psychopharmacology. 2004;171:441–49. doi: 10.1007/s00213-003-1598-4. [DOI] [PubMed] [Google Scholar]
- 199.Tanda G, Goldberg SR. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms - a review of recent preclinical data. Psychopharmacology. 2003;169:115–34. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- 200.Devane WA, Dysarz FA, III, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–13. [PubMed] [Google Scholar]
- 201.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 202.Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology: XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 203.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 204.Chen J, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Δ9-Tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology. 1990;102:156–62. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- 205.Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common μ1 opioid receptor mechanism. Science. 1997;276:2048–50. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- 206.Matyas F, Yanovsky Y, Mackie K, Kelsch W, Misgeld U, Freund TF. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 2006;137:337–61. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 207.Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–63. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- 208.Patel S, Rademacher DJ, Hillard CJ. Differential regulation of the endocannabinoids anandamide and 2-arachidonylglycerol within the limbic forebrain by dopamine receptor activity. J Pharmacol Exp Ther. 2003;306:880–88. doi: 10.1124/jpet.103.054270. [DOI] [PubMed] [Google Scholar]
- 209.Centonze D, Battista N, Rossi S, et al. A critical interaction between dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal GABAergic transmission. Neuropsychopharmacology. 2004;29:1488–97. doi: 10.1038/sj.npp.1300458. [DOI] [PubMed] [Google Scholar]
- 210.Caillé S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Xi Z-X, Li X, Gardner EL. Pharmacological actions of the CB1 receptor antagonists AM251 and SR141716A in animal models of psychostimulant addiction. Neurosci Biobehav Rev. 2008 in press. [Google Scholar]
- 212.Rinaldi-Carmona M, Barth F, Heaulme M, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–44. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 213.Le Foll B, Goldberg SR. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther. 2005;312:875–83. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- 214.Beardsley PM, Thomas BF. Current evidence supporting a role of cannabinoid CB1 receptor (CB1R) antagonists as potential pharmacotherapies for drug abuse disorders. Behav Pharmacol. 2005;16:275–96. doi: 10.1097/00008877-200509000-00003. [DOI] [PubMed] [Google Scholar]
- 215.Fattore L, Spano MS, Deiana S, et al. An endocannabinoid mechanism in relapse to drug seeking: a review of animal studies and clinical perspectives. Brain Res Rev. 2007;53:1–16. doi: 10.1016/j.brainresrev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 216.Arnold JC. The role of endocannabinoid transmission in cocaine addiction. Pharmacol Biochem Behav. 2005;81:396–406. doi: 10.1016/j.pbb.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 217.De Vries TJ, Shaham Y, Homberg JR, et al. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7:1151–4. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- 218.Filip M, Golda A, Zaniewska M, et al. Involvement of cannabinoid CB1 receptors in drug addiction: effects of rimonabant on behavioral responses induced by cocaine. Pharmacol Rep. 2006b;58:806–19. [PubMed] [Google Scholar]
- 219.Soria G, Mendizabal V, Tourino C, et al. Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology. 2005;30:1670–80. doi: 10.1038/sj.npp.1300707. [DOI] [PubMed] [Google Scholar]
- 220.Cheer JF, Wassum KM, Sombers LA, et al. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–95. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cossu G, Ledent C, Fattore L, et al. Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behav Brain Res. 2001;118:61–65. doi: 10.1016/s0166-4328(00)00311-9. [DOI] [PubMed] [Google Scholar]
- 222.Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3:1073–74. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- 223.Lesscher HMB, Hoogveld E, Burbach JPH, van Ree JM, Gerrits MAFM. Endogenous cannabinoids are not involved in cocaine reinforcement and development of cocaine-induced behavioural sensitization. Eur Neuropsychopharmacol. 2005;15:31–37. doi: 10.1016/j.euroneuro.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 224.Xi Z-X, Spiller K, Pak AC, Gilbert J, Dillon C, Li X, Peng X-Q, Gardner EL. Cannabinoid CB1 Receptor antagonists attenuate cocaine’s rewarding effects: experiments with self-administration and brain-stimulation reward in rats. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301552. Epub ahead of print 2008, 33: 1735–1745. [DOI] [PubMed] [Google Scholar]
- 225.Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Cocaine, but not morphine, induces conditioned place preference and sensitization to locomotor responses in CB1 knockout mice. Eur J Neurosci. 2000;12:4038–46. doi: 10.1046/j.1460-9568.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- 226.Lan R, Liu Q, Fan P, et al. Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem. 1999;42:769–76. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- 227.Krishnamurthy M, Li W, Moore BM., II Synthesis, biological evaluation, and structural studies on N1 and C5 substituted cycloalkyl analogues of the pyrazole class of CB1 and CB2 ligands. Bioorg Med Chem. 2004;12:393–404. doi: 10.1016/j.bmc.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 228.Vinklerova J, Novakova J, Sulcova A. Inhibition of methamphetamine self-administration in rats by cannabinoid receptor antagonist AM 251. J Psychopharmacol. 2002;16:139–43. doi: 10.1177/026988110201600204. [DOI] [PubMed] [Google Scholar]
- 229.Xi Z-X, Gilbert JG, Peng X-Q, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006;26:8531–36. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Corbille AG, Valjent E, Marsicano G, et al. Role of cannabinoid type 1 receptors in locomotor activity and striatal signaling in response to psychostimulants. J Neurosci. 2007;27:6937–47. doi: 10.1523/JNEUROSCI.3936-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Boctor SY, Martinez JL, Jr, Koek W, France CP. The cannabinoid CB1 receptor antagonist AM251 does not modify methamphetamine reinstatement of responding. Eur J Pharmacol. 2007;571:39–43. doi: 10.1016/j.ejphar.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- 233.Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- 234.Schramm-Sapyta NL, Olsen CM, Winder DG. Cocaine self-administration reduces excitatory responses in the mouse nucleus accumbens shell. Neuropsychopharmacology. 2006;31:1444–51. doi: 10.1038/sj.npp.1300918. [DOI] [PubMed] [Google Scholar]
- 235.Miguéns M, Del Olmo N, Higuera-Matas A, Torres I, García-Lecumberri C, Ambrosio E. Glutamate and aspartate levels in the nucleus accumbens during cocaine self-administration and extinction: a time course microdialysis study. Psychopharmacology. 2008;196:303–13. doi: 10.1007/s00213-007-0958-x. [DOI] [PubMed] [Google Scholar]
- 236.Kozell B, Meshul K. The effects of acute or repeated cocaine administration on nerve terminal glutamate within the rat mesolimbic system. Neuroscience. 2001;106:15–25. doi: 10.1016/s0306-4522(01)00274-3. [DOI] [PubMed] [Google Scholar]
- 237.Kozell B, Meshul K. Alterations in nerve terminal glutamate immunoreactivity in the nucleus accumbens and ventral tegmental area following single and repeated doses of cocaine. Psychopharmacology. 2003;165:337–45. doi: 10.1007/s00213-002-1296-7. [DOI] [PubMed] [Google Scholar]
- 238.Carlezon WA, Jr, Wise RA. Microinjections of phencyclidine (PCP) and related drugs into nucleus accumbens shell potentiate medial forebrain bundle brain stimulation reward. Psychopharmacology. 1996;128:413–20. doi: 10.1007/s002130050151. [DOI] [PubMed] [Google Scholar]
- 239.Clements RL, Greenshaw AJ. Facilitation of brain stimulation reward by MK-801 (dizocilpine) may be independent of D2-like dopamine receptor stimulation in rats. Psychopharmacology. 2005;182:65–74. doi: 10.1007/s00213-005-0039-y. [DOI] [PubMed] [Google Scholar]
- 240.Carlezon WA, Jr, Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci. 1996;16:3112–22. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ranaldi R, French E, Roberts DCS. Systemic pretreatment with MK-801 (dizocilpine) increases breaking points for self-administration of cocaine on a progressive-ratio schedule in rats. Psychopharmacology. 1996;128:83–88. doi: 10.1007/s002130050113. [DOI] [PubMed] [Google Scholar]
- 242.Kim JH, Austin JD, Tanabe L, Creekmore E, Vezina P. Activation of group II mGlu receptors blocks the enhanced drug taking induced by previous exposure to amphetamine. Eur J Neurosci. 2005;21:295–300. doi: 10.1111/j.1460-9568.2004.03822.x. [DOI] [PubMed] [Google Scholar]
- 243.Gerdjikov TV, Beninger RJ. Place preference induced by nucleus accumbens amphetamine is impaired by local blockade of Group II metabotropic glutamate receptors in rats. BMC Neurosci. 2006;7:43. doi: 10.1186/1471-2202-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Chiamulera C, Epping-Jordan MP, Zocchi A, et al. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–74. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- 245.Levy D, Shabat-Simon M, Shalev U, Barnea-Ygael N, Cooper A, Zangen A. Repeated electrical stimulation of reward-related brain regions affects cocaine but not “natural” reinforcement. J Neurosci. 2007;27:14179–189. doi: 10.1523/JNEUROSCI.4477-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Todtenkopf MS, Parsegian A, Naydenov A, Neve RL, Konradi C, Carlezon WA., Jr Brain reward regulated by AMPA receptor subunits in nucleus accumbens shell. J Neurosci. 2006;26:11665–669. doi: 10.1523/JNEUROSCI.3070-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–65. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Smith JA, Mo Q, Guo H, Kunko PM, Robinson SE. Cocaine increases extraneuronal levels of aspartate and glutamate in the nucleus accumbens. Brain Res. 1995;683:264–69. doi: 10.1016/0006-8993(95)00383-2. [DOI] [PubMed] [Google Scholar]
- 249.Reid MS, Hsu K, Jr, Berger SP. Cocaine and amphetamine preferentially stimulate glutamate release in the limbic system: studies on the involvement of dopamine. Synapse. 1997;27:95–105. doi: 10.1002/(SICI)1098-2396(199710)27:2<95::AID-SYN1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 250.Cornish JL, Duffy P, Kalivas PW. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience. 1999;93:1359–67. doi: 10.1016/s0306-4522(99)00214-6. [DOI] [PubMed] [Google Scholar]
- 251.Layer RT, Uretsky NJ, Wallace LJ. Effects of the AMPA/kainate receptor antagonist DNQX in the nucleus accumbens on drug-induced conditioned place preference. Brain Res. 1993;617:267–73. doi: 10.1016/0006-8993(93)91094-9. [DOI] [PubMed] [Google Scholar]
- 252.Hyytiä P, Bäckström P, Liljequist S. Site-specific NMDA receptor antagonists produce differential effects on cocaine self-administration in rats. Eur J Pharmacol. 1999;378:9–16. doi: 10.1016/s0014-2999(99)00446-x. [DOI] [PubMed] [Google Scholar]
- 253.Maldonado C, Rodriguez-Arias M, Castillo A, Aguilar MA, Minarro J. Effect of memantine and CNQX in the acquisition, expression and reinstatement of cocaineinduced conditioned place preference. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:932–39. doi: 10.1016/j.pnpbp.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 254.Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–60. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Baker DA, McFarland K, Lake RW, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–49. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 256.Bell K, Duffy P, Kalivas PW. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology. 2000;23:335–44. doi: 10.1016/S0893-133X(00)00100-7. [DOI] [PubMed] [Google Scholar]
- 257.Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You Z-B. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–96. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wang B, You Z-B, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF2 receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology. 2007;193:283–94. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- 259.Madayag A, Lobner D, Kau KS, et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27:13968–976. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25:6389–93. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Xi Z-X, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther. 2002;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- 262.Fukami G, Hashimoto K, Koike K, Okamura N, Shimizu E, Iyo M. Effect of antioxidant N-acetyl-L-cysteine on behavioral changes and neurotoxicity in rats after administration of methamphetamine. Brain Res. 2004;1016:90–95. doi: 10.1016/j.brainres.2004.04.072. [DOI] [PubMed] [Google Scholar]
- 263.Hashimoto K, Tsukada H, Nishiyama S, et al. Protective effects of N-acetyl-L-cysteine on the reduction of dopamine transporters in the striatum of monkeys treated with methamphetamine. Neuropsychopharmacology. 2004;29:2018–23. doi: 10.1038/sj.npp.1300512. [DOI] [PubMed] [Google Scholar]
- 264.Wan F-J, Tung C-S, Shiah I-S, Lin H-C. Effects of alpha-phenyl-N-tert-butyl nitrone and N-acetylcysteine on hydroxyl radical formation and dopamine depletion in the rat striatum produced by d-amphetamine. Eur Neuropsychopharmacol. 2006;16:147–53. doi: 10.1016/j.euroneuro.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 265.Achat-Mendes C, Anderson KL, Itzhak Y. Impairment in consolidation of learned place preference following dopaminergic neurotoxicity in mice is ameliorated by N-acetylcysteine but not D1 and D2 dopamine receptor agonists. Neuropsychopharmacology. 2007;32:531–41. doi: 10.1038/sj.npp.1301119. [DOI] [PubMed] [Google Scholar]
- 266.LaRowe SD, Mardikian P, Malcolm R, et al. Safety and tolerability of N-acetylcysteine in cocaine-dependent individuals. Am J Addict. 2006;15:105–10. doi: 10.1080/10550490500419169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm RJ. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:389–94. doi: 10.1016/j.pnpbp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 268.Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–04. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- 269.Lea PM, IV, Faden AI. Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Rev. 2006;12:149–66. doi: 10.1111/j.1527-3458.2006.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol. 2004;499:121–33. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 271.Xi Z-X, Gilbert J, Campos A, Ashby CR, Jr, Gardner EL. The metabotropic glutamate receptor 5 antagonist MPEP blocks reinstatement of drug-seeking triggered by cocaine, but not by stress or cues. Abstract at the 66th Annual Meeting of the College on Problems of Drug Dependence; San Juan, Puerto Rico. June 2004. [Google Scholar]
- 272.Xi Z-X, Gilbert J, Campos AC, Peng X-Q, Ashby CR, Jr, Gardner EL. The mGluR5 antagonist MPEP lowers the progressive-ratio break-point for cocaine self-administration, and inhibits reinstatement of drug-seeking triggered by cocaine but not by stress or cues. Abstract at the 34th Annual Meeting of the Society for Neuroscience; San Diego, California. October 23–27, 2004; p. Abstract 691.9. [Google Scholar]
- 273.Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology. 2005;179:255–61. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- 274.Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology. 2005;179:247–54. doi: 10.1007/s00213-004-2069-2. [DOI] [PubMed] [Google Scholar]
- 275.Kenny PJ, Paterson NE, Boutrel B, et al. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Ann N Y Acad Sci. 2003;1003:415–18. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- 276.Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD. Attenuation of behavioral effects of cocaine by the Metabotropic Glutamate Receptor 5 Antagonist 2-Methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: comparison with dizocilpine. J Pharmacol Exp Ther. 2005;312:1232–40. doi: 10.1124/jpet.104.078733. [DOI] [PubMed] [Google Scholar]
- 277.McGeehan AJ, Olive MF. The mGluR5 antagonist MPEP reduces the conditioned rewarding effects of cocaine but not other drugs of abuse. Synapse. 2003;47:240–42. doi: 10.1002/syn.10166. [DOI] [PubMed] [Google Scholar]
- 278.Herzig V, Capuani EM, Kovar KA, Schmidt WJ. Effects of MPEP on expression of food-, MDMA-or amphetamine-conditioned place preference in rats. Addict Biol. 2005;10:243–49. doi: 10.1080/13556210500223272. [DOI] [PubMed] [Google Scholar]
- 279.McGeehan AJ, Janak PH, Olive MF. Effect of the mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine (MPEP) on the acute locomotor stimulant properties of cocaine, D-amphetamine, and the dopamine reuptake inhibitor GBR12909 in mice. Psychopharmacology. 2004;174:266–73. doi: 10.1007/s00213-003-1733-2. [DOI] [PubMed] [Google Scholar]
- 280.Herzig V, Schmidt WJ. Effects of MPEP on locomotion, sensitization and conditioned reward induced by cocaine or morphine. Neuropharmacology. 2004;47:973–84. doi: 10.1016/j.neuropharm.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 281.Pietraszek M, Rogoz Z, Wolfarth S, Ossowska K. Opposite influence of MPEP, an mGluR5 antagonist, on the locomotor hyperactivity induced by PCP and amphetamine. J Physiol Pharmacol. 2004;55:5875–93. [PubMed] [Google Scholar]
- 282.Bäckström P, Hyytiä P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–86. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- 283.Bäckström P, Hyytiä P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2007;192:571–80. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- 284.Marek GJ. Metabotropic glutamate 2/3 receptors as drug targets. Curr Opin Pharmacol. 2004;4:18–22. doi: 10.1016/j.coph.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 285.Baker DA, Xi Z-X, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–41. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Xi Z-X, Baker DA, Shen H, Kalivas PW. Group II metabotropic glutamate receptors modulate glutamate release in the nucleus accumbens. J Pharmacol Exp Ther. 2002;300:162–72. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- 287.Hu G, Duffy P, Swanson C, Ghasemzadeh MB, Kalivas PW. The regulation of dopamine transmission by metabotropic glutamate receptors. J Pharmacol Exp Ther. 1999;289:412–16. [PubMed] [Google Scholar]
- 288.Spooren W, Ballard T, Gasparini F, Amalric M, Mutel V, Schreiber R. Insight into the function of Group I and Group II metabotropic glutamate (mGlu) receptors: behavioural characterization and implications for the treatment of CNS disorders. Behav Pharmacol. 2003;14:257–77. doi: 10.1097/01.fbp.0000081783.35927.8f. [DOI] [PubMed] [Google Scholar]
- 289.Cartmell J, Monn JA, Schoepp DD. The mGlu2/3 receptor agonist LY379268 selectively blocks amphetamine ambulations and rearing. Eur J Pharmacol. 2000;400:221–24. doi: 10.1016/s0014-2999(00)00423-4. [DOI] [PubMed] [Google Scholar]
- 290.Kim J-H, Vezina P. The mGlu2/3 receptor agonist LY379268 blocks the expression of locomotor sensitization by amphetamine. Pharmacol Biochem Behav. 2002;73:333–37. doi: 10.1016/s0091-3057(02)00827-4. [DOI] [PubMed] [Google Scholar]
- 291.Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–27. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine-and food-seeking behavior in rats. Psychopharmacology. 2006;186:143–49. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- 293.Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–8. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 294.Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Robinson MB, Blakely RD, Couto R, Coyle JT. Hydrolysis of the brain dipeptide N-acetyl-L-aspartyl-L-glutamate. Identification and characterization of a novel N-acetylated alpha-linked acidic dipeptidase activity from rat brain. J Biol Chem. 1987;262:14498–506. [PubMed] [Google Scholar]
- 297.Neale JH, Bzdega T, Wroblewska B. N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J Neurochem. 2000;75:443–52. doi: 10.1046/j.1471-4159.2000.0750443.x. [DOI] [PubMed] [Google Scholar]
- 298.Burlina AP, Skaper SD, Mazza MR, Ferrari V, Leon A, Burlina AB. N-acetylaspartylglutamate selectively inhibits neuronal responses to N-methyl-D-aspartic acid in vitro. J Neurochem. 1994;63:1174–77. doi: 10.1046/j.1471-4159.1994.63031174.x. [DOI] [PubMed] [Google Scholar]
- 299.Tsai G, Slusher BS, Sim L, Coyle JT. Immunocytochemical distribution of N-acetylaspartylglutamate in the rat forebrain and glutamatergic pathways. J Chem Neuroanat. 1993;6:277–92. doi: 10.1016/0891-0618(93)90033-z. [DOI] [PubMed] [Google Scholar]
- 300.Passani LA, Vonsattel JP, Coyle JT. Distribution of N-acetylaspartylglutamate immunoreactivity in human brain and its alteration in neurodegenerative disease. Brain Res. 1997;772:9–22. doi: 10.1016/s0006-8993(97)00784-1. [DOI] [PubMed] [Google Scholar]
- 301.Tsai G, Stauch BL, Vornov JJ, Deshpande JK, Coyle JT. Selective release of N-acetylaspartylglutamate from rat optic nerve terminals in vivo. Brain Res. 1990;518:313–16. doi: 10.1016/0006-8993(90)90989-o. [DOI] [PubMed] [Google Scholar]
- 302.Slusher BS, Tsai G, Yoo G, Coyle JT. Immunocytochemical localization of the N-acetyl-aspartyl-glutamate (NAAG) hydrolyzing enzyme N-acetylated α-linked acidic dipeptidase (NAALADase) J Comp Neurol. 1992;315:217–29. doi: 10.1002/cne.903150208. [DOI] [PubMed] [Google Scholar]
- 303.Berger UV, Luthi-Carter R, Passani LA, et al. Glutamate carboxypeptidase II is expressed by astrocytes in the adult rat nervous system. J Comp Neurol. 1999;415:52–64. doi: 10.1002/(sici)1096-9861(19991206)415:1<52::aid-cne4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 304.Xi Z-X, Gilbert J, Peng X-Q, et al. Effects of the NAALADase inhibitors 2-PMPA, GPI-16476 and GPI-16477 on cocaine self-administration and reinstatement of drug-seeking behavior. Paper presented at NIDA-sponsored Mini-Convention on “Frontiers in Addiction Research,” Satellite scientific conference of the meetings of the Society for Neuroscience; San Diego. October 2004. [Google Scholar]
- 305.Gardner EL, Ashby CR, Jr, Gilbert JG, et al. Inhibition of NAALADase attenuates cocaine’s reinforcement and cocaine-triggered reinstatement of cocaine-seeking behavior in rats. Abstract at the 35th Annual Meeting of the Society for Neuroscience; Washington, DC. November 12–16, 2005; p. Abstract 227.9. [Google Scholar]
- 306.Slusher BS, Thomas A, Paul M, Schad CA, Ashby CR., Jr Expression and acquisition of the conditioned place preference response to cocaine in rats is blocked by selective inhibitors of the enzyme N-acetylated-α-linked-acidic dipeptidase (NAALADASE) Synapse. 2001;41:22–28. doi: 10.1002/syn.1056. [DOI] [PubMed] [Google Scholar]
- 307.Shippenberg TS, Rea W, Slusher BS. Modulation of behavioral sensitization to cocaine by NAALADase inhibition. Synapse. 2000;38:161–66. doi: 10.1002/1098-2396(200011)38:2<161::AID-SYN7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 308.Mitsukawa K, Yamamoto R, Ofner S, et al. A selective metabotropic glutamate receptor 7 agonist: activation of receptor signaling via an allosteric site modulates stress parameters in vivo. Proc Natl Acad Sci USA. 2005;102:18712–17. doi: 10.1073/pnas.0508063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Makoff A, Pilling C, Harrington K, Emson P. Human metabotropic glutamate receptor type 7: molecular cloning and mRNA distribution in the CNS. Brain Res Mol Brain Re. 1996;40:165–70. doi: 10.1016/0169-328x(96)00110-6. [DOI] [PubMed] [Google Scholar]
- 310.Xi Z-X, Shen H, Baker DA, Kalivas PW. Inhibition of non-vesicular glutamate release by group III metabotropic glutamate receptors in the nucleus accumbens. J Neurochem. 2003;87:1204–12. doi: 10.1046/j.1471-4159.2003.02093.x. [DOI] [PubMed] [Google Scholar]
- 311.Mao L, Wang JQ. Distinct inhibition of acute cocaine-stimulated motor activity following microinjection of a group III metabotropic glutamate receptor agonist into the dorsal striatum of rats. Pharmacol Biochem Behav. 2000;67:93–101. doi: 10.1016/s0091-3057(00)00307-5. [DOI] [PubMed] [Google Scholar]
- 312.Mao L, Lau Y-S, Wang JQ. Activation of group III metabotropic glutamate receptors inhibits basal and amphetamine-stimulated dopamine release in rat dorsal striatum: an in vivo microdialysis study. Eur J Pharmacol. 2000;404:289–97. doi: 10.1016/s0014-2999(00)00633-6. [DOI] [PubMed] [Google Scholar]
- 313.Li X, Peng X-Q, Gilbert J, Pak AC, Xi Z-X, Gardner EL. Activation of metabotropic glutamate receptor 7 (mGluR7) by AMN082 attenuates the rewarding effects of cocaine by a DA-independent mechanism in rats. Abstract at the 36th Annual Meeting of the Society for Neuroscience; Atlanta, Georgia. October 14–18, 2006; p. Abstract 21.7. [Google Scholar]
- 314.Li X, Gardner EL, Xi Z-X. The metabotropic glutamate receptor 7 (mGluR7) allosteric agonist AMN082 modulates nucleus accumbens GABA and glutamate, but not dopamine, in rats. Neuropharmacology. 2008;54:542–51. doi: 10.1016/j.neuropharm.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Wise MS, Arand DL, Auger RR, Brooks SN, Watson NF. Treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30:1712–27. doi: 10.1093/sleep/30.12.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Ballon JS, Feifel D. A systematic review of modafinil: potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–66. doi: 10.4088/jcp.v67n0406. [DOI] [PubMed] [Google Scholar]
- 317.Madras BK, Xie Z, Lin Z, et al. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319:561–69. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- 318.Dopheide MM, Morgan RE, Rodvelt KR, Schachtman TR, Miller DK. Modafinil evokes striatal [3H]dopamine release and alters the subjective properties of stimulants. Eur J Pharmacol. 2007;568:112–23. doi: 10.1016/j.ejphar.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 319.Gold LH, Balster RL. Evaluation of the cocaine-like discriminative stimulus effects and reinforcing effects of modafinil. Psychopharmacology. 1996;126:286–92. doi: 10.1007/BF02247379. [DOI] [PubMed] [Google Scholar]
- 320.Deroche-Gamonet V, Darnaudéry M, Bruins-Slot L, Piat F, Le Moal M, Piazza PV. Study of the addictive potential of modafinil in naive and cocaine-experienced rats. Psychopharmacology. 2002;161:387–95. doi: 10.1007/s00213-002-1080-8. [DOI] [PubMed] [Google Scholar]
- 321.Rush CR, Kelly TH, Hays LR, Baker RW, Wooten AF. Acute behavioral and physiological effects of modafinil in drug abusers. Behav Pharmacol. 2002;13:105–15. doi: 10.1097/00008877-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 322.Rush CR, Kelly TH, Hays LR, Wooten AF. Discriminative-stimulus effects of modafinil in cocaine-trained humans. Drug Alcohol Depend. 2002;67:311–22. doi: 10.1016/s0376-8716(02)00082-0. [DOI] [PubMed] [Google Scholar]
- 323.Dackis CA, Lynch KG, Yu E, et al. Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend. 2003;70:29–37. doi: 10.1016/s0376-8716(02)00335-6. [DOI] [PubMed] [Google Scholar]
- 324.Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- 325.Malcolm R, Swayngim K, Donovan JL, et al. Modafinil and cocaine interactions. Am J Drug Alcohol Abuse. 2006;32:577–87. doi: 10.1080/00952990600920425. [DOI] [PubMed] [Google Scholar]
- 326.Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked Cocaine Self-Administration is Decreased by Modafinil. Neuropsychopharmacology. 2008;33:761–68. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- 327.O’Brien CP, Dackis CA, Kampman K. Does modafinil produce euphoria? Am J Psychiatry. 2006;163:1109. doi: 10.1176/ajp.2006.163.6.1109. [DOI] [PubMed] [Google Scholar]
- 328.Jasinski DR, Kovaceviæ-Ristanoviæ R. Evaluation of the abuse liability of modafiniland other drugs for excessive daytime sleepiness associated with narcolepsy. Clin Neuropharmacol. 2000;23:149–56. doi: 10.1097/00002826-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 329.Prisinzano TE, Tidgewell K, Harding WW. κ opioids as potential treatments for stimulant dependence. AAPS J. 2005;7:E592–9. doi: 10.1208/aapsj070361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Wadenberg ML. A review of the properties of spiradoline: a potent and selective κ-opioid receptor agonist. CNS Drug Rev. 2003;9:187–98. doi: 10.1111/j.1527-3458.2003.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Maisonneuve IM, Archer S, Glick SD. U50,488, a κ opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopamine in the nucleus accumbens of rats. Neurosci Lett. 1994;181:57–60. doi: 10.1016/0304-3940(94)90559-2. [DOI] [PubMed] [Google Scholar]
- 332.Glick SD, Maisonneuve IM, Raucci J, Archer S. κ opioid inhibition of morphine and cocaine self-administration in rats. Brain Res. 1995;681:147–52. doi: 10.1016/0006-8993(95)00306-b. [DOI] [PubMed] [Google Scholar]
- 333.Kuzmin AV, Semenova S, Gerrits MA, Zvartau EE, Van Ree JM. κ-Opioid receptor agonist U50,488H modulates cocaine and morphine self-administration in drug-naive rats and mice. Eur J Pharmacol. 1997;321:265–71. doi: 10.1016/s0014-2999(96)00961-2. [DOI] [PubMed] [Google Scholar]
- 334.Negus SS, Mello NK, Portoghese PS, Lin C-E. Effects of κ opioids on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1997;282:44–55. [PubMed] [Google Scholar]
- 335.Kantak KM, Riberdy A, Spealman RD. Cocaine-opioid interactions in groups of rats trained to discriminate different doses of cocaine. Psychopharmacology. 1999;147:257–65. doi: 10.1007/s002130051165. [DOI] [PubMed] [Google Scholar]
- 336.Negus SS, Mello NK. Effects of kappa opioid agonists on the discriminative stimulus effects of cocaine in rhesus monkeys. Exp Clin Psychopharmacol. 1999;7:307–17. doi: 10.1037//1064-1297.7.4.307. [DOI] [PubMed] [Google Scholar]
- 337.Negus SS. Effects of the kappa opioid agonist U50,488 and the kappa opioid antagonist nor-binaltorphimine on choice between cocaine and food in rhesus monkeys. Psychopharmacology. 2004;176:204–13. doi: 10.1007/s00213-004-1878-7. [DOI] [PubMed] [Google Scholar]
- 338.Schenk S, Partridge B, Shippenberg TS. U69593, a κ-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology. 1999;144:339–46. doi: 10.1007/s002130051016. [DOI] [PubMed] [Google Scholar]
- 339.Schenk S, Partridge B, Shippenberg TS. Reinstatement of extinguished drug-taking behavior in rats: effect of the kappa-opioid receptor agonist, U69593. Psychopharmacology. 2000;151:85–90. doi: 10.1007/s002130000476. [DOI] [PubMed] [Google Scholar]
- 340.Schenk S, Partridge B, Shippenberg TS. Effects of the kappa-opioid receptor agonist, U69593, on the development of sensitization and on the maintenance of cocaine self-administration. Neuropsychopharmacology. 2001;24:441–50. doi: 10.1016/S0893-133X(00)00190-1. [DOI] [PubMed] [Google Scholar]
- 341.Vanderschuren LJMJ, Schoffelmeer ANM, Wardeh G, De Vries TJ. Dissociable effects of the κ-opioid receptor agonists bremazocine, U69593, and U50488H on locomotor activity and long-term behavioral sensitization induced by amphetamine and cocaine. Psychopharmacology. 2000;150:35–44. doi: 10.1007/s002130000424. [DOI] [PubMed] [Google Scholar]
- 342.El Daly E, Chefer V, Sandill S, Shippenberg TS. Modulation of the neurotoxic effects of methamphetamine by the selective κ opioid receptor agonist U69593. J Neurochem. 2000;74:1553–62. doi: 10.1046/j.1471-4159.2000.0741553.x. [DOI] [PubMed] [Google Scholar]
- 343.Schenk S, Partridge B. Effect of the κ-opioid receptor agonist, U69593, on reinstatement of extinguished amphetamine self-administration behavior. Pharmacol Biochem Behav. 2001;68:629–34. doi: 10.1016/s0091-3057(00)00478-0. [DOI] [PubMed] [Google Scholar]
- 344.Powell KR, Holtzman SG. Modulation of the discriminative stimulus effects of d-amphetamine by mu and kappa opioids in squirrel monkeys. Pharmacol Biochem Behav. 2000;65:43–51. doi: 10.1016/s0091-3057(99)00183-5. [DOI] [PubMed] [Google Scholar]
- 345.Nagase H, Hayakawa J, Kawamura K, et al. Discovery of a structurally novel opioid κ-agonist derived from 4,5-epoxymorphinan. Chem Pharm Bull. 1998;46:366–69. doi: 10.1248/cpb.46.366. [DOI] [PubMed] [Google Scholar]
- 346.Hasebe K, Kawai K, Suzuki T, et al. Possible pharmacotherapy of the opioid κ receptor agonist for drug dependence. Ann N Y Acad Sci. 2004;1025:404–13. doi: 10.1196/annals.1316.050. [DOI] [PubMed] [Google Scholar]
- 347.Endoh T, Matsuura H, Tajima A, et al. Potent antinociceptiveeffectsof TRK-820, a novel κ-opioid receptor agonist. Life Sci. 1999;65:1685–1694. doi: 10.1016/s0024-3205(99)00417-8. [DOI] [PubMed] [Google Scholar]
- 348.Wang Y, Tang K, Inan S, et al. Comparison of pharmacological activities of three distinct κ-ligands (Salvinorin A, TRK-820 and 3FLB) on κ opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J Pharmacol Exp Ther. 2004;312:220–30. doi: 10.1124/jpet.104.073668. [DOI] [PubMed] [Google Scholar]
- 349.Mori T, Nomura M, Nagase H, Narita M, Suzuki T. Effects of a newly synthesized κ-opioid receptor agonist, TRK-820, on the discriminative stimulus and rewarding effects of cocaine in rats. Psychopharmacology. 2002;161:17–22. doi: 10.1007/s00213-002-1028-z. [DOI] [PubMed] [Google Scholar]
- 350.Mori T, Nomura M, Yoshizawa K, et al. Differentialpropertiesbetween TRK-820 and U-50,488H on the discriminative stimulus effects in rats. Life Sci. 2004;75:2473–82. doi: 10.1016/j.lfs.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 351.Sorbera L, Castaner J, Leeson P. Nalfurafine hydrochloride. Antipruritic, analgesic, kappa opioid agonist. Drugs Future. 2003;28:237–42. [Google Scholar]
- 352.Mello NK, Negus SS. Interactions between κ opioid agonists and cocaine. Preclinical studies. Ann N Y Acad Sci. 2000;909:104–32. doi: 10.1111/j.1749-6632.2000.tb06678.x. [DOI] [PubMed] [Google Scholar]
- 353.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–54. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 354.Bubar MJ, Cunningham KA. Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr Top Med Chem. 2006;6:1971–85. doi: 10.2174/156802606778522131. [DOI] [PubMed] [Google Scholar]
- 355.Sorensen SM, Kehne JH, Fadayel GM, et al. Characterization of the 5-HT2 receptor antagonist MDL 100907 as a putative atypical antipsychotic: behavioral, electrophysiological and neurochemical studies. J Pharmacol Exp Ther. 1993;266:684–91. [PubMed] [Google Scholar]
- 356.Offord SJ, Wong DF, Nyberg S. The role of positron emission tomography in the drug development of M100907, a putative antipsychotic with a novel mechanism of action. J Clin Pharmacol. 1999;(Suppl):17S–24S. doi: 10.1002/j.1552-4604.1999.tb05933.x. [DOI] [PubMed] [Google Scholar]
- 357.Kehne JH, Ketteler HJ, McCloskey TC, Sullivan CK, Dudley MW, Schmidt CJ. Effects of the selective 5-HT2A receptor antagonist MDL 100,907 on MDMA-induced locomotor stimulation in rats. Neuropsychopharmacology. 1996;15:116–24. doi: 10.1016/0893-133X(95)00160-F. [DOI] [PubMed] [Google Scholar]
- 358.Palfreyman MG, Schmidt CJ, Sorensen SM, et al. Electrophysiological, biochemical and behavioral evidence for 5-HT2 and 5-HT3 mediated control of dopaminergic function. Psychopharmacology. 1993;112(Suppl 1):S60–67. doi: 10.1007/BF02245008. [DOI] [PubMed] [Google Scholar]
- 359.Schmidt CJ, Fadayel GM, Sullivan CK, Taylor VL. 5-HT2 receptors exert a state-dependent regulation of dopaminergic function: studies with MDL 100,907 and the amphetamine analogue, 3,4-methylenedioxymethamphetamine. Eur J Pharmacol. 1992;223:65–74. doi: 10.1016/0014-2999(92)90819-p. [DOI] [PubMed] [Google Scholar]
- 360.Moser PC, Moran PM, Frank RA, Kehne JH. Reversal of amphetamine- induced behaviours by MDL 100,907, a selective 5-HT2A antagonist. Behav Brain Res. 1996;73:163–67. doi: 10.1016/0166-4328(96)00090-3. [DOI] [PubMed] [Google Scholar]
- 361.O’Neill MF, Heron-Maxwell CL, Shaw G. 5-HT2 receptor antagonism reduces hyperactivity induced by amphetamine, cocaine, and MK-801 but not D1 agonist C-APB. Pharmacol Biochem Behav. 1999;63:237–43. doi: 10.1016/s0091-3057(98)00240-8. [DOI] [PubMed] [Google Scholar]
- 362.McMahon LR, Cunningham KA. Antagonism of 5-hydroxytryplainine2A receptors attenuates the behavioral effects of cocaine in rats. J Pharmacol Exp Ther. 2001;297:357–63. [PubMed] [Google Scholar]
- 363.Fletcher PJ, Grottick AJ, Higgins GA. Differential effects of the 5-HT2A receptor antagonist M100907 and the 5-HT2C receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology. 2002;27:576–86. doi: 10.1016/S0893-133X(02)00342-1. [DOI] [PubMed] [Google Scholar]
- 364.Martin JR, Bös M, Jenck F, et al. 5-HT2C receptor agonists: pharmacological characteristics and therapeutic potential. J Pharmacol Exp Ther. 1998;286:913–24. [PubMed] [Google Scholar]
- 365.Cussac D, Newman-Tancredi A, Quentric Y, et al. Characterization of phospholipase C activity at h5-HT2C compared with h5-HT2B receptors: influence of novel ligands upon membrane-bound levels of [3H]phosphatidylinositols. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:242–52. doi: 10.1007/s00210-001-0505-y. [DOI] [PubMed] [Google Scholar]
- 366.Navailles S, De Deurwaerdère P, Porras G, Spampinato U. In vivo evidence that 5-HT2C receptor antagonist but not agonist modulates cocaine-induced dopamine outflow in the rat nucleus accumbens and striatum. Neuropsychopharmacology. 2004;29:319–26. doi: 10.1038/sj.npp.1300329. [DOI] [PubMed] [Google Scholar]
- 367.Navailles S, Moison D, Cunningham KA, Spampinato U. Differential regulation of the mesoaccumbens dopamine circuit by serotonin2C receptors in the ventral tegmental area and the nucleus accumbens: an in vivo microdialysis study with cocaine. Neuropsychopharmacology. 2008;33:237–46. doi: 10.1038/sj.npp.1301414. [DOI] [PubMed] [Google Scholar]
- 368.Grottick AJ, Fletcher PJ, Higgins GA. Studies to investigate the role of 5-HT2C receptors on cocaine-and food-maintained behavior. J Pharmacol Exp Ther. 2000;295:1183–91. [PubMed] [Google Scholar]
- 369.Burbassi S, Cervo L. Stimulation of serotonin2C receptors influences cocaine-seeking behavior in response to drug-associated stimuli in rats. Psychopharmacology. 2008;196:15–27. doi: 10.1007/s00213-007-0916-7. [DOI] [PubMed] [Google Scholar]
- 370.Fletcher PJ, Chintoh AF, Sinyard J, Higgins GA. Injection of the 5-HT2C receptor agonist Ro 60–0175 into the ventral tegmental area reduces cocaine-induced locomotor activity and cocaine self-administration. Neuropsychopharmacology. 2004;29:308–18. doi: 10.1038/sj.npp.1300319. [DOI] [PubMed] [Google Scholar]
- 371.Bromidge SM, Duckworth M, Forbes IT, et al. 6-Chloro-5-methyl-1-[[2-[(2-methyl-3-pyridyl)oxy]-5-pyridyl]carbamoyl]-indoline (SB-242084): the first selective and brain penetrant 5-HT2C receptor antagonist. J Med Chem. 1997;40:3494–96. doi: 10.1021/jm970424c. [DOI] [PubMed] [Google Scholar]
- 372.Neisewander JL, Acosta JI. Stimulation of 5-HT2C receptors attenuates cue and cocaine-primed reinstatement of cocaine-seeking behavior in rats. Behav Pharmacol. 2007;18:791–800. doi: 10.1097/FBP.0b013e3282f1c94b. [DOI] [PubMed] [Google Scholar]