Abstract
Better tumor markers are needed for early diagnosis and staging of prostate cancer, and for monitoring therapeutic response than the currently used prostate specific antigen (PSA). Prostate specific membrane antigen (PSMA) is highly expressed on the surface of prostatic epithelial cells making it a good target for prostate cancer. In this study, mAb 3C6, specific for the extracellular epitope of PSMA, was evaluated both in vitro and in vivo for PSMA-targeting. Immunoreactivity and specificity of mAb 3C6 was evaluated by flow cytometry using prostate cell lines expressing PSMA such as LNCaP and 22Rv1 and a cell line, DU145, that expresses very little PSMA. 3C6 was conjugated with the acyclic CHX-A” DTPA chelate, radiolabeled with 111In, and its in vitro and in vivo properties were assessed. The biodistribution of the radioimmunoconjugate evaluated in athymic mice bearing xenografts of three human prostate carcinoma cell lines shows high uptake after 72 hr in LNCaP tumors (%ID/g 22.93 ± 6.32) and 22Rv1 (%ID/g 10.44 ± 2.32) in contrast to low uptake by the DU145 tumors (%ID/g 4.27 ± 0.37). Planar γ-scintigraphic images obtained for xenografted tumor bearing mice demonstrated targeting for PSMA positive tumors suggesting possible applications in imaging and for targeted radiation therapy.
Keywords: prostate cancer, prostate membrane specific antigen, 3C6, monoclonal antibody, gamma scintigraphy
Introduction
Prostate cancer has the highest incidence rate in men and accounts for 29% of all new cancer diagnosis. It is the second-leading cause of cancer deaths in the adult male population [1]. In 2007, approximately 218,890 new cases of prostate cancer and 27,050 deaths due to prostate cancer were estimated in the US alone [1]. Early detection through increased screening has reduced the mortality rate of prostate cancer. However, major efforts are still needed to increase the sensitivity and specificity of the tests.
Since its discovery and isolation in seminal fluid, prostatic tissue and human serum, prostate specific antigen (PSA) has been the most clinically useful tumor marker for the early detection and staging of prostate cancer, as well as for monitoring therapeutic response. Development of the PSA assay represented major advances for the detection of prostate cancer as well as in the monitoring and management of patients during therapy. Monitoring of PSA levels alone has led to a greater number of prostate malignancies being diagnosed as compared to the digital rectal exam (DRE) [2]. PSA as a tumor marker and target for imaging and therapy, however, has limitations. It is not cancer-specific, it is dependent on age and race and does not discriminate prostate cancer from other benign prostate pathologies such as benign prostate hyperplasia (BPH) and prostatitis [3]. Additionally, at least 15 % of prostate cancers, confirmed by biopsy, have serum PSA levels within the normal reference range (i.e., false-negatives) [4,5]. As a result, serum PSA levels within the reference range are not indicative of the absence of cancer, nor are values that are above normal necessarily indicative of cancer. This lack of sensitivity, specificity and reliability has encouraged researchers to continue to develop improved means for diagnosis of prostate cancer.
Prostate specific membrane antigen (PSMA), a type II transmembrane glycoprotein with a Mr of ∼100 kD, is found in metastatic prostate cancer, hormone refractory tumors, the vascular endothelium of some carcinomas and sarcomas, and limited expression in normal cells and benign prostatic secretory-acinar epithelium [6-8]. Unlike PSA, prostatic acid phosphatase (PAP) or prostate secretory protein (PSP), PSMA is not normally released into the serum [9]. This property in combination with its expression pattern renders PSMA a potentially useful molecular target for diagnosis, imaging and/or therapy of prostate cancer. Monoclonal antibodies (mAbs) specific for PSMA have been developed for use in cancer diagnosis. One example is 7E11.C5, the mAb that originally defined PSMA, and currently used in Prostascint™ (Cytogen, Inc., NJ) for the detection of prostate cancer [10]. One drawback of this mAb is that it targets an intracellular epitope of PSMA [11], thus it does not react or bind with viable cells, which greatly limits it potential as a diagnostic imaging agent.
Identification of this shortcoming prompted development of a second generation of monoclonal antibodies that specifically target extracellular epitopes of PSMA. The first of these was the IgM mAb designated 3F5.4G6 which recognizes PSMA, but does not compete with 7E11.C5, and therefore was proposed to target the extracellular domain of PSMA [12]. Murine mAbs J415 and J591 were developed and characterized by Smith-Jones et al and demonstrated a high affinity for an extracellular epitope of PSMA [13]. The J591 mAb has been modified with up to five DOTA (1,4,7,10-tetraazacyclododecane-N,N’,N”,N’”-tetraacetic acid) chelates without loss of its immunoreactivity [13]. As a result, the evaluation of mAb J591 was extended to assess its ability to target PSMA in vivo. When labeled with 90Y- or 213Bi-, J591 demonstrated positive results in the pre-clinical evaluation of J591 for PSMA-targeted radioimmunotherapy (RIT) of prostate cancer [14-17]. Its humanized counterpart is now being assessed in clinical trials [9].
Monoclonal antibody 3C6, a murine IgG1 is another second-generation mAb that reacts with an extracellular epitope of PSMA [18]. The studies described herein are a continuation in the evaluation of mAb 3C6. Previous studies with this mAb have demonstrated its specificity for PSMA [18]. In contrast to other PSMA-targeting antibody such as 7E11.C5, 3C6 does not recognize linear amino acid sequence epitopes in PSMA, but rather binds to the conformational epitope of PSMA found in the extracellular domain of the protein [18]. This particular specificity of 3C6 to bind live cells expressing PSMA in its natural conformation suggests utility in diagnostic and/or therapeutic applications. In this study, the in vivo biodistribution, blood pharmacokinetics, and imaging characteristics of 111In-labeled 3C6 for possible applications in PSMA targeting are described.
Results
Flow Cytometry
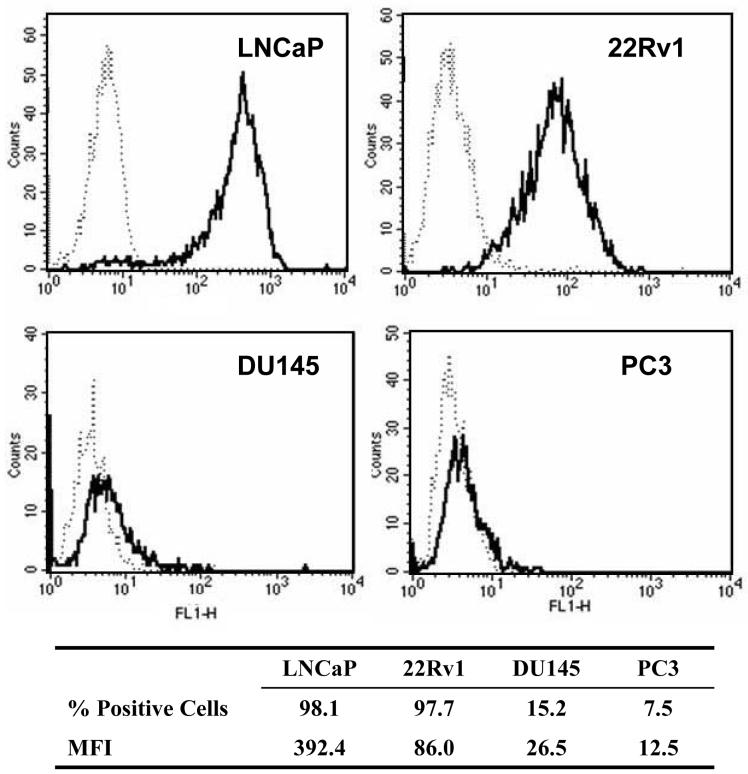
Flow cytometric analyses, depicted in Fig. (1), confirms that the LNCaP and 22Rv1 cell lines both express PSMA with mAb 3C6 reacting with 98.1% and 97.7% of the cells, respectively. The mean fluorescence intensity (MFI) of the LNCaP cells was 392.4 while the MFI of the 22Rv1 was much lower at 86.0, indicating that PSMA is expressed at much higher levels per cell in LNCaP. In contrast, very low expression was observed with both DU145 and PC3. The respective cell population positive for PSMA expression was 15.2 % and 7.5% with concomitantly low MFI (26.5 and 12.5) for both cell lines.
Figure 1.
Immunoreactivity of mAb 3C6 with human prostate carcinoma cell lines; mAb 3C6, solid line, isotype control, dotted line.
Conjugation and radiolabeling
Conjugation of mAb 3C6 with the acyclic CHX-A” DTPA was performed at a 10:1 and 20:1 chelate-to-antibody ratio (molar excess). These reactions resulted in final products in which the chelate-to-mAb ratio was 0.5 and 0.7, respectively (Table 1). The overall recovery for each of the reactions was 75% and 67%.
Table 1.
Summary of conjugation and radiolabeling procedures with mAb 3C6
| Reactiona | |||
|---|---|---|---|
| 10-fold | 20-fold | ||
| Conjugation | Chelate:Protein | 0.5 | 0.7 |
| Recovery | 75% | 67% | |
| Radiolabeling | Specific Activity | 9.6 | 10.7 |
| Labeling Efficiency | 87% | 91% | |
The conjugation reaction of CHX-A”-DTPA with mAb 3C6 was performed at two different molar excess amounts chelate as described in Materials and Methods.
Radiolabeling of each immunoconjugate preparation was efficient and yielded products with excellent specific activities (9.6 and 10.7 mCi/mg). Size-exclusion HPLC of the isolated radioimmunoconjugate confirmed that the radioactivity was associated with the mAb peak as shown in Fig. (2). Subsequent studies were performed with the immunoconjugate preparation from the 10-fold reaction that resulted in a chelate-to-mAb ratio of 0.5.
Figure 2.
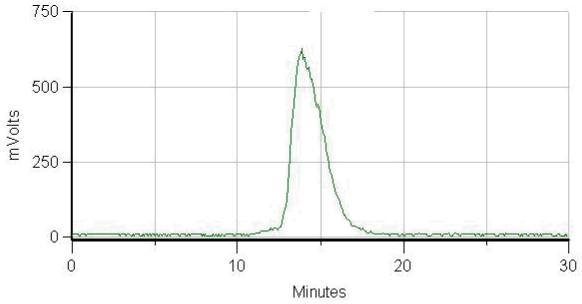
Size-exclusion HPLC radiochromatogram of 111In-CHX-A”-3C6
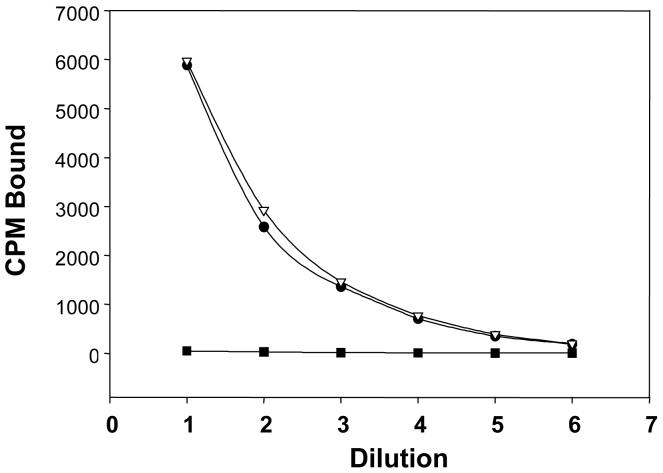
The immunoreactivity of mAb 3C6 was found to be retained following the radiolabeling procedure. Immunoreactivity of 111In-3C6 with the cell lines, LNCaP and 22Rv1, that express PSMA was found to be identical as illustrated in Fig. (3). Specificity of the binding is demonstrated by the lack of 111In-3C6 binding with the DU145 cell line in which PSMA expression is very low or absent.
Figure 3.
Radioimmunoassay evaluating the immunoreactivity of 111In-CHX-A”-3C6. Binding was tested using LNCaP (●), 22Rv1 (△) and DU145 (■).
In vivo studies
Athymic mice (n = 5 per group) bearing LNCaP, 22Rv1, or DU145 (s.c.) xenografts were given i.v. injections of 111In-CHX-A”-3C6 to establish and define tumor targeting and normal organ distribution of the radioimmunoconjugate (Table 2). Data indicate excellent in vivo PSMA-targeting of 111In-CHX-A”-3C6 with a % ID/g of 22.93 ± 6.23 and 10.44 ± 2.32 (mean ± SD) for LNCaP and 22Rv1 tumors, respectively, at 72 hr. At this same time point a %ID/g of 4.27 ± 0.37 for the DU145 was observed (Table 2). The tumor %ID/g remained high until the end of the study (168 hr) with a value of 22.62 ± 9.56 for LNCaP tumors and 14.12 ± 6.13 for 22Rv1, while remaining low for DU145 with a tumor %ID/g of 4.29 ± 2.62. With the exception of tumor and blood, no significantly high uptake of the radioimmunoconjugate in any normal organ was observed for all three sets of prostate tumor types. Hepatic and splenic uptake of 111In-3C6 for all three tumor types was low with the %ID/g ≤ 6. The lowest %ID/g throughout the entire study was observed in the femur. The highest recorded value for the femur was at 24 hr in those mice bearing 22Rv1 xenografts with a %ID/g value of 1.96 ± 0.60.
Table 2.
Tumor and normal organ distribution of 111In-CHXA”-3C6 following i.v. injection: Percent Injected dose/gram (%ID/g)a
| Time point (hr) | |||||
|---|---|---|---|---|---|
| Tumor | Tissue | 24 | 48 | 72 | 168 |
| LNCaP | blood | 13.51a ± 1.78b | 11.90 ± 2.21 | 9.92 ± 1.32 | 7.84 ± 1.95 |
| tumor | 18.72 ± 5.08 | 20.40 ± 3.81 | 22.93 ± 6.32 | 22.62 ± 9.56 | |
| liver | 4.75 ± 0.63 | 4.77 ± 0.17 | 5.33 ± 1.69 | 3.90 ± 0.96 | |
| spleen | 4.20 ± 0.68 | 4.29 ± 1.47 | 3.77 ± 1.20 | 4.26 ± 1.18 | |
| kidney | 2.81 ± 0.29 | 2.63 ± 0.44 | 2.19 ± 0.46 | 1.83 ± 0.42 | |
| lung | 5.13 ± 1.02 | 5.45 ± 1.60 | 4.29 ± 0.82 | 4.12 ± 1.60 | |
| femur | 1.49 ± 0.32 | 1.31 ± 0.31 | 1.04 ± 0.20 | 1.06 ± 0.16 | |
| 22Rv1 | blood | 11.47 ± 3.53 | 10.72 ± 0.89 | 7.61 ± 1.01 | 9.27 ± 2.88 |
| tumor | 8.42 ± 2.32 | 12.92 ± 3.90 | 10.44 ± 2.32 | 14.12 ± 6.13 | |
| liver | 4.46 ± 1.55 | 3.85 ± 0.92 | 3.48 ± 0.75 | 5.10 ± 1.21 | |
| spleen | 3.61 ± 1.49 | 3.40 ± 0.68 | 2.91 ± 0.44 | 7.39 ± 3.00 | |
| kidney | 2.98 ± 0.93 | 2.89 ± 0.31 | 2.32 ± 0.43 | 3.53 ± 1.17 | |
| lung | 5.34 ± 1.65 | 3.54 ± 1.12 | 3.00 ± 0.49 | 4.91 ± 1.30 | |
| femur | 1.96 ± 0.60 | 1.64 ± 0.09 | 1.48 ± 0.19 | 1.34 ± 0.25 | |
| DU145 | blood | 9.34 ± 1.42 | 8.85 ± 1.70 | 6.83 ± 0.76 | 4.92 ± 1.36 |
| tumor | 3.80 ± 0.45 | 4.01 ± 1.06 | 4.27 ± 0.37 | 4.29 ± 0.62 | |
| liver | 3.43 ± 0.56 | 3.39 ± 0.55 | 4.08 ± 0.76 | 3.03 ± 0.52 | |
| spleen | 2.00 ± 0.39 | 2.96 ± 1.46 | 2.47 ± 0.35 | 2.17 ± 0.38 | |
| kidney | 2.30 ± 0.45 | 2.11 ± 0.54 | 1.74 ± 0.24 | 1.24 ± 0.27 | |
| lung | 3.81 ± 0.55 | 3.45 ± 0.72 | 3.13 ± 0.42 | 2.18 ± 0.78 | |
| femur | 1.21 ± 0.21 | 1.18 ± 0.49 | 1.10 ± 0.22 | 0.98 ± 0.34 | |
Athymic male mice bearing s.c.human prostate carcinoma xenografts were injected i.v. with approximately 7.5 μCi of 111In-CHXA”-3C6. The mice (n=5) were sacrificed by exsanguination as described in Materials and Methods. The blood, tumor and major organs were collected, wet-weighed and the radioactivity measured. The %ID/g were calculated for each tissue as well as the standard deviation. The values represent the average %ID/g (percent injected dose/gram).
Values are standard deviation of the %ID/g.
Tissue-to-blood ratios were calculated to the specificity and stability of the injected radioimmunoconjugate (Table 3). As expected, tumor-to-blood ratios for 111In-CHX-A”-3C6 in both the LNCaP and 22Rv1 tumors increased over time from a ratio at 1.73 and 0.75 at 24 hr to attain ratios of 2.94 and 1.48 at 168 hr, respectively. The tumor-to-blood ratio for DU145 tumor ranges from 0.41 at 24 hr to 0.90 at 168 hr. The lack of radioactivity accumulating in the DU145 tumors attests to the PSMA specificity of mAb 3C6 (at 24 hr ρ = 0.005 for DU145 to LNCaP and ρ = 0.0002 for DU145 to 22Rv1; at 168 hr ρ = 0.035 for DU145 to LNCaP and ρ = 0.004 for DU145 to 22Rv1). The specificity ratio, defined as the ratio of the tumor-to-blood value for LNCaP to the tumor-to-blood value for DU145 is 3.5 at 72 hr which decreases to ∼2.5 at 168 hr. Tissue-to-blood ratios for the normal organs in all three sets of prostate carcinoma xenografts remained below unity, suggesting that the 111In-CHX-A”-3C6 was stable. No differences between the high PSMA-expressing and low PSMA-expressing tumor bearing mice in normal organ uptake are discernible.
Table 3.
Tissue-to-blood ratios of 111In-CHXA”-3C6 following i.v. injection in male athymic mice bearing s.c. prostate cancer xenografts
| Time point (hr) | |||||
|---|---|---|---|---|---|
| Ratio | 24 | 48 | 72 | 168 | |
| LNCaP | tumor | 1.73 ± 0.24 | 1.73 ± 0.24 | 2.28 ± 0.36 | 2.94 ± 1.44 |
| liver | 0.41 ± 0.09 | 0.41 ± 0.09 | 0.53 ± 0.11 | 0.50 ± 0.05 | |
| spleen | 0.36 ± 0.08 | 0.36 ± 0.08 | 0.37 ± 0.09 | 0.54 ± 0.10 | |
| kidney | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.23 ± 0.01 | |
| lung | 0.38 ± 0.22 | 0.38 ± 0.22 | 0.43 ± 0.03 | 0.51 ± 0.08 | |
| femur | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.10 ± 0.01 | 0.14 ± 0.02 | |
| 22Rv1 | tumor | 0.75 ± 0.08 | 1.20 ± 0.31 | 1.36 ± 0.16 | 1.48 ± 0.28 |
| liver | 0.39 ± 0.08 | 0.36 ± 0.08 | 0.46 ± 0.10 | 0.56 ± 0.07 | |
| spleen | 0.32 ± 0.11 | 0.32 ± 0.06 | 0.38 ± 0.03 | 0.78 ± 0.13 | |
| kidney | 0.26 ± 0.01 | 0.27 ± 0.03 | 0.31 ± 0.04 | 0.38 ± 0.01 | |
| lung | 0.52 ± 0.32 | 0.34 ± 0.12 | 0.39 ± 0.03 | 0.54 ± 0.03 | |
| femur | 0.17 ± 0.04 | 0.15 ± 0.02 | 0.20 ± 0.02 | 0.15 ± 0.02 | |
| DU145 | tumor | 0.41 ± 0.04 | 0.45 ± 0.09 | 0.63 ± 0.10 | 0.90 ± 0.19 |
| liver | 0.37 ± 0.02 | 0.39 ± 0.07 | 0.60 ± 0.13 | 0.63 ± 0.90 | |
| spleen | 0.21 ± 0.02 | 0.35 ± 0.22 | 0.36 ± 0.05 | 0.45 ± 0.06 | |
| kidney | 0.25 ± 0.07 | 0.24 ± 0.02 | 0.25 ± 0.02 | 0.26 ± 0.03 | |
| lung | 0.41 ± 0.03 | 0.39 ± 0.01 | 0.46 ± 0.02 | 0.44 ± 0.03 | |
| femur | 0.13 ± 0.04 | 0.13 ± 0.03 | 0.16 ± 0.02 | 0.21 ± 0.08 | |
Athymic male mice bearing s.c.human prostate carcinoma xenografts were injected i.v. with approximately 7.5 μCi of 111In-CHXA”-3C6. The mice (n=5) were sacrificed by exsanguination as described in Materials and Methods. The blood, tumor and major organs were collected, wet-weighed and the radioactivity measured. The blood-to-tissue ratios were calculated using the %ID/g for each tissue as well as the standard deviation. The values represent the average ratio.
Values are standard deviation of the %ID/g.
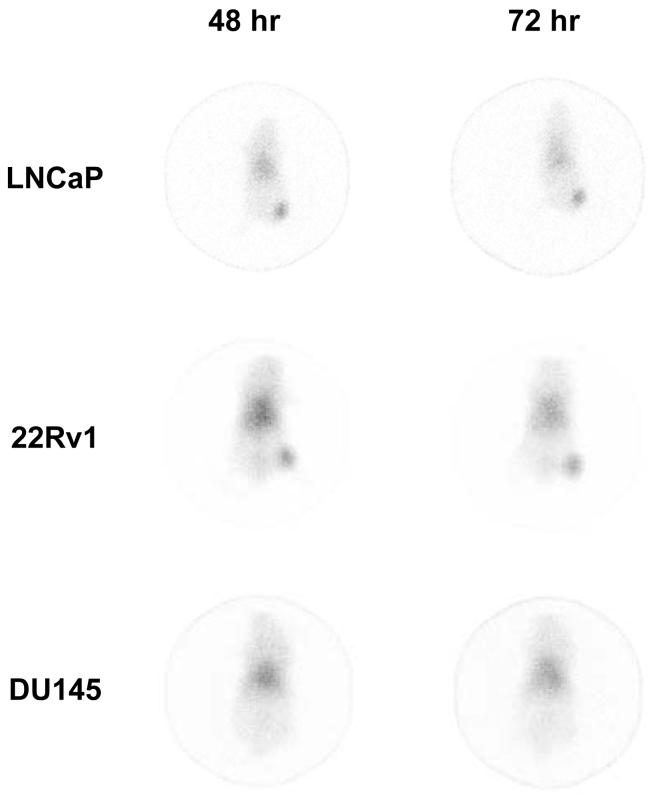
Clear visualization of the high PSMA-expressing tumors (LNCaP and 22Rv1) was obtained following i.v. injection of 100 μCi of 111In-CHX-A”-3C6 throughout the 168 hr study period using planar γ-scintigraphy. Images obtained at 48 and 72 hr are presented in Fig. (4) for comparative purposes. As expected for a murine mAb administered to a mouse, the radioimmunoconjugate is evident in the blood pool with a high signal observed in the heart, lung, and liver [19]. Clearance of the 111In-CHX-A”-3C6 from the blood pool is evident from 48 to 72 hr and as the background to signal ratio improves the radioimmunoconjugate is seen to remain at the tumor. These imaging results were in accordance with the biodistribution data. In comparison, targeting of the DU145 xenografts, low PSMA-expressing tumors, is absent.
Figure 4.
Planar γ-scintigraphic images obtained at 48, 72, and 168 hr after i.v. injection of 111In-CHX-A”-3C6 in LNCaP, 22Rv1 or DU145-tumor bearing male athymic mouse.
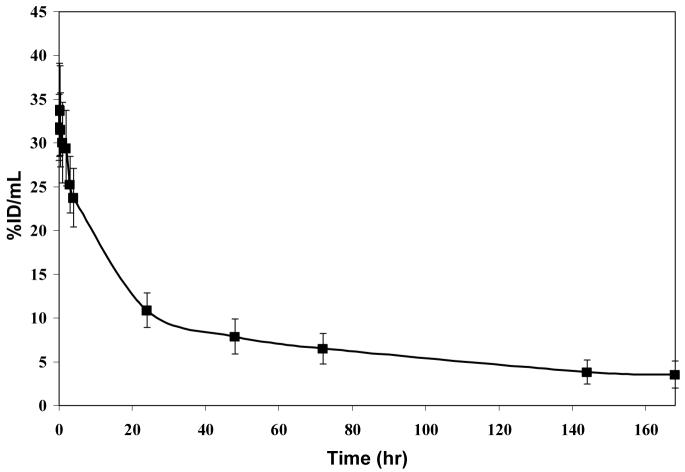
Clearance of the 111In-CHX-A”-3C6 from the blood compartment was found to be consistent with a murine mAb in an athymic mouse tumor model (Fig. (5)). At 24 hr, the %ID/mL is still 10.9, 34% of the %ID/mL at 1 min after injection. The %ID/mL follows a biphasic clearance and at 168 hr has decreased to 3.5, 11% of the initial level in the blood. The blood pharmacokinetics studies estimates the t½α of the radioimmunoconjugate to be ∼5.3 hr with the t½β then to be about ∼92.3 hr.
Figure 5.
Blood pharmacokinetics for 111In-CHX-A”-3C6 in a LNCaP-tumor bearing male athymic mouse showing the average %ID/mL at each time point. Error bars are reported as standard deviations.
Discussion
Use of antibodies as targeting agents for imaging and for therapy is not a novel concept. Radiolabeled antibodies provide highly specific localization to the target in vivo i.e. tumor based on the antibody-antigen recognition and act as delivery system for the attached radionuclide for detection/imaging and/or treatment [20]. Due to the sensitivity of this method, the amount of radioactivity used is small and toxicity issues are rarely a concern. However, there are a few disadvantages such as the low tumor uptake that is usually obtained, tumor ∼0.005% ID/g, and the development of antibodies against the murine mAb, preventing its usefulness in follow-up or in multi-cycle therapeutic regimens. Advances in antibody engineering, production, and screening have greatly improved the field with better targeting capabilities which has increased the tumor %ID/g. The development of human-murine chimeras or humanized mAb has the potential to reduce the immune response and enable repeated treatments which is usually required for the management of the disease.
The 3C6 mAb, a murine IgG1 was developed to target the prostate specific membrane antigen (PSMA) which is regarded to be a better tumor target than PSA for diagnosis, staging, and monitoring of prostate cancer [21]. High PSMA expression is found in ∼66% of prostate cancers and is expressed in poorly differentiated, metastatic, and hormone refractory cancers [22,23].
Monoclonal antibody 3C6 has been shown to react with conformational epitopes of the native protein in the extracellular domain of PSMA; it has also been shown to react with viable prostatic cells. In fact, the preliminary experiment described herein was to confirm the reactivity and specificity of mAb 3C6. Flow cytometric analyses demonstrated excellent immunoreactivity of the mAb with viable LNCap cells as well as with 22Rv1, another prostate carcinoma cell line that expresses PSMA, although not to the same degree. Reactivity with cell lines expressing either very low or no PSMA (DU-145 and PCS) was absent. The histogram that was obtained for LNCap was consistent with previously published data [12]. This may suggest better in vivo specificity than a mAb such as 7E11.C5, which reacts with a linear epitope as can be observed from previous flow cytometric studies made in comparison with 3C6 [17,18].
As with any mAb, modification of amino acid residues can alter the conformation or binding sites which in turn can greatly impact its recognition capability. Previous modification of 3C6 by addition of FITC has been shown to have no affect on its immunoreactivity [18]. Hence, only a small and non-critical alteration is probable when hopefully incorporated the requisite chelates for radiolabeling this mAb. The choice of the chelate is also important to avoid toxicity issues. In this study, the acyclic CHX-A” DTPA was chosen due to its established stability with several radionuclides useful for either imaging (eg., 111In, 177Lu for SPECT, 86Y for PET) or therapeutic purposes (eg., 177Lu 90Y, 213Bi,) [24,25].
To validate the bioconjugate CHX-A”-3C6, evaluation of its immunoreactivity towards both the target (LNCaP and 22Rv1) and control (DU145 and PC3) cell lines is necessary. In this case, radiolabeling with 111In to assay this step in vitro and in vivo provides both the sensitivity and means to perform the evaluation. Evident from the in vitro cell binding assay, conjugation with CHX-A” DTPA does not inhibit binding or alter the specificity of mAb 3C6 for PSMA as demonstrated by the ability of the radioimmunocojugate 111In-CHX-A”-3C6 to bind to live high PSMA-expressing cells (LNCaP and 22Rv1), but not to cells (DU145) with low PSMA expression.
Biodistribution studies also demonstrated targeting and specificity for tumor xenografts with the higher expression of PSMA as shown by the high uptake (22.9 %ID/g at 72 hr) versus a peak tumor %ID/g of 4.29 at 168 hr with the low PSMA expressing tumor. The in vivo data obtained with 111In-CHX-A”-3C6 resulted in higher tumor %ID/g than J591, the best characterized of the anti-PSMA mAb. Murine J591 was radiolabeled with 131I and 111In and its tumor targeting was compared to mAb 7E11. The highest tumor %ID/g for the 131I-J591 was 11.4 at 4 days and 17.4 at 6 days with the 111In-J591 [26]. In the data presented here, 111In-CHX-A”-3C6 realized a tumor %ID/g of 22.6 at 168 hr. This is not an unusual or unexpected result since considerable differentials in tumor uptake versus what one might have expected extrapolating from in vitro target expression levels. Cells in vitro may not be very predictive of in vivo antigen expression and empirical targeting studies may be the only technique to validate actual tumor targeting as opposed to cell culture results.
The blood clearance of 111In-CHX-A”-3C6 was also relatively fast with a t½α of 5.3 hr and t½β of 92.3 hr. The t½β is comparable to the 2.3 d that was reported for 111In-J591.[26] Interestingly, the investigators reported a longer residence time (4.2 d) in the blood for 131I-J591. A similar pattern, although longer, was also obtained with 131I- and 111In-labeled 7E11with t½β of 7.1 and 5 days. Stability of the radioimmunoconjugate is also observed as noted by the fast clearance and low accumulation in liver and spleen.
γ-Scintigraphy corroborated the biodistribution data in that tumors were readily visualized at 24 hr and remained clearly visible throughout the 168 hr study period. Meanwhile there is a clearing of radioactivity in the blood and hence an improved signal-to-noise ratio. Specificity was also demonstrated by the lack of localization of the radioactivity in the DU-145 xenografts. In this instance, the images obtained with 111In-3C6 are comparable to those obtained with 111In-J591 [26].
Generation of mAbs, either intact or in forms such as scFvs, directed against the extracellular domain of PSMA continues to be pursued. In 2006, Biele et al used purified PSMA to generate three intact mAb and 4 scFvs [27]. Their characterization of the mAbs revealed that the mAbs reacted only with purified PSMA and did not recognize PSMA in the context of the cell membrane. The group proceeded to generate additional mAbs using LNCap cell lysates and were successful in generating three more intact IgGs and one scFV that did recognize and react with viable LNCap cells [27]. Other anti-PSMA mAbs have been generated and are at different stages of characterization [28-30].
Anti-PSMA mAbs are currently under evaluation in clinical trials for targeting therapeutic radionuclides for the treatment of prostate cancer. When radiolabeled with 177Lu, mAb J591was found to be tolerated by patients at up to 75 mCi/m2, 70 mCi/m2 was determined to be the single maximum tolerated dose; 16 of 35 patients received up to 3 doses [15]. Targeting of known sites of disease was confirmed by imaging in 30 of the patients. Some efficacy was observed in that 4 of the patients experienced a >50% decrease in their PSA levels which lasted from 3 to 8 months. Sixteen other patients had a stabilization of their PSA levels that ranged from 1 to 21 months.
The potential of anti-PSMA mAbs such as 3C6 may also extend beyond the management and treatment of prostate cancer patients. PSMA is also expressed in the vascular endothelium of other cancers. A phase I trial has been conducted with 27 patients whose tumors, breast, colon, bladder, kidney, lung, pancreas and melanoma, were known to express PSMA [31]. In a dose escalation study, patients received J591 to which 111In-J591 was added for the purposes of confirming tumor targeting and for pharmacokinetic determinations. Tumor targeting was demonstrated in 74% of the patients with at least one known site of disease being imaged. Due to the lack of toxicity (Grade 3 or 4 reactions), the first nine patients received 2 doses of J591, i.e., progressed to the next dose level. The 18 other patients were given J591 weekly for a total of 6 doses. The investigators reported no objective regressions were observed in these patients, although as a phase I trial none were expected. They did however report that two patients at the 10 mg dose had stable disease and one patient with metastastic colon cancer experienced a 53% decrease in carcinoembryonic antigen levels. Overall, the results were positive and certainly suggested that anti-PSMA mAb may very well serve as vascular targeting agents.
In conclusion, the feasibility of using monoclonal antibody 3C6 for targeting PSMA in vivo has been demonstrated. 3C6 conjugation with CHX-A” and radiolabeling with the appropriate radiometal provides a useful means to image for PSMA-expressing prostate cancer demonstrating its potential as imaging or diagnostic agent.
Experimental Section
Cell Lines and Cell Culture
DU145 and PC3 cells were grown in DMEM (Dulbecco’s modified Eagle’s medium) and RPMI-1640, respectively. LNCaP cells were grown in RPMI-1640 containing 2 mM L-glutamine. 22Rv1 cells were grown in RPMI-1640 supplemented with 10 mM HEPES, 1 mM sodium pyruvate, 1.5 g/L sodium bicarbonate, and 4.5 g/L glucose. In addition to the supplements mentioned, all cell lines were also grown in the presence of 10% fetal bovine sera and 1 mM non-essential amino acids. All media and supplements were obtained from Quality Biological (Gaithersburg, MD). All four cell lines are derived from human prostate carcinoma cell lines and were obtained from ATCC (Manassas, VA).
Flow Cytometric Analysis
Reactivity of mAb 3C6 was assessed using the LNCaP, 22Rv1, DU145 and PC3 cell lines using flow cytometric techniques by methods previously described [32]. Briefly, cells were trypsinized, pelleted at 1,500 x g for 5 min and re-suspended in PBS containing 1% bovine serum albumin, pH 7.2 (PBS/BSA). The cells (1 × 106 cells/100 μL PBS/BSA) were added to 12×75mm polypropylene tubes (Evergreen Scientific, Los Angeles, CA) along with 1 μg mAb 3C6 (generously supplied byNorthwest Biotherapeutics, Bothell, WA). The cells were incubated for 1 hr at 4°C, washed three times by adding 3 mL of PBS/BSA, pelleting the cells at 1,500 x g for 5 minutes and decanting the supernatant. Following the last wash, the cells were resuspended in 100 μL of PBS/BSA containing 0.5 μg Alexa Fluor 488 goat anti-mouse IgG (H+L) (Invitrogen, Carlsbad, CA) and incubated for an additional 1 hr at 4°C. MOPC-21, an IgG1 mouse myeloma protein (Sigma Diagnostic, St. Louis, MO) with no known reactivity with mouse or human tissues, served as a non-specific isotype control antibody. The cells were washed three times as before, resuspended in 1 mL PBS and analyzed using a FACSCalibur with CellQuest software (BD Biosciences, San Jose, CA); 10,000 events were collected for each sample.
Chelate synthesis and antibody conjugation
The synthesis, characterization, and purification of 2-(p-isothiocyanatobenzyl)-N-(2-aminoethyl)-trans-1,2-diaminocyclohexane-N,N’,N”-pentaacetic acid, CHX-A” DTPA, has been previously described [24]. The CHX-A” DTPA was conjugated to mAb 3C6 following established methods using a molar excess of ligand to antibody [32,33]. The amount of protein was quantified by the Lowry method using a BSA standard [34]. The number of CHX-A” DTPA molecules linked to the antibody was determined using an yttrium(III)-Arsenazo III-based assay [35,36].
Radiolabeling of CHX-A”-3C6
Radiolabeling of CHX-A”-3C6 with 111In (PerkinElmer, Shelton, CT) was achieved using methods detailed elsewhere [37]. Briefly, 1 mCi of 111In chloride (in 0.05 M HCl) was added to CHX-A”-3C6 (100 μg in 0.15 M ammonium acetate buffer, pH 7.0). The reaction mixture was incubated for 1 hr at 37°C and quenched by adding 3 μL of 0.05M EDTA to scavenge any free radio-metal. The radiolabeled antibody was purified through a PD-10 desalting column (GE Healthcare Bio-Sciences, Piscataway, NJ). Fractions were collected, pooled and analyzed by size exclusion radio-HPLC (Lab Alliance, State College, PA) equipped with a γ-RAM radio flow gamma detector (IN/US Systems, Inc., Tampa, FL) using a TSK G3000SW column 10 μm, 7.8 mm × 30 cm column (Tosoh Bioscience, Montgomeryville, PA) eluted with PBS at 0.5 mL/min.
Radioimmunoassay
Immunoreactivity of the 111In-labeled 3C6 was tested against three prostate cancer cell lines, LNCaP, 22Rv1 and DU145 in a live cell radioimmunoassay by methods previously described [37]. Briefly, after trypsinization, 1× 106 cells of each of the cell lines,in 100 μL of PBS/BSA, was added to 12×75mm polypropylene tubes, in duplicate. Serial dilutions of the 111In-3C6 were then added to each tube (100 μL each). Following an overnight incubation at 4 °C, the cells were washed with 4 mL of PBS/BSA, pelleted, the supernatant decanted and counted in a γ-counter (1480 Wizard 3”, PerkinElmer, Shelton, CT). The average cpm bound for each dilution was calculated and plotted.
In vivo studies
All procedures were performed in accordance with the National Institutes of Health guidelines on the use of animals in research and were approved by the Animal Care and Use Committee of the National Cancer Institute. Animal studies were performed using 4-6 week old male athymic (nu/nu) mice obtained from Charles River Laboratories (Wilmington, MA). The animals were allowed to rest for one week and then injected subcutaneously in the right flank with either 5 × 106 cells (LNCaP) or 4 × 106 cells (DU145 and 22Rv1). Injected volumes were 200 μL in media with 20% Matrigel™ (BD Biosciences, Bedford, MA). The tumor xenografts were allowed to attain a size of 0.4 to 0.6 cm before the mice were used for in vivo studies.
For the biodistribution studies, mice were injected i.v. with approximately 7.5 μCi of 111In-CHX-A”-3C6 (0.5-1.0 μg) in 200 μL of PBS. Mice were sacrificed via CO2 inhalation at 24, 48, 72, and 168 hour time points (n = 5 per time point). Blood, tumor and major organs were harvested, wet-weighed, and counted in a γ-scintillation counter (1480 Wizard, PerkinElmer, Shelton, CT). The percent injected dose per gram (%/ID/g) and standard deviation (SD) were calculated.
Blood pharmacokinetics were conducted by injecting (i.v.) athymic male mice (n = 5) bearing s.c. LNCaP tumor xenografts with approximately 7.5 μCi of 111In-CHX-A”-3C6 in 200 μL of PBS. Blood (10 μL) was collected at various time points via a tail snip using heparinized capillary tubes (Drummond Scientific, Broomall, PA). The samples were transferred to cotton filters, placed in 12×75 polypropylene tubes, and counted in a γ-scintillation counter (1480 Wizard). The percent injected dose per mL (%/ID/mL) and standard deviation (SD) were calculated.
Gamma Scintigraphy
Imaging studies was performed on athymic male mice (n = 5) bearing s.c. LNCaP xenografts injected i.v. with 100 μCi of 111In-CHX-A”-3C6 (200 μL PBS). Images were collected 48, 72 and 168 hr post-injection of the radioimmunoconjugate. The mice were chemically restrained using 2.5% isoflurane (Abbott Laboratories, NJ) in O2 using a SurgiVet Vetinerary Surgical Products vaporizer (Waukesha, WI) at a flow rate of ∼1.0 mL/min. Images were obtained at 48, 72, and 168 hr using a large field of view (LFOV) γ-camera (Siemens Medical Solutions USA, Inc., Malvern, PA) equipped with a pinhole collimator at 6 cm height and a 15% window centered on both photopeaks (173 and 247 keV) of 111In with 100,000 counts per imaging being collected.
Acknowledgement
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- [1].Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- [2].Catalona WJ, Richie JP, Ahmann FR, Hudson MA, Scardino PT, Flanigan RC, deKernion JB, Ratliff TL, Kavoussi LR, Dalkin BL. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J. Urol. 1994;151:1283. doi: 10.1016/s0022-5347(17)35233-3. [DOI] [PubMed] [Google Scholar]
- [3].Murphy GP, Barren RJ, Erickson SJ, Bowes VA, Wolfert RL, Bartsch G, Klocker H, Pointner J, Reissigl A, McLeod DG, Douglas T, Morgan T, Kenny GM, Ragde H, Boynton AL, Holmes EH. Evaluation and comparison of two new prostate carcinoma markers. Free-prostate specific antigen and prostate specific membrane antigen. Cancer. 1996;78:809. doi: 10.1002/(SICI)1097-0142(19960815)78:4<809::AID-CNCR18>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- [4].Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, Crowley JJ, Coltman CA. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N. Engl. J. Med. 2004;350:2239. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- [5].Myrtle JF. Normal levels of prostate-specific antigen (PSA) In: Catalona WJ, Coffey DS, Karr JP, editors. Clinical aspects of prostate cancer: assessment of new diagnostic and management procedures. Elsevier; New York: 1989. pp. 183–189. [Google Scholar]
- [6].Sokoloff RL, Norton KC, Gasior CL, Marker KM, Grauer LS. A dual-monoclonal sandwich assay for prostate-specific membrane antigen: Levels in tissues, seminal fluid and urine. Prostate. 2000;43:150. doi: 10.1002/(sici)1097-0045(20000501)43:2<150::aid-pros10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- [7].Israeli RS, Powell CT, Corr JG, Fair WR, Heston WD. Expression of the prostate-specific membrene antigen. Cancer Res. 1994;54:1807. [PubMed] [Google Scholar]
- [8].Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52:637. doi: 10.1016/s0090-4295(98)00278-7. [DOI] [PubMed] [Google Scholar]
- [9].Nanus DM, Milowsky MI, Kostakoglu L, Smith-Jones P, Vallabhajosula S, Goldsmith SJ, Bander NH. Clinical use of monoclonal antibody HuJ591 therapy: Targeting prostate specific membrane antigen. J. Urol. 2003;170:S84. doi: 10.1097/01.ju.0000095151.97404.7c. [DOI] [PubMed] [Google Scholar]
- [10].Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticaner Res. 1987;7:927. [PubMed] [Google Scholar]
- [11].Troyer JK, Feng Q, Beckett ML, Wright GL., Jr. Biochemical characterization and mapping of the 7E11.C5 epitope of the prostate-specific membrane antigen. Urol. Oncol. 1995;1:29. doi: 10.1016/1078-1439(95)00004-2. [DOI] [PubMed] [Google Scholar]
- [12].Murphy GP, Tino WT, Holmes EH, Boynton AL, Erickson SJ, Bowes VA, Barren RJ, Tjoa BA, Misrock SL, Raqde H, Kenny GM. Measurement of prostate-specific membrane antigen in the serum with a new antibody. Prostate. 1996;28:266. doi: 10.1002/(SICI)1097-0045(199604)28:4<266::AID-PROS7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- [13].Smith-Jones PM, Vallabhajosula S, Goldsmith SJ, Navarro V, Hunter CJ, Bastidas D, Bander NH. In vitro characterization of radiolabeled monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen. Cancer Res. 2000;60:5237. [PubMed] [Google Scholar]
- [14].Li Y, Tian Z, Rizvi SM, Bander NH, Allen BJ. In vitro and preclinical targeted alpha therapy of human prostate cancer with Bi-213 labeled J591 antibody against the prostate specific membrane antigen. Prostate Cancer Prostatic Dis. 2002;5:36. doi: 10.1038/sj.pcan.4500543. [DOI] [PubMed] [Google Scholar]
- [15].Bander NH, Trabulsi EJ, Kostakoglu L, Yao D, Vallabhajosula S, Smith-Jones P, Joyce MA, Milowsky M, Nanus DM, Goldsmith SJ. Targeting metastatic prostate cancer with radiolabeled monoclonal antibody J591 to the extracellular domain of prostate specific membrane antigen. J. Urol. 2003;170:1717. doi: 10.1097/01.ju.0000091655.77601.0c. [DOI] [PubMed] [Google Scholar]
- [16].Smith-Jones P, Vallabhajosula S, Navarro V, Bastidas D, Goldsmith SJ, Bander NH. Radiolabeled monoclonal antibodies specific to the extracellular domain of prostate-specific membrane antigen: Preclinical studies in nude mice bearing LnCaP human prostate tumor. J. Nucl. Med. 2003;44:610. [PubMed] [Google Scholar]
- [17].Vallabhajosula S, Smith-Jones P, Navarro V, Goldsmith SJ, Bander NH. Radioimmunotherapy of prostate cancer in human xenografts using monoclonal antibodies to prostate specific membrane antigen (PSMA): studies in nude mice. Prostate. 2004;58:145. doi: 10.1002/pros.10281. [DOI] [PubMed] [Google Scholar]
- [18].Tino WT, Huber MJ, Lake TP, Greene TG, Murphy GP, Holmes EH. Isolation and characterization of monoclonal antibodies specific for protein conformational epitopes present in prostate-specific membrane antigen (PSMA) Hybridoma. 2000;19:249. doi: 10.1089/02724570050109648. [DOI] [PubMed] [Google Scholar]
- [19].Wu AM, Senter PD. Arming antibodies: prospects and challenges for immunoconjugates. Nature Biotechnol. 2005;23:1137. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- [20].Wu AM. Tools for pretargeted radioimmunotherapy. Cancer Biother. Radiopharm. 2001;16:103. doi: 10.1089/108497801300189191. [DOI] [PubMed] [Google Scholar]
- [21].Chang SS. Overview of prostate-specific membrane antigen. Rev. Urol. 2004;6:S13. [PMC free article] [PubMed] [Google Scholar]
- [22].Chang SS, O’Keefe DS, Bacich DJ, Reuter VE, Heston WDW, Gaudin PB. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin. Cancer Res. 1999;5:2674. [PubMed] [Google Scholar]
- [23].Mhawech-Fauceglia P, Zhang S, Terracciano L, Sauter G, Chadhuri A, Hermann FR, Penetrante R. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using multiple tumour tissue microarray technique. Hispathology. 2007;50:472. doi: 10.1111/j.1365-2559.2007.02635.x. [DOI] [PubMed] [Google Scholar]
- [24].Wu C, Kobayashi H, Sun B, Yoo TM, Paik CH, GAnsow OA, Carrasquillo JA, Pastan I, Brechbiel MW. Stereochemical influence on the stability of radio-metal complexes in vivo. Synthesis and evaluation of the four stereoisomers of 2-(p-nitrobenzyl)-trans-CyDTPA. Bioorg. Med. Chem. 1997;5:1925. doi: 10.1016/s0968-0896(97)00130-2. [DOI] [PubMed] [Google Scholar]
- [25].Wu C, Brechbiel MW, Kozak RW, GAnsow OA. Metal-chelate-dendrimer-antibody constructs for use in radioimmunotherapy and imaging. Bioorg. Med. Chem. Lett. 1994;4:449. [Google Scholar]
- [26].Smith-Jones PM, Vallabhajosula S, Navarro V, Bastidas D, Goldsmith SJ, Bander NH. Radiolabeled monoclonal antibodies specific to the extracellular domain of prostate-specific membrane antigen: Preclinical studies in nude mice bearing LNCaP human prostate tumor. J. Nucl. Med. 2003;44:610. [PubMed] [Google Scholar]
- [27].Elsässer-Beile U, Wolf P, Gierschner D, Bühler P, Schultze-Seeman W, Wetterauer U. A new generation of monoclonal and recombinant antibodies against cell-adherent prostate specific membrane antigen for diagnostic and therapeutic targeting of prostate cancer. Prostate. 2006;66:1359. doi: 10.1002/pros.20367. [DOI] [PubMed] [Google Scholar]
- [28].Henry MD, Wen S, Silva MD, Chandra S, Milton M, Worland PJ. A prostate-specific membrane antigen-targeted monoclonal antibody-chemotherapeutic conjugate designed for the treatment of prostate cancer. 64. 2004;21:8001. doi: 10.1158/0008-5472.CAN-04-1722. [DOI] [PubMed] [Google Scholar]
- [29].Moffett S, Mélançon D, DeCrescenzo G, St-Pierre C, Deschénes F, Saragovi HU, Gold P, Cuello AC. Preparation and characterization of new anti-PSMA monoclonal antibodies with potential clinical use. Hybridoma. 2007;26:363. doi: 10.1089/hyb.2007.0522. [DOI] [PubMed] [Google Scholar]
- [30].Brown LG, Wegner SK, Wang H, Buhler KR, Arfman EW, Lange PH, Vessella RL. A novel monoclonal antibody 107-1A4 with high prostate specificity: generation, characterization of antigen expression, and targeting of human prostate cancer xenografts. Pros Canc Pros Dis. 1998;1:208. doi: 10.1038/sj.pcan.4500233. [DOI] [PubMed] [Google Scholar]
- [31].Milowsky MI, Nanus DM, Kostakoglu L, Sheehan CE, Vallabhajosula S, Goldsmith SJ, Ross JS, Bander NH. Vascular targeted therapy with anti-prostate-specific membrane antigen monoclonal antibody J591 in advanced solid tumors. J. Clin. Oncol. 2007;25:540. doi: 10.1200/JCO.2006.07.8097. [DOI] [PubMed] [Google Scholar]
- [32].Garmestani K, Milenic DE, Plascjak PS, Brechbiel MW. A new and convenient method for purification of 86Y using a Sr(II) selective resin and comparison of biodistribution of 86Y and 111In labeled Herceptin. Nucl. Med. Biol. 2002;29:599. doi: 10.1016/s0969-8051(02)00322-0. [DOI] [PubMed] [Google Scholar]
- [33].Kozak RW, Raubitschek A, Mirzadeh S, Brechbiel MW, Junghaus R, GAnsow OA, Waldmann TA. Nature of the bifunctional chelating agent used for radioimmunotherapy with ytrrium-90 monoclonal antibodies: critical factors in determining in vivo survival and organ toxicity. Cancer Res. 1989;49:2639. [PubMed] [Google Scholar]
- [34].Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265. [PubMed] [Google Scholar]
- [35].Pippin CG, Parker TA, McMurry TJ, Brechbiel MW. Spectrophotometric method for the determination of a bifunctional DTPA ligand in DTPA-monoclonal antibody conjugates. Bioconjugate Chem. 1992;3:342. doi: 10.1021/bc00016a014. [DOI] [PubMed] [Google Scholar]
- [36].Dadachova E, Chappell LL, Brechbiel MW. Spectrophotometric method for determination of bifunctional macrocyclic ligands in macrocyclic ligand-protein conjugates. Nucl. Med. Biol. 1999;26:977. doi: 10.1016/s0969-8051(99)00054-2. [DOI] [PubMed] [Google Scholar]
- [37].Milenic DE, Garmestani K, Brady ED, Albert PS, Ma D, Abdulla A, Brechbiel MW. Targeting of Her2 antigen for the treatment of disseminated peritoneal disease. Clin. Cancer Res. 2004;10:7834. doi: 10.1158/1078-0432.CCR-04-1226. [DOI] [PubMed] [Google Scholar]