Abstract
The anxiolytic effects of opiates active at the mu-opioid receptor (μ-OR) may be ascribed, in part, to suppression of neurons that are responsive to the stress-associated peptide, corticotropin releasing factor (CRF), in the central amygdala (CeA) and bed nucleus of the stria terminalis (BNST). The CRF receptor (CRFr) and μ-OR are expressed in both the CeA and BNST, but their subcellular relationship to each other is not known in either region. To address this question, we used dual electron microscopic immunolabeling of μ-OR and CRFr in the mouse lateral CeA and anterolateral BNST. Immunolabeling for each receptor was detected in the same as well as in separate somatic, dendritic and axonal profiles of neurons in each region. CRFr had a plasmalemmal or cytoplasmic distribution in many dendrites, including those co-expressing μ-OR. The co-expression of CRFr and μ-OR also was seen near excitatory-type synapses on dendritic spines. In both the CeA and BNST, over 50% of the CRFr-labeled dendritic profiles (dendrites and spines) contained immunoreactivity for the μ-OR. However, less than 25% of the dendritic profiles containing the μ-OR were labeled for CRFr in either region, suggesting that opiate activation of the μ-OR affects many neurons in addition to those responsive to CRF. The dendritic profiles containing CRFr and/or μ-OR received asymmetric, excitatory-type synapses from unlabeled or CRFr-labeled axon terminals. In contrast, the μ-OR was identified in terminals forming symmetric, inhibitory-type synapses. Thus, in both the CeA and BNST, μ-OR and CRFr have strategic locations for mediation of CRF and opioid effects on the postsynaptic excitability of single neurons, and on the respective presynaptic release of excitatory and inhibitory neurotransmitters. The commonalities in the synaptic location of both receptors in the CeA and BNST suggest that this is a fundamental cellular association of relevance to both drug addiction and stress-induced disorders.
Keywords: stress, addiction, relapse, withdrawal, anxiety, electron microscopic immunolabeling
Endogenous opioids strongly influence neuroendocrine, autonomic and behavioral responses to stress in a variety of species (Morris et al, 1990; McCubbin et al, 1993; McCubbin, 1993; Sufka et al, 1994; Janssens et al, 1995; Drolet et al, 2001). These effects are primarily mediated by the inhibitory, G-protein-coupled mu-opioid receptor (μ-OR) (Buckingham & Cooper, 1984; Marson et al, 1989; degli Uberti et al, 1995; Liberzon et al, 2002; Xu et al, 2004; Ribeiro et al, 2005). The μ-OR is activated not only by endogenous opioids, but also by the opiates, morphine and heroin (Contet et al, 2004). Relapse to opiate dependence can be induced by stress or by opiate-priming in rodents, and this relapse is attenuated by blockade of the largely stimulatory, G-protein-coupled corticotropin releasing factor receptor (CRFr) (Shaham et al, 1997, Shaham et al, 1998; Lu et al, 2000; Wang et al, 2006). Increased activity in CRF systems within the brain is also thought to underlie the negative affective states and somatic/autonomic symptoms associated with morphine withdrawal (Lu et al, 2000; Iredale et al, 2000; Contarino & Papaleo, 2005; Stinus et al, 2005; Skelton et al, 2007). Together, these observations suggest that opiate withdrawal symptoms may, in part, reflect upregulation of the brain CRF system chronically suppressed by opiates.
The reciprocally-connected central nucleus of the amygdala (CeA) and bed nucleus of the stria terminalis (BNST) are among the primary brain regions implicated in both the withdrawal symptoms and relapse occurring in animal models of opiate addiction (Heinrichs et al, 1995; Watanabe et al, 2002; Veinante et al, 2003; Nakagawa et al, 2005; Wang et al, 2006; Harris & Aston-Jones, 2007). The emotional responses mediated through the BNST are thought to be slower-onset and longer-lasting, while those involving the CeA are suggested to be rapid-onset and short-duration (Walker et al, 2003; Walker & Davis, 2008). However, the extensive bidirectional anatomical connections between these regions indicate that neither region is independent of the other (Sun et al, 1991; Poulin et al, 2006).
There are many GABAergic and peptidergic neurons throughout the CeA and BNST (Sun & Cassell, 1993; Day et al, 1999). Neurons in each region contain an abundance of opioid peptides and opioid receptors (Moskowitz & Goodman, 1984; Arluison et al, 1994; Mansour et al, 1994; Mansour et al, 1995; Ding et al, 1996; Moriwaki et al, 1996; Day et al, 1999; Abbadie et al, 2000; Chieng et al, 2006; Poulin et al, 2006; Marchant et al, 2007). Opioid receptors, and specifically the μ-OR, show major medial and lateral differences in expression levels (Mansour et al, 1994; Mansour et al, 1995; Abbadie et al, 2000; Poulin et al, 2006). CRF and CRF receptors also are distributed throughout the CeA and BNST (Swanson et al, 1983; Sakanaka et al, 1986; Phelix & Paull, 1990; Uryu et al, 1992; Gray, 1993; Chen et al, 2000; Treweek et al, 2009; Van Pett et al, 2000). CRF is similar to opioid peptides in being differentially expressed in medial and lateral subdivisions (Swanson et al, 1983; Sakanaka et al, 1986; Phelix & Paull, 1990; Uryu et al, 1992; Treweek et al, 2009).
The lateral subdivision of the CeA (CeL) and the anterolateral subdivision of the BNST (BSTal) are homologous regions that are highly interconnected (Sakanaka et al, 1986; Han & Ju, 1990; Sun et al, 1991; Roder & Ciriello, 1993; Sun & Cassell, 1993; Veinante & Freund-Mercier, 1998; Erb et al, 2001; Nakagawa et al, 2005; Poulin et al, 2006). Each of these major subdivisions project extensively to the reward-associated ventral tegmental area (Georges & Aston-Jones, 2002; Dong & Swanson, 2004; Rodaros et al, 2007), as well as to autonomic-related brainstem regions (Moga & Gray, 1985; Moga et al, 1989; Gray & Magnuson, 1987; Gray & Magnuson, 1992; Dong et al, 2001; Dong & Swanson, 2004). The output from the lateral as well as medial subdivisions of both regions is also controlled largely by glutamatergic inputs from the cerebral cortex and basolateral amygdala (Yasui et al, 1991;Schmued, 1994; Walker et al, 2003; Zhu & Pan, 2004; Li and Kirouac, 2008). Interestingly, the CeL and BSTal are among a select group of regions that receive dense, likely glutamatergic, afferent input from the posterior paraventricular thalamus (Frassoni et al, 1997; Li & Kirouac, 2008), a critical regulator of neuroendocrine responses to repeated stress (Jaferi & Bhatnagar, 2006).
Individual activation of the CRFr or μ-OR can modulate excitatory transmission (Liu et al, 2004; Rainnie et al, 2004; Zhu & Pan, 2004; Zhu & Pan, 2005; Orozco-Cabal et al, 2008). It remains unclear, however, whether CRF and opiates modulate the postsynaptic excitability, or presynaptic neurotransmitter release onto single neurons in the CeA or BNST. We examined the dual electron microscopic immunolabeling of the μ-OR and CRFr in single sections through the CeL and BSTal of mouse brain to determine whether these receptors are present in the same or in separate, synaptically-linked neurons in each of these regions. This species was chosen due to the increasing use of mice with genetic manipulations that are of direct relevance to opiate addiction and stress (Kieffer & Gavériaux-Ruff, 2002; Keck et al, 2005). Our results provide the first ultrastructural evidence that the μ-OR and CRFr have subcellular distributions that would enable their participation in the respective opioid and CRF-induced modulation of the postsynaptic excitability of single dendrites and dendritic spines in both the CeL and BSTal. They also suggest that, in each of these regions, there are major differences between the μ-OR and CRFr in their respective involvement in presynaptic release of excitatory compared with inhibitory neurotransmitters. The results have important implications for stress-induced relapse in opiate addicts and for anxiety-related opiate withdrawal symptoms.
Experimental Procedures
Animals and tissue preparation
All experiments were performed in accordance with NIH regulations of animal care, and were approved by the Institutional Animal Care and Use Committee of Weill Medical College of Cornell University. Six adult male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were individually housed and left undisturbed in a quiet facility on a 12-hour light/dark cycle. These mice were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.), and their brain tissue fixed by vascular perfusion through the left ventricle of the heart with sequential delivery of (1) 5 ml of heparin-saline (1000 U/ml), (2) 30 ml of 3.75% acrolein and 2% paraformaldehyde in 0.1M phosphate buffer (PB; pH 7.4), and (3) 100 ml of 2% paraformaldehyde in PB. Brains were removed from the cranium, post-fixed with 2% PFA in 0.1M PB for 30min, and then transferred to PB. These brains were then cut coronally into 40 um sections on a Vibratome (Leica, Deerfield, IL). The coronal sections were collected through the rostrocaudal extent of the CeA and BNST based on the mouse brain atlas by Hof et al (2000).
Antisera
For immunolabeling of the CRFr, we used a rabbit polyclonal antiserum raised against the epitope corresponding to amino acids 230–444 mapping at the C-terminus of the CRFr-1 of human origin (CRF-R1/R2 (H-215): sc-5543 Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The specificity of this antiserum for the CRFr has previously been demonstrated by the diminished immunoreactivity in brain tissue processed after prior adsorption with the antigenic peptide (Treweek et al, 2009), and in brain sections of CRFr-1 (−/−) knockout compared with CRFr-1 (+/+) wildtype mice (Reyes et al, 2008; Treweek et al, 2009).
For immunolabeling of the μ-OR, we used a polyclonal guinea pig antiserum directed against a synthetic peptide corresponding to amino acids 384–398 of the C-terminus of the cloned rat μ-OR (AB1774; Chemicon, Temecula, CA). The specificity of this antiserum has previously been shown by the absence of immunoreactivity in adsorption controls (Rodriguez et al, 2001; Drake & Milner, 2002; Chemicon, 2005). In the present study, we examined immunolabeling for the guinea pig antiserum, as well as for a rabbit μ-OR antiserum (24216; Immunostar Inc, Hudson, WI), in brain sections from μ-OR (+/+) wildtype compared to μ-OR (−/−) knockout mice, each with deletions at distinct exons of the μ-OR gene. These knockouts had deletions at exon 1, exons 2/3 or exon 11, each of which were generously donated by Drs. John Pintar, Sabita Roy and Gavril Pasternak, respectively.
Immunocytochemical labeling
Brain sections were processed for dual immunoperoxidase and immunogold-silver labeling of the CRFr and μ-OR (Chan et al, 1990; Wang et al, 1999; Drake & Milner, 2002; Pickel et al, 2004; Hara & Pickel, 2005; Lane et al, 2008). For this dual-labeling, the vibratome sections were placed in 1% sodium borohydride in 0.1 M PB for 30 minutes to remove excess aldehydes, and then washed thoroughly in 0.1 M PB wash. To enhance the penetration of tissue by immunoreagents, the sections were soaked in a cryoprotectant solution (25% sucrose and 3.5% glycerol in 0.05M PB) for 15 minutes, and rapidly freeze-thawed by sequential immersion in liquid chlorodifluoromethane (Freon, Refron Inc., NY), liquid nitrogen, and 0.1 M PB at room temperature. The sections were then rinsed in 0.1 M Tris-buffered saline (TBS). To reduce non-specific binding of the antisera, the sections were incubated in 0.5% bovine serum albumin (BSA) in 0.1 M TBS, pH 7.6, for 30 minutes before placement in the primary antisera solution. The sections were incubated for 48 hours at 4 C in a solution containing both primary antisera (1:100 rabbit anti-CRFr for immunogold-silver labeling and 1:800 guinea pig anti-μ-OR for immunoperoxidase labeling.) For the reverse labeling scheme, the primary antisera solution consisted of 1:500 rabbit anti-CRFr for immunoperoxidase labeling and 1:500 guinea pig anti-μ-OR for immunogold-silver labeling. All antisera dilutions were prepared in 0.1% BSA in 0.1 M TBS. Incubations with primary as well as secondary antisera were with shaking on a rotator (OS-500; VWR, West Chester, PA).
For immunoperoxidase labeling, the sections were rinsed in 0.1 M TBS and then placed for 30 min in a 1:400 dilution of biotinylated anti-guinea pig (for μ-OR) or biotinylated anti-rabbit (for CRFr). These were then washed in 0.1 M TBS and incubated for 30 min in avidin-biotin peroxidase complex (Vectastain Elite Kit, Vector Labs, Burlingame, CA). This incubation was followed by a wash in 0.1 M TBS, and reaction for 6 min in 0.022% 3,3′-diaminobenzidine (DAB, Aldrich, Milwaukee, WI) and 0.003% hydrogen peroxide in 0.1 M TBS.
For dual detection of antigens, the sections were rinsed in 0.01M PBS (pH 7.4) after the DAB reaction, and blocked in 0.8% BSA and 0.1% gelatin in PBS for 10 minutes. The sections were subsequently incubated with secondary immunogold conjugates for 2 hours at room temperature. These secondary conjugates were a 1:50 dilution of anti-rabbit (for CRFr) or anti-guinea pig (for μ-OR) IgG conjugated with one nm colloidal gold (Amersham, Arlington Heights, IL). The sections were washed several times with 0.1 M TBS. To aid in the binding of gold particles conjugated to IgG, the sections were incubated in 2% glutaraldehyde for 10 minutes. Silver intensification was performed using the IntenS-EM kit (Amersham) with a 6 minute reaction in the silver solution at room temperature. The sections were post-fixed in 2% osmium tetroxide in 0.1 M PB for 60 minutes, washed in PB, and dehydrated by passing through an increasingly concentrated series of ethanol solutions, followed by 100% propylene oxide. The sections were incubated overnight in a 1:1 mixture of propylene oxide and Epon (Electron Microscopy Sciences, Fort Washington, PA), and then transferred to 100% Epon for 2–3 hours before flat-embedding between sheets of Aclar plastic (Electron Microscopy Sciences, Fort Washington, PA). These flat-embedded sections were viewed with a light microscope to select immunolabeled portions of the CeL at Bregma −1.6 mm, and of the dorsal BSTal at Bregma 0.2 mm for collection of ultra-thin sections according to the mouse brain atlas by Hof et al, 2000(Fig. 1). Ultra-thin sections of approximately 70nm thickness were cut from the superficial surface of the section using a diamond knife (Diatome, Fort Washington, PA) on an ultratome (NOVA LKV-Productor AB, Bromma, Sweden). The thin sections were collected on 400-mesh copper grids (Electron Microscopy Sciences), and counterstained with uranyl acetate and lead citrate (Reynolds, 1963). These grids were rinsed and allowed to air dry before viewing at 60 kV with a Philips CM10 transmission electron microscope (FEI, Hillsboro, OR). Those areas showing immunolabeling of μ-OR and CRFr were then magnified and captured as digital images using AMT Advantage HR/HR-B CCD Camera System (Advanced Microscopy Techniques, Danvers, MA).
Fig. 1.
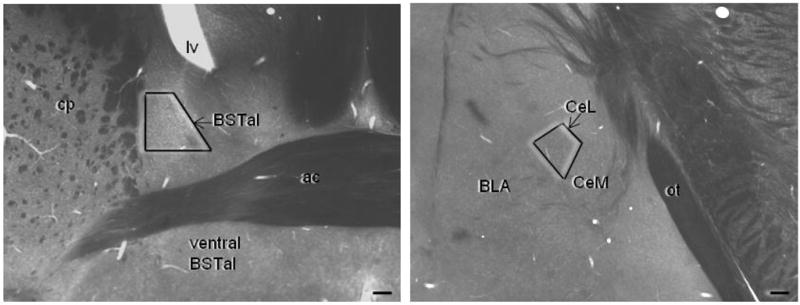
The BNST and CeA subregions sampled for electron microscopy are shown in plastic-embedded vibratome hemisections of a mouse brain at the level 0.2 mm anterior to Bregma for the BNST and 1.6mm posterior to Bregma for the CeA (Hof et al, 2000). The trapezoids indicate the locations from which the ultrathin sections were collected for electron microscopic image analysis. Scale bar = 100 μm. Lv= lateral ventricle, BSTal= anterolateral bed nucleus of the stria terminalis, ac= anterior commisure, cp= caudate putamen, BLA= basolateral amygdala, CeL= lateral subdivision of the central amygdala, CeM= medial subdivision of the central amygdala, ot= optic tract.
Electron Microscopic Analysis
Electron microscopic analysis was carried out in ultra-thin sections of six vibratome sections (three each of CeA and BNST from three mice). To confirm that the μ-OR and CRFr have similar labeling patterns regardless of the immuno-markers used, we repeated this analysis using the reverse labeling scheme in an additional six vibratome sections from three mice. Micrographs containing μ-OR and CRFr immunoreactivity at magnifications of 19000X to 25000X were analyzed in a total tissue area of 4889 μm2 in the CeL and 4881 μm2 in the BSTal. We exclusively examined tissue at the surface of vibratome sections at the Epon-tissue interface, where there was optimal penetration of primary and secondary antisera. All electron microscope images were imported into an IBM computer, where Adobe Photoshop (version 7.0.1, Adobe Systems Inc., Mountain View, CA) was used to sharpen and enhance contrast as needed. These images were then imported into PowerPoint (Microsoft Office, 2007), which was used to assemble figures and add lettering.
Profiles containing μ-OR and/or CRFr immunoreactivity were classified as neuronal (dendrites, dendritic spines, axons, axon terminals) or glial processes based on criteria described in Peters et al (1991). Immunoperoxidase labeling was regarded as positive when the electron-dense reaction product in individual profiles was greater than that seen in other morphologically similar profiles in the neuropil. Profiles were considered to be immunogold-labeled when they contained one or more gold particles. Profiles considered immunogold-labeled when containing only one gold particle included small (< 0.6 μm) structures, and were always located in a neuropil in which no presumed extraneous particles were seen in a comparable area of neuropil. This approach was validated by examining spurious gold particles overlying myelin, a structure not known to express receptors. Out of a total of 122 myelinated axons in 2,448 μm2 in the CeL and 2,428 μm2 in the BSTal examined, none displayed CRFr immunogold particles over myelin. In each subregion, we quantified the number of neuronal processes that were single- or dual-labeled as well as the number and types of synaptic associations between profiles that were separately labeled for each receptor. Asymmetric synapses were recognized by thick postsynaptic densities, while symmetric synapses were recognized by equally thin pre- and postsynaptic specializations. Appositions/non-synaptic contacts were recognized by closely spaced, parallel plasma membranes that lacked discernible synaptic densities.
Results
Regional and cellular location of μ-OR
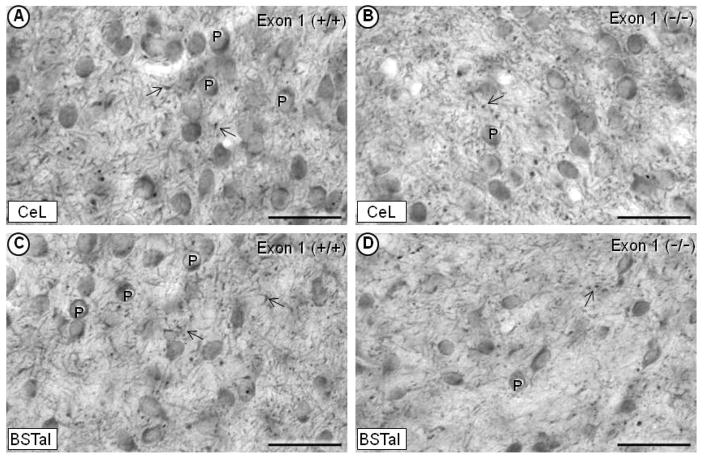
The light microscopic immunoperoxidase labeling of the μ-OR was prevalent in perikarya and processes of the CeL as well as medial and capsular subdivisions of the CeA, and in the anterolateral subdivision of the BNST. In each region, the μ-OR immunoreactivity was detected in fewer perikarya and processes in knockout mice with deletions of Exon 1 (Fig. 2B, 2C), Exons 2/3 or Exon 11 (not shown). The residual labeling suggests that the guinea pig μ-OR antiserum recognizes these and possibly other exons within the μ-OR (Abbadie et al, 2000). Like the guinea pig antiserum, the commonly used rabbit μ-OR antiserum also produced residual immunoperoxidase labeling of perikarya and processes in the CeA and BNST of Exon 1, 2/3 and 11 knockout mice. However, with the rabbit antiserum, immunolabeling in these knockout mice was equivalent to, if not more than, labeling seen in μ-OR wildtype mice, suggesting cross reactivity of the antiserum with proteins other than the mu-opioid receptor. The CRFr was less abundant, but otherwise similar to the μ-OR in being expressed in both perikarya and processes in the CeL and BSTal.
Fig. 2.
Light micrographs showing immunoperoxidase labeling of the μ-OR in the CeL (A, B) and BSTal (C, D). In an exon-1 wildtype (+/+) mouse, dense immunoperoxidase labeling of the μ-OR is seen in perikarya (P) as well as in thick, smooth dendritic-like structures and in punctate processes (arrow) resembling varicosities (A, C). In an exon-1 (−/−) mouse, the μ-OR labeling is seen in fewer perikarya and processes in each of the respective regions (B, D). BSTal= anterolateral BNST; CeL= lateral subdivision of central amygdala. Scale bars = 50 μm.
Electron microscopy showed the presence of the μ-OR and CRFr in the same as well as separate somatic, dendritic and axonal profiles in the CeL and BSTal. In contrast with the prominent neuronal localization, less than 4% of the μ-OR or CRFr was seen in glial profiles of either region.
Somatodendritic distributions of μ-OR and CRFr
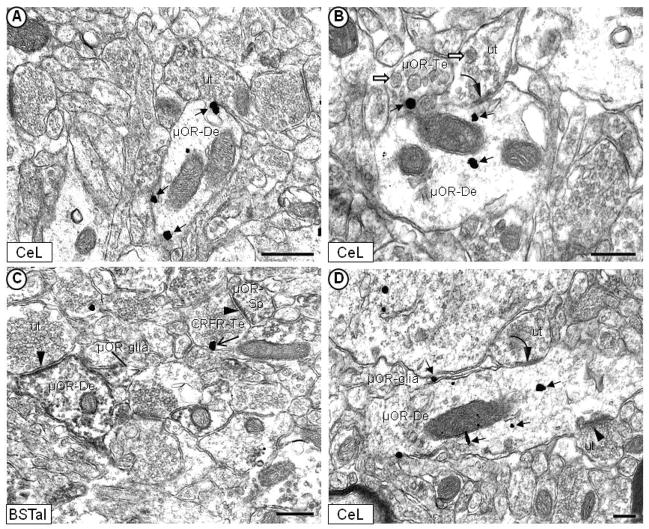
The pattern of immunolabeling for the μ-OR and/or CRFr in somatic and dendritic profiles was similar in the CeL and BSTal. Thus, the described subcellular distributions are equally applicable to both regions except when specifically referred to in the following descriptions. The μ-OR was prominent on non-synaptic portions of plasma membrane (Fig. 3A), and within cytoplasm (Fig. 3B), as shown by immunogold or peroxidase labeling (Fig. 3C). Immunoreactivity for the μ-OR (Fig. 3C, 3D) or CRFr (Fig. 4, 5A) was observed in dendrites and dendritic spines. Seventy-seven percent (270/350) of all μ-OR-labeled neuronal (dendritic + axonal) processes observed in the CeA were dendrites and dendritic spines. Similarly, in the BSTal, 79% (309/389) of the total neuronal processes containing the μ-OR were dendrites and spines.
Fig. 3.
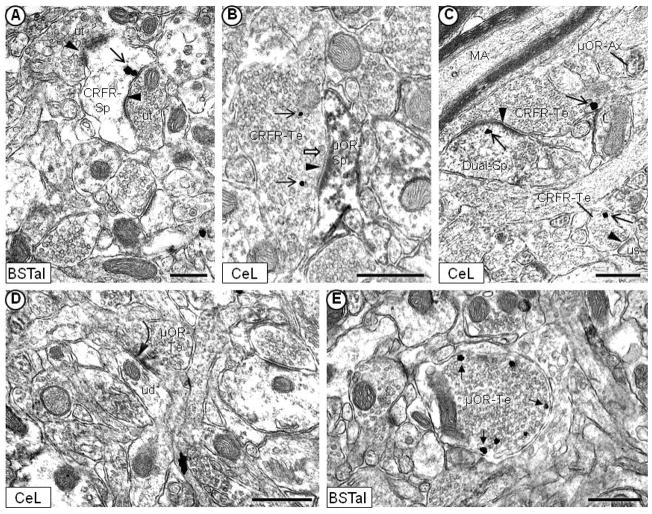
Electron micrographs showing immunogold or immunoperoxidase labeling of the μ-OR in neuronal and glial profiles without detectable CRFr in the mouse CeL (A, B, D) and BSTal (C). μ-OR immunogold particles (filled-head arrow) are distributed in dendrites (μOR-De) along the plasma membrane (A) or in the cytoplasm (B). In (B), μ-OR immunogold labeling is observed on the plasma membrane in an axon terminal (μOR-Te) that contains a few small, clear vesicles, and many large, dense core vesicles (white block arrow). In (C), μ-OR immunoperoxidase labeling is diffusely distributed along the plasma membrane, and in the cytoplasm of a dendrite and dendritic spine (μOR-Sp). Plasmalemmal CRFr immunogold labeling (open-head arrow) in (C) is seen in an axon terminal (CRFr-Te) that contains densely packed small, clear vesicles, and forms an asymmetric synapse with a dendritic spine that contains the μ-OR (μOR-Sp). Asymmetric, excitatory-type (arrowhead) and/or symmetric, inhibitory-type (curved arrow) synapses are formed by unlabeled terminals (ut) presynaptic to μ-OR-labeled dendrites and spines in (B), (C) and (D). The μ-OR immunoperoxidase (C) or immunogold (D) labeling is also seen in glial processes that appose μ-OR-labeled dendrites. Scale bars = 0.5 μm.
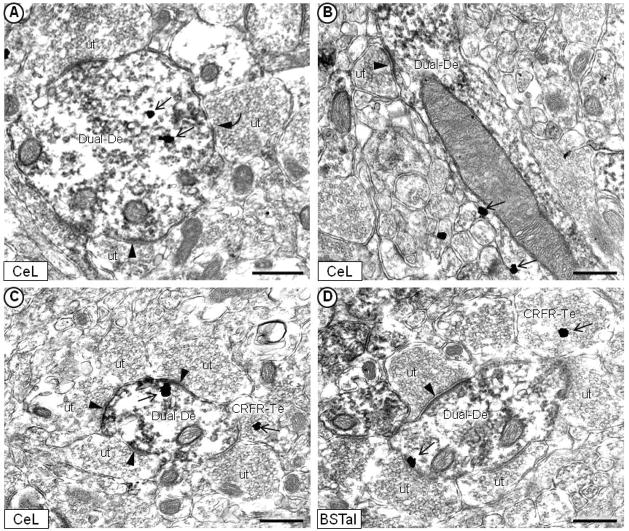
Fig. 4.
Electron micrographs showing immunogold labeling of the CRFr (open-head arrow) and immunoperoxidase labeling of the μ-OR within the same dendrites of the mouse CeL (A, B, C) and BSTal (D). CRFr immunogold particles are located in the cytoplasm (A) and on the plasma membrane (B, C, D) of dendrites that are immunoperoxidase labeled for the μ-OR (Dual-De). In each micrograph, the dual-labeled dendrites receive asymmetric, excitatory-type synapses (arrowhead) from unlabeled axon terminals (ut). Dual-labeled dendrites also appose terminals that contain CRFr immunogold labeling (CRFR-Te) in (C) and (D). In (A), the dual-labeled dendrite receives a symmetric, inhibitory-type synapse (curved arrow) from an unlabeled terminal. Scale bars = 0.5 μm.
Fig 5.
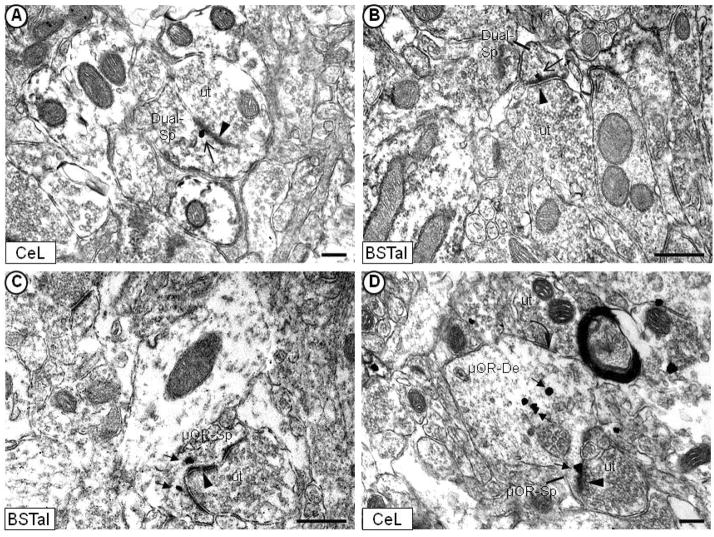
Immunogold localization of the CRFr (open-head arrow) or μ-OR (filled-head arrow) near asymmetric, excitatory-type synapses on dendritic spines of the mouse CeL (A, D) and BSTal (B, C). In (A) and (B), the CRFr immunogold labeling is seen in the region of the asymmetric postsynaptic membrane (arrowhead) on spines that are immunoperoxidase labeled for the μ-OR (Dual-Sp). μ-OR immunogold particles have a similar location near asymmetric synapses in dendritic spines (μOR-Sp) in (C) and (D). Spines containing the μ-OR (C, D) or both receptors (A, B) receive asymmetric synapses from unlabeled axon terminals (ut). In (D), a dendrite that is immunoperoxidase labeled for the μ-OR receives a symmetric, inhibitory-type synapse (curved arrow) from an unlabeled terminal. Scale bars = 0.5 μm.
Like μ-OR-containing profiles, the majority of observed CRFr-containing neuronal profiles were dendrites and dendritic spines [62% (88/141) for CeL; 63% (90/143) for BSTal]. Many dendrites (Fig. 4) and spines (Fig. 5A, 5B, 6C) containing immunogold labeling for the CRFr also displayed immunoperoxidase labeling for the μ-OR. Although the numbers of dendritic profiles containing the CRFr and/or μ-OR varied between individual mice (Table I), the proportion of single- or dual-labeled dendritic profiles were similar between mice as well as on average. Out of all CRFr-labeled dendritic profiles in the CeL, approximately 66% (58/88) contained immunoreactivity for the μ-OR (Table II). Similarly, in the BSTal, approximately 63% (56/90) of all dendritic profiles containing CRFr immunoreactivity also displayed μ-OR labeling. Conversely, when considering the total number of μ-OR-labeled dendritic profiles that contained the CRFr, 21% (58/270) were dual-labeled in the CeL, and 18% (56/309) were dual-labeled in the BSTal (Table II).
Fig. 6.
Electron micrographs showing the post- and/or presynaptic localization of the CRFr or μ-OR in axon terminals of the mouse CeL (B, C, D) and BSTal (A, E). Unlabeled or CRFr-labeled axon terminals (CRFR-Te) form asymmetric, excitatory-type synapses (arrowhead) with dendritic spines containing the CRFr (A), the μ-OR (B), or both receptors (C). In (B), CRFr immunogold particles (open-head arrow) have cytoplasmic locations in an axon terminal that contains a large, dense core vesicle (white block arrow) near the synapse. In (C), CRFr immunogold particles are seen on the plasma membrane of axon terminals (CRFR-Te), one of which forms a clearly defined asymmetric, excitatory-type synapse on a large spine head that is dual-labeled (Dual-Sp) with CRFr immunogold and μ-OR peroxidase. μ-OR immunoperoxidase labeling is seen in a small axon (μOR-Ax) in (C), and at a presynaptic location in an axon terminal that forms a symmetric synapse with an unlabeled dendrite (ud) in (D). μ-OR immunogold particles (filled-head arrow) are distributed on the plasma membrane of a non-synaptic axon terminal in (E). MA = myelinated axons. Us = unlabeled spine. Scale bars = 0.5 μm.
Table I. Summary of Samples.
Summary of the number of neuronal profiles sampled from each mouse. Sections of the CeL or BSTal from mice 1–3 were processed for immunogold labeling of the CRFr and immunoperoxidase labeling of the μ-OR. Sections from mice 4–6 were processed with the reverse markers (CRFr peroxidase and μ-OR immunogold).
| Mouse #1 | Mouse #2 | Mouse #3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| μOR | CRFr | CRFr+ μOR | μOR | CRFr | CRFr+ μOR | μOR | CRFr | CRFr+ μOR | |
| CeL | |||||||||
| # of Dendritic Profiles | 146 | 29 | 52 | 262 | 24 | 67 | 230 | 38 | 54 |
| # of Axonal Profiles | 61 | 71 | 4 | 118 | 30 | 0 | 55 | 53 | 2 |
| BSTal | |||||||||
| # of Dendritic Profiles | 308 | 30 | 64 | 263 | 41 | 53 | 188 | 29 | 52 |
| # of Axonal Profiles | 76 | 49 | 4 | 84 | 57 | 7 | 63 | 40 | 4 |
| Reverse Markers | Mouse #4 | Mouse #5 | Mouse #6 | ||||||
| μOR | CRFr | CRFr+ μOR | μOR | CRFr | CRFr+ μOR | μOR | CRFr | CRFr+ μOR | |
|
| |||||||||
| CeL | |||||||||
| # of Dendritic Profiles | 122 | 27 | 42 | 106 | 29 | 45 | 172 | 32 | 45 |
| # of Axonal Profiles | 98 | 49 | 0 | 64 | 31 | 1 | 64 | 48 | 1 |
| BSTal | |||||||||
| # of Dendritic Profiles | 79 | 46 | 45 | 78 | 27 | 35 | 70 | 40 | 43 |
| # of Axonal Profiles | 19 | 41 | 3 | 27 | 26 | 1 | 23 | 28 | 0 |
Table II. Distribution of CRFr and μ OR within neuronal profiles.
Summary of the mean percentages of axonal (axons and axon terminals) and dendritic (dendrites and spines) profiles that were immunogold labeled for the CRFr, immunoperoxidase labeled for the μ-OR, or labeled for both receptors in sections of the mouse CeL or BSTal.
| Dendritic profiles (single label) | Dendritic profiles (dual label) | Axonal profiles (single label) | Axonal profiles (dual label) | |
|---|---|---|---|---|
| CeL | ||||
| CRF receptor | 34% (30/88) | 66% (58/88) | 96% (51/53) | 4% (2/53) |
| Mu-opioid receptor | 79% (213/270) | 21% (58/270) | 97% (78/80) | 3% (2/80)) |
| BSTal | ||||
| CRF receptor | 37% (33/90) | 63% (56/90) | 91% (49/54) | 9% (5/54) |
| Mu-opioid receptor | 82% (253/309) | 18% (56/309) | 94% (74/79) | 6% (5/79) |
These patterns of labeling in the CeL and BSTal were confirmed by repeating the immunolabeling procedure with the reverse markers (Fig. 5C, 5D). In both the CeL and BSTal, more than half of all dendritic profiles that were immunoperoxidase-labeled for the CRFr also contained μ-OR immunogold particles [60% (44/73) for CeL; 52% (41/79) for BSTal]. In contrast, a minority of all μ-OR-labeled dendritic profiles contained CRFr immunoreactivity [25% (44/177) for CeL; 35% (41/117)] for BSTal].
Immunogold particles identifying the μ-OR or CRFr were often observed within or near the postsynaptic membrane specializations at asymmetric synapses on dendritic spines in both the CeL and BSTal (Fig. 5). Out of 59 unlabeled terminals in contact with dendrites containing one or both receptors in the CeL, approximately 31% (18/59) formed asymmetric synapses with dual-labeled dendrites, and 10% (6/59) formed symmetric, inhibitory-type synapses with dual-labeled dendrites (Table III). Forty-seven percent of these terminals (28/59) formed asymmetric synapses, and 7% (4/59) formed symmetric synapses with μ-OR-labeled dendritic profiles. Five percent (3/59) formed asymmetric synapses with CRFr-labeled dendritic profiles. The BSTal was remarkably similar to the CeL with respect to types of synaptic associations of dendrites containing one or both receptors. For example, in the BSTal, approximately 30% (19/65) of the unlabeled terminals formed asymmetric synapses, and 8% (5/65) formed symmetric synapses with dual-labeled dendrites (Table III). This suggests that the CRFr and μ-OR are mainly segregated to those dendritic segments that are receptive to excitatory inputs as indicated by the asymmetry of the postsynaptic membranes. It should be noted, however, that in some cases as in Figure 4A, the symmetry/asymmetry of the synapses can be difficult to judge due to the accumulation of peroxidase product at postsynaptic densities.
Table III. Synaptic input to CRFr- and/or μ OR-labeled dendritic profiles.
Summary of the types of synapses (asymmetric, excitatory-type or symmetric, inhibitory-type) that CRFr- and/or μ-OR-containing dendritic profiles received from unlabeled axon terminals. These dendritic profiles were immunogold-labeled for the CRFr, immunoperoxidase-labeled for the μ-OR, or labeled for both receptors in sections of the mouse CeL or BSTal. The number of axon terminals observed in the CeL (n=59) was averaged across three mice where n=43 for mouse 1, n=58 for mouse 2, and n=77 for mouse 3. The number of axon terminals observed in the BSTal (n=65) was also averaged across three mice where n=79 for mouse 1, n=72 for mouse 2, and n=44 for mouse 3.
| CRFr- labeled dendritic profiles | μOR-labeled dendritic profiles | Dual-labeled dendritic profiles | |
|---|---|---|---|
| CeL | |||
| Asymmetric synapse | 5% (3/59) | 47% (28/59) | 31% (18/59) |
| Symmetric synapse | 0% (0/59) | 7% (4/59) | 10% (6/59) |
| BSTal | |||
| Asymmetric synapse | 11% (7/65) | 42% (27/65) | 29% (19/65) |
| Symmetric synapse | 2% (1/65) | 8% (5/65) | 8% (5/65) |
Axonal distributions of μ-OR and CRFr
The pattern of immunolabeling for the μ-OR or CRFr in axonal profiles was similar in the CeL and BSTal. These included small axons (Fig. 6C) and axon terminals (6B-6E). The axonal immunogold particles identifying the μ-OR or CRFr were in contact with the plasma membrane (Fig. 6C, 6E), or were distributed over vesicular structures in the cytoplasm of axon terminals (Fig. 6B). μ-OR or CRFr-immunoreactive axon terminals contained mainly small, clear synaptic vesicles that typically store amino acid neurotransmitters (i.e., glutamate, GABA) (Peters & Palay, 1996; Torrealba & Carrasco, 2004). Some of these terminals, however, contained a mixture of small clear and large, dense core vesicles (Fig. 3B, 6B), the latter of which typically store monamines or peptides (i.e., opioids, CRF) (Nirenberg et al, 1995; Peters & Palay, 1996; Torrealba & Carrasco, 2004).
The axonal profiles containing each of these receptors were less numerous than the labeled dendritic profiles described above. In the CeL, small axons or axon terminals comprised approximately 23% (80/350) of all identified μ-OR-labeled neuronal profiles, and 20% (79/389) of those in the BSTal. Out of all CRFr-labeled neuronal processes, 38% (53/141) were axonal profiles in the CeL, and similarly, 38% (54/143) were axonal profiles in the BSTal.
Dual-labeling of the CRFr and μ-OR was rarely observed in axon terminals in either the CeL or BSTal. The number of axonal profiles labeled for the CRFr and/or μ-OR varied between mice (Table I), but the proportion of single- and dual-labeled axonal profiles was similar between mice, and on average. Approximately 4% (2/53) of all CRFr-labeled axonal profiles in the CeL contained immunoreactivity for the μ-OR (Table II). In the BSTal, 9% (5/54) of the axonal profiles that contained the CRFr also showed μ-OR-labeling (Table II). Conversely, 3% (2/80) of all μ-OR-labeled axonal profiles also contained the CRFr in the CeL, and 6% (5/79) contained both receptors in the BSTal. This rarity of dual-labeling of the CRFr and μ-OR in axonal profiles was confirmed by immunolabeling with the reverse markers. The largely separate distribution of the CRFr and μ-OR in axon terminals is consistent with their respective preferential localization in axon terminals that form asymmetric compared with symmetric synapses.
In both the CeL and BSTal, axon terminals that were CRF-immunoreactive or unlabeled often formed asymmetric synapses with dendritic profiles containing exclusively the μ-OR (Fig. 3C, 5D, 6B) or CRFr (Fig. 6A) as well as those containing both receptors (Fig. 4C, 5A, 6C). The asymmetric synapses are typical of axon terminals containing glutamate (Farb et al, 1992). In contrast, the μ-OR-labeled terminals in each region commonly showed non-synaptic appositions or formed symmetric synapses with unlabeled dendrites (Fig. 6D). Numerous non-synaptic appositions were observed between μ-OR and/or CRFr-immunoreactive axon terminals, and dendrites containing both receptors in the CeL and BSTal. In the CeL, 106 non-synaptic appositional contacts were observed between dual-labeled dendrites and axon terminals. Approximately 67% (71/106) of these terminals were unlabeled in the CeL. In the BSTal, approximately 61% (47/77) of the appositional contacts were between dual-labeled dendrites and unlabeled terminals.
Discussion
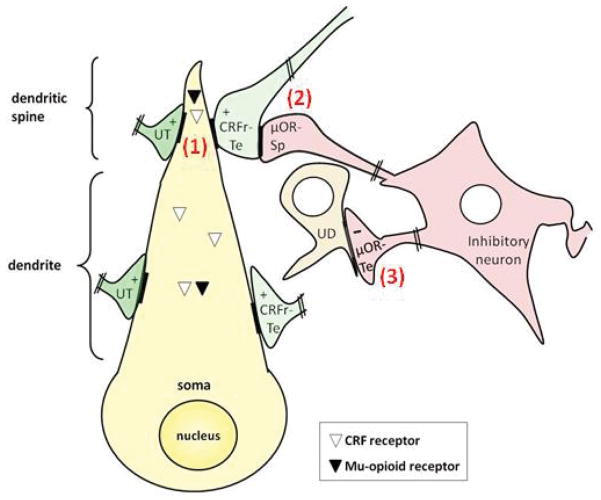
The present study provides the first ultrastructural evidence that in the mouse CeL and BSTal, the μ-OR and CRFr are co-expressed in dendritic profiles receiving asymmetric, excitatory-type synapses (Fig. 7.1). These results suggest that activation of the μ-OR or CRFr may independently or opposingly affect the postsynaptic excitability of neurons in two brain regions that are critical mediators of the stress response. In addition, the observed presence of the CRFr within some of the excitatory-type terminals that are presynaptic to μ-OR and/or CRFr-containing dendrites in each region suggests that the CRFr can also affect the output of these neurons through presynaptic mechanisms independent of the μ-OR (Fig. 7.2). Instead, the μ-OR is identified in axon terminals forming inhibitory-type synapses with mainly unlabeled dendrites (Fig. 7.3). Collectively, these results have important implications for the well-documented functional associations between stress and opiate abuse.
Fig. 7.
Schematic diagram showing the distribution of the CRFr and μ-OR in the same postsynaptic, and separate presynaptic, neurons of the CeL and BSTal. Both the μ-OR and CRFr are present within or near excitatory-type synapses (+) on dendritic spines (1), suggesting that the activation of the μ-OR or CRFr can independently or opposingly affect the postsynaptic excitability of single neurons in each of these regions. These excitatory-type synapses are formed by axon terminals that are unlabeled or that contain the CRFr (CRFr-Te). Axon terminals that contain the CRFr also form excitatory-type synapses with μ-OR-expressing dendritic spines (2), suggesting that the CRFr can affect the output of opiate-sensitive spines in the CeL and BSTal through presynaptic mechanisms. The μOR-containing axon terminals (μOR-Te) are illustrated as arising from intrinsic or extrinsic inhibitory neurons whose local dendrites express μ-OR, but not CRFr, and as forming inhibitory-type synapses (−) with dendrites not expressing either receptor (3). In contrast, the CRFr is shown in axon terminals that form excitatory-type synapses, suggesting distinct roles for the μ-OR and CRFr in the release of inhibitory and excitatory neurotransmitters, respectively.
Methodological considerations
The CRFr antiserum has previously (Reyes et al, 2008; Treweek et al, 2009), and the guinea pig μ-OR antiserum has presently, been shown to recognize proteins that are absent or attenuated in the respective CRFr-1 and μ-OR knockout mice. Our present results indicate that the μ-OR antiserum recognizes multiple exons, since no one gene knockout completely eliminated the immunolabeling. However, we cannot totally exclude the possibility that at least some of the residual immunolabeling observed in knockout mice reflects cross-reaction of the antiserum with structurally similar proteins. The potential cross reaction, or recognition of multiple exons at the c-terminus of the cloned μ-OR with the guinea pig antiserum makes it necessary to consider the labeling as μ-OR-like immunoreactivity, a caveat also applicable to the CRFr antiserum which may partially recognize the CRFr-2 (Treweek et al, 2009). Both antisera do, however, meet other important criteria of specificity including absence of immunoreactivity when pre-adsorbed with the antigenic peptide (Rodriguez et al, 2001; Drake & Milner, 2002; Chemicon, 2005; Treweek et al, 2009). The pattern of labeling obtained with the guinea pig μ-OR antiserum is also consistent with μ-OR autoradiographical localization (Mansour et al, 1987), and with the distribution of other μ-OR antisera that have been used extensively in rodent brain (Arvidsson et al, 1995; Mansour et al, 1995; Wang et al, 1996; Wang et al, 1999).
In most cases, only a few (1–4) immunogold particles were seen in neuronal profiles that were considered to be labeled for either receptor. This is a valid approach only in samples of brain tissue in which structures such as myelin show few, if any, spurious gold particles, and when there is a correspondence between the subcellular location seen with immunogold and immunoperoxidase labeling methods (Wang & Pickel, 2001; Wang & Pickel, 2002; Hara & Pickel, 2005). The moderate to low amount of CRFr labeling in the CeA is consistent with known low levels of receptor mRNA in this brain region (Van Pett et al, 2000), which contains an abundance of CRF immunoreactive neurons (Swanson et al, 1983; Uryu et al, 1992; Gray, 1993; Treweek et al, 2009). Low expression levels of the CRFr and/or μ-OR may have contributed to an underestimation of co-localization of these receptors in single neurons, as well as the synaptic associations occurring between neurons that differentially express these receptors.
Co-localization of μ-OR and CRFr in dendrites receiving excitatory-type synapses
In both CeL and BSTal, a similar percentage of CRFr-expressing dendrites contain the μ-OR. In addition, μ-OR and/or CRFr-expressing dendrites in each brain region more often receive excitatory-type than inhibitory-type synapses. These regional similarities in receptor distribution and synaptic associations in the CeL and BSTal are in line with previous findings of a correspondence between the CeA and BNST in cytologic features (Cassell & Gray, 1989; Moga et al, 1989), anatomical connectivity (Gray and Magnunson, 1987; Gray & Magnunson, 1992; de Olmos & Heimer, 1999; McDonald et al, 1999; Cassell et al, 1999; Shammah-Lagnado et al, 2000), and neurochemical attributes (Shimada et al, 1989; Sun & Cassell, 1993; Day et al, 1999), as well as functional roles in emotional and autonomic processes (Saper, 1995; Koob, 2003).
Our present findings provide evidence that the CRFr is prevalent within or near excitatory synapses in dendritic compartments that also contain the μ-OR in the CeL and BSTal. The presence of both the μ-OR and CRFr at these synapses suggests that the activation of one or both receptors can affect the postsynaptic excitability of neurons in each of these regions. The activation of the μ-OR and CRFr may, however, have opposing effects on intracellular signaling. This is consistent with the prior suggestion that endogenous opioids may counterbalance the excitatory effects of CRF on stress systems in order to limit excessive activation of the stress response (Curtis et al, 2001; Valentino & Van Bockstaele, 2001). The functional opposition is supported by distinct intracellular signaling mechanisms. Opioids, specifically enkephalin, act on the Gi/Go-coupled μ-OR to inhibit the cAMP pathway, while CRF preferentially acts on the Gs-coupled CRFr to stimulate the cAMP pathway (Ueda et al, 1988; Grammatopoulos et al, 2001).
Presynaptic distributions of μ-OR and/or CRFr
Co-localization of the μ-OR and CRFr was rarely seen in CeL or BSTal axons or terminals, although numerous axonal profiles showing single labeling were seen in each region. The μ-OR immunoreactivity was evident on plasma membranes of axon terminals that contained dense core vesicles, suggesting a role for the μ-OR in regulation of the release of monoamines or neuropeptides that are frequently stored in these vesicles. The endogenous opioids and CRF are among the many neuropeptides in axon terminals of the CeL and BSTal (Swanson et al, 1983; Uryu et al, 1992; Poulin et al, 2006; Treweek et al, 2009). Presynaptic μ-OR autoreceptor in axon terminals that contain enkephalin-type opioids has been described in other brain regions such as the ventral tegmental area, nucleus accumbens and caudate-putamen as well as the spinal cord dorsal horn (Cheng et al, 1996; Svingos et al, 1996; Wang et al, 1996; Garzon & Pickel, 2002). In addition, however, we also observed the μ-OR in axon terminals that contained small, clear vesicles that are typical of those that release amino acid neurotransmitter (Sesack & Pickel, 1995; Peters & Palay, 1996; Torrealba & Carrasco, 2004), and often forming symmetric synapses. Together, these observations suggest that enkephalin and/or GABA or other inhibitory amino acids are the neurotransmitters in many of the neurons that express the μ-OR in their axon terminals. The CRFr-containing axon terminals formed excitatory-type synapses, a type that rarely contained the μ-OR.
Implications
The level of co-expression of the CRFr and μ-OR in the CeL and BSTal is above that for most other receptors within any brain region thus far examined (Huang & Pickel, 2003; Delle Donne et al, 2004; Pickel et al, 2004; Pickel et al, 2006). The ultrastructural findings of the present study suggest that the CRFr in these regions acts synergistically with glutamate largely by amplifying postsynaptic responses (Liu et al, 2004; Fu & Neugebauer, 2008), but also by enhancing glutamate release from axon terminals presynaptic to neurons that co-express the μ-OR and CRFr (Liu et al, 2004; Orozco-Cabal et al, 2008). The prevalence of the CRFr and μ-OR in the same postsynaptic dendrites of the CeL and BSTal is consistent with the respective CRFr or μ-OR facilitation and inhibition of excitatory transmission within the amygdala circuitry. The activation of the CRFr can facilitate or depress excitatory glutamatergic transmission depending on the brain region involved, and on prior stress or drug treatment (Liu et al, 2004; Rainnie et al, 2004; Orozco-Cabal et al, 2008). In the CeA, μ-OR agonists inhibit postsynaptic and presynaptic glutamatergic transmission (Zhu & Pan, 2004; Zhu & Pan, 2005). However, we rarely saw the μ-OR in terminals forming excitatory-type synapses, thus suggesting that the presynaptic inhibition may be mediated through indirect presynaptic mechanisms involving the excitatory-type terminals that express the CRFr. Alternatively, excitatory modulators (e.g. CRF) may be present in at least some of the unlabeled neurons that receive input from μ-OR-expressing terminals.
Most of the neurons in the CeA and BNST are GABAergic (Sun & Cassell, 1993), implying that many excitatory-type terminals observed in these regions are extrinsic in origin. Extrinsic excitatory input to CRFr- and μ-OR-expressing dendrites could be provided by afferents from cortical areas (Yasui et al, 1991), brainstem sensory regions such as the parabrachial nucleus (Lopez de Armentia & Sah, 2007) as well as from other amygdaloid nuclei such as the basolateral amygdala and lateral amygdala (Schmued, 1994; Zhu & Pan, 2004). Given the involvement of excitatory transmission in opiate dependence (Gonzalez et al, 1997; Bossert et al, 2004; LaLumiere & Kalivas, 2008), excitatory inputs to μ-OR and CRFr-expressing dendrites within the CeL and BSTal may play an important role in stress-induced relapse in opiate addicts (Koob & Kreek, 2007).
Chronic activation of the μ-OR results in up-regulation or sensitization of the cAMP pathway as a compensatory adaptation to opiate-induced inhibition of cAMP (Duman et al, 1988; Terwilliger et al, 1991; Nestler, 1996). Complementary functional data show that chronic, but not acute, morphine treatment sensitizes electrophysiological responses of neurons to CRF in the locus coeruleus (Xu et al, 2004). Similarly, in the CeL and BSTal, where we found extensive postsynaptic co-localization of the μ-OR and CRFr, persistent activation of the Gi-coupled μ-OR may lead to changes in the activity of the Gs-coupled CRFr. This mechanism would be equally important for enabling chronic stress to affect μOR-dependent opioid systems as well as contributing to anxiety-like states during opiate withdrawal.
Acknowledgments
We thank Drs. Gavril Pasternak, John Pintar and Sabita Roy for generous donation of brain tissue from mu-opioid receptor knockout mice.
Supporting Grants: This work was supported by NIDA (DA00727416) to AJ, DA04600 and NIMH (MH40342) to VMP, and DA016735 to Mary Jeane Kreek (The Laboratory of the Biology of Addictive Diseases, The Rockefeller University, New York, NY.)
Abbreviations
- BNST
bed nucleus of the stria terminalis
- BSA
bovine serum albumin
- BSTal
anterolateral bed nucleus of the stria terminalis
- CeA
central amygdala
- CeL
lateral central amygdala
- CRF
corticotropin-releasing-factor
- CRFr
corticotropin-releasing-factor receptor
- CRFr-1
corticotropin-releasing-factor receptor 1
- CRFr-2
corticotropin-releasing-factor receptor 2
- i.p.
intraperitoneal
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- TBS
Tris-buffered saline
- μ-OR
mu-opioid receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbadie C, Pan YX, Pasternak GW. Differential distribution in rat brain of mu opioid receptor carboxy terminal splice variants MOR-1C-like and MOR-1-like immunoreactivity: evidence for region-specific processing. J Comp Neurol. 2000;419(2):244–56. doi: 10.1002/(sici)1096-9861(20000403)419:2<244::aid-cne8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Arluison M, Brochier G, Vankova M, Leviel V, Villalobos J, Tramu G. Demonstration of peptidergic afferents to the bed nucleus of the stria terminalis using local injections of colchicine. A combined immunohistochemical and retrograde tracing study. Brain Res Bull. 1994;34(4):319–37. doi: 10.1016/0361-9230(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995;15(5 Pt 1):3328–41. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24(47):10726–30. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham JC, Cooper TA. Differences in hypothalamo-pituitary-adrenocortical activity in the rat after acute and prolonged treatment with morphine. Neuroendocrinology. 1984;38(5):411–7. doi: 10.1159/000123927. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Gray TS. Morphology of peptide-immunoreactive neurons in the rat central nucleus of the amygdala. J Comp Neurol. 1989;281(2):320–33. doi: 10.1002/cne.902810212. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci. 1999;877:217–41. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- Chan J, Aoki C, Pickel VM. Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J Neurosci Methods. 1990;33(2–3):113–27. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemicon. Guinea-pig anti-μ opioid receptor-1 polyclonal antibody AB1774 Product Information. 2005 Downloaded from http://www.chemicon.com/webfiles/PDF/AB1774.pdf on 11/26/08.
- Chen Y, Brunson KL, Muller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF(1))-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. J Comp Neurol. 2000;420(3):305–23. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PY, Moriwaki A, Wang JB, Uhl GR, Pickel VM. Ultrastructural localization of mu-opioid receptors in the superficial layers of the rat cervical spinal cord: extrasynaptic localization and proximity to Leu5-enkephalin. Brain Res. 1996;731:141–154. doi: 10.1016/0006-8993(96)00492-1. [DOI] [PubMed] [Google Scholar]
- Chieng BC, Christie MJ, Osbourne PB. Characterization of neurons in the rat central nucleus of the amygdala: cellular physiology, morphology, and opioid sensitivity. J Comp Neurol. 2006;497(6):910–27. doi: 10.1002/cne.21025. [DOI] [PubMed] [Google Scholar]
- Contarino, Papaleo The corticotropin-releasing factor receptor-1 pathway mediates the negative affective states of opiate withdrawal. Proc Natl Acad Sci U S A. 2005;102(51):18649–54. doi: 10.1073/pnas.0506999102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C, Kieffer BL, Befort K. Mu opioid receptor: a gateway to drug addiction. Curr Opin Neurobiol. 2004;14(3):370–8. doi: 10.1016/j.conb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bello NT, Valentino RJ. Evidence for functional release of endogenous opioids in the locus ceruleus during stress termination. J Neurosci. 2001;21(13):RC152. doi: 10.1523/JNEUROSCI.21-13-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Curran EJ, Watson SJ, Jr, Akil H. Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1beta. J Comp Neurol. 1999;413(1):113–28. [PubMed] [Google Scholar]
- degli Uberti EC, Petraglia F, Bondanelli M, Guo AL, Valentini A, Salvadori S, Criscuolo M, Nappi RE, Genazzani AR. Involvement of mu-opioid receptors in the modulation of pituitary-adrenal axis in normal and stressed rats. J Endocrinol Invest. 1995;18(1):1–7. doi: 10.1007/BF03349688. [DOI] [PubMed] [Google Scholar]
- Delle Donne KT, Chan J, Boudin H, Pélaprat D, Rostène W, Pickel VM. Electron microscopic dual labeling of high-affinity neurotensin and dopamine D2 receptors in the rat nucleus accumbens shell. Synapse. 2004;52(3):176–87. doi: 10.1002/syn.20018. [DOI] [PubMed] [Google Scholar]
- De Olmos JS, Heimer L. The concepts of the ventral striatopallidal system and extended amygdala. Ann N Y Acad Sci. 1999;877:1–32. doi: 10.1111/j.1749-6632.1999.tb09258.x. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Kaneko T, Nomura S, Mizuno N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol. 1996;367(3):375–402. doi: 10.1002/(SICI)1096-9861(19960408)367:3<375::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001;436(4):430–55. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol. 2004;468(2):277–98. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA. Mu opioid receptors are in discrete hippocampal interneuron subpopulations. Hippocampus. 2002;12(2):119–36. doi: 10.1002/hipo.1107. [DOI] [PubMed] [Google Scholar]
- Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25(4):729–41. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- Duman RS, Tallman JF, Nestler EJ. Acute and chronic opiate-regulation of adenylate cyclase in brain: specific effects in locus coeruleus. J Pharmacol Exp Ther. 1988;246(3):1033–9. [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158(4):360–5. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Farb C, Aoki C, Milner T, Kaneko T, LeDoux J. Glutamate immunoreactive terminals in the lateral amygdaloid nucleus: a possible substrate for emotional memory. Brain Res. 1992;593(2):145–58. doi: 10.1016/0006-8993(92)91303-v. [DOI] [PubMed] [Google Scholar]
- Frassoni C, Spreafico R, Bentivoglio M. Glutamate, aspartate and co-localization with calbindin in the medial thalamus. An immunohistochemical study in the rat. Exp Brain Res. 1997;115(1):95–104. doi: 10.1007/pl00005689. [DOI] [PubMed] [Google Scholar]
- Fu Y, Neugebauer V. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J Neurosci. 2008;28(15):3861–76. doi: 10.1523/JNEUROSCI.0227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22(12):5173–87. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González P, Cabello P, Germany A, Norris B, Contreras E. Decrease of tolerance to, and physical dependence on morphine by, glutamate receptor antagonists. Eur J Pharmacol. 1997;332(3):257–62. doi: 10.1016/s0014-2999(97)01099-6. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK, Randeva HS, Levine MA, Kanellopoulou KA, Hillhouse EW. Rat cerebral cortex corticotropin-releasing hormone receptors: evidence for receptor coupling to multiple G-proteins. J Neurochem. 2001;76(2):509–19. doi: 10.1046/j.1471-4159.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnunson DJ. Neuropeptide neuronal efferents from the bed nucleus of the stria terminalis and central amygdaloid nucleus to the dorsal vagal complex in the rat. J Comp Neurol. 1987;262(3):365–74. doi: 10.1002/cne.902620304. [DOI] [PubMed] [Google Scholar]
- Gray TS, Magnunson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13(3):451–60. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- Gray TS. Amygdaloid CRF pathways. Role in autonomic, neuroendocrine, and behavioral responses to stress. Ann N Y Acad Sci. 1993;697:53–60. doi: 10.1111/j.1749-6632.1993.tb49922.x. [DOI] [PubMed] [Google Scholar]
- Han ZS, Ju G. Effects of electrical stimulation of the central nucleus of the amygdala and the lateral hypothalamic area on the oval nucleus of the bed nucleus of the stria terminalis and its adjacent areas in the rat. Brain Res. 1990;536:56–62. doi: 10.1016/0006-8993(90)90008-y. [DOI] [PubMed] [Google Scholar]
- Hara Y, Pickel VM. Overlapping intracellular and differential synaptic distributions of dopamine D1 and glutamate N-methyl-D-aspartate receptors in rat nucleus accumbens. J Comp Neurol. 2005;492(4):442–55. doi: 10.1002/cne.20740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Activation in extended amygdala corresponds to altered hedonic processing during protracted morphine withdrawal. Behav Brain Res. 2007;176(2):251–8. doi: 10.1016/j.bbr.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Schulteis G, Koob GF, Stinus L. Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behav Pharmacol. 1995;6(1):74–80. [PubMed] [Google Scholar]
- Hof PR, Young WG, Bloom FE, Belichenko PV, Celio MR. Comparative Cytoarchitectonic Atlas of the C57BL/6 and 129/Sv Mouse Brains. Amsterdam: Elsevier Science; 2000. [Google Scholar]
- Huang J, Pickel VM. Ultrastructural localization of serotonin 2A and N-methyl-D-aspartate receptors in somata and dendrites of single neurons within rat dorsal motor nucleus of the vagus. J Comp Neurol. 2003;455(2):270–80. doi: 10.1002/cne.10497. [DOI] [PubMed] [Google Scholar]
- Iredale PA, Alvaro JD, Lee Y, Terwilliger R, Chen YL, Duman RS. Role of corticotropin-releasing factor receptor-1 in opiate withdrawal. J Neurochem. 2000;74(1):199–208. doi: 10.1046/j.1471-4159.2000.0740199.x. [DOI] [PubMed] [Google Scholar]
- Jaferi A, Bhatnagar S. Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology. 2006;147(10):4917–30. doi: 10.1210/en.2005-1393. [DOI] [PubMed] [Google Scholar]
- Janssens CJ, Helmond FA, Loyens LW, Schouten WG, Wiegant VM. Chronic stress increases the opioid-mediated inhibition of the pituitary-adrenocortical response to acute stress in pigs. Endocrinology. 1995;136(4):1468–73. doi: 10.1210/endo.136.4.7895656. [DOI] [PubMed] [Google Scholar]
- Keck ME, Ohl F, Holsboer F, Müller MB. Listening to mutant mice: a spotlight on the role of CRF/CRF receptor systems in affective disorders. Neurosci Biobehav Rev. 2005;29(4–5):867–89. doi: 10.1016/j.neubiorev.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Gavériaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66(5):285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13(6):442–52. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164(8):1149–59. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28(12):3170–7. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane DA, Lessard AA, Chan J, Colago EE, Zhou Y, Schlussman SD, Kreek MJ, Pickel VM. Region-specific changes in the subcellular distribution of AMPA receptor GluR1 subunit in the rat ventral tegmental area after acute or chronic morphine administration. J Neurosci. 2008;28(39):9670–81. doi: 10.1523/JNEUROSCI.2151-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol. 2008;506(2):263–87. doi: 10.1002/cne.21502. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Zubieta JK, Fig LM, Phan KL, Koeppe RA, Taylor SF. mu-Opioid receptors and limbic responses to aversive emotional stimuli. Proc Natl Acad Sci U S A. 2002;99(10):7084–9. doi: 10.1073/pnas.102174799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu B, Neugebauer V, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher JP. Corticotropin-releasing factor and Urocortin I modulate excitatory glutamatergic synaptic transmission. J Neurosci. 2004;24(16):4020–9. doi: 10.1523/JNEUROSCI.5531-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Armentia M, Sah P. Bidirectional synaptic plasticity at nociceptive afferents in the rat central amygdala. J Physiol. 2007;58(Pt 3):961–70. E. doi: 10.1113/jphysiol.2006.121822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Liu D, Ceng X, Ma L. Differential roles of corticotropin-releasing factor receptor subtypes 1 and 2 in opiate withdrawal and in relapse to opiate dependence. Eur J Neurosci. 2000;12(12):4398–404. [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7(8):2445–64. [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350(3):412–38. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Akil H, Watson SJ. Immunohistochemical localization of the cloned mu opioid receptor in the rat CNS. J Chem Neuroanat. 1995;8(4):283–305. doi: 10.1016/0891-0618(95)00055-c. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Densmore VS, Osbourne PB. Coexpression of prodynorphin and corticotrophin-releasing hormone in the rat central amygdala: Evidence of two distinct endogenous opioid systems in the lateral division. J Comp Neurol. 2007;504(6):702–15. doi: 10.1002/cne.21464. [DOI] [PubMed] [Google Scholar]
- Marson L, Kiritsy-Roy JA, Van Loon GR. mu-Opioid peptide modulation of cardiovascular and sympathoadrenal responses to stress. Am J Physiol. 1989;257(4 Pt 2):R901–8. doi: 10.1152/ajpregu.1989.257.4.R901. [DOI] [PubMed] [Google Scholar]
- McCubbin JA. Stress and endogenous opioids: behavioral and circulatory interactions. Biol Psychol. 1993;35(2):91–122. doi: 10.1016/0301-0511(93)90008-v. [DOI] [PubMed] [Google Scholar]
- McCubbin JA, Kaplan JR, Manuck SB, Adams MR. Opioidergic inhibition of circulatory and endocrine stress responses in cynomolgus monkeys: a preliminary study. Psychosom Med. 1993;55(1):23–8. doi: 10.1097/00006842-199301000-00005. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann N Y Acad Sci. 1999;877:309–38. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- Moga MM, Gray TS. Evidence for corticotropin-releasing factor, neurotensin, and somatostatin in the neural pathway from the central nucleus of the amygdala to the parabrachial nucleus. J Comp Neurol. 1985;241(3):275–84. doi: 10.1002/cne.902410304. [DOI] [PubMed] [Google Scholar]
- Moga MM, Saper CB, Gray TS. Bed nucleus of the stria terminalis: cytoarchitecture, immunohistochemistry, and projection to the parabrachial nucleus in the rat. J Comp Neurol. 1989;283(3):315–32. doi: 10.1002/cne.902830302. [DOI] [PubMed] [Google Scholar]
- Moriwaki A, Wang JB, Svingos A, van Bockstaele E, Cheng P, Pickel V, Uhl GR. mu Opiate receptor immunoreactivity in rat central nervous system. Neurochem Res. 1996;21(11):1315–31. doi: 10.1007/BF02532373. [DOI] [PubMed] [Google Scholar]
- Morris M, Salmon P, Steinberg H, Sykes EA, Bouloux P, Newbould E, McLoughlin L, Besser GM, Grossman A. Endogenous opioids modulate the cardiovascular response to mental stress. Psychoneuroendocrinology. 1990;15(3):185–92. doi: 10.1016/0306-4530(90)90029-9. [DOI] [PubMed] [Google Scholar]
- Moskowitz AS, Goodman RR. Light microscopic autoradiographic localization of mu and delta opioid binding sites in the mouse central nervous system. J Neurosci. 1984 May;4(5):1331–42. doi: 10.1523/JNEUROSCI.04-05-01331.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Yamamoto R, Fujio M, Suzuki Y, Minami M, Satoh M, Kaneko S. Involvement of the bed nucleus of the stria terminalis activated by the central nucleus of the amygdala in the negative affective component of morphine withdrawal in rats. Neuroscience. 2005;134(1):9–19. doi: 10.1016/j.neuroscience.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Nirenberg MJ, Liu Y, Peter D, Edwards RH, Pickel VM. The vesicular monoamine transporter 2 is present in small synaptic vesicles and preferentially localizes to large dense core vesicles in rat solitary tract nuclei. Proc Natl Acad Sci U S A. 1995;92(19):8773–7. doi: 10.1073/pnas.92.19.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cabal L, Liu J, Pollandt S, Schmidt K, Shinnick-Gallagher P, Gallagher JP. Dopamine and corticotropin-releasing factor synergistically alter basolateral amygdala-to-medial prefrontal cortex synaptic transmission: functional switch after chronic cocaine administration. J Neurosci. 2008;28(2):529–42. doi: 10.1523/JNEUROSCI.2666-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HD. The Fine Structure of the Nervous System. Oxford University Press; New York: 1991. [Google Scholar]
- Peters A, Palay SL. The morphology of synapses. J Neurocytol. 1996;25(12):687–700. doi: 10.1007/BF02284835. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Paull WK. Demonstration of distinct corticotropin releasing factor--containing neuron populations in the bed nucleus of the stria terminalis. A light and electron microscopic immunocytochemical study in the rat. Histochemistry. 1990;94(4):345–64. doi: 10.1007/BF00266441. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kash TL, Rodríguez JJ, MacKie K. Compartment-specific localization of cannabinoid 1 (CB1) and mu-opioid receptors in rat nucleus accumbens. Neuroscience. 2004;127(1):101–12. doi: 10.1016/j.neuroscience.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kearn CS, Mackie K. Targeting dopamine D2 and cannabinoid-1 (CB1) receptors in rat nucleus accumbens. J Comp Neurol. 2006;495(3):299–313. doi: 10.1002/cne.20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin JF, Chevalier B, Laforest S, Drolet G. Enkephalinergic afferents of the centromedial amygdala in the rat. J Comp Neurol. 2006;496(6):859–76. doi: 10.1002/cne.20956. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24(14):3471–9. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149(1):122–30. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–12. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro SC, Kennedy SE, Smith YR, Stohler CS, Zubieta JK. Interface of physical and emotional stress regulation through the endogenous opioid system and mu-opioid receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1264–80. doi: 10.1016/j.pnpbp.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150(1):8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Roder S, Ciriello J. Contribution of bed nucleus of the stria terminalis to the cardiovascular responses elicited by stimulation of the amygdala. J Auton Nerv Syst. 1993;45(1):61–75. doi: 10.1016/0165-1838(93)90362-x. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. Journal of Neuroscience. 2001;21(3):823–33. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Ultrastructural relationships between terminals immunoreactive for enkephalin, GABA, or both transmitters in the rat ventral tegmental area. Brain Res. 1995;672(1–2):261–75. doi: 10.1016/0006-8993(94)01391-t. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17(7):2605–14. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 1998;137(2):184–90. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Beltramino CA, McDonald AJ, Miselis RR, Yang M, de Olmos J, Heimer L, Alheid GF. Supracapsular bed nucleus of the stria terminalis contains central and medial extended amygdala elements: evidence from anterograde and retrograde tracing experiments in the rat. J Comp Neurol. 2000;422(4):533–55. doi: 10.1002/1096-9861(20000710)422:4<533::aid-cne5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Shimada S, Inagaki S, Kubota Y, Ogawa N, Shibasaki T, Takagi H. Coexistence of peptides (corticotropin releasing factor/neurotensin and substance P/somatostatin) in the bed nucleus of the stria terminalis and central amygdaloid nucleus of the rat. Neuroscience. 1989;30(2):377–83. doi: 10.1016/0306-4522(89)90259-5. [DOI] [PubMed] [Google Scholar]
- Skelton KH, Oren D, Gutman DA, Easterling K, Holtzman SG, Nemeroff CB, Owens MJ. The CRF(1) receptor antagonist, R121919, attenuates the severity of precipitated morphine withdrawal. Eur J Pharmacol. 2007;571(1):17–24. doi: 10.1016/j.ejphar.2007.05.041. [DOI] [PubMed] [Google Scholar]
- Stinus L, Cador M, Zorrilla EP, Koob GF. Buprenorphine and a CRF1 antagonist block the acquisition of opiate withdrawal-induced conditioned place aversion in rats. Neuropsychopharmacology. 2005;30(1):90–8. doi: 10.1038/sj.npp.1300487. [DOI] [PubMed] [Google Scholar]
- Sufka KJ, Hughes RA, McCormick TM, Borland JL. Opiate effects on isolation stress in domestic fowl. Pharmacol Biochem Behav. 1994;49(4):1011–5. doi: 10.1016/0091-3057(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Sun N, Roberts L, Cassell MD. Rat central amygdaloid nucleus projections to the bed nucleus of the stria terminalis. Brain Res Bull. 1991;27(5):651–62. doi: 10.1016/0361-9230(91)90041-h. [DOI] [PubMed] [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol. 1993;330(3):381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Moriwaki A, Wang JB, Uhl GB, Pickel VM. Ultrastructural immunocytochemical localization of mu-opioid receptors in rat nucleus accumbens: extrasynaptic plasmalemmal distribution and association with Leu5-enkephalin. J Neurosci. 1996;16:4162–4173. doi: 10.1523/JNEUROSCI.16-13-04162.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36(3):165–86. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991;548(1–2):100–10. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- Torrealba F, Carrasco MA. A review on electron microscopy and neurotransmitter systems. Brain Res Brain Res Rev. 2004;47(1–3):5–17. doi: 10.1016/j.brainresrev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Treweek JB, Jaferi A, Colago EE, Zhou P, Pickel VM. Electron Microscopic Localization of Corticotropin Releasing Factor (CRF) and CRF Receptor in Rat and Mouse Central Nucleus of the Amygdala. J Comp Neurol. 2009;512:323–335. doi: 10.1002/cne.21884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Harada H, Nozaki M, Katada T, Ui M, Satoh M, Takagi H. Reconstitution of rat brain mu opioid receptors with purified guanine nucleotide-binding regulatory proteins, Gi and Go. Proc Natl Acad Sci U S A. 1988;85(18):7013–7. doi: 10.1073/pnas.85.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uryu K, Okumura T, Shibasaki T, Sakanaka M. Fine structure and possible origins of nerve fibers with corticotropin-releasing factor-like immunoreactivity in the rat central amygdaloid nucleus. Brain Res. 1992;577(1):175–9. doi: 10.1016/0006-8993(92)90554-m. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Opposing regulation of the locus coeruleus by corticotropin-releasing factor and opioids. Potential for reciprocal interactions between stress and opioid sensitivity. Psychopharmacology (Berl) 2001;158(4):331–42. doi: 10.1007/s002130000673. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428(2):191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ. Intrinsic and extrinsic connections of the rat central extended amygdala: an in vivo electrophysiological study of the central amygdaloid nucleus. Brain Res. 1998;794(2):188–98. doi: 10.1016/s0006-8993(98)00228-5. [DOI] [PubMed] [Google Scholar]
- Veinante P, Stoeckel M, Lasbennes F, Freund-Mercier MJ. c-Fos and peptide immunoreactivities in the central extended amygdala of morphine-dependent rats after naloxone-precipitated withdrawal. Eur J Neurosci. 2003;18(5):1295–305. doi: 10.1046/j.1460-9568.2003.02837.x. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463(1–3):199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008 doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Wang H, Moriwaki A, Wang JB, Uhl GR, Pickel VM. Ultrastructural immunocytochemical localization of mu opioid receptors and Leu5-enkephalin in the patch compartment of the rat caudate-putamen nucleus. J Comp Neurol. 1996;375(4):659–74. doi: 10.1002/(SICI)1096-9861(19961125)375:4<659::AID-CNE7>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Wang H, Gracy KN, Pickel VM. Mu-opioid and NMDA-type glutamate receptors are often colocalized in spiny neurons within patches of the caudate-putamen nucleus. J Comp Neurol. 1999;412(1):132–46. doi: 10.1002/(sici)1096-9861(19990913)412:1<132::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Wang H, Pickel VM. Preferential cytoplasmic localization of delta-opioid receptors in rat striatal patches: comparison with plasmalemmal mu-opioid receptors. J Neurosci. 2001;21(9):3242–50. doi: 10.1523/JNEUROSCI.21-09-03242.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Pickel VM. Dopamine D2 receptors are present in prefrontal cortical afferents and their targets in patches of the rat caudate-putamen nucleus. J Comp Neurol. 2002;442(4):392–404. doi: 10.1002/cne.10086. [DOI] [PubMed] [Google Scholar]
- Wang J, Fang Q, Liu Z, Lu L. Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place pr eference in rats. Psychopharmacology (Berl) 2006;185(1):19–28. doi: 10.1007/s00213-005-0262-6. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M. Involvement of glutamate receptors within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Jpn J Pharmacol. 2002;88:399–406. doi: 10.1254/jjp.88.399. [DOI] [PubMed] [Google Scholar]
- Xu G, Van Bockstaele E, Reyes B, Bethea T, Valentino RJ. Chronic morphine sensitizes the brain norepinephrine system to corticotropin-releasing factor and stress. J Neurosci. 2004;24(38):8193–7. doi: 10.1523/JNEUROSCI.1657-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y, Breder CD, Saper CB, Cechetto DFA. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol. 1991;303:355–374. doi: 10.1002/cne.903030303. [DOI] [PubMed] [Google Scholar]
- Zhu W, Pan ZZ. Synaptic properties and postsynaptic opioid effects in rat central amygdala neurons. Neuroscience. 2004;127(4):871–9. doi: 10.1016/j.neuroscience.2004.05.043. [DOI] [PubMed] [Google Scholar]
- Zhu W, Pan ZZ. Mu-opioid-mediated inhibition of glutamate synaptic transmission in rat central amygdala neurons. Neuroscience. 2005;133(1):97–103. doi: 10.1016/j.neuroscience.2005.02.004. [DOI] [PubMed] [Google Scholar]