To the editor: Obtaining diffraction-quality crystals is a major bottleneck in protein X-ray crystallography. For example, the current success rate for protein structure solution at the Midwest Center for Structural Genomics (starting from purified protein) is ~10%. Protein crystallization is influenced by many factors, and many methods have been developed to enhance crystallization. In particular, reductive methylation of proteins has been successfully applied to obtain high-quality crystals1-4. Several studies3,5,6 have indicated that methylating the solvent-exposed ε-amino group of lysines changes protein properties (pI, solubility and hydropathy)7,8, which may promote crystallization via improving crystal packing. Reductive methylation of proteins is a simple, generic method; it is fast, specific and requires few steps under relatively mild buffer and chemical conditions and can be executed for several proteins in parallel. Native and methylated proteins have very similar structures, and, in most cases, methylated proteins maintain their biochemical function2,5,9. Some proteins can only be crystallized after methylation3,10, and crystals of modified proteins often diffract to higher resolution3,9. The efficacy of the method has been previously tested on 10 proteins, with a 30% success rate3.
Here we investigated the application of reductive methylation on a large scale. We applied a previously described reductive methylation protocol2,11 (Supplementary Methods online) to 370 sequence-diverse proteins selected from protein families that had no structural homologs with >30% sequence identity. We expressed 370 recombinant proteins and purified them using standard methods12 and screened them using standard crystal screening methods (Supplementary Methods). Of the 370 proteins, 269 proteins had not previously yielded crystals suitable for structure determination (crystals were too small, poorly ordered, twinned, highly mosaic or multiple), 85 proteins had previously failed to crystallize and 16 proteins were a reference set (not previously screened for crystallization; Table 1 and Supplementary Tables 1 and 2 online). After reductive methylation, we obtained diffraction-quality crystals for 40 of the 370 proteins, and so far we solved 26 crystal structures (Table 1). The crystallization success rate of methylated proteins did not correlate with the number of lysines, pI, hydropathy or molecular weight (Supplementary Table 1).
Table 1.
Summary of methylation results for protein sets used in this study
| Description of protein set | Number of proteins in the set | Macroscopic crystals after methylation | Diffraction-quality crystals after methylation | Structures solved | Overall success rate (percentage of structures solved) |
|---|---|---|---|---|---|
| Crystallized previously but crystals unsuitable for structure determination | 269 | 59 | 33 | 20 | 7.4 |
| Screened previously but no crystals obtained | 85 | 8 | 5 | 5 | 5.9 |
| Proteins not previously screened | 16 | 5 | 2 | 1 | 6.3 |
| Total | 370 | 72 | 40 | 26 | 7.0 |
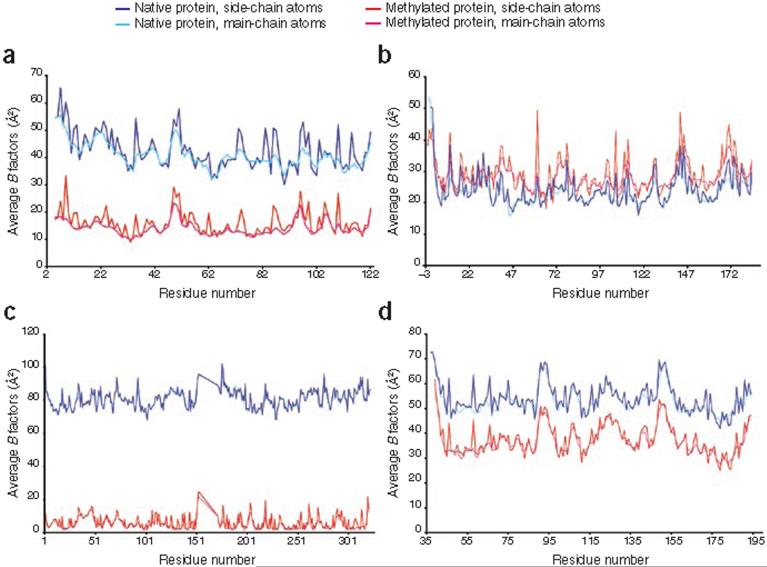
We also determined the structures of 4 proteins in their native as well as their methylated states (Supplementary Methods). By comparing these structures, we obtained insight into how methylation affects protein crystallization. We observed a decrease in the isotropic B factor (Fig. 1), which is likely a result of more ordered crystal packing and which leads to better diffraction limits. Indeed, the resolution of the methylated structures (average, 2.07 Å) was better than that of their native counterparts (average, 3.05 Å; Supplementary Table 1). The methylated lysines were engaged in various intra- and intermolecular interactions with protein and solvent (carboxylates and main chain carbonyls as reported earlier2-4 as well as with histidine and arginine residues and water; Supplementary Figs. 1 and 2 online). Adding methyl groups effectively increases the lysine interaction radius by 1-1.2 Å, allowing a weak, long-distance ε-amine (>4.2 Å) interaction with oxygen or nitrogen to be replaced with stronger (3.3 Å) ε-amine-(N-methyl) oxygen or nitrogen interactions (Supplementary Fig. 3 online). Methylation of lysine may be considered as similar to replacing it with an arginine, which has a higher propensity for interactions and is found more often on protein-protein13 and crystal packing interfaces14-16 (Supplementary Figs. 1, 4 and 5 online). Ab initio quantum mechanical calculations using ethylamine and N,N-dimethylethyl amine model compounds (Supplementary Methods) provided an estimate of the binding energy between N-methyl groups. The computation results predicted the optimal methylated amine interaction distance with the water oxygen to be ~3.3 Å (Supplementary Fig. 3), consistent with observations in the crystal structures.
Figure 1.
Plots of isotropic B factors calculated for protein structures solved in both the native and methylated states. (a) Putative HopJ protein VP0580 (Protein Data Bank (PDB) identifiers 2QHQ (methylated) and 2QM2 (native)). (b) Flavodoxin FldA (PDB identifier 2ARK (methylated)). (c) Gfo/ Idh/MocA family oxidoreductase SP1482 (PDB identifier 2HO3 (methylated) and 2H05 (native)). (d) Extracellular domain of arabinofuranosyltransferase DIP0159 (PDB identifier 2IDL (methylated)).
We showed in this large-scale study that reductive methylation of proteins provides a generally useful, simple, inexpensive and efficient method to alter protein-surface properties that can improve the crystallizability of proteins. We achieved an overall success rate, from purified protein to structure, of 7.0% for proteins that did not yield a structure in initial attempts, thus demonstrating that reductive methylation can be an effective method for increasing the chances of obtaining a structure.
Supplementary Material
ACKNOWLEDGMENTS
We thank all members of the Structural Biology Center and Midwest Center for Structural Genomics at Argonne National Laboratory for their help in conducting these experiments, S. Li, C.B. Lindberg and C. Giometti for running mass spectrometry experiments, A. Edwards for reading and commenting on the drafts, and L. Butler for preparing the manuscript. This work was supported by US National Institutes of Health (GM62414, GM074942) and by the US Department of Energy, Office of Biological and Environmental Research, under contract DE-AC02-06CH11357.
Footnotes
Note: Supplementary information is available on the Nature Methods website.
References
- 1.D'Arcy A, Stihle M, Kostrewa D, Dale G. Acta Crystallogr. D Biol. Crystallogr. 1999;55:1623–1625. doi: 10.1107/s0907444999008136. [DOI] [PubMed] [Google Scholar]
- 2.Rypniewski WR, Holden HM, Rayment I. Biochemistry. 1993;32:9851–9858. doi: 10.1021/bi00088a041. [DOI] [PubMed] [Google Scholar]
- 3.Walter TS, et al. Structure. 2006;14:1617–1622. doi: 10.1016/j.str.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw N, et al. BMC Struct. Biol. 2007;7:46. doi: 10.1186/1472-6807-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Means GE. Methods Enzymol. 1977;47:469–478. doi: 10.1016/0076-6879(77)47047-2. [DOI] [PubMed] [Google Scholar]
- 6.Means GE, Feeney RE. Anal. Biochem. 1995;224:1–16. doi: 10.1006/abio.1995.1001. [DOI] [PubMed] [Google Scholar]
- 7.Canaves JM, Pagea R, Wilson IA, Stevens RC. J. Mol. Biol. 2004;344:977–991. doi: 10.1016/j.jmb.2004.09.076. [DOI] [PubMed] [Google Scholar]
- 8.Kyte J, Doolittle RF. J. Mol. Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi M, Kubota M, Matsuura Y. Acta Crystallogr. D Biol. Crystallogr. 1999;55:931–933. doi: 10.1107/s0907444999002115. [DOI] [PubMed] [Google Scholar]
- 10.Schubot FD, Waugh DS. Acta Crystallogr. D Biol. Crystallogr. 2004;60:1981–1986. doi: 10.1107/S0907444904023005. [DOI] [PubMed] [Google Scholar]
- 11.Means GE, Feeney RE. Bioconjug. Chem. 1990;1:2–12. doi: 10.1021/bc00001a001. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y, et al. J. Struct. Funct. Genomics. 2004;5:111–118. doi: 10.1023/B:JSFG.0000029206.07778.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magalhaes A, et al. J. Protein Chem. 1994;13:195–215. doi: 10.1007/BF01891978. [DOI] [PubMed] [Google Scholar]
- 14.Anashkina A, Kuznetso E, Esipova N, Tumanyan V. Proteins. 2007;67:1060–1077. doi: 10.1002/prot.21363. [DOI] [PubMed] [Google Scholar]
- 15.Glaser F, Steinberg DM, Vakser IA, Ben-Tal N. Proteins. 2001;43:89–102. [PubMed] [Google Scholar]
- 16.Juers DH, Matthews BW. J. Mol. Biol. 2001;311:851–862. doi: 10.1006/jmbi.2001.4891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.