Abstract
Glial-derived neurotrophic factor (GDNF) promotes both sensory and motor neuron survival. The delivery of GDNF to the peripheral nervous system has been shown to enhance regeneration following injury. In this study we evaluated the effect of affinity-based delivery of GDNF from a fibrin matrix in a nerve guidance conduit on nerve regeneration in a 13 mm rat sciatic nerve defect. Seven experimental groups were evaluated which received GDNF or nerve growth factor (NGF) with the delivery system within the conduit, control groups excluding one or more components of the delivery system, and nerve isografts. Nerves were harvested 6 weeks after treatment for analysis by histomorphometry and electron microscopy. The use of the delivery system (DS) with either GDNF or NGF resulted in a higher frequency of nerve regeneration vs. control groups, as evidenced by a neural structure spanning the 13 mm gap. The GDNF DS and NGF DS groups were also similar to the nerve isograft group in measures of nerve fiber density, percent neural tissue and myelinated area measurements, but not in terms of total fiber counts. In addition, both groups contained a significantly greater percentage of larger diameter fibers, with GDNF DS having the largest in comparison to all groups, suggesting more mature neural content. The delivery of GDNF via the affinity-based delivery system can enhance peripheral nerve regeneration through a silicone conduit across a critical nerve gap and offers insight into potential future alternatives to the treatment of peripheral nerve injuries.
Keywords: Drug delivery, growth factor, nerve guidance conduit, eripheral nerve graft, tissue engineering
1. Introduction
Despite recent advances in the understanding of peripheral nerve injury and regeneration, functional outcomes are still suboptimal. In nerve transection injuries, the current standard of care is a primary end-to-end repair. In nerve gap injuries, when tension precludes a primary repair, an autograft is used to provide a scaffold for the regenerating nerve. This procedure, however, has limitations due to donor site availability and morbidity [1,2]. One alternative to autografting is the use of a nerve guidance conduit (NGC). NGCs facilitate bridging the gap between a proximal and a distal nerve, protect regenerating axons from infiltrating scar tissue, and allow the microenvironment of the regenerating nerve to be manipulated by controlling biochemical and physical contents [1,3].
A variety of materials have been investigated for use as scaffolds to fill the lumen of a NGC, including the extracellular matrix proteins collagen [4,5], fibronectin [6] and laminin [5], as well as naturally derived matrices such as agarose [7,8] and alginate [9,10]. Fibrin has also been used as a biomaterial scaffold to support neural regeneration within an NGC [11–13] and may offer an advantage over other materials because it naturally forms within an empty silicone conduit connecting the damaged ends of rat sciatic nerve [14]. Furthermore, fibrin contains sites for cell binding via integrin receptors [15], including cell binding sites for Schwann cells [16], which may facilitate cellular migration.
Numerous drug delivery methods have also been used with NGCs [17–22]. However, diffusion-based release of growth factors from degradable polymers is the most common delivery method [17,18,23]. One shortcoming of this approach is that the release rate cannot be modulated or controlled by cells during regeneration. One alternative is to use an affinity-based delivery system (DS) that allows the release of growth factors to be controlled by cell-based degradation of the delivery system [24]. Our laboratory has developed an affinity-based delivery system that sequesters heparin-binding proteins within a fibrin matrix using non-covalent interactions [25,26]. This system contains a bi-domain peptide containing a transglutaminase substrate domain and a heparin-binding domain. Based on the α2-plasmin inhibitor substrate [27,28], the peptide is able to crosslink into the fibrin matrix during polymerization via the transglutaminase activity of Factor XIIIa, leaving the other domain free to interact [25,26]. This heparin-binding domain has the capability to sequester various neurotrophic factors due to their ability to bind to heparin via the sulfated domains on the heparin [29]. This delivery system has been used with a variety of growth factors in many potential treatment applications [11,24,30–33]. Specifically, we have characterized the effect of affinity-based delivery of nerve growth factor (NGF) on peripheral nerve regeneration [11].
Glial-derived neurotrophic factor (GDNF) has shown promise in the treatment of peripheral nerve injuries. While GDNF has been found to promote the survival of both sensory and motor neurons, multiple studies report it to be the most potent motor neuron trophic and survival factor [34–39]. GDNF expression in peripheral nerves is also up-regulated significantly in the distal stump of injured sciatic nerve, as well as in the corresponding muscle [40,41]. Given the ability of GDNF to enhance peripheral nerve regeneration [17,18,20], we chose to examine controlled delivery of GDNF from our affinity-based delivery system in vitro and found that GDNF could be retained and released from the delivery system in a biologically active form [42].
In the present study, we evaluated the effects of controlled release of GDNF from a fibrin matrix containing our affinity-based delivery system within a NGC on nerve regeneration in vivo using a rat sciatic nerve injury model. We included NGF in the current study for comparison to our previous study. We hypothesized that controlled delivery of GDNF would enhance nerve regeneration and have histomorphometric equivalence to a nerve isograft.
2. Materials and Methods
2.1. Experimental animals
Adult male Lewis rats (Harlan Sprague-Dawley, Indianapolis, IN), each weighing 250–300 g, were used in this study. All surgical procedures and peri-operative care measures were performed in strict accordance with the National Institutes of Health guidelines and were approved by the Washington University Animal Studies Committee. All animals were housed in a central animal facility, given a rodent diet (PicoLab Rodent Diet 20 #5053, PMI Nutrition International) and water ad libitum. After surgical procedures, animals recovered in a warm environment and were closely monitored for 2 h. Animals were then returned to the animal facility and monitored for weight loss, infection and other morbidities.
2.2. Experimental design
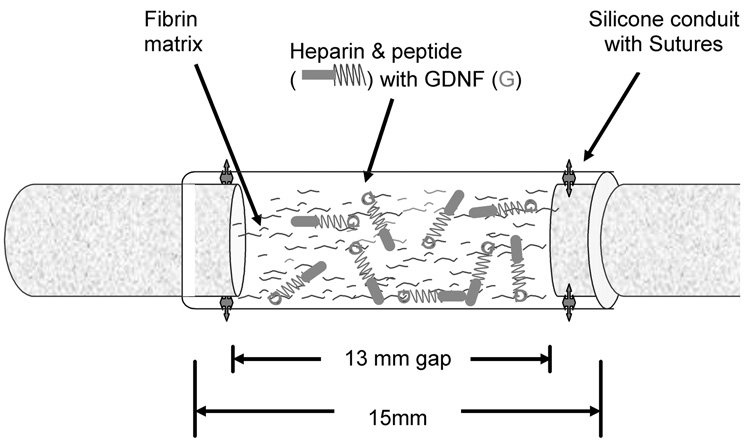
Eighty-four animals were randomized into seven groups (n = 12), as shown in Table 1. An additional six animals served as sciatic nerve isograft donors. In all experimental groups, the sciatic nerve was transected and a 5 mm segment was excised just proximal to the trifurcation of the nerve. The nerve was repaired with a 15 mm silicone conduit containing fibrin matrices with or without the delivery system and growth factor. One millimeter of nerve was incorporated into each end of the conduit to create a 13 mm nerve gap, exceeding the “critical gap” of spontaneous rat sciatic regeneration through silicone conduits by 3 mm [14,43] (Fig. 1). Group I served as the untreated control group and received an empty conduit. Groups II, III and IV were additional control groups, receiving conduits containing fibrin alone, fibrin with the delivery system (no growth factor) or fibrin with the growth factor but no delivery system, respectively. These groups examined the isolated effects of the delivery system components. The remaining groups (V, VI) were implanted with conduits containing the fibrin matrix containing the delivery system with doses of GDNF or NGF, which were selected based upon in vitro DRG dose studies [42] and preliminary data obtained from dose–response pilot studies in the sciatic nerve model (test doses included 25, 50, 100, and 250 ng ml−1 GDNF with the delivery system). Group VII served as a positive control receiving reversed nerve isografts from syngeneic donor animals.
Table 1.
Experimental design
| Group | GF dose (ng ml−1) | Delivery system | Fibrin matrix | Number of rats | Number of rats with neural regeneration |
|---|---|---|---|---|---|
| I | 0 | No | No | 12 | 3 |
| II | 0 | No | Yes | 12 | 5 |
| III | 0 | Yes | Yes | 12 | 3 |
| IV | 100 – GDNF | No | Yes | 12 | 1 |
| V | 100 – GDNF | Yes | Yes | 12 | 6 |
| VI | 50 – NGF | Yes | Yes | 12 | 7 |
| VII | Isograft | No | No | 12 | 12 |
GF, growth factor; GDNF, glial cell line-derived neurotrophic factor; NGF, nerve growth factor.
Fig. 1.
Schematic representation of surgical implantation of nerve guidance conduit containing the affinity-based delivery system. A 13 mm nerve gap was repaired with a 15 mm silicone conduit containing fibrin matrices with or without delivery system and growth factor and sutured to the transected proximal and distal stumps, incorporating 1 mm of nerve on either end. The delivery system consisted of a bi-domain peptide cross-linked into the fibrin matrix at one domain while the other binds heparin by electrostatic interactions. The growth factor can then bind to the bound heparin, creating a matrix-bound, non-diffusible complex, which can be retained for cell mediated degradation of the fibrin matrix.
2.3. Preparation of fibrin matrices
Fibrinogen solutions were prepared by dissolving human plasminogen-free fibrinogen in deionized water at 8 mg ml−1 for 1 h and dialyzing vs. 4 l of Tris-buffered saline (TBS) (33 mM Tris, 8 g l−1 NaCl, 0.2 g l−1 KCl) at pH 7.4 overnight to exchange salts present in the protein solution. The resulting solution was sterilized by filtration through 5.0 and 0.22 µm syringe filters, and the final fibrinogen concentration was determined by measuring absorbance at 280 nm. For the delivery system, a bi-domain peptide (ATIII) based on a modified version of the antithrombin III-heparin binding domain ((AcG)NQEQVSPK(βA)FAKLAAR-LYRKA, where AcG denotes N-acetyl-glycine and the tranglutaminase substrate is given in italics) [25,44] was synthesized as described previously [42]. Fibrin matrices were also prepared as previously described. Components were mixed to obtain the following final solution concentrations: 4 mg ml−1 fibrinogen, 2.5 mM Ca2+, 2 NIH units ml−1 of thrombin, 0.25 mM peptide (which results in 8 mol of cross-linked peptide per mol of fibrinogen [25,26]), 62.5 µM heparin (sodium salt) and recombinant human GDNF or NGF (at the correct dose, Peprotech; see Table 1).
Silicone tubing (SF Medical, Hudson, MA) (1.5 mm inside diameter × 0.3 mm wall thickness) was autoclaved overnight, cut into 15 mm segments and soaked in 70% ethyl alcohol. Prior to filling, the tubes were rinsed with sterile saline solution. The fibrinogen solution was drawn into the silicone tube using a pipette and allowed to polymerize for 10 min prior to implantation.
2.4. Operative procedure
All surgical procedures were performed using aseptic technique and microsurgical dissection and repairs. Under subcutaneous anesthesia with ketamine (75 mg kg−1) and medetomidine (0.5 mg kg−1), the hind leg of the rat was prepped and the sciatic nerve was exposed through a dorsolateral gluteal muscle splitting incision. A 5 mm nerve segment was excised proximal to the trifurcation of the sciatic nerve and a 15 mm silicone tube, with the fibrin matrices with or without delivery system and growth factor, was sutured to the transected proximal and distal stumps, incorporating 1 mm of nerve on either end as described above (Fig. 1). Four 9-0 nylon interrupted microepineurial sutures were used to secure the conduit. In animals receiving the isograft control, a 13 mm segment of sciatic nerve was harvested from a syngeneic donor animal and inserted into the recipient animal. Wounds were irrigated with saline, dried and closed with a running 5-0 vicryl suture in muscle fascia, and then interrupted 4-0 nylon skin sutures.
Anesthesia in experimental animals was then reversed with a subcutaneous injection of atipamezole HCl (1 mg kg−1) (Pfizer Animal Health, Exton, PA), and the animals recovered in a warm environment. After recovery, the animals were returned to the housing facility.
Six weeks postoperatively, all animals were re-anesthetized and nerve harvests were performed by reopening the prior muscle splitting incision. The nerve conduit and a 5 mm portion of native nerve both proximally and distally were harvested. The specimens were marked with a proximal suture and stored in 3% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2) at 4°C until histomorphometric analysis was performed. Under anesthesia, the animals were then euthanized with intracardiac injection of Euthasol (150 mg kg−1) (Delmarva Laboratories, Des Moines, IA).
2.5. Histomorphometric and electron microscopic evaluation
The tissues were post-fixed with 1% osmium tetroxide, ethanol dehydrated and embedded in Araldite 502 (Polyscience Inc., Warrington, PA). Thin (1 µm) sections were made from the tissue using a LKB II Ultramicrotome (LKB-Produckter A.B., Broma, Sweden) and then stained with 1% toluidine blue for examination by light microscopy. The slides were evaluated by an observer blinded to the experimental groups for overall nerve architecture, quantity of regenerated nerve fibers, degree of myelination and the presence of Wallerian degeneration [45].
Proximal and distal cross sections from the host nerve, and sections through the conduit or graft were evaluated. At ×1000 magnification, six representative fields per nerve were evaluated with an automated digital image-analysis system linked to morphometry software using previously described methods to measure nerve morphometry [45]. Briefly, total fascicular area and total fiber number were measured. At least 80% of the nerve area was measured to determine the fiber diameters and density. From these primary measurements the following morphometric indices were calculated: total number of nerve fibers, nerve fiber density (fiber number mm−2), percent neural tissue (100 × neural area/intrafascicular area) and nerve fiber width. Morphometric indices from experimental neural specimens were compared to the isograft controls.
For electron microscopy, ultrathin 70 nm sections of the embedded tissues were cut by a LKB III Ultramicrotome and stained with uranyl acetate–lead citrate. These sections were examined with a Zeiss 902 electron microscope (Zeiss Instruments, Chicago, IL). The quality of myelination, relative prevalence of unmyelinated fibers, and the area of myelinated and unmyelinated fibers were evaluated.
2.6. Statistical analysis
All results are reported as mean ± standard error of the mean. Statistical analyses were performed using Statistica version 6 (Statsoft Inc., Tulsa, OK). Power analysis was performed to determine the minimum sample size for p<0.05 and a statistical power level of 0.8 with an effect of 1.25. All data were evaluated for differences between groups using the Kruskal–Wallis analysis of variance (ANOVA) and median test. Post hoc Mann–Whitney tests were used for determining differences between groups with significance set at α = 0.05.
3. Results
3.1. Nerve guidance conduit harvest
The efficacy of GDNF in promoting nerve regeneration across a critical nerve gap was evaluated in vivo after sciatic nerve transaction and NGC implantation. After 42 days, groups with the delivery system and growth factor showed more successful regeneration, as evidenced by a neural structure spanning the 13 mm gap with higher frequency. Six of 12 conduits from the 100 ng ml−1 GDNF with delivery system (GDNF DS) and 7 of 12 conduits from 50 ng ml−1 NGF with DS (NGF DS) groups contained regenerated nerve cables. Five of 12 conduits from the group containing fibrin, 3 of 12 conduits from DS alone with no growth factor (DS alone (no GF)) group, 1 of 12 of the 100 ng ml−1 GDNF (GDNF (no DS)) group and 3 of 12 from the empty group demonstrated nerve regeneration (Table 1). All 12 animals in the isograft group demonstrated regeneration. The gross appearance of the regenerated nerves in the GDNF DS group exhibited a larger, more robust nerve cable in comparison to the other experimental groups. The neural structure was centered compactly in the conduit, away from the walls, in all conduit specimens. All conduit specimens demonstrated intact connections to the proximal or distal sciatic nerve, despite variability in regeneration.
3.2. Histology
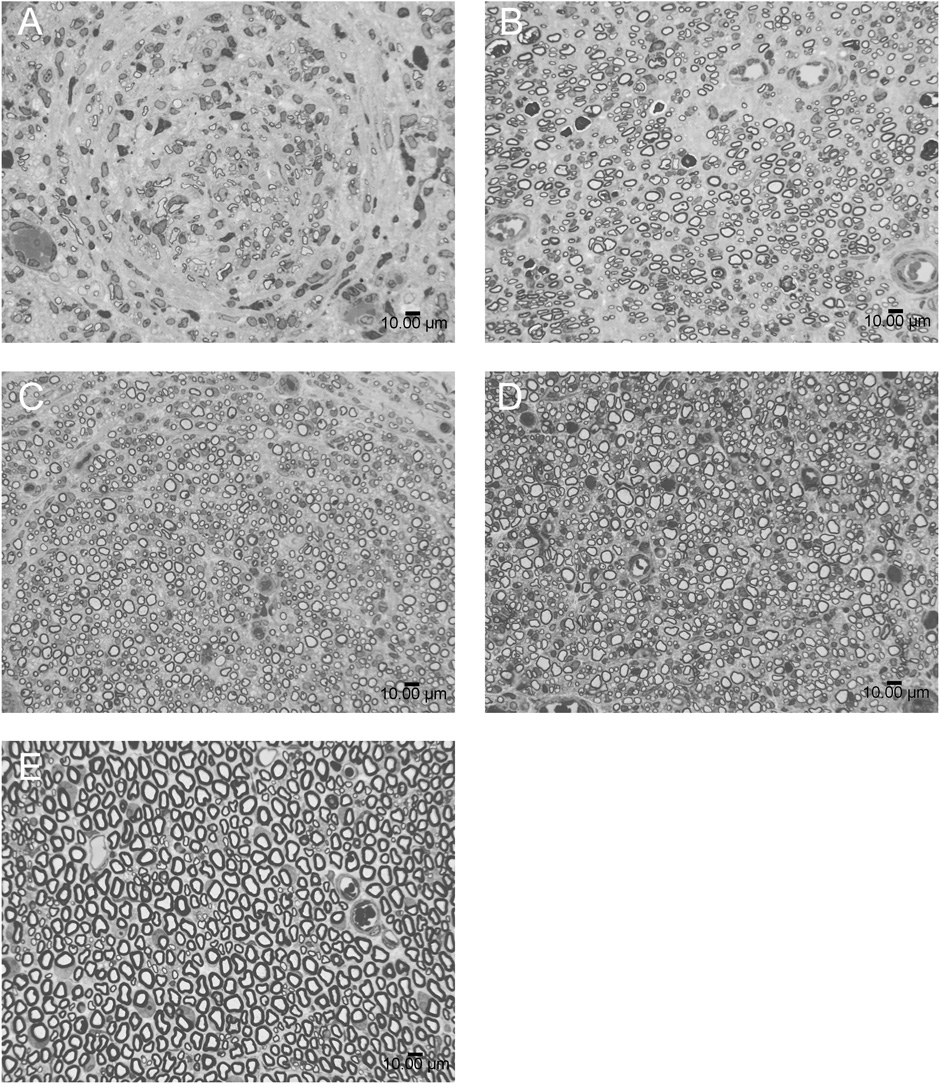
Qualitative examination of the midline of the conduits or grafts by light microscopy revealed differences in nerve architecture, as reflected by the arrangement and description of the regenerating axons (Fig. 2). In particular, the normal rat sciatic nerve contains myelinated fibers in a packed, semi-symmetric, uniform arrangement with fibers that are relatively similar in size and shape. Overall, this arrangement can be described as “organized” architecture. The isograft, GDNF DS and NGF DS reflected this organized appearance, while the fibrin alone group demonstrated more random spacing and swirling of fibers, as well as less symmetrically shaped individual myelinated fibers. In addition, groups with growth factor and the delivery system appeared to have more tightly packed fibers than the isograft, which was likely due to the compact area for neural regeneration in the silicone tube. No inflammatory response or residual fibrin was appreciated.
Fig. 2.
Histological sections of regenerating nerves at the midline of the conduit (or graft). (A) Fibrin alone; (B) isograft; (C) delivery system incorporating GDNF (GDNF DS); (D) delivery system incorporating NGF (NGF DS); (E) normal uninjured nerve. Thin (1 µm) sections of sciatic nerve specimens were stained with 1% toluidine blue for qualitative examination of the midline of the conduits by light microscopy. GDNF DS and NGF DS groups demonstrated more organized neural architecture, closely approximating the isograft, in comparison to the fibrin alone group. Scale bar, 10 µm.
3.3. Histomorphometry
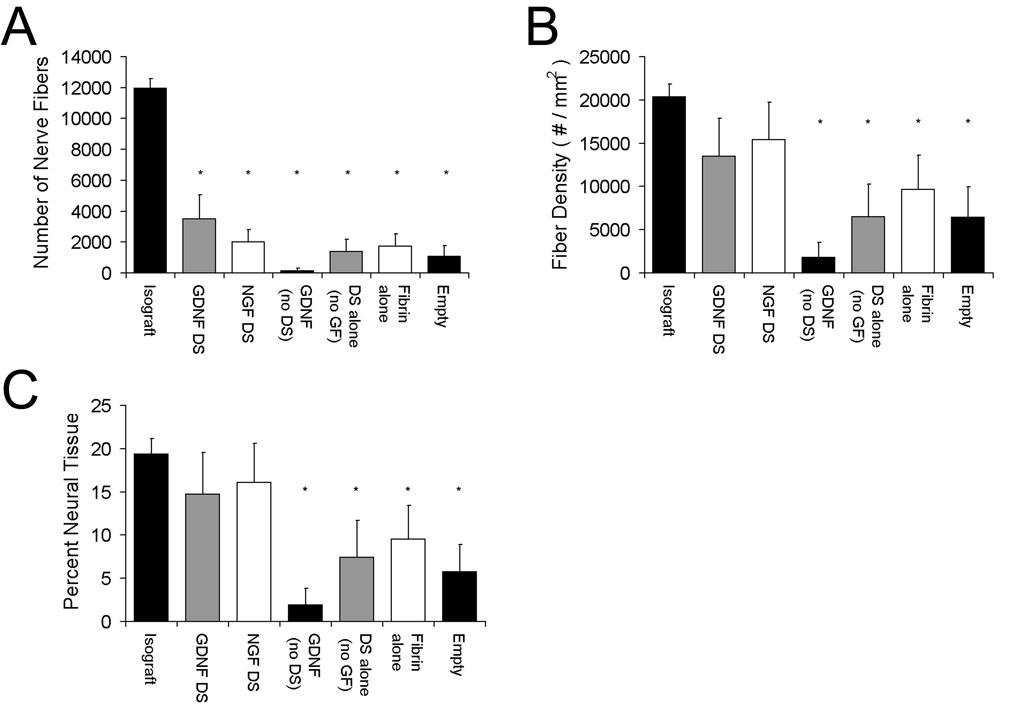
At 6 weeks, the average total myelinated fiber count, one measure of the effectiveness of neural regeneration, was 12,000 ± 600 fibers (n = 12) for the isograft, while the conduits with GDNF DS contained 3500 ± 1500 fibers (n = 12) (Fig. 3a). The NGF DS group had 2000 ± 770 fibers (n = 12) and the fibrin alone group had 1700 ± 800 fibers. The GDNF alone, DS alone (no GF) and empty conduit groups had little regeneration and resulted in fiber counts of less than 1700. The isograft had significantly more fibers than all other groups. The average number of fibers in a normal rat sciatic nerve is approximately 7200 ± 410 [46].
Fig. 3.
Histomorphometric analysis of nerves at the midline of the conduit (or graft). The total number of myelinated nerve fibers, density and percent neural tissue were measured by quantitative histomorphometry. No groups were similar to the isograft group in terms of total number of nerve fibers (A), but the delivery system with GDNF (GDNF DS) or NGF (NGF DS) was similar to the isograft in terms of fiber density (B) and percent neural tissue (C). Data (n = 12) are shown as mean ± SEM. *Statistical significance (p < 0.05) compared to the isograft.
Nerve fiber density is another measure of neural regeneration. The nerve fiber density at the midline for conduits was highest for the GDNF DS (~13,000 fibers mm−2) and NGF DS (~15,000 fibers mm−2) groups, and were not significantly different from the isograft controls (~20,000 fibers mm−2) (Fig. 3b). For normal sciatic nerve, the fiber density is ~12,000 fibers mm−2 [46]. Both groups incorporating the delivery system and growth factor also had the highest percentage of neural tissue at the midline of the conduit (15–16%) and were also not significantly different from the isografts (~19%) (Fig. 3c). This assessment provides a measure of quality of the regenerating nerve and suggests that the quality of nerve regeneration is better in the groups containing both the delivery system and growth factor compared to the control groups. According to these two measures, these two groups are also similar to the isograft group.
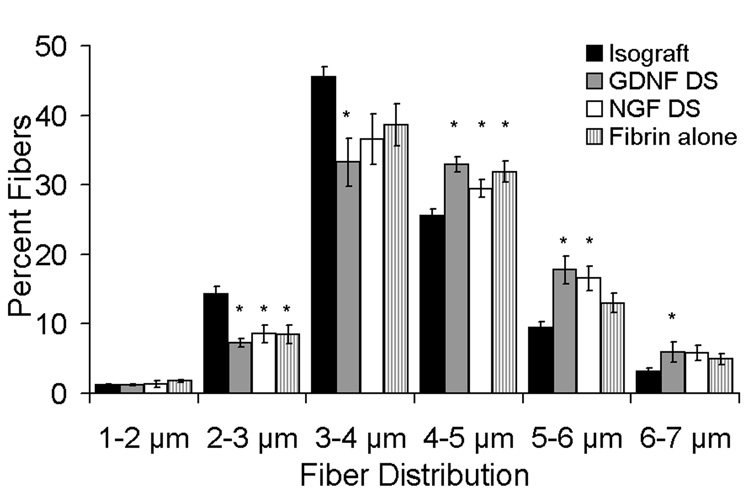
The myelinated nerve fiber width was assessed as a measure of maturity of the regenerating nerve fibers, and groups that were most effective in promoting neural regeneration were assessed for their fiber width distribution. All groups utilizing fibrin within conduits for regeneration contained fewer smaller nerve fibers (2–3 µm) compared to isograft controls (Fig. 4); furthermore, the GDNF DS group also contained fewer nerve fibers than the isograft in the 3–4 µm distribution. Both groups containing the delivery system and growth factor demonstrated higher percentages of larger nerve fibers (4–5, 5–6 µm) compared to the isograft, but only the GDNF DS group promoted the higher percentages of large-caliber nerve fibers (6–7 µm) (p<0.05) compared to the isograft, suggesting more mature regenerating fibers. The normal median rat sciatic nerve fiber width is ~6.5 µm [46].
Fig. 4.
Myelinated fiber size distribution of regenerating nerves at the midline of the conduit (or graft). The nerve fiber width was measured by quantitative histomorphometry. The percentage of large regenerating nerve fibers (4–5, 5–6 µm) were larger in the GDNF DS and NGF DS groups compared to the isograft group. GDNF DS had significantly the greatest percentage of the largest fibers (6–7µm) compared to the isograft. Data (n = 12) are shown as mean ± SEM. *Statistical significance (p < 0.05) compared to the isograft.
3.4. Electron microscopy
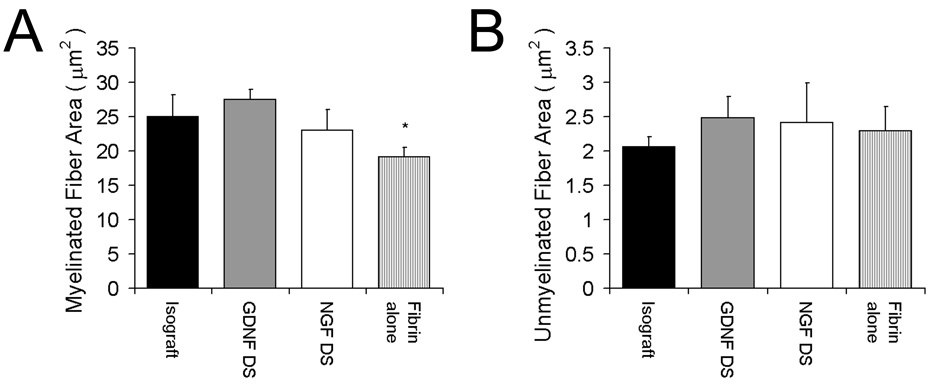
Electron microscopy was performed to evaluate the regenerative nerve ultrastructure. Representative sections from groups that were most effective in promoting nerve regeneration (GDNF DS, NGF DS, fibrin alone and isograft) are shown in Fig. 5. The myelinated and unmyelinated fiber areas were determined from randomly selected specimens from each group by a researcher blinded to the groups (n = 3). Qualitatively, the GDNF DS group again appeared to have more organized structure and more uniform fiber shape with larger myelinated fibers than the other experimental groups including the isograft, while fibrin alone had the most disorganized appearance and less uniform fiber shapes. The myelinated area of the GDNF DS (27 ± 2.4 µm2) and NGF DS (23 ± 5.1 µm2) groups were equivalent to the isograft (25 ± 5.5 µm2), whereas fibrin alone (19 ± 1.4 µm2) had significantly less myelinated fiber area (Fig. 6a). There were no differences among groups for unmyelinated fiber area (Fig. 6b).
Fig. 5.
Electron micrographs of regenerating nerves at the midline of the conduit (or graft). (A) Fibrin alone; (B) isograft; (C) delivery system incorporating GDNF (GDNF DS); (D) delivery system incorporating NGF (NGF DS); (E) normal uninjured nerve. Ultrathin (70 nm) sections of the embedded tissues were cut and stained with uranyl acetate-lead citrate. Qualitatively, GDNF DS and NGF DS appeared to have more uniform fiber structures with larger myelinated fibers than fibrin alone, which had the most disorganized fiber appearance. Scale bar, 5 µm.
Fig. 6.
Myelinated and unmyelinated fiber areas of regenerating nerves at the midline of the conduit (or graft). The myelinated and unmyelinated fiber areas were determined from randomly selected specimens by electron microscopy from each group. The myelinated areas of for groups with the delivery system incorporating GDNF (GDNF DS) or NGF (NGF DS) were equivalent to the isograft, whereas the fibrin alone group had a lower myelinated fiber area (A). There were no differences among groups in unmyelinated fiber area (B). Data (n = 3) are shown as mean ± SEM. *Statistical significance (p < 0.05) compared to the isograft.
4. Discussion
Peripheral nerve injuries are devastating and alternatives to standard repairs of nerve gaps rarely lead to complete clinical recovery. In this study we examined the effect of growth factor delivery using an NGC to bridge and enhance nerve regeneration across a critical nerve gap length. We used an affinity-based delivery system within a fibrin matrix to immobilize growth factors, slow their diffusion from the matrix and allow release by cell-mediated degradation of the matrix, thus controlling their delivery to the regenerative site. This “cell-mediated” matrix degradation and subsequent growth factor release may be facilitated through the processes of axonal outgrowth and cell-induced protease activation (e.g. plasmin activation on the growth cone) [47–51].
We have previously examined controlled release of NGF with this delivery system in vivo and found that affinity-based release of NGF increased myelinated nerve fiber sprouting and outgrowth compared to diffusion-based release from fibrin matrices alone [11]. In the present study, we focused on the controlled delivery of another neurotrophic factor, GDNF, to investigate its effect on peripheral nerve regeneration in a sciatic nerve injury model. We found that controlled delivery of GDNF was superior to controls in aspects of nerve regeneration, including neural fiber size and organized nerve architecture, suggesting more mature neural content. The use of our affinity-based delivery system directly affected regeneration across a 13 mm gap and the inclusion of the delivery system sequestering GDNF had a greater effect on eliciting neural regeneration than providing GDNF in an unbound form to the regenerative site. This effect may be explained by the delivery’s system’s ability to sequester GDNF and avoid an initial burst of drug release, as found in vitro [42]. Others have cited initial bursts of GDNF release to be detrimental to nerve regeneration [17].
We anticipated the ability of an exogenous fibrin matrix, with or without an affinity-based delivery system, to facilitate bridging a critical nerve defect because a fibrin matrix naturally forms within an empty silicone conduit connecting the damaged ends of rat sciatic nerve over a 1 week period [14]. However, we were surprised to see that three out of 12 empty conduits had regeneration across this critical defect, although the neural content was histologically poorly organized. Nerve regeneration has been shown to be inconsistent beyond a 10 mm defect without the addition of other components, such as extracellular matrix molecules, Schwann cells, plasma or neurotrophic factors [14,43]. The regeneration in our empty conduits may be a result of superior neural regeneration for rats, as it has been shown that the rat is able to spontaneously regenerate into an unfilled 4.5 cm nerve gap to a distance of 2.4 cm after 5 months [52]. Despite the regeneration in some of the controls, the inclusion of our affinity-based delivery system incorporating GDNF or NGF more closely approximated neural regeneration of an isograft across a critical gap defect.
The efficacy of affinity-based delivery of GDNF or NGF to the regenerative site was also observed in the histomorphometry and electron microscopy results. Both groups containing the delivery system and neurotrophic factors were not statistically different from the isograft controls with regards to fiber density and percent neural tissue, both measures of nerve quality. Beyond enhancing the quality of regeneration, we found that the delivery of GDNF and NGF from the affinity-based delivery system improved the maturity of the regenerating fibers. It is well known that axon size and myelin thickness are measures of maturity [53–56]. Both growth factors increased fiber maturity, as seen in histomorphometric fiber distributions as well as myelinated fiber area from electron microscopy. Our findings suggest that, although not equivalent in fiber number, the delivery of GDNF by our delivery system produces larger, mature nerve fibers. Of particular importance, the GDNF group had more 6–7 µm diameter fibers than all other groups, including the isograft. In the normal, uninjured sciatic rat nerve, the average myelinated fiber width is 6–7 µm. Furthermore, studies have shown that larger axon diameter and myelination result in larger conduction velocity and can be correlated to greater function as compared to smaller, less myelinated fibers [55,56]. Although we did not look at functional outcomes in this study, parallel studies are underway to explore if these larger diameter fibers result in greater return of function.
Our study corroborates previous findings in the literature on the effect of GDNF on peripheral nerve regeneration. Barras et al. [17] evaluated the effects of GDNF delivery from an ethylene vinyl acetate polymer rod across an 8 mm rat facial nerve defect. Similar to our results, they found that GDNF delivery increased myelination and fiber size. In this pure motor model, they also found that GDNF promoted increased motor neuron labeling in comparison to neurotrophin-3, suggesting that GDNF has a significant role in motor nerve regeneration. Given the strong evidence of GDNF in motor neuron survival and enhancement of peripheral nerve regeneration, further studies looking at the affinity-based delivery of GDNF to specific motor and sensory nerves will be performed to elucidate this neurotrophic factor’s impact on modality specific regeneration.
In addition, Fine et al. [18] evaluated axonal regeneration across a 15 mm long gap in the rat sciatic nerve in the presence of GDNF or NGF provided by synthetic nerve guidance channels continuously releasing the neurotrophic factors. They found that the average number of myelinated axons at the midpoint of the regenerated nerves was greater in the presence of GDNF than in the presence of NGF. The GDNF group also had significantly greater numbers of retrograde labeled motoneurons. Thus, these authors report GDNF to be more effective than NGF in overall regeneration. In contrast, we did not find significant differences between GDNF and NGF, except in fiber size distribution. The difference in our findings may be attributed to our method of delivery. In particular, Fine et al. used diffusion-based delivery of GDNF or NGF from a biodegradable conduit where the dose delivered to the regenerating nerve was constant for a period of time. Our delivery method relies on cell-mediated release of sequestered growth factor from a luminal matrix, which allows the release rate to vary based on the presence of cells and position within the NGC. This result suggests that this method of growth factor delivery may play a role in regeneration. The effect of delivery rate has been noted by others, such as Piquilloud et al. [20], who used a resorbable conduit that released GDNF at three different delivery rates and found differences in neural regeneration due to release rate.
We examined our delivery system with a silicone NGC because of the biocompatibility of the product, its mechanical stability and the well known critical defect length in our surgical model. However, clinically, silicone conduits are not ideal and have associated morbidities. Silicone conduits have been reported to cause chronic nerve compression, irritation at the implantation site requiring removal, and inflammatory and fibrotic reactions impacting nerve regeneration [3,57,58]. Thus, the combination of our drug delivery system with a biodegradable conduit would be more desirable for clinical peripheral nerve injury repairs. Future studies directed toward this goal would be of certain benefit in translating our delivery system into clinical practice.
Finally, previous studies have demonstrated that both NGF and GDNF can enhance peripheral nerve regeneration [18,59]. However, NGF has been shown to primarily promote sensory nerve regeneration in the peripheral nervous system [59,60] as motor neurons do not express NGF or its receptors [59]. Our study further confirms the assertion that the delivery of these neurotrophic factors enhances peripheral nerve regeneration in a sciatic nerve model, a model that contains both sensory and motor fibers. Given that that GDNF and NGF affect different cell populations [59] and in vitro work demonstrates increased neurite outgrowth with the combination of the two factors compared to either alone [61], an interesting investigation would be to evaluate the possible synergistic effects of delivering a combination of both neurotrophic factors on peripheral nerve regeneration.
5. Conclusions
The goal of this study was to evaluate and elucidate the efficacy of affinity-based delivery of GDNF to the regenerative site in a critical size defect model. We examined histological outcomes of this neurotrophic factor and compared it to controls as well as NGF. Given the increased maturity and organized architecture of the regenerating nerve under the influence GDNF, we believe affinity-based delivery of GDNF offers an insight into potential future alternatives for the treatment of peripheral nerve injuries.
Acknowledgements
The authors thank the WF Coulter Foundation Translational Research Award, the American Association of Plastic Surgeons Academic Scholar Award and the National Institutes of Health (R01NS051706) for funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No benefit of any kind will be received either directly or indirectly by the authors.
References
- 1.Meek MF, Coert JH. Clinical use of nerve conduits in peripheral-nerve repair: review of the literature. J Reconstr Microsurg. 2002;18:97–109. doi: 10.1055/s-2002-19889. [DOI] [PubMed] [Google Scholar]
- 2.Chen MB, Zhang F, Lineaweaver WC. Luminal fillers in nerve conduits for peripheral nerve repair. Ann Plast Surg. 2006;57:462–471. doi: 10.1097/01.sap.0000237577.07219.b6. [DOI] [PubMed] [Google Scholar]
- 3.Battiston B, Geuna S, Ferrero M, Tos P. Nerve repair by means of tubulization: literature review and personal clinical experience comparing biological and synthetic conduits for sensory nerve repair. Microsurgery. 2005;25:258–267. doi: 10.1002/micr.20127. [DOI] [PubMed] [Google Scholar]
- 4.Chamberlain LJ, Yannas IV, Arrizabalaga A, Hsu HP, Norregaard TV, Spector M. Early peripheral nerve healing in collagen and silicone tube implants: myofibroblasts and the cellular response. Biomaterials. 1998;19:1393–1403. doi: 10.1016/s0142-9612(98)00018-0. [DOI] [PubMed] [Google Scholar]
- 5.Labrador RO, Buti M, Navarro X. Influence of collagen and laminin gels concentration on nerve regeneration after resection and tube repair. Exp Neurol. 1998;149:243–252. doi: 10.1006/exnr.1997.6650. [DOI] [PubMed] [Google Scholar]
- 6.Chen YS, Hsieh CL, Tsai CC, Chen TH, Cheng WC, Hu CL, Yao CH. Peripheral nerve regeneration using silicone rubber chambers filled with collagen, laminin and fibronectin. Biomaterials. 2000;21:1541–1547. doi: 10.1016/s0142-9612(00)00028-4. [DOI] [PubMed] [Google Scholar]
- 7.Labrador RO, Buti M, Navarro X. Peripheral nerve repair: role of agarose matrix density on functional recovery. Neuroreport. 1995;6:2022–2026. [PubMed] [Google Scholar]
- 8.Yu XJ, Bellamkonda RV. Tissue-engineered scaffolds are effective alternatives to autografts for bridging peripheral nerve gaps. Tissue Engineering. 2003;9:421–430. doi: 10.1089/107632703322066606. [DOI] [PubMed] [Google Scholar]
- 9.Ohta M, Suzuki Y, Chou H, Ishikawa N, Suzuki S, Tanihara M, Mizushima Y, Dezawa M, Ide C. Novel heparin/alginate gel combined with basic fibroblast growth factor promotes nerve regeneration in rat sciatic nerve. J Biomed Mater Res A. 2004;71:661–668. doi: 10.1002/jbm.a.30194. [DOI] [PubMed] [Google Scholar]
- 10.Mohanna PN, Terenghi G, Wiberg M. Composite phb-ggf conduit for long nerve gap repair: a long-term evaluation. Scand J Plast Reconstr Surg Hand Surg. 2005;39:129–137. doi: 10.1080/02844310510006295. [DOI] [PubMed] [Google Scholar]
- 11.Lee AC, Yu VM, Lowe JB, 3rd, Brenner MJ, Hunter DA, Mackinnon SE, Sakiyama-Elbert SE. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp Neurol. 2003;184:295–303. doi: 10.1016/s0014-4886(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 12.Galla TJ, Vedecnik SV, Halbgewachs J, Steinmann S, Friedrich C, Stark GB. Fibrin/schwann cell matrix in poly-epsilon-caprolactone conduits enhances guided nerve regeneration. Int J Artif Organs. 2004;27:127–136. doi: 10.1177/039139880402700208. [DOI] [PubMed] [Google Scholar]
- 13.Marcol W, Kotulska K, Larysz-Brysz M, Matuszek I, Olakowska E, Lewin-Kowalik J. Extracts obtained from predegenerated nerves improve functional recovery after sciatic nerve transection. Microsurgery. 2005;25:486–494. doi: 10.1002/micr.20155. [DOI] [PubMed] [Google Scholar]
- 14.Williams LR, Longo FM, Powell HC, Lundborg G, Varon S. Spatial–temporal progress of peripheral nerve regeneration within a silicone chamber: parameters for a bioassay. J Comp Neurol. 1983;218:460–470. doi: 10.1002/cne.902180409. [DOI] [PubMed] [Google Scholar]
- 15.Thiagarajan P, Rippon AJ, Farrell DH. Alternative adhesion sites in human fibrinogen for vascular endothelial cells. Biochemistry. 1996;35:4169–4175. doi: 10.1021/bi952532b. [DOI] [PubMed] [Google Scholar]
- 16.Chernousov MA, Carey DJ. Alphavbeta8 integrin is a schwann cell receptor for fibrin. Exp Cell Res. 2003;291:514–524. doi: 10.1016/s0014-4827(03)00409-9. [DOI] [PubMed] [Google Scholar]
- 17.Barras FM, Pasche P, Bouche N, Aebischer P, Zurn AD. Glial cell line-derived neurotrophic factor released by synthetic guidance channels promotes facial nerve regeneration in the rat. J Neurosci Res. 2002;70:746–755. doi: 10.1002/jnr.10434. [DOI] [PubMed] [Google Scholar]
- 18.Fine EG, Decosterd I, Papaloizos M, Zurn AD, Aebischer P. Gdnf and ngf released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur J Neurosci. 2002;15:589–601. doi: 10.1046/j.1460-9568.2002.01892.x. [DOI] [PubMed] [Google Scholar]
- 19.Whittlesey KJ, Shea LD. Delivery systems for small molecule drugs, proteins, and DNA: the neuroscience/biomaterial interface. Exp Neurol. 2004;190:1–16. doi: 10.1016/j.expneurol.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Piquilloud G, Christen T, Pfister LA, Gander B, Papaloizos MY. Variations in glial cell line-derived neurotrophic factor release from biodegradable nerve conduits modify the rate of functional motor recovery after rat primary nerve repairs. Eur J Neurosci. 2007;26:1109–1117. doi: 10.1111/j.1460-9568.2007.05748.x. [DOI] [PubMed] [Google Scholar]
- 21.Dodla MC, Bellamkonda RV. Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps. Biomaterials. 2008;29:33–46. doi: 10.1016/j.biomaterials.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfister LA, Alther E, Papaloizos M, Merkle HP, Gander B. Controlled nerve growth factor release from multi-ply alginate/chitosan-based nerve conduits. Eur J Pharm Biopharm. 2008 doi: 10.1016/j.ejpb.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Burdick JA, Ward M, Liang E, Young MJ, Langer R. Stimulation of neurite outgrowth by neurotrophins delivered from degradable hydrogels. Biomaterials. 2006;27:452–459. doi: 10.1016/j.biomaterials.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 24.Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. Journal of Controlled Release. 2000;69:149–158. doi: 10.1016/s0168-3659(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 25.Sakiyama SE, Schense JC, Hubbell JA. Incorporation of heparin-binding peptides into fibrin gels enhances neurite extension: an example of designer matrices in tissue engineering. Faseb J. 1999;13:2214–2224. doi: 10.1096/fasebj.13.15.2214. [DOI] [PubMed] [Google Scholar]
- 26.Schense JC, Hubbell JA. Cross-linking exogenous bifunctional peptides into fibrin gels with factor xiiia. Bioconjugate Chemistry. 1999;10:75–81. doi: 10.1021/bc9800769. [DOI] [PubMed] [Google Scholar]
- 27.Ichinose A, Tamaki T, Aoki N. Factor xiii-mediated cross-linking of NH2-terminal peptide of alpha 2-plasmin inhibitor to fibrin. FEBS Letters. 1983;153:369–371. doi: 10.1016/0014-5793(83)80645-0. [DOI] [PubMed] [Google Scholar]
- 28.Kimura S, Tamaki T, Aoki N. Acceleration of fibrinolysis by the N-terminal peptide of alpha 2-plasmin inhibitor. Blood. 1985;66:157–160. [PubMed] [Google Scholar]
- 29.Yamada KM. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]
- 30.Taylor SJ, McDonald JW, Sakiyama-Elbert SE. Controlled release of neurotrophin-3 from fibrin gels for spinal cord injury. Journal of Controlled Release. 2004;98:281–294. doi: 10.1016/j.jconrel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Taylor SJ, Sakiyama-Elbert SE. Effect of controlled delivery of neurotrophin-3 from fibrin on spinal cord injury in a long term model. J Control Release. 2006;116:204–210. doi: 10.1016/j.jconrel.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gelberman RH, Thomopoulos S, Sakiyama-Elbert SE, Das R, Silva MJ. The early effects of sustained platelet-derived growth factor administration on the functional and structural properties of repaired intrasynovial flexor tendons: an in vivo biomechanic study at 3 weeks in canines. J Hand Surg [Am] 2007;32:373–379. doi: 10.1016/j.jhsa.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Wood MD, Sakiyama-Elbert SE. Release rate controls biological activity of nerve growth factor released from fibrin matrices containing affinity-based delivery systems. J Biomed Mater Res A. 2008;84:300–312. doi: 10.1002/jbm.a.31269. [DOI] [PubMed] [Google Scholar]
- 34.Henderson CE, Camu W, Mettling C, Gouin A, Poulsen K, Karihaloo M, Rullamas J, Evans T, McMahon SB, Armanini MP, et al. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 1993;363:266–270. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Wu W, Lin LF, Lei M, Oppenheim RW, Houenou LJ. Rescue of adult mouse motoneurons from injury-induced cell death by glial cell line-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:9771–9775. doi: 10.1073/pnas.92.21.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oppenheim RW, Houenou LJ, Johnson JE, Lin LF, Li L, Lo AC, Newsome AL, Prevette DM, Wang S. Developing motor neurons rescued from programmed and axotomy-induced cell death by gdnf. Nature. 1995;373:344–346. doi: 10.1038/373344a0. [DOI] [PubMed] [Google Scholar]
- 37.Yan Q, Matheson C, Lopez OT. In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature. 1995;373:341–344. doi: 10.1038/373341a0. [DOI] [PubMed] [Google Scholar]
- 38.Oppenheim RW, Houenou LJ, Parsadanian AS, Prevette D, Snider WD, Shen L. Glial cell line-derived neurotrophic factor and developing mammalian motoneurons: regulation of programmed cell death among motoneuron subtypes. J Neurosci. 2000;20:5001–5011. doi: 10.1523/JNEUROSCI.20-13-05001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoke A, Gordon T, Zochodne DW, Sulaiman OA. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term schwann cell denervation. Exp Neurol. 2002;173:77–85. doi: 10.1006/exnr.2001.7826. [DOI] [PubMed] [Google Scholar]
- 40.Trupp M, Ryden M, Jornvall H, Funakoshi H, Timmusk T, Arenas E, Ibanez CF. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J Cell Biol. 1995;130:137–148. doi: 10.1083/jcb.130.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naveilhan P, ElShamy WM, Ernfors P. Differential regulation of mRNAs for GDNF and its receptors RET and GDNFR alpha after sciatic nerve lesion in the mouse. Eur J Neurosci. 1997;9:1450–1460. doi: 10.1111/j.1460-9568.1997.tb01499.x. [DOI] [PubMed] [Google Scholar]
- 42.Wood MD, Borschel GH, Sakiyama-Elbert SE. Controlled release of glial-derived neurotrophic factor from fibrin matrices containing an affinity-based delivery system. J Biomed Mater Res A. 2008 doi: 10.1002/jbm.a.32043. [DOI] [PubMed] [Google Scholar]
- 43.Lundborg G, Dahlin LB, Danielsen N, Gelberman RH, Longo FM, Powell HC, Varon S. Nerve regeneration in silicone chambers: Influence of gap length and of distal stump components. Exp Neurol. 1982;76:361–375. doi: 10.1016/0014-4886(82)90215-1. [DOI] [PubMed] [Google Scholar]
- 44.Tyler-Cross R, Sobel M, Marques D, Harris RB. Heparin binding domain peptides of antithrombin iii: analysis by isothermal titration calorimetry and circular dichroism spectroscopy. Protein Science. 1994;3:620–627. doi: 10.1002/pro.5560030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunter DA, Moradzadeh A, Whitlock EL, Brenner MJ, Myckatyn TM, Wei CH, Tung TH, Mackinnon SE. Binary imaging analysis for comprehensive quantitative histomorphometry of peripheral nerve. J Neurosci Methods. 2007;166:116–124. doi: 10.1016/j.jneumeth.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mackinnon SE, Dellon AL, O'Brien JP. Changes in nerve fiber numbers distal to a nerve repair in the rat sciatic nerve model. Muscle Nerve. 1991;14:1116–1122. doi: 10.1002/mus.880141113. [DOI] [PubMed] [Google Scholar]
- 47.Kalderon N. Schwann cell proliferation and localized proteolysis: expression of plasminogen-activator activity predominates in the proliferating cell populations. Proc Natl Acad Sci U S A. 1984;81:7216–7220. doi: 10.1073/pnas.81.22.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krystosek A, Seeds NW. Peripheral neurons and schwann cells secrete plasminogen activator. J Cell Biol. 1984;98:773–776. doi: 10.1083/jcb.98.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alvarez-Buylla A, Valinsky JE. Production of plasminogen activator in cultures of superior cervical ganglia and isolated schwann cells. Proc Natl Acad Sci U S A. 1985;82:3519–3523. doi: 10.1073/pnas.82.10.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pittman RN, Buettner HM. Degradation of extracellular matrix by neuronal proteases. Dev Neurosci. 1989;11:361–375. doi: 10.1159/000111912. [DOI] [PubMed] [Google Scholar]
- 51.Herbert CB, Bittner GD, Hubbell JA. Effects of fibrinolysis on neurite growth from dorsal root ganglia cultured in two- and three-dimensional fibrin gels. Journal of Comparative Neurology. 1996;365:380–391. doi: 10.1002/(SICI)1096-9861(19960212)365:3<380::AID-CNE4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 52.Mackinnon SE, Hudson AR, Hunter DA. Histologic assessment of nerve regeneration in the rat. Plast Reconstr Surg. 1985;75:384–388. doi: 10.1097/00006534-198503000-00014. [DOI] [PubMed] [Google Scholar]
- 53.Aitken JT, Sharman M, Young JZ. Maturation of regenerating nerve fibres with various peripheral connexions. J Anat. 1947;81:1–22. 22. [PubMed] [Google Scholar]
- 54.Young JZ. Narrowing of nerve fibres at the nodes of ranvier. J Anat. 1949;83:55. [PubMed] [Google Scholar]
- 55.Williams PL, Wendell-Smith CP. Some additional parametric variations between peripheral nerve fibre populations. J Anat. 1971;109:505–526. [PMC free article] [PubMed] [Google Scholar]
- 56.Fraher J, Dockery P. A strong myelin thickness–axon size correlation emerges in developing nerves despite independent growth of both parameters. J Anat. 1998;193(Pt 2):195–201. doi: 10.1046/j.1469-7580.1998.19320195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Merle M, Dellon AL, Campbell JN, Chang PS. Complications from silicon-polymer intubulation of nerves. Microsurgery. 1989;10:130–133. doi: 10.1002/micr.1920100213. [DOI] [PubMed] [Google Scholar]
- 58.Dellon AL. Use of a silicone tube for the reconstruction of a nerve injury. J Hand Surg [Br] 1994;19:271–272. doi: 10.1016/0266-7681(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 59.Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27:277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- 60.Terenghi G. Peripheral nerve regeneration and neurotrophic factors. J Anat. 1999;194(Pt 1):1–14. doi: 10.1046/j.1469-7580.1999.19410001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deister C, Schmidt CE. Optimizing neurotrophic factor combinations for neurite outgrowth. J Neural Eng. 2006;3:172–179. doi: 10.1088/1741-2560/3/2/011. [DOI] [PubMed] [Google Scholar]