Abstract
Rationale
Neuropeptides are linked to the psychopathology of stimulants of abuse, principally through dopamine mechanisms. Substance P (SP) is one of these neuropeptides and is associated with both limbic and extrapyramidal dopaminergic pathways and likely contributes to the pharmacology of these stimulants. The effects of nicotine on these dopamine systems has also been extensively studied, however, its effects on the associated SP pathways, have received little attention.
Objectives
In the present study, we elucidated the effects of nicotine treatment on limbic and extrapyramidal SP systems by measuring changes in associated SP tissue concentrations.
Materials and methods
Male Sprague-Dawley rats received (±) nicotine 4.0 mg/kg/day (0.8 mg/kg, intraperitoneally; five injections at 2-h intervals) in the presence or absence of selective dopamine D1 and D2 receptor antagonists or a nonselective nicotinic acetylcholine receptor antagonist.
Results
The nicotine treatment significantly but temporarily decreased substance P-like immunoreactivity (SPLI) content in the ventral tegmental area (VTA) and substantia nigra 12–18 h after drug exposure. The nicotine-mediated changes in SPLI were selectively blocked by pretreatment with mecamylamine as well as a dopamine D1, D2, or both receptor antagonists. Other brain areas that also selectively demonstrated nicotine-related declines in SPLI content included prefrontal cortex, the nucleus accumbens shell and the very posterior caudate.
Conclusions
These findings indicate that some limbic and basal ganglia SP systems are significantly affected by exposure to nicotine through processes mediated by nicotinic and dopaminergic receptors, suggesting a role for SP pathways in nicotine’s limbic and extrapyramidal effects.
Keywords: substance P, nicotine, mecamylamine, dopamine receptor, nicotinic receptor, ventral tegmental area, substantia nigra, prefrontal cortex, nucleus accumbens
Introduction
Cigarette smoking is responsible for approximately one in five deaths in the United States accounting for approximately 438,000 deaths annually. In addition, each year cigarette smoking costs the economy of this country over $42 billion in health care costs and lost productivity (for review, see Center for Disease Control and Prevention 2005). Nicotine is widely regarded as the active pharmacological ingredient of tobacco that is principally responsible for addiction to this substance. The cause of addiction, as well as rewarding and cognitive properties of nicotine, is thought to be associated with the midbrain dopamine (DA) systems (Nisell et al. 1996). Thus, the limbic DA pathways have been identified as key components in these responses due to the presence of nicotinic receptors (Aghajnian and Bunney 1977; Jones et al. 2001; Zoli et al. 2002; Picciotto 2003; Laviolette and van der Kooy 2004; Hamada et al. 2004; David et al. 2006; Quarta et al. 2007) and their influence on related DA functions. These DA pathways consist of cell bodies in the ventral tegmental area (VTA) that project mainly to the nucleus accumbens in the ventral striatum (Adinoff 2004) and the frontal cortex (Nisell et al. 1996; Cao et al. 2005) and are activated by exposure to nicotine (Role and Berg 1996; Wonnacott 1997; Marshall et al. 1997; Hamada et al. 2004; Quarta et al. 2007). Another midbrain DA projection that is also activated by nicotine and likely contributes to the pharmacological effects of this stimulant, originates in the substantia nigra, an extrapyramidal structure, and primarily projects to the dorsal striatum (Laviolette and van der Kooy 2004).
While considerable research on the interaction between nicotinic and mesolimbic and mesocortical dopaminergic systems has been reported with the exception of a recent report that neurotensin systems are affected by nicotine treatment (Alburges et al. 2007), there has been little study of the potential role of DA-related neuropeptide systems in mediating the neuropharmacological effects of nicotine (Naftchi et al. 1988; Singer et al. 2004; Alburges et al. 2007). Because of this lack of information concerning the influence of nicotine on neuropeptides, in the present research we investigated the effects of nicotine on substance P (SP), an undecapeptide that is considered to have neurotransmitter functions and is closely aligned with both basal ganglia and limbic dopaminergic neurons.
Studies have indicated that SP is associated with cell bodies of medium spiny striatal neurons (Gerfen et al. 1990; Kawaguchi et al. 1990) that project ipsilaterally to the substantia nigra where its fibers terminate primary within the zone reticulata of this brain region (Hong et al. 1977; Gale et al. 1977; Brownstein et al. 1977; Mroz et al. 1977; Kanazawa et al. 1977) with collateral projections to the globus pallidus. The role of SP in the striatonigral pathway has been extensively investigated (Davies and Dray 1976; Cheramy et al. 1977; Waldmeier et al. 1978; James and Starr 1979; Kelley and Iversen 1979) and is thought to tonically excite ascending nigrostriatal dopaminergic neurons (Davies and Dray 1976; Cheramy et al. 1977; Waldmeier et al. 1978; James and Starr 1979; Kelley and Iversen 1979). This type of interaction between SP and central dopaminergic systems is proposed to play an important role in mediating behavior under basal ganglia dopamine regulation (James and Starr 1979; Kelley and Iversen 1979). In addition, because the injection of this tachykinin into the VTA (Cador et al. 1989; Kelley et al. 1989; Kelley and Delfs 1991; West and Michael 1991), frontal cortex (Krasnova et al. 2000), nucleus accumbens (Kalivas and Miller 1984; Nikolaev et al. 2004), striatum (Tang et al. 1998; Preston et al. 2000; Krasnova et al. 2000), or substantia nigra (Tan and Tsou 1988a,b) increases neuronal firing and dopamine turnover, it is not surprising that drugs which alter dopamine activity associated with these systems would also influence associated SP pathways. In fact, previous studies demonstrated that changes in activity of the dopaminergic pathways induced by amphetamines (Ritter et al. 1984, 1985; Sonsalla et al. 1984, 1986; Hanson et al. 1986a,b, 2002; Bannon et al. 1987; Ujike et al. 1988) or cocaine (Alburges et al. 2000; Kraft et al. 2001) significantly alter SP systems in limbic and basal ganglia regions. Thus, as part of a previous report on the responses of neurotensin systems to nicotine, we also indicated that this drug reduces SP levels in the VTA (Alburges et al. 2007). However, in this earlier study the response of SP pathways to nicotine was only briefly mentioned and not examined nor characterized.
To appreciate how SP function in the central nervous system (CNS) is altered by nicotine exposure, the responses of the mesocortical and mesolimbic SP systems to treatment by this stimulant were examined in detail and the underlying mechanism studied. This was accomplished by measuring the contents of substance P-like immunoreactivity (SPLI) in brain regions associated with these limbic areas, following multiple administrations of nicotine alone and in combination with other drugs. Based on previous reports it has been demonstrated that drug-induced changes in SPLI tissue levels likely reflect alterations in SP turnover due to its release and/or synthesis (Hanson et al. 2002; Kraft et al. 2001; Loonam et al. 2003; Adams et al. 2001).
In the present study, we observed that multiple nicotine administrations significantly reduced SPLI content in the VTA and substantia nigra as well as in prefrontal cortex, nucleus accumbens shell, and very posterior caudate 12–18 hours after treatment. All of these nicotine-mediated SP changes recovered by 48 hours. In the VTA this nicotine effect was selectively blocked by pretreatment with either a dopamine D1 or D2 receptor antagonist. Furthermore, in the substantia nigra the nicotine effect was selectively blocked by a specific dopamine D2, but not a dopamine D1, receptor antagonist. In addition, pretreatment with the nonselective nicotinic acetylcholine receptor antagonist, mecamylamine, completely prevented the nicotine-induced effect on all affected SP systems examined. The significance of these findings is discussed.
Materials and Methods
Animals
Male Sprague-Dawley rats (Charles River Laboratories, Raleigh, NC) weighing 250–330 g were maintained in a temperature-controlled environment and cared for according to National Institutes of Health guidelines. Animals were kept on a 12-h light/dark cycle with food and water available ad libitum. Animals were allowed to acclimate for at least 2 weeks before their use. All the experiments were performed according to the guidelines of the University of Utah Institutional Animal Care and Use Committee.
Drug treatment and tissue dissection
Following the acclimatization period, two experimental protocols were used in this study: (a) animals were injected with 5 administrations of either saline (1.0 ml/kg, intraperitoneally [i.p.]) or (±) nicotine freebase 4.0 mg/kg/day (0.8 mg/kg, i.p.; 5 injections at 2-h intervals) and were killed 12, 18 or 48 h after treatment; (b) animals were pretreated with dopamine D1 (SCH 23390; 0.5 mg/kg/injection , i.p.), dopamine D2 (eticlopride; 0.5 mg/kg/injection, i.p.), or nicotine (mecamylamine; 3.0 mg/kg/injection, subcutaneously [s.c.]) receptor antagonists 15 min prior to each of the five administrations of (±) nicotine or saline and killed 18 h after treatment. Brains were removed rapidly, frozen immediately on dry ice and stored at −80 °C. For regional studies, brain areas were dissected from consecutive 1-mm thick coronal slices as previously described (Alburges et al. 2001a,b). Based on the atlas of Paxinos and Watson (1986), the caudate was dissected into medial anterior caudate (1.20 mm anterior to bregma), medial posterior caudate (0.20 mm anterior to bregma), and very posterior caudate (0.80 mm posterior to bregma) regions. The prefrontal cortex, nucleus acumbens (shell and core), ventral tegmental area, and substantia nigra regions were also removed. All tissue samples were subsequently stored at −80 °C until assayed for SPLI. The selection of the nicotine dose (0.8 mg/kg, i.p.) was based on preliminary experiments (data not shown), has been previously used (Kane et al. 2000, 2001, 2005; Li and Kane 2003; Li et al. 2000; Matta et al. 2007; Alburges et al. 2007), and is intended to mimic a drug exposure pattern expected from average human smokers. The time response paradigm selected in this study was based on previous studies (Ritter et al. 1984, 1985; Sonsalla et al. 1984, 1986; Hanson et al. 1986a,b, 2002; Alburges et al. 2000), which demonstrated that administration of methamphetamine or cocaine produce significant changes in extrapyramidal and limbic SP systems.
Drugs
Eticlopride hydrochloride (S(-)-3-Chloro-5-ethyl-N-[(1-ethyl-2-pyrrolidinyl)methyl]-6-hydroxy-2-methoxy-benzamide hydrochloride), SCH 23390 hydrochloride (R(+)-7-Chloro-8-hydroxy-3-methyl-1-phenyl-2, 3, 4, 5-tetrahydro-1H-3-benzazepine hydrochloride), mecamylamine hydrochloride, and (±) nicotine were acquired from Sigma-Aldrich (St. Louis, MO). All doses were calculated as freebase of the drug and were freshly prepared in physiological saline solution (0.9 % w/v NaCl, pH 7.4).
Radioimmunoassay
The solid-phase radioimmunoassay used to analyze the neuropeptide levels in this study was adapted from the methods previously described (Maidment et al. 1991; Alburges et al. 2001a,b). Tissue samples were homogenized in 300 µl of 0.01 N HCl. The resulting homogenate was then placed in boiling water for 10 min in order to inactivate peptidases. Homogenates were centrifuged (17,000 × g) for 30 min. Supernatant was then collected and an aliquot was used to determine the total protein for each tissue sample by the method of Bradford (1976). Remaining sample was lyophilized overnight and stored at −80°C until the radioimmunoassay was performed. The concentration of neuropeptide was determined with a modified solid-phase radioimmunoassay technique described for neurotensin by Maidment et al. (1991). Lyophilized samples were reconstituted in 300 µl phosphate-buffered saline (pH 7.4) containing 0.1% (w/v/) gelatin and 0.1% (v/v) Triton X-100. Nunc-Immunoplates (ISC BioExpress, Kaysville, UT) were incubated overnight at 4°C with 50 µl of protein G solution (50 ng/100 µl in 0.1 M sodium bicarbonate; pH 9.0). After washing the wells three times with wash buffer [0.15 M K2HPO4, 0.02 M NaH2PO4, 0.2 mM ascorbic acid, 0.2% (v/v) Tween-20 and 0.1% (w/v) sodium azide; pH 7.5], 25 µl of a highly selective antiserum for SP was diluted in assay buffer [same as wash buffer containing 0.1% (w/v) gelatin] to 1:200,000. Following addition of SP antisera, wells were incubated for 4 h at room temperature in order to allow the attachment of antibody to the protein G-coated surface. After incubation, wells were washed 3 times and 25 µl of sample or standard(s) were added to each well and incubated at room temperature overnight. The next day, 25 µl of the labeled peptide ([125I]substance P) diluted with assay buffer to approximately 6500 dpm per 25 µl, were added to the wells and incubated for 2 h at room temperature. After incubation, wells were washed, separated and placed in 12×75-mm polypropylene tubes and counted in a five-channel Packard Cobra II Auto-Gamma counter (Packard Instrument Co., Meriden, CT). The total and nonspecific binding were defined by adding 25 µl of the labeled peptide to protein G-untreated and -treated wells, respectively. Quantities of neuropeptide immunoreactivity were determined by comparing bound to free [125I]substance P in each sample to a standard curve (from 0.5 to 125 pg/assay tube). The reproducibility of the assay was evaluated using cerebellum tissue spiked with 62 and 250 pg of each peptide. This technique has been demonstrated to be very reproducible, resulting in less than 10% variability between assays and less than 5% between sample and standard duplicates. The procedure allowed reliable detection of 500 fg of SPLI per sample.
Antiserum
The SP anstiserum was raised in New Zealand White rabbits as previously described (Letter et al. 1987; Ritter et al. 1984; Wagstaff et al. 1996). This antiserum recognizes the SP carboxy terminus and is highly selective, expressing no cross-reactivity with 1000-fold excess concentrations of other endogenous neuropeptides such as dynorphin A, metenkephalin, cholecystockinin or substance K.
Statistical analysis
Results from these experiments are expressed as percentages of their respective controls in order to facilitate comparisons between groups (mean values ± S.E.M.). The control values (picograms of SPLI per milligram of protein) for each experiment are indicated in the corresponding figure legend. Differences between means were analyzed using one-way analysis of variance followed by Fisher-Protected Least Significant Difference (PLSD). Differences were considered significant when the probability that they were zero was less than 5%.
Results
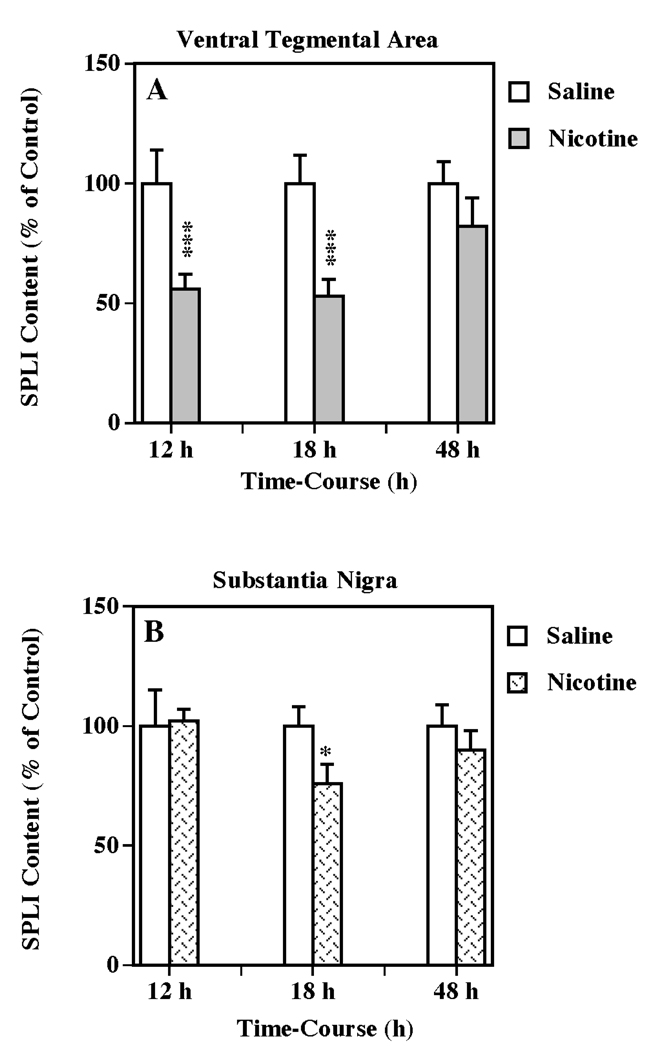
In order to determine if nicotine treatment influences limbic and/or basal ganglia SP systems, the effect of multiple administrations (five injections, 2-h intervals) of (±) nicotine (0.8 mg/kg/injections, i.p.) on SPLI contents in VTA and substantia nigra were evaluated after 12, 18 and 48 h (Fig. 1). This nicotine treatment significantly reduced SPLI in the VTA at both 12 and 18 h after drug administration (56 % and 53 % of control, respectively; one-way ANOVA: P < 0.001 in both cases) (Fig. 1a). In the adjacent substantia nigra, the nicotine treatment also significantly decreased SPLI concentrations (76 % of control; one-way ANOVA: P < 0.05) at 18 h following injection (Fig. 1b). After 48 h, the SPLI contents in both brain regions returned to control. In contrast, a single dose of (±) nicotine (from 0.4 to 3.2 mg/kg, i.p.) did not cause any significant changes in SPLI concentrations in these brain regions up to 24 h after treatment (data not shown).
Figure 1.
Temporal effects of multiple nicotine administration on SPLI content in VTA (a) and substantia nigra (b). Animals were administered five injections of (±) nicotine (0.8 mg/kg/injection, i.p., 2-h intervals) or saline (control) and killed 12, 18 or 48 h following treatment. Results are expressed as percentages of control and represent mean values ± S.E.M. (n= 8 for saline and 9 for drug-treated animals per group). The average control value of SPLI concentration for ventral tegmental area and substantia nigra were 1,131 ± 132 and 7,420 ± 779 pg/mg protein, respectively. *P < 0.05, ***P < 0.001 vs. control.
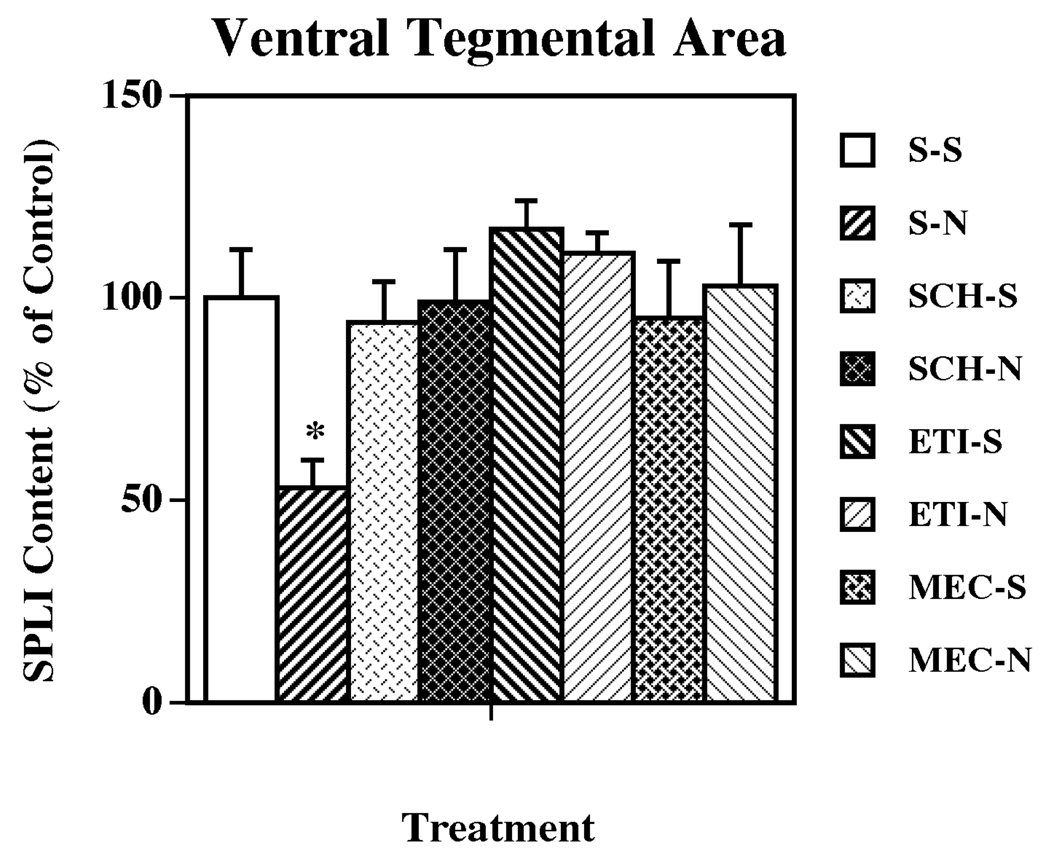
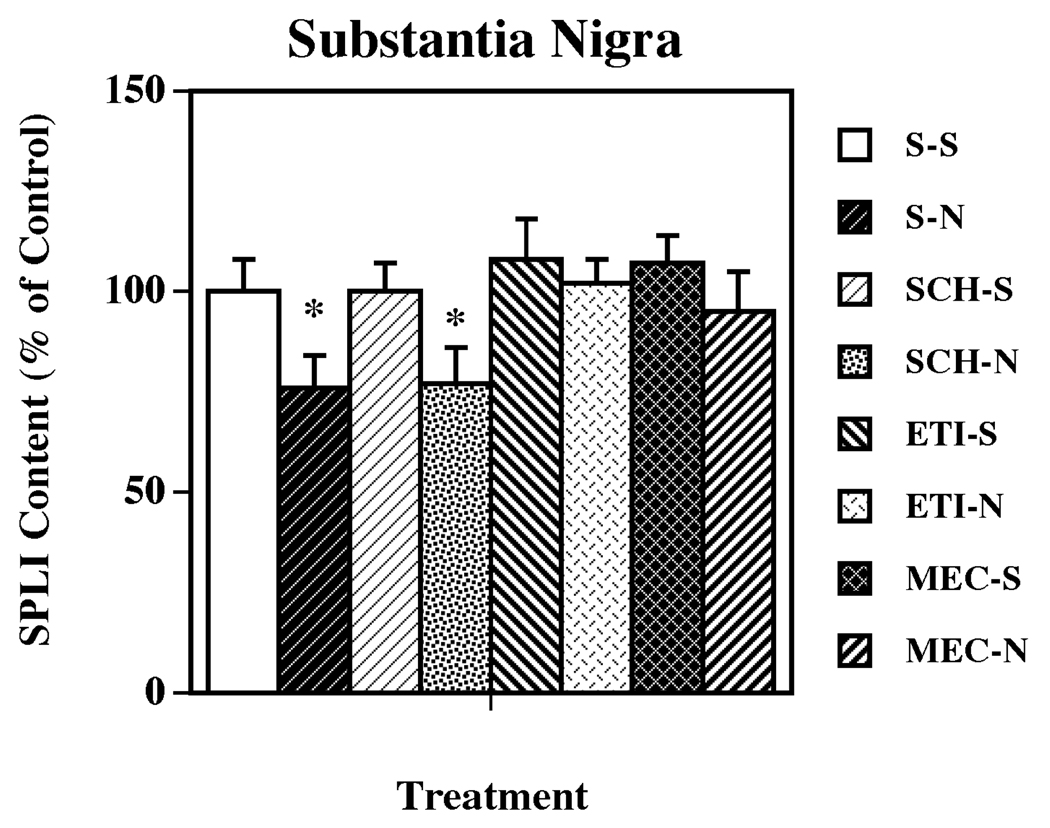
The role of DA subtypes, and nicotinic, receptors in the nicotine-induced changes in limbic and basal ganglia SP systems was evaluated by pretreating animals with selective DA D1 (SCH 23390) and DA D2 (eticlopride) receptor antagonists, and with the nonselective nicotinic receptor antagonist mecamylamine prior to each nicotine injection. The DA receptor antagonists and the nicotinic receptor antagonist alone did not significantly affect SPLI levels in any of the regions examined. As previously observed, the administration of nicotine significantly decreased the content of SPLI in VTA (Fig. 2) and substantia nigra (Fig. 3). In the VTA, pretreatment with either a dopamine D1 or D2 receptor antagonist completely prevented the nicotine-induced SP effect (Fig. 2). However, in the substantia nigra the antagonism of dopamine D2, but not dopamine D1, receptors completely blocked the nicotine-induced decreases in SPLI concentrations (Fig. 3). Pretreatment with the nonselective nicotinic acetylcholine receptor antagonist mecamylamine, also prevented the nicotine-induced decreases in SPLI content in both VTA and substantia nigra (Fig. 2 and Fig. 3, respectively).
Figure 2.
Effects of selective dopamine receptor and nicotinic acetylcholine receptor antagonists on nicotine-induced changes in SPLI content in the VTA. Animals were given five administrations of (±) nicotine (N; 0.8 mg/kg/injection, i.p., 2-h intervals) or saline (S; control), alone or 15 min after administration of SCH 23390 (SCH; dopamine D1 receptor antagonist; 0.5 mg/kg/injection, i.p.), eticlopride (ETI; dopamine D2 receptor antagonist; 0.5 mg/kg/injection, i.p.), or mecamylamine (MEC; nicotinic acetylcholine receptor antagonist; 3.0 mg/kg/injection, s.c.). Animals were killed 18 h following the last treatment. Values represent the means ± S.E.M. expressed as percentages of control (n= 8 for control and 9 for drug-treated animals per group). The control value ± S.E.M. for SPLI concentrations (picograms per milligram protein) was 1,094 ± 130. *P < 0.05 vs. all other groups.
Figure 3.
Effects of selective dopamine receptor and nicotinic acetylcholine receptor antagonists on nicotine-induced changes in SPLI content in substantia nigra. Animals were given five administrations of (±) nicotine (N; 0.8 mg/kg/injection, i.p., 2-h intervals) or saline (S; control), alone or 15 min after administration of dopamine D1, D2 or nicotinic acetylcholine receptor antagonists (as described for Fig. 2). Animals were killed 18 h following the last treatment. Values represent the means ± S.E.M. expressed as percentages of control (n= 8 for control and 9 for drug-treated animals per group). The control value ± S.E.M. for SPLI concentrations (picograms per milligram protein) was 7,325 ± 565. *P < 0.05 vs. all other groups.
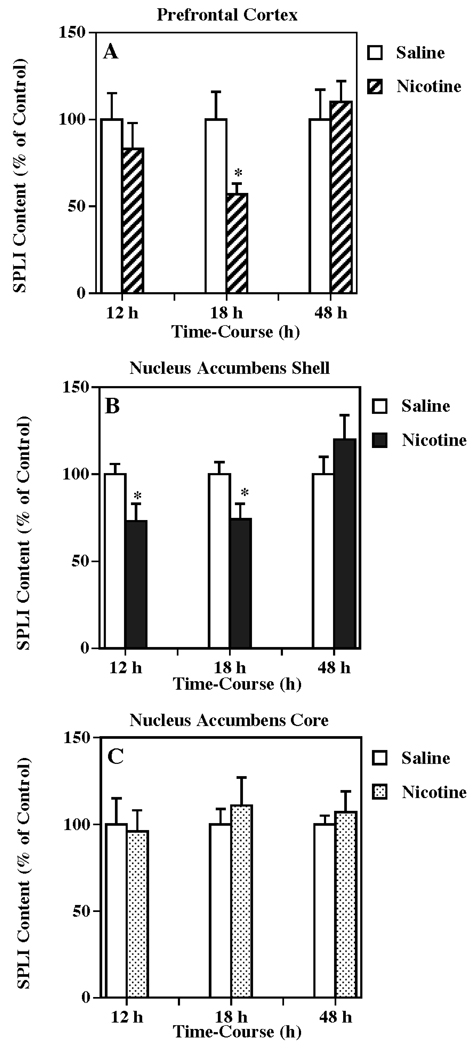
The temporal response of SP systems to nicotine administration in the terminal region of mesocortical projections (i.e., prefrontal cortex), was evaluated using the same nicotine treatment described in Fig. 1. The tissue content of SPLI in prefrontal cortex (Fig. 4a) was significantly decreased to 57 % of control (one-way ANOVA: P < 0.05) 18 h following the last nicotine administration. In order to assess the SP response in the terminal region of mesolimbic DA projections, the shell of the nucleus accumbens was examined (Fig. 4b), and SPLI concentrations were significantly reduced to 73 % and 74 % of control (one-way ANOVA: P < 0.05 in both cases) at 12 and 18 h after nicotine administrations, respectively. In contrast, no significant changes in SPLI concentrations were observed in the core of the nucleus accumbens from the same animals (Fig. 4c).
Figure 4.
Temporal response of multiple nicotine administration on SPLI content in VTA-related brain regions such as the prefrontal cortex (a), nucleus accumbens shell (b), and nucleus accumbens core (c). Animals were given injections of (±) nicotine (as described for Fig. 1) or saline (control) and killed 12, 18 or 48 h following treatment. Results are expressed as percentages of control and represent mean values ± S.E.M. (n= 8 for control and 9 for drug-treated animals per group). The average control value of SPLI concentration for prefrontal cortex, nucleus accumbens shell and nucleus accumbens core were 319 ± 46, 2,189 ± 159, and 1,418 ± 153 pg/mg protein, respectively. *P < 0.05 vs. control.
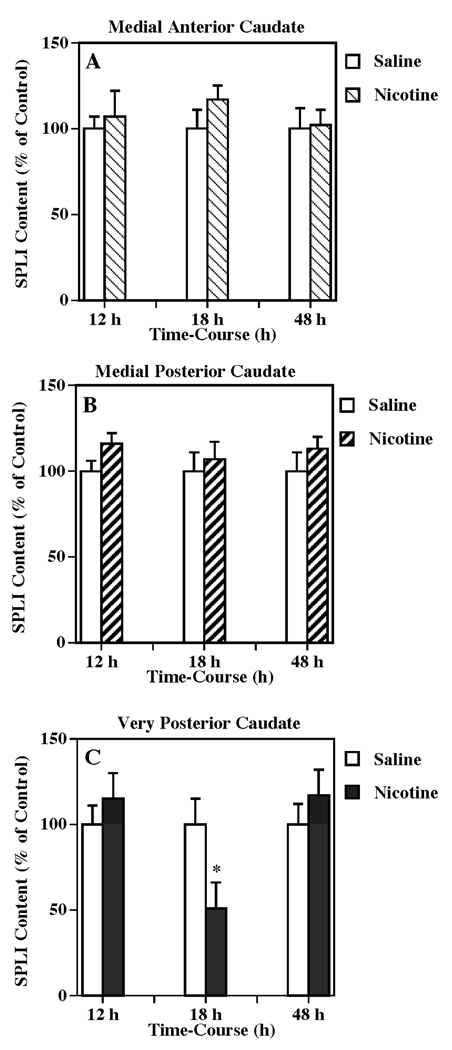
The time-related effects of nicotine treatment on the SPLI content in the parallel terminal regions of the basal ganglia system were also evaluated. The tissue content of SPLI was significantly decreased in the very posterior caudate to 51 % of the control (one-way ANOVA: P < 0.05) 18 h after drug treatment, but returned to the control levels by 48 h (Fig. 5c). However, SPLI contents in either the anterior or posterior of the medial caudate were not significantly different from control at any of the times examined (Fig, 5a, b).
Figure 5.
Temporal response of multiple nicotine administration on SPLI content in substantia nigracaudate nucleus projection such as the medial anterior caudate (a), medial posterior caudate (b) and very posterior caudate (c). Animals were given injections of (±) nicotine (as described for Fig. 1) or saline (control) and killed 12, 18 or 48 h following treatment. Results are expressed as percentages of control and represent mean values ± S.E.M. (n= 8 for control and 9 for drug-treated animals per group). The average control value of SPLI concentration for medial anterior caudate, medial posterior caudate, and very posterior caudate were 1,617 ± 157, 1,197 ± 113, and 1,846 ± 277 pg/mg protein, respectively. *P < 0.05 vs. control.
Discussion
It is known that nicotine exposure through tobacco smoking or by administration of the pure drug has significant effects on the activity of limbic systems contributing to both the rewarding (and abuse liability) and cognitive features of this stimulant (Maskos et al. 2005; Singer et al. 2004). These properties of nicotine treatment are almost certainly associated with DA release from limbic structures such as the nucleus accumbens (Nisell et al. 1994; Sziraki et al. 2002; Ferrari et al. 2002; Rowell and Volk 2004) and the prefrontal cortex (Nisell et al. 1996; Cao et al. 2005), due to a high concentration of nicotinic receptors subunits such as alpha 4 and 7, and beta 2 on DA neurons originating from the VTA (Maskos et al. 2005; Klink et al. 2001; Wu et al. 2004; Nisell et al. 1994; Quarta et al. 2007).
While considerable details have been reported about nicotinic and dopaminergic interactions in limbic structures, there has been little research examining nicotine’s effects on other VTA-related neuroregulatory systems, such as the neuropeptides, that have also been demonstrated to help regulate DA efferent pathways and associated functional outcomes. In this regard, we recently reported a study that demonstrated limbic and extrapyramidal neurotensin systems are significantly altered in the VTA, prefrontal cortex and substantia nigra after exposure to nicotine (Alburges et al. 2007).
To compliment the neurotensin observations, and to follow up an observation in that report that nicotine can also alter SPLI levels in the VTA (Alburges et al. 2007), the present studies examined in detail which limbic and extrapyramidal SP systems are influenced by nicotine treatment and determined the role of dopamine mechanisms in these effects. Substance P in limbic and extrapyramidal structures has been associated with biochemical and behavioral changes when injected into the VTA (Kelley et al. 1979; Elliott et al. 1986; Cador et al. 1989; West and Michael 1991; Kelley and Delfs 1991; Zhou and Nyberg 2002), prefrontal cortex (Krasnova et al. 2000), nucleus accumbens (Iversen 1982; Kalivas and Miller 1984; Huston and Hasenohrl 1995; Schildein et al. 1998; Nikolaev et al. 2004), and substantia nigra (Tan and Tsou 1988a,b). There is also evidence that SP plays a central role in mediating the abstinence reaction to psychoactive drugs such as opioids (Tiong et al. 1992; Kreeger and Larson 1996; Murtra et al. 2000) and antidepressants (Jones and Olpe 1984). Thus, alteration of withdrawal symptoms from these drugs may be mediated by the interaction of SP with the mesocorticolimbic dopaminergic systems (Zhou and Nyberg 2002; Zhou et al. 2003, 2004) suggesting the possibility of using SP-targeted psychopharmacology to treat the adverse effects of other DA-related psychoactive drugs such as nicotine.
The rationale for selecting limbic and extrapyramidal structures to study the nicotine effect was based on the close association between these dopamine projections and substance P-containing neurons (Cheramy et al. 1977; Gerfen et al. 1990; Lu et al. 1998), and the possible involvement of SP with both limbic and extrapyramidal dopaminergic pathways in the reward and addiction process of stimulant of abuse. Thus, a SP pathway comparable to that associated with the basal ganglia is also linked with mesolimbic dopaminergic neurons. These SP-containing cell bodies are found in the medial habenular nucleus and project to the VTA and terminate near mesolimbic dopaminergic neurons (Lindvall et al. 1977; Mroz et al. 1977; Kanazawa et al. 1977). These biochemical and electrophysiological evidences suggest that SP induces some of its effects through activation of the mesocortical and mesolimbic dopamine systems.
In order to understand better the function of SP systems in the VTA, especially as it relates to nicotine effects, we determined the actions of nicotine injections on SPLI tissue levels. We selected the nicotine dose (0.8 mg/kg/injection) based on: (1) our preliminary studies where 0.4 to 3.2 mg/kg, i.p. nicotine was administered and doses of 1.6 and 3.2 mg/kg, i.p. were found to cause motor dysfunction and even sometimes seizures (data not shown); and (2) an administration paradigm previously reported (Kane et al. 2000, 2001, 2005; Li and Kane 2003; Li et al. 2000; Matta et al. 2007; Alburges et al. 2007) and intended to mimic the pattern of nicotine exposure that would occur in the typical heavy smoker.
We observed that exposure to nicotine rapidly (12 to 18 h), but reversibly (back to control levels by 48 h) reduced SPLI content in the VTA (Fig. 1a). We confirmed this SP change was linked to the nicotinic and dopaminergic systems by the observation that the effect was blocked by a nicotinic and both DA D1 and D2, receptor antagonists (Fig. 2). These conclusions were supported by the previous finding that the DA-releasing drug methamphetamine also reduces SPLI levels in the VTA (Hanson et al. 1986b). For comparison, a similar treatment by nicotine reduced the level of neurotensin in this same limbic structure through nicotinic and dopaminergic mechanisms, although in contrast to the corresponding SP response, the neurotensin decreases were mediated by only D2, but not D1, receptor mechanisms (Alburges et al. 2007).
Our observation that nicotine treatment reduced the SPLI levels in the VTA is consistent with the interpretation that stimulation of nicotinic receptors releases SP, resulting in an increase in SP turnover and depletion of tissue levels. Because SCH 23390 or eticlopride pretreatments blocked this response, it is likely that the nicotine-induced SP changes were mediated by DA release activating D1 and D2 receptors (Hanson et al. 2002; Alburges et al. 2007). If stimulation of nicotinic receptors does increase SP release in the VTA as suggested by these findings, it is possible that SP plays a significant role in mediating some of the consequences of nicotine consumption on associated limbic function. This conclusion is consistent with findings that SP in the VTA: (1) has an excitatory effect on neurons found in this brain region (Korotkova et al. 2006); (2) is linked with drug-seeking behavior (Placenza et al. 2004); (3) may contribute to the regulation of DA release in the nucleus accumbens caused by nicotine treatment (Zhou and Nyberg 2002); (4) contributes to locomotor activity mediated by mesolimbic systems (Elliott et al. 1992); and (5) affects feeding behavior (Cador et al. 1986). Since nicotine administration can influence all of the properties listed above, perhaps SP systems contribute to these effects.
Due to similarities with the limbic systems, the SP projections associated with the striatonigral pathway (Cheramy et al. 1977; Waldmeier et al. 1978; Haber and Nauta 1983; Gerfen et al. 1990; Kawaguchi et al. 1990) were also evaluated by measuring nigral SPLI levels after nicotine treatment. Although the nigral effect did not appear to be as robust as the SP changes in the VTA, nicotine exposure also did reversibly reduce SPLI levels in this tissue at 18 h (Fig. 1b). Like the SPLI changes in the VTA, the nigral SP response was blocked both by the nicotinic and D2 antagonists, but differed in that blockade of the D1 receptor did not alter the nigral response (Fig. 3). These findings suggest the nigral SP effects were mediated by nicotinic receptors likely causing release of DA, stimulating D2 receptors and effecting changes in the striatonigral SP pathway.
Because nicotine caused changes in SPLI content through DA receptor mechanisms in both the VTA and substantia nigra, we tested the possibility that exposure to nicotine also alters SP systems in other brain regions where nicotine exposure results in DA release (Emmett and Greenfield 2005; Singer et al. 2004; Janhunen and Ahtee 2004). Specifically, we measured the effects of nicotine treatment on SPLI levels in DA terminal regions for cell bodies originating in the VTA and the substantia nigra. Our findings revealed that the same multiple nicotine administrations that influenced SPLI levels in the regions of the DA cell bodies similarly reduced SPLI content in the prefrontal cortex (Fig. 4a), nucleus accumbens shell (Fig. 4b), and very posterior caudate (Fig. 5c) in a reversible manner, but had no significant effect on SPLI content in the nucleus accumbens core (Fig. 4c), or the medial anterior and posterior caudate (Fig. 5a, b). These observations demonstrate that the SP responses to nicotine exposure are selective, even within limbic and extrapyramidal systems. It may be relevant that previous reports suggest acute nicotine exposure (such as the paradigm used in the present study) tends to increase release of DA in the nucleus accumbens shell and other limbic areas, whereas chronic exposure causes a shift in DA responses to more of the accumbens core region (De Chiara 2000). This possibly explains why we observed SP changes in the shell, but not the core, after this acute exposure to nicotine. As previously described, nicotine administration has been shown to induce DA release in the frontal cortex (Nisell et al. 1996; Cao et al. 2005) explaining why SP systems are affected in this cognitive-related brain region. As for the pattern of SP responses in extrapyramidal structures, there are no known studies that have attempted to compare variable effects of nicotine on DA release in the different caudate regions.
The decreases in SPLI concentration (30–50%) in prefrontal cortex, VTA and substantia nigra seen after multiple nicotine administration are in contrast with the findings from Naftchi et al. (1988). They found no changes in SPLI content in these brain regions following nicotine treatment. These distinctions are likely due to differences in the experimental drug paradigm used. In our study, we administered a multiple dosing protocol (more consistent with clinical exposures associated with traditional smoking patterns) in which animals were injected every 2 h for five doses and then killed 12, 18 and 48 h after last nicotine treatment. Naftchi et al. (1988) only administered a single injection of nicotine and sacrificed the animals 10 min after the nicotine treatment. It seems likely that multiple administrations of nicotine are required to induce SPLI changes in these brain areas. On the other hand, in agreement with the previous finding (Naftchi et al. 1988), we found that this nicotine treatment also decreased SPLI content in posterior caudate and nucleus accumbens shell (but not core).
Although the present study examined the effect of multiple nicotine administrations on limbic and extrapyramidal SP systems, previous studies demonstrated that other psychostimulants such as the amphetamines and cocaine also alter SP pathways in these CNS regions via dopamine mechanisms. For example, different from the nicotine effects reported herein, high doses of methamphetamine and cocaine elevate SPLI levels in the caudate nucleus and nucleus accumbens (Sonsalla et al. 1984, 1986; Ritter et al. 1984, 1985; Alburges et al. 2000; Hanson et al. 1986a, 2002) through dopamine mechanisms (Ritter et al. 1985; Sonsalla et al. 1986; Alburges et al. 2000). Similar high-dose treatment with these drugs also stimulates the synthesis of SP as demonstrated by an increase in the mRNA for preprotachykinin (Adams et al. 2001), possibly contributing to the accompanying elevation in peptide levels. It is noteworthy and likely relevant to these current findings that a similar high dose of methamphetamine (10 mg/kg) does not alter the release of SP in the substantia nigra. In contrast, a low dose of methamphetamine (0.5 mg/kg) which doubles the release of SP in the substantia nigra has an opposite effect and reduces the SPLI levels in several extrapyramidal brain regions (Hanson et al. 2002). As shown in the current study nicotine also decreased SPLI tissue levels in the substantia nigra as well as other extrapyramidal and limbic structures, suggesting that these nicotine-induced changes were associated with SP release, as was the case with the low-dose methamphetamine treatment.
In summary, administration of nicotine in a dose and pattern resembling that associated with heavy smoking, substantially influences SP systems linked with mesocortical and mesolimbic DA pathways. Thus, these treatments of nicotine caused 30–50% reductions in both the regions of DA cell bodies (VTA) and terminals (prefrontal cortex and nucleus accumbens shell). In a similar, but somewhat diminished fashion, we observed that these nicotine treatments also reduced SPLI in some regions associated with the extrapyramidal DA cell bodies and terminal. These reductions in SPLI tissue content likely reflect a nicotine-induced release of SP and increased turnover, suggesting that these SP systems contribute to the limbic and extrapyramidal functional consequences of heavy nicotine consumption.
Acknowledgements
This work was supported by U.S. Public Health Service Grants DA09407 and DA00378. The authors would like to thank Mr. Lloyd Bush for his technical assistance. The experiments described within this publication are all in compliance with the laws of the USA.
References
- Adams D, Hanson GR, Keefe K. Differential effects of cocaine and methamphetamine on neurotensin/neuromedin and preprotachykinin messenger RNA expression in unique regions of the striatum. Neuroscience. 2001;102:843–851. doi: 10.1016/s0306-4522(00)00530-3. [DOI] [PubMed] [Google Scholar]
- Adinoff B. Neurobiologic processes in drug reward and addiction. Harv Rev Psychiatry. 2004;12:305–320. doi: 10.1080/10673220490910844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanian GK, Bunney BS. Dopamine ‘autoreceptors’: pharmacological characterization by microiontophoretic single cell recording studies. Naunyn Schmiedeberg’s Arch Pharmacol. 1977;297:1–7. doi: 10.1007/BF00508803. [DOI] [PubMed] [Google Scholar]
- Alburges ME, Ramos BP, Bush L, Hanson GR. Responses of the extrapyramidal and limbic substance P systems to ibogaine and cocaine treatments. Eur J Pharmacol. 2000;390:119–126. doi: 10.1016/s0014-2999(99)00919-x. [DOI] [PubMed] [Google Scholar]
- Alburges ME, Keefe KA, Hanson GR. Unique responses of limbic met-enkephalin systems to low and high doses of methamphetamine. Brain Res. 2001a;905:120–126. doi: 10.1016/s0006-8993(01)02514-8. [DOI] [PubMed] [Google Scholar]
- Alburges ME, Keefe KA, Hanson GR. Contrasting response by basal ganglia metenkephalin systems to low and high doses of methamphetamine in a rat model. J Neurochem. 2001b;76:721–729. doi: 10.1046/j.1471-4159.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- Alburges ME, Hoonakker AJ, Hanson GR. Nicotinic and dopamine D2 receptors mediate nicotine-induced changes in ventral tegmental area neurotensin systems. Eur J Pharmacol. 2007;573:124–132. doi: 10.1016/j.ejphar.2007.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon MJ, Elliott PJ, Bunney EB. Striatal tachykinin biosynthesis: regulation of mRNA and peptide levels by dopamine agonists and antagonists. Brain Res. 1987;427:31–37. doi: 10.1016/0169-328x(87)90041-6. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brownstein MJ, Mroz EA, Tappaz ML, Leeman SE. On the origin of substance P and glutamic acid decarboxylase (GAD) in the substantia nigra. Brain Res. 1977;135:315–323. doi: 10.1016/0006-8993(77)91034-4. [DOI] [PubMed] [Google Scholar]
- Cador M, Kelley AE, Le Moal M, Stinus L. Ventral tegmental area infusion of substance P, neurotensin and enkephalin: differential effects on feeding behavior. Neuroscience. 1986;18:659–669. doi: 10.1016/0306-4522(86)90061-8. [DOI] [PubMed] [Google Scholar]
- Cador M, Rivet J, Kelley A, Le Moal M, Stinus L. Substance P, neurotensin and enkephalin injections into the ventral tegmental area: comparative study on dopamine turnover in several forebrain structures. Brain Res. 1989;486:357–363. doi: 10.1016/0006-8993(89)90523-4. [DOI] [PubMed] [Google Scholar]
- Cao YJ, Surowy CS, Puttfarcken PS. Different nicotinic acetylcholine receptor subtypes mediating striatal and prefrontal cortical [3H]dopamine release. Neuropharmacology. 2005;48:72–79. doi: 10.1016/j.neuropharm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. Annual smoking-attributable mortality, years of potential life lost, and productivity losses-U.S., 1997–2001. MMWR Morb Mortal Wkly Rep. 2005;54(25):625–628. [PubMed]
- Cheramy A, Nieoullon A, Michelot R, Glowinski J. Effects of intranigral application of dopamine and substance P on the in vivo release of newly synthesized [3H]-dopamine in the ipsilateral caudate nucleus of the cat. Neurosci Lett. 1977;48:105–109. doi: 10.1016/0304-3940(77)90152-5. [DOI] [PubMed] [Google Scholar]
- David V, Besson M, Changeux J, Granon S, Cazala P. Reinforcing effects of nicotine microinjections into the ventral tegmental area of mice: dependence on cholinergic nicotinic and dopaminergic D1 receptors. Neuropharmacology. 2006;50:1030–1040. doi: 10.1016/j.neuropharm.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Davies J, Dray A. Substance P in the substantia nigra. Brain Res. 1976;107:623–627. doi: 10.1016/0006-8993(76)90150-5. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Elliott PJ, Alpert JE, Bannon MJ, Iversen SD. Selective activation of mesolimbic and mesocortical dopamine metabolism in rat brain by infusion of a stable subastance P analogue into the ventral tegmental area. Brain Res. 1986;363:145–147. doi: 10.1016/0006-8993(86)90667-0. [DOI] [PubMed] [Google Scholar]
- Elliott PJ, Mason GS, Graham EA, Turpin MP, Hagan RM. Modulation of the rat mesolimbic dopamine pathway by neurokinins. Behav Brain Res. 1992;51(1):77–82. doi: 10.1016/s0166-4328(05)80314-6. [DOI] [PubMed] [Google Scholar]
- Emmett S, Greenfield S. Correlation between dopaminergic neurons, acetylcholinesterase and nicotinic acetylcholine receptors containing the alpha 3-or alpha 5-subunit in the rat substantia nigra. J Chem Nuroanat. 2005;30:34–44. doi: 10.1016/j.jchemneu.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Le Novera N, Picciotto MR, Changeux JP, Zoli M. Acute and long-term changes in the mesolimbic dopamine pathway after systemic or local single nicotine injections. Eur J Neurosci. 2002;15:1810–1818. doi: 10.1046/j.1460-9568.2001.02009.x. [DOI] [PubMed] [Google Scholar]
- Gale K, Hong J, Guidott A. Presence of substance P and GABA in separate striatonigral neurons. Brain Res. 1977;136:371–375. doi: 10.1016/0006-8993(77)90813-7. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan IC, Susel Z, Chase TN, Monsma FJ, Sibley DR. D-1 and D-2 dopamine receptor-regulated gene expression of striatonigral and striatopalidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Haber SN, Nauta WJ. Ramifications of the globus pallidus in the rat as indicated by patterns of immunohistochemistry. Neuroscience. 1983;9:245–260. doi: 10.1016/0306-4522(83)90291-9. [DOI] [PubMed] [Google Scholar]
- Hamada M, Higashi H, Nairn AC, Greengard P, Nishi A. Differential regulation of dopamine D1 and D2 signaling by nicotine in neostriatal neurons. J Neurochemistry. 2004;90:1094–1103. doi: 10.1111/j.1471-4159.2004.02574.x. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Letter AA, Merchant K, Gibb JW. Comparison of responses by striatonigral substance P and neurokinin A systems to methamphetamine treatment. Peptides. 1986a;7:983–987. doi: 10.1016/0196-9781(86)90125-7. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Ritter JK, Schmidt CJ, Gibb JW. Response of mesolimbic substance P systems to methamphetamine treatment. Eur J Pharmacol. 1986b;128:265–268. doi: 10.1016/0014-2999(86)90775-2. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Bush L, Keefe KA, Alburges ME. Distinct responses of basal ganglia substance P systems to low and high doses of methamphetamine. J Neurochem. 2002;82:1171–1178. doi: 10.1046/j.1471-4159.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- Hong J, Yang H, Racagni G, Costa E. Projections of substance P-containing neurons from neostriatum to substantia nigra. Brain Res. 1977;122:541–544. doi: 10.1016/0006-8993(77)90464-4. [DOI] [PubMed] [Google Scholar]
- Huston JP, Hasenohrl RU. The role of neuropeptides in learning: focus on the neurokinin substance P. Behav Brain Res. 1995;66:117–127. doi: 10.1016/0166-4328(94)00132-y. [DOI] [PubMed] [Google Scholar]
- Iversen SD. Behavioural effects of substance P through dopaminergic pathways in the brain. Ciba Found Symp. 1982;91:307–324. doi: 10.1002/9780470720738.ch18. [DOI] [PubMed] [Google Scholar]
- James TA, Starr MS. Effects of substance P injected into the substantia nigra. Br J Pharmacol. 1979;65:423–429. doi: 10.1111/j.1476-5381.1979.tb07846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janhunen S, Ahtee L. Comparison of the effects of nicotine and epibatidine on the striatal extracellular dopamine. Eur J Pharmacol. 2004;494:167–177. doi: 10.1016/j.ejphar.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Jones RS, Olpe HR. An increase in sensitivity of rat cingulated cortical neurons to substance P occurs following withdrawal of chronic administration of antidepressant drugs. Br J Pharmac. 1984;81:659–664. doi: 10.1111/j.1476-5381.1984.tb16132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones IW, Bolam JP, Wonnacott S. Presynaptic localization of the nicotinic acetylcholine receptor beta2 subunit immunoreactivity in rat nigrostriatal dopaminergic neurons. J Comp Neurol. 2001;439:235–247. doi: 10.1002/cne.1345. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Miller JS. Substance P modulation of dopamine in the nucleus accumbens. Neurosci Lett. 1984;48:55–59. doi: 10.1016/0304-3940(84)90288-x. [DOI] [PubMed] [Google Scholar]
- Kanazawa I, Emson PC, Cuello AC. Evidence for the existence of substance P-containing fibers in striatonigral and pallidonigral pathways in rat brain. Brain Res. 1977;119:447–453. doi: 10.1016/0006-8993(77)90323-7. [DOI] [PubMed] [Google Scholar]
- Kane JK, Parker SL, Matta SG, Fu Y, Sharp BM, Li MD. Nicotine up-regulates expression of orexin and its receptors in rat brain. Endocrinology. 2000;141:3623–3629. doi: 10.1210/endo.141.10.7707. [DOI] [PubMed] [Google Scholar]
- Kane JK, Parker SL, Li MD. Hypothalamic orexin-A binding are downregulated by chronic nicotine treatment in the rat. Neurosci Lett. 2001;298:1–4. doi: 10.1016/s0304-3940(00)01730-4. [DOI] [PubMed] [Google Scholar]
- Kane JK, Hwang Y, Konu O, Loughlin SE, Leslie FM, Li MD. Regulation of homer and group I metabotropic glutamate receptors by nicotine. Eur J Neurosci. 2005;21:1145–1154. doi: 10.1111/j.1460-9568.2005.03945.x. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injections of biocytin. J Neurosci. 1990;10:3421–3438. doi: 10.1523/JNEUROSCI.10-10-03421.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Iversen SD. Substance P infusion into substantia nigra of rat: behavioral analysis and involvement of striatal dopamine. Eur J Pharmacol. 1979;60:171–179. doi: 10.1016/0014-2999(79)90216-4. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Delfs JM. Dopamine and conditioned reinforcement. II. Contrasting effects of amphetamine microinjection into the nucleus accumbens with peptide microinjection into the ventral tegmental area. Psychopharmacology (Berl) 1991;103:197–203. doi: 10.1007/BF02244203. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Stinus L, Iversen SD. Behavioural activation induced in the rat by substance P infusion into ventral tegmental area: implication of dopaminergic A10 neurones. Neurosci Lett. 1979;11:335–339. doi: 10.1016/0304-3940(79)90018-1. [DOI] [PubMed] [Google Scholar]
- Kelley A, Cador M, Stinus, Le Moal M. Neurotensin, substance P, neurokinin-alpha, and enkephalin: injection into ventral tegmental area in the rat produces differential effects on operant responding. Psychopharmacology (Berl.) 1989;97:243–252. doi: 10.1007/BF00442258. [DOI] [PubMed] [Google Scholar]
- Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux J. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci. 2006;23:2677–2685. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- Kraft M, Noailles P, Angulo JA. Substance P modulates cocaine-evoked dopamine overflow in the striatum of the rat brain. Ann N Y Acad Sci. 2001;937:121–131. doi: 10.1111/j.1749-6632.2001.tb03561.x. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Bychkov ER, Lioudyno VI, Zubareva OE, Dambinova SA. Intracerebroventricular administration of substance P increases dopamine content in the brain of 6-hydroxydopamine-lesioned rats. Neuroscience. 2000;95:113–117. doi: 10.1016/s0306-4522(99)00400-5. [DOI] [PubMed] [Google Scholar]
- Kreeger JS, Larson AA. The substance P amino-terminal metabolite substance P (1–7), administered peripherally, prevents the development of acute morphine tolerance and attenuates the expression of withdrawal in mice. J Pharmacol Exp Ther. 1996;279:662–667. [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- Letter AA, Merchant KM, Gibb JW, Hanson GR. Effect of methamphetamine on neurotensin concentrations in rat brain regions. J Pharmacol Exp Ther. 1987;24:443–447. [PubMed] [Google Scholar]
- Li MD, Kane JK. Effect of nicotine on the expression of leptin and forebrain leptin receptors in the rat. Brain Res. 2003;991:222–231. doi: 10.1016/j.brainres.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Li MD, Kane JK, Parker SL, McAllen K, Matta SG, Sharp BM. Nicotine administration enhances NPY expression in the rat hypothalamus. Brain Res. 2000;867:157–164. doi: 10.1016/s0006-8993(00)02283-6. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A, Divac I. Organization of mesencephalic dopamine neurons projecting to neocortex and septum. In: Costa E, Gessa G, editors. Non-striatal dopaminergic neurons. New York: Raven Press; 1977. pp. 39–46. [PubMed] [Google Scholar]
- Loonam TM, Noailles PA, YU J, Angulo JA. Substance P and cholecystokinin regulate neurochemical responses to cocaine and methamphetamine in the striatum. Life Sci. 2003;73:727–739. doi: 10.1016/s0024-3205(03)00393-x. [DOI] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- Maidment NT, Siddall BJ, Rudolph VR, Erdelyi E, Evans CJ. Dual determination of extracellular cholecystokinin and neurotensin fragments in rat forebrain: microdialysis combined with a sequential multiple antigen radioimmunoassay. Neuroscience. 1991;45:81–93. doi: 10.1016/0306-4522(91)90105-w. [DOI] [PubMed] [Google Scholar]
- Marshall DL, Redfern PH, Wonnacott S. Presynaptic nicotinic modulation of dopamine release in the three ascending pathways studied by in vivo microdialysis: comparison of naïve and chronic nicotine-treated rats. J Neurochem. 1997;68:1511–1519. doi: 10.1046/j.1471-4159.1997.68041511.x. [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles B, Pons S, Besson M, Guiard B, Guilloux J, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloez-Tayarani I, Bemelmans A, Mallet J, Gardier A, David V, Faure P, Granon S, Changeux J. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;435:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Prameswaran N, Perkins KA, Picciotto MR, Ouik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Mroz EA, Brownstein MJ, Leeman SE. Evidence for substance P in the striatonigral tract. Brain Res. 1977;125:305–311. doi: 10.1016/0006-8993(77)90623-0. [DOI] [PubMed] [Google Scholar]
- Murtra P, Sheasby AM, Hunt SP, De Felipe C. Rewarding effects of opiates are absent in mice lacking the receptor for substance P. Nature. 2000;405:180–183. doi: 10.1038/35012069. [DOI] [PubMed] [Google Scholar]
- Naftchi NE, Maker H, Lapin E, Sleis J, Lajtha A, Leeman S. Acute reduction of brain substance P induced by nicotine. Neurochem Res. 1988;13:305–309. doi: 10.1007/BF00972478. [DOI] [PubMed] [Google Scholar]
- Nikolaev SV, Lebedev AA, Bychkov ER, Oblyapin AV, Dambinova SA, Shabanov PD. The effect of substance P after central administration on the activity of the mesolimbic system of the rat brain as studied by microdialysis. Neurosci Behav Physiol. 2004;34:743–746. doi: 10.1023/b:neab.0000036016.65208.27. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Hertel P, Panagis G, Svensson TH. Condition-independent sensitization of locomotor stimulation and mesocortical dopamine release following chronic nicotine treatment in rat. Synapse. 1996;22:369–381. doi: 10.1002/(SICI)1098-2396(199604)22:4<369::AID-SYN8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. San Diego, California: Academic Press; 1986. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Picciotto MR. Nicotine as a modulator of behavior: beyond the inverted U. Trends Pharmacol Sci. 2003;24:493–499. doi: 10.1016/S0165-6147(03)00230-X. [DOI] [PubMed] [Google Scholar]
- Placenza FM, Fletcher PJ, Rotzinger S, Vaccarino FJ. Infusion of the substance P analogue, DiMe-C7, into the ventral tegmental area induces reinstatement of cocaine-seeking behaviour in rats. Psychopharmacology (Berl) 2004;177:111–120. doi: 10.1007/s00213-004-1912-9. [DOI] [PubMed] [Google Scholar]
- Preston Z, Lee K, Widdowson L, Richardson PJ, Pinnock RD. Tachykinins increases [3H]acetylcholine release in mouse striatum through multiple receptor subtypes. Neuroscience. 2000;95:367–376. doi: 10.1016/s0306-4522(99)00440-6. [DOI] [PubMed] [Google Scholar]
- Quarta D, Ciruela F, Patkar K, Borycz J, Solinas M, Lluis C, Franco R, Wise RA, Goldberg SR, Hope BT, Woods AS, Ferré S. Heteromeric nicotinic acetylcholine-dopamine autoreceptor complexes modulate striatal dopamine release. Neuropsychopharmacology. 2007;32:35–42. doi: 10.1038/sj.npp.1301103. [DOI] [PubMed] [Google Scholar]
- Ritter JK, Schmidt CJ, Gibb JW, Hanson GR. Increases of substance P-like immunoreactivity within striatal-nigral structures after subacute methamphetamine treatment. J Pharmacol Exp Ther. 1984;229:233–240. [PubMed] [Google Scholar]
- Ritter JK, Schmidt CJ, Gibb JW, Hanson GR. Dopamine-mediated increases in nigral substance P-like immunoreactivity. Biochem Pharmacol. 1985;34:3161–3166. doi: 10.1016/0006-2952(85)90163-7. [DOI] [PubMed] [Google Scholar]
- Role LW, Berg DK. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Rowell P, Volk K. Nicotinic activation of mesolimbic neurons assessed by rubidium efflux in rat accumbens and ventral tegmentum. Neurosignals. 2004;13:114–121. doi: 10.1159/000076564. [DOI] [PubMed] [Google Scholar]
- Schildein S, Agmo A, Huston JP, Schwarting RK. Intraaccumbens injections of substance P, morphine and amphetamine: effects on conditioned place preference and behavioral activity. Brain Res. 1998;790:185–194. doi: 10.1016/s0006-8993(98)00062-6. [DOI] [PubMed] [Google Scholar]
- Singer S, Rossi S, Verzosa S, Hashim A, Lonow R, Cooper T, Sershen H, Lajtha A. Nicotine-induced changes in neurotransmitter levels in brain areas associated with cognitive function. Neurochem Res. 2004;29:1779–1792. doi: 10.1023/b:nere.0000035814.45494.15. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Gibb JW, Hanson GR. Opposite responses in the striato-nigral substance P system to D1 and D2 receptor activation. Eur J Pharmacol. 1984;105:185–187. doi: 10.1016/0014-2999(84)90666-6. [DOI] [PubMed] [Google Scholar]
- Sonsalla PK, Gibb JW, Hanson GR. Nigrostriatal dopamine actions on the D2 receptors mediate methamphetamine effects on the striatonigral substance P system. Neuropharmacology. 1986;25:1221–1230. doi: 10.1016/0028-3908(86)90139-5. [DOI] [PubMed] [Google Scholar]
- Sziraki I, Sershen H, Hashim A, Lajtha A. Receptors in the ventral tegmental area mediating nicotine-induced dopamine release in the nucleus accumbens. Neurochem Res. 2002;27:253–261. doi: 10.1023/a:1014844823534. [DOI] [PubMed] [Google Scholar]
- Tan DP, Tsou K. Intranigral injection of dynorphin in combination with substance P on striatal dopamine metabolism in the rat. Brain Res. 1988a;443:310–314. doi: 10.1016/0006-8993(88)91624-1. [DOI] [PubMed] [Google Scholar]
- Tan DP, Tsou K. Differential effects of tachykinins injected intranigrally on striatal dopamine metabolism. J Neurochem. 1988b;51:1333–1337. doi: 10.1111/j.1471-4159.1988.tb01093.x. [DOI] [PubMed] [Google Scholar]
- Tang FI, Chiu TH, Wang Y. Electrochemical studies of the effects of substance P on dopamine terminals in the rat striatum. Exp Neurol. 1998;152:41–49. doi: 10.1006/exnr.1998.6834. [DOI] [PubMed] [Google Scholar]
- Tiong GK, Pierce TL, Olley JE. Sub-chronic exposure to opiates in the rat: effects on brain levels of substance P and calcitonin gene-related peptide during dependence and withdrawal. J Neurosci Res. 1992;32:569–575. doi: 10.1002/jnr.490320412. [DOI] [PubMed] [Google Scholar]
- Ujike H, Ogawa N, Otsuki S. Effects of acute and long-term treatment with methamphetamine on substance P concentration and receptor numbers in the rat brain. Brain Res. 1988;453:136–142. doi: 10.1016/0006-8993(88)90151-5. [DOI] [PubMed] [Google Scholar]
- Wagstaff J, Gibb JW, Hanson GR. Dopamine D2-receptors regulate neurotensin release from nucleus accumbens and striatum as measured by in vivo microdialysis. Brain Res. 1996;721:196–203. doi: 10.1016/0006-8993(96)00132-1. [DOI] [PubMed] [Google Scholar]
- Waldmeier PC, Kam R, Stocklin K. Increased dopamine metabolism in rat striatum after infusion of substance P into the substantia nigra. Brain Res. 1978;159:223–227. doi: 10.1016/0006-8993(78)90124-5. [DOI] [PubMed] [Google Scholar]
- West CH, Michael RP. Substance P injections into the ventral tegmentum effect unit activity in mesolimbic terminal regions. Brain Res Bull. 1991;26:229–233. doi: 10.1016/0361-9230(91)90232-9. [DOI] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic Ach receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Wu J, George AA, Schroeder KM, Xu L, Marxer-Miller S, Lucero L, Lukas RJ. Electrophysiological, pharmacological, and molecular evidence for alpha 7-nicotinic acethylcholine receptors in rat midbrain dopamine neurons. J Pharmacol Exp Ther. 2004;311:80–91. doi: 10.1124/jpet.104.070417. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Nyberg F. Injection of substance P (SP) N-terminal fragment SP(1–7) into the ventral tegmental area modulates the levels of nucleus accumbens dopamine and dihydroxyphenylacetic acid in male rats during morphine withdrawal. Neurosci Lett. 2002;320:117–120. doi: 10.1016/s0304-3940(01)02564-2. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Frandberg PA, Kindlundh AM, Le Greves P, Nyberg F. Substance P (1–7) affects the expression of dopamine D2 receptors mRNA in male rat brain during morphine withdrawal. Peptides. 2003;24:147–153. doi: 10.1016/s0196-9781(02)00287-5. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Kindlundh AM, Hallberg M, Nyberg F. The substance P (SP) heptapeptide fragment SP1-7 alters the density of dopamine receptors in rat brain mesocorticolimbic structures during morphine withdrawal. Peptides. 2004;25:1951–1957. doi: 10.1016/j.peptides.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22:8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]