Abstract
Background and Purpose:
Instability is a significant risk factor for falls in individuals with a bilateral labyrinthine deficit. The purpose of this case report is to describe an intervention that we found to improve balance in a patient with bilateral labyrinthine deficit using a training paradigm based on the sensory reweighting hypothesis.
Case Description:
The participant was a female and 10 years post-onset of bilateral labyrinthine deficit. The participant was instructed to focus on the motion of her hips and knees while standing on a dynamic platform that was either stationary or matched to the excursion of her center of mass (COM) but in the opposite direction and with gradually increasing amplitude. She was tested for her ability to maintain her balance under conditions of sensory conflict both before the training and on two periods after training.
Outcomes:
Decreases in anteroposterior and mediolateral motion of the COM were observed between the pretest and both posttests with a stationary and a moving platform when in the dark and under conditions of sensory conflict. Using the method of approximate entropy, we found that the complexity of the center of pressure (COP) response increased in both the anteroposterior and medolateral directions from the pretest to both posttests when on the platform matched to the COM motion.
Summary:
Results indicated that training on a dynamic platform diminished the destabilizing effect of conflicting sensory signals. Additionally, a relationship was observed between decreased COM motion and increased complexity in COP, which represents a more self-organized system. This finding suggests that improved stability may be associated with an increased complexity in the COP trajectory.
Keywords: virtual environment, labyrinthine deficient, approximate entropy, posture
INTRODUCTION
Posture and balance are controlled by the vestibular, visual, and somatosensory systems, and the emergent motor outcome has been described as a “weighted sum” of all sensory cues.1,2 The sensory weighting hypothesis suggests that each sensory channel is multiplied by some weight and then the weighted variables are summed to produce a response modulated to the relevancy of the incoming afferents.2 If the central nervous system (CNS) is using a weighted sum of all inputs in the production of postural behaviors, we might expect that when one sensory cue is absent or inappropriate, other more reliable cues become more heavily weighted.3-6 Thus, the situation may arise in which an individual will rely on slower sensory feedback (eg, vision) to produce postural stabilization if faster, more direct feedback (eg, proprioception) has become distorted or diminished.7,8 Furthermore, if a sensory pathway is damaged or lost, then we might expect that, through adaptation to this dysfunction, the CNS will weight the remaining inputs more heavily and rely on those signals to generate motor responses. For example, an individual having bilateral labyrinthine deficit would more heavily weight visual and somatosensory inputs to maintain postural orientation in space.
A potential problem with this adaptive strategy arises when the remaining inputs become mismatched. Indeed, there is a large body of evidence indicating that individuals having unilateral labyrinthine deficit become more unstable and experience greater oscillopsia when moving within visually demanding environments such as busy streets and supermarkets.9 A possible method to overcome such a problem is to experimentally manipulate the environment to exploit the remaining sensory inputs to create an adaptation to the environmental conditions. In this case report, we used a virtual environment (VE) combined with a dynamic platform10 on three separate occasions to test the outcome of a robust sensory conflict on an individual having bilateral labyrinthine deficit before and after a novel training paradigm that engaged sensory reweighting as part of the rehabilitation process.
During the training procedure, balance demands were created by placing the subject in the dark, thus eliminating any visual input, and then a physical perturbation was provided via a dynamic platform. Because our subject was bilaterally labyrinthine deficient, we assumed that any perturbation detected by the vestibular system was minimal and that the response to the dynamic perturbation would be encoded in intrinsic coordinates.10 Thus, to increase focus on this intrinsic feedback, we instructed the subject to direct her attention to her hip and knee responses to the instability. We then tested whether the subject did indeed improve her response to instability by placing her in an environment that presented conflicting sensory information and required that she perform an upper extremity tracking task while maintaining balance. The aim of this case report was to evaluate whether asking the subject to focus attention on her lower limb somatosensory feedback could improve her response to instability. We hypothesized that this would be achieved by increasing the sensory weighting of the somatosensory inputs, thereby reducing the destabilizing effects of sensory conflict.
CASE REPORT
History
Our subject was a 73-year-old woman who was 10 years post-onset of bilateral labyrinthine deficit after the administration of intravenous gentamicin. She gave informed consent in accordance with the Institutional Review Board of the Feinberg School of Medicine, Northwestern University.
The subject was an active, community-dwelling individual with no other significant health problems. Symptoms of dizziness were mainly elicited by rapid head movements and walking in a dark room. She was not bothered by positional vertigo. She also had trouble walking on irregular surfaces and turning quickly or slowly, and she had complaints of falling at least once a month. She was unable to stand in tandem Romberg, even with eyes open, and was unsteady in the regular Romberg position with eyes open. Sensory examination revealed that lower extremity responses to vibration and proprioceptive testing were intact.
Responses to caloric irrigation introduced into the ear canal at 7°C above and below body temperature revealed a reduced total response of 16 degrees/s (normal responses are 27 degrees/s11), suggestive of bilateral labyrinthine hypofunction. This was investigated further with sinusoidal rotary chair tests. When tested from 0.01 to 0.32 Hz, the gain (the ratio of the amplitude of eye movement to the amplitude of the head movement) of the vestibulo-ocular reflex was below normal limits, except at the highest test frequency (Fig. 1), indicating that the patient had some remaining labyrinthine function in the horizontal (yaw) plane.12 However, eye movement responses were lower than normal for a sum-of-sines stimulus, which was indicative of bilateral labyrinthine pare-sis. An abnormal phase lead was also present, which was consistent with a peripheral vestibular system abnormality.
FIGURE 1.
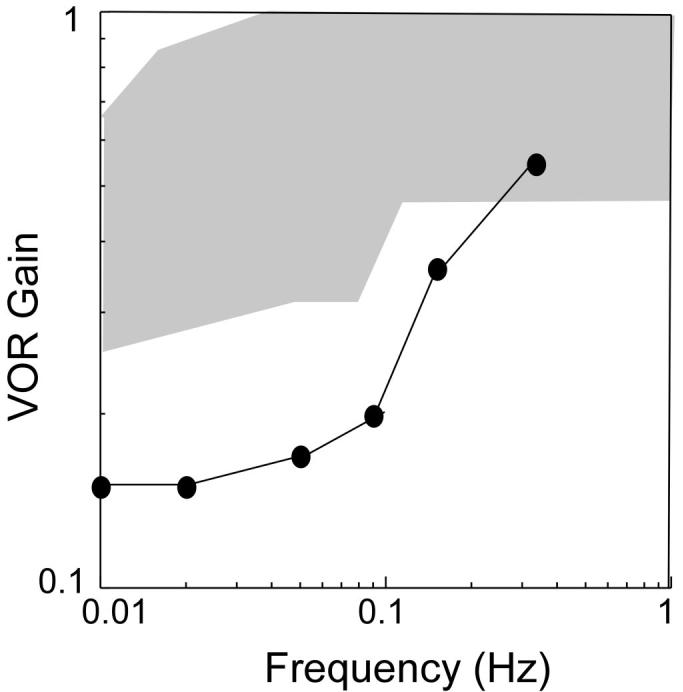
Results of the rotary chair test for our subject demonstrates a decreased gain at low frequencies. The gain point at the highest frequency is in the range of normal (shaded area) responses. Abbreviation: VOR, vestibulo-ocular reflex.
Testing and Intervention
The subject stood on a dynamic platform (Neurocom, Inc., Clackamas, OR) controlled by D/A outputs from an online PC. The platform was placed within a single-wall VE system 1.5 m from the center of the screen. The VE and the hardware and software responsible for its generation have previously been reported13 (Fig. 2). The platform was matched to the motion of the subject's center of mass (COM) for translation in the anteroposterior (AP) and mediolateral (ML) directions. To calculate the motion of the COM, reflective markers were attached bilaterally on the second metatarsophalangeal joint, lateral malleolus, lateral epicondyle of the tibia, greater trochanter, acromion process, lateral epicondyle of the humerus, styloid process of the ulna, second metacarpophalangeal joint, zygomatic arch, and at C7 and L4-5. COM was then computed by Winter's14 equations. The equations were coded and run using commercial software (Matlab, MathWorks Inc., Natick, MA).
FIGURE 2.

Screen shot of an individual performing the pointing task within the virtual environment. See Test Protocol Video on jnptextra.org.
There were three phases to this protocol: pretest, training, and two posttests. During the three testing periods, the subject performed a total of ten 120-second trials with two different platform motions (stationary and matched to the motion of the COM) and five visual scenes. The platform was stationary for five trials, and for five trials it was matched to the excursion of the COM in the opposite direction. Each platform condition was paired with five different visual conditions: standing in the dark, viewing a virtual scene with motion matched to motion of the subject's head, pointing to an object that repeatedly moved 1 m from the left to the right while viewing a head matched scene, viewing a virtual scene that rotated counterclockwise in the frontal plane with a constant velocity of 30 degrees/s, and, finally, pointing to an object that moved one meter from the left to the right while viewing a scene that rotated in counterclockwise in the frontal plane at a constant velocity of 30 degrees/s. The order of the trials was determined at random before the experiment.
The training period took place in the dark over a two-week period for five consecutive days each week and consisted of a series of eight 15-minute blocks each day. The platform gain was initially set at −0.35 at the start of the first training session and gradually increased according to the subject's ability to maintain double-leg stance during the period of a block. A gain of 1.0 would move the platform to a maximum of 100% of the movement of COM of the subject in the same direction. The initial gain of −0.35 indicated that the platform was set to move in the opposite direction, up to a maximum of 35% of COM motion (Fig. 3). The first block each day started with a gain at which the subject was able to maintain her balance on the previous day. The gain was then increased incrementally after each block until the subject was no longer able to maintain balance. If the subject lost balance during the period of a block, the platform was shut off, allowing the subject to regain balance. The platform gain was then turned back to the value at which the subject was able to maintain balance and increased accordingly.
FIGURE 3.
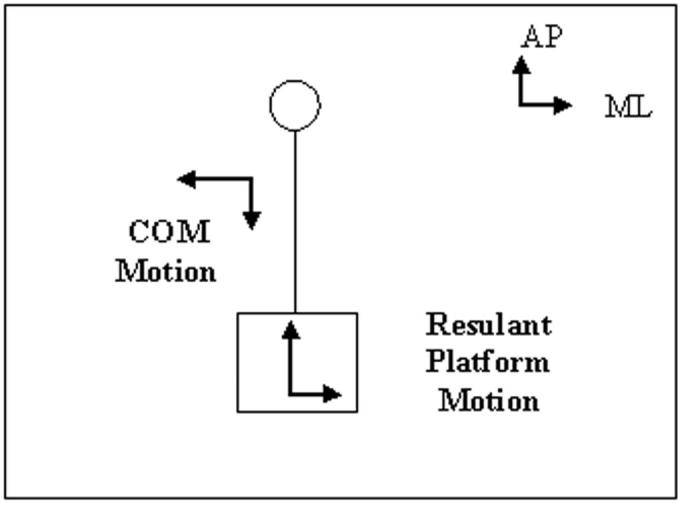
An illustration of the direction of platform movement when matched to movement of the center of mass (COM) of the subject. Abbreviations: AP, anteroposterior; ML, mediolateral.
During each block, the subject was verbally instructed to focus on the motion of her hips and knees and to move them to try to maintain her balance. She was instructed to use this information to remain as stable as possible. The goal of the training was for the subject to attend to her somatosensory feedback, thereby, we assumed, increasing the central weighting of that feedback. At the end of the two-week training period, the first posttest was given; the second posttest took place two weeks later.
Measures
Three-dimensional kinematic data were recorded at 120 Hz using the Motion Analysis Hawk System (Motion Analysis, Inc., Santa Rosa, CA) and low-pass filtered at 4 Hz using a fourth-order Butterworth filter. Displacement of the COM for the entire body in the AP and ML directions were derived from a theoretical model using commercial software (Matlab).14 The root mean square (RMS) was then calculated for each time series of the COM in each direction. RMS represents the average spread of a time series distribution relative to its mean. Higher RMS values indicate greater variability and are traditionally interpreted as greater postural instability or error.15 For the purpose of this case report, RMS values were averaged for the dark, head-matched motion and counterclockwise rotating visual conditions for each platform condition.
Center of pressure (COP) recordings were collected at a rate of 200 Hz from two force plates (AMTI, Watertown, MA) incorporated into the platform. Resultant vectors in the AP and ML directions were calculated as a weighted sum from the individual signals from the right and left force plates. The raw COP time series in each direction for each trial was analyzed with Matlab files available on Physionet16 using a nonlinear regularity statistic known as approximate entropy (ApEn). ApEn is a probability statistic based on the logarithmic likelihood that a sample of data will remain within a tolerance window that defines the criterion of similarity (r = 0.2) for subsequent data increments of two data points (m = 2).17-19 ApEn values tend to range between 0 and 2, with values closer to 2 indicating more unpredictable motion and, therefore, a more complex response organization.18 Values closer to 0 indicate a higher level of regularity and a system response that is more predictable and less complex.18 ApEn values for the AP and ML directions were then calculated for each trial for each visual condition in each platform condition.
Outcomes
This case report limited our ability to draw conclusions about the specific effects of the different visual scenes. Therefore, we collapsed the trials with and without pointing into one measure of the rotating visual condition and one of the head-matched visual condition, each having two outcome measures (COM and COP) for the two conditions of platform motion (stationary or matched). During training, we were able to increase platform gain from −0.35 in the first training session to −1.05 in the last training session. Thus, the platform was moved in the opposite direction from the COM at an initial 35% of COM motion to 105% of COM motion at the end of the training period.
When the platform was stationary, excursion of the COP decreased in both the AP and ML directions (Fig. 4a) from the pretest to both posttests. A concomitant large decrease (>50%) was observed between the pretest and both posttests in the COM RMS in the AP direction when the scene was either dark or head matched (Fig. 5). Decreases in COM RMS approached 50% in the second posttest when the scene rotated counterclockwise. COM RMS actually increased in the dark in the ML direction, whereas responses to the rotating and head-matched scenes decreased by 15% and 40%, respectively. Small (10%–30%) decreases were also exhibited in the ApEn of the COP from pretest to posttests for both directions (Table 1).
FIGURE 4.
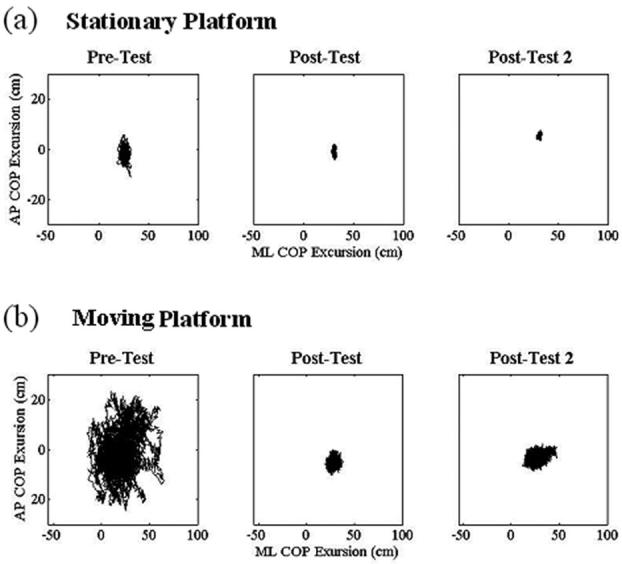
Plots of anteroposterior (AP; y axis) and mediolateral (ML; x axis) excursions of center of pressure (COP) while standing on a stationary (a) or moving (b) platform within a virtual scene that rotated in a counterclockwise direction at a constant velocity of 30 degrees/s and pointing to an object that repeatedly moved one meter from the left to the right for each of the three test periods.
FIGURE 5.
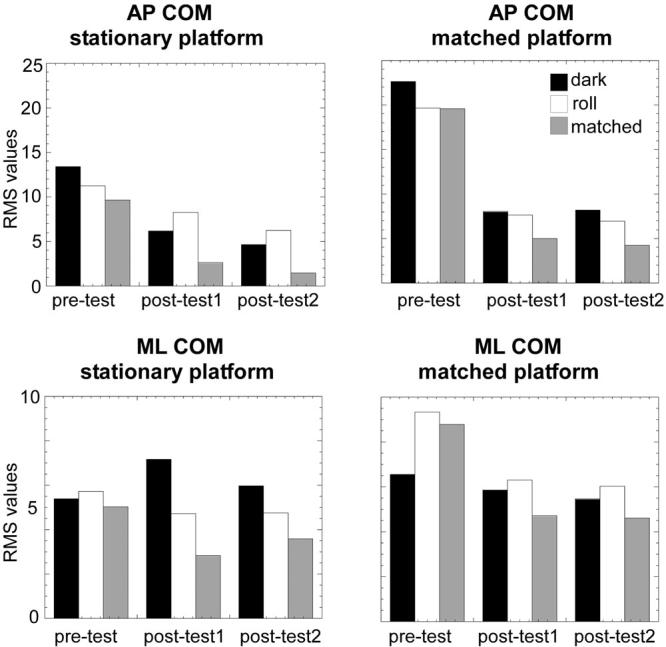
Bar graphs of the center of mass (COM) root mean square (RMS) in the anteroposterior (AP; top plots) and mediolateral (ML; bottom plots) directions when the platform was stationary (left) or matched to the motion of the COM (right) and the scene was dark (black bars), rotating counterclockwise (white bars), and matched to the motion of the head (gray bars) for each of the three test periods.
TABLE 1.
Center of Pressure Approximate Entropy
| Plane | Test Period |
Platform | Mean | SD |
|---|---|---|---|---|
| AP | Pre | Stationary | 0.6454 | 0.0884 |
| AP | Post 1 | Stationary | 0.5177 | 0.1563 |
| AP | Post 2 | Stationary | 0.4744 | 0.1160 |
| ML | Pre | Stationary | 1.2995 | 0.1848 |
| ML | Post 1 | Stationary | 1.1762 | 0.3086 |
| ML | Post 2 | Stationary | 1.2269 | 0.1071 |
| AP | Pre | Moving | 1.3140 | 0.2080 |
| AP | Post 1 | Moving | 1.6743 | 0.0687 |
| AP | Post 2 | Moving | 1.6874 | 0.2185 |
| ML | Pre | Moving | 1.1080 | 0.2445 |
| ML | Post 1 | Moving | 1.4046 | 0.1776 |
| ML | Post 2 | Moving | 1.4153 | 0.3356 |
Abbreviations: SD, standard deviations; AP, anteroposterior; ML, mediolateral.
When the platform moved opposite the COM, COP excursion greatly diminished in both the AP and ML directions (Fig. 4b) between the pretests and posttests. There was a 65% decrease from pretests to posttests in COM RMS with dark and rotating visual scenes and a 75% decrease with a head-matched scene in the AP direction (Fig. 5). In the ML direction with a platform matched to motion of the COM, RMS responses to both the head-matched and rotating visual scenes were even greater than those in the dark. ML RMS values in the dark did not decrease more than 15% in the posttests, but a more than 30% decrease was observed with the rotating scene and a 45% decrease emerged with the head-matched scene (Fig. 5). COM RMS values were greater in the AP direction, as expected from previous reports on healthy subjects.20 As COM motion decreased in the posttests when compared with the pretest, ApEn of the COP exhibited increases in both the AP (about 30%) and ML (about 25%) directions (Fig. 6).
FIGURE 6.
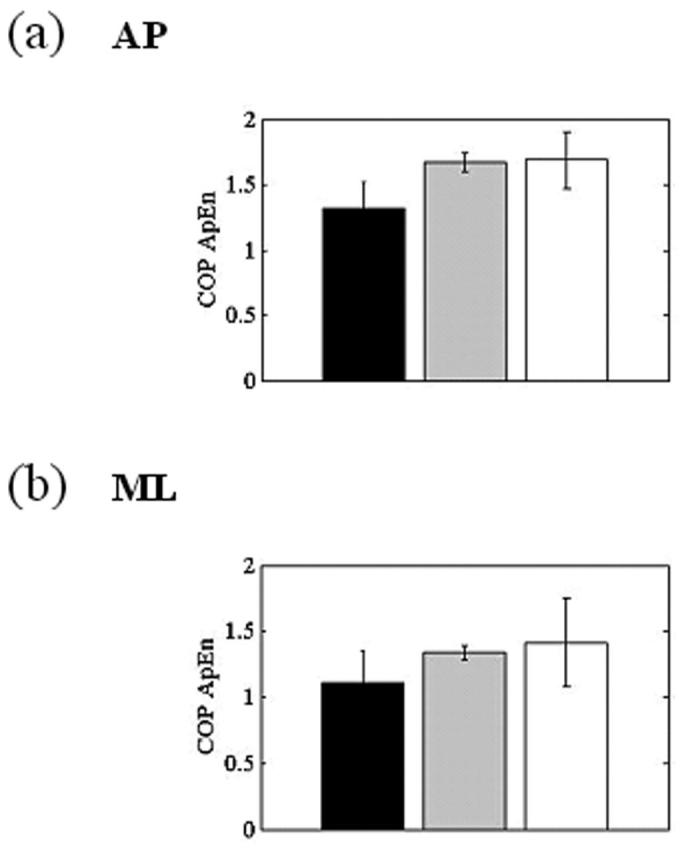
Bar graphs of the mean and standard deviations of the center of pressure (COP) approximate entropy responses for the three test periods in the anteroposterior (AP; a) and mediolateral (ML; b) directions when motion of the platform was matched to motion of the center of mass.
DISCUSSION
This training paradigm provided some intriguing results about the potential for influencing sensory weighting by the CNS via self-directed attention to physical motion. Our patient improved in her ability to maintain balance with both a stationary and a moving platform after the training paradigm. During the pretest, the subject exhibited large excursions of the COM that corresponded with large trajectories of COP. Associating these two outcome measures signifies the ability of the postural control system to adapt to an external perturbation based on the degree of improvement in the steadiness of standing.21,22 With each posttest, motion of the COM and the corresponding COP trajectory decreased, indicating increasing postural stabilization. We believe that reduced excursions of the principal outcome measures for postural stability (COM and COP) are a direct result of implementing our training paradigm with this patient, particularly because she had experienced significant instability for the 10 years after damage to her vestibular system. We are not able to address whether this treatment would have been more or less beneficial had it taken place at an earlier point in her recovery. We speculate that administration of the intervention immediately after the insult may have been able to more strongly bias the CNS adaptation that occurred in response to the loss of labyrinthine input.
We implemented an unusually intense balance training paradigm (10 days over two weeks) and the large differences emerging between the pretests and posttests might be considered only a change in confidence when standing on the moving platform. However, modulated responses to the three visual conditions (dark, rotating, and head matched) in the two posttests imply that some perceptual-motor learning must have occurred, allowing the individual to attend to and modify her responses appropriately to each visual task.
Current approaches to motor relearning often rely on explicit feedback about goal attainment. It has been hypothesized, however, that although kinematics were learned from knowledge of results about errors in extent and direction, response dynamics were learned from the intrinsic proprioceptive errors relating to joint motion.23 In fact, it has been shown in patients with stroke that explicit information was detrimental to implicit motor-sequence learning.24 We therefore chose to have our subject direct her focus to her somatosensory feedback rather to some arbitrary endpoint, which we defined as “upright.”
We believe that this attention to self-motion produced a central reweighting of the sensory modalities. First, there was an improvement in postural stabilization to the stationary as well as the moving base of support. A stationary platform does not produce instability; thus, we must assume that the subject's instability on the stationary platform during the pretest was elicited by the absence of vision or the visual field motion. The improved balance responses after our training paradigm were strongly indicative of an improved ability to respond to the specific parameters of the visual field. In fact, the greatest decrease in COM motion occurred with the head-matched visual field that supplies cues to a vertical orientation. Balance was also greatly improved when motion of the visual world did not match the subject's physical motion (ie, while having to maintain balance on a moving platform with visual field motion that did not match motion of the body).
Dual-task paradigms have been used to study the effects of distraction on posture control and have demonstrated that, particularly in elderly individuals, instability increases during multiple tasks.25 Our subject was able to maintain improvements in balance, even with multisensory demands on her attention, from which we infer that she had incorporated the training into an implicit memory of action with the relevant body parts.24 We conclude from our results that a new focus on her somatosensory feedback (ie, increased weighting of those inputs) allowed our subject to improve her postural stability in the dark. This increased reliance on somatosensory feedback would also allow her to select more effectively the visual cues that could assist with postural stabilization or to decrease the impact (ie, downweight) the destabilizing effects of a rotating visual field.
Even though we were training our subject to attend to the motion of her lower limbs, we did not expect a single optimal kinematic strategy to emerge. Previous research suggested that a healthy and adaptive motor system exhibits advantageous variability, which implies more complexity in the control system.15,26-28 A more complex control system would have more interactions between system components and environmental conditions, which are exhibited as a more chaotic output signal.15,26-28 A less complex postural control system is associated with fewer interactions, reflected by more periodic or noisy signal, making the system too rigid or unstable.15,26-28
In order to explore the presence of complex multisensory processing in this subject's response behaviors, we measured fluctuations in the COP through the measure of ApEn.15,26,27,29 We found that, when the base of support was unstable, as variability of COM motion decreased between the pretests and posttests, there was a corresponding increase in ApEn. Thus, as variability in whole body movement decreased, the complexity of the COP trajectory increased. This finding suggests that improved stability of the whole body (or less variation at the COM) under dynamic circumstances may actually be associated with more chaotic fluctuations of the force trajectories at the base of support. This finding is of particular importance as few studies have established a potential correlation between a linear measure of motion (ie, RMS) and a nonlinear measure of response outcome (ApEn). When the platform was stationary, variability in the COM did not change across sessions, from which we may infer that the patient had identified a successful strategy for postural stability under stable support conditions and that there was no need for the postural control system to recalibrate the COP trajectories.
In conclusion, our results suggest that practice on a dynamic platform, with clear instructions about attending to self-motion, produced an increased reliance on the faster somatosensory feedback and a diminished response to conflicting visual inputs.21,30,31 This technique provided the subject with a more complex and more adaptable movement strategy when she was presented with destabilizing sensory conflicts. Anecdotally, the patient reported that after the training period she felt “more secure” while walking in the community. Future research could be directed toward determining whether there is a lower limit to the amount of variability emerging at the COM and an upper limit to the amount of complexity of the COP in individuals with compromised motor systems.
Supplementary Material
ACKNOWLEDGMENTS
Supported by NIH-NIA grant AG26470 and NIH-NIDCD grant DC05235. We gratefully acknowledge VRCO, Inc., for the use of the CAVELIB to develop the visual environment. We thank Drs. Jefferson Streepey and Leo Wu for their assistance with data collection.
Footnotes
Supplemental information (videos) for this article can be found at www.jnptextra.org
REFERENCES
- 1.Herdman SJ, Schubert MC, Tisa RJ. Strategies for Balance rehabilitation: fall risk and treatment. Ann N Y Acad Sci. 2001;942:413–427. [PubMed] [Google Scholar]
- 2.Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002;3:1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- 3.Carver S, Kiemel T, Jeka JJ. Modeling the dynamics of sensory reweighting. Biol Cybern. 2006;2:123–134. doi: 10.1007/s00422-006-0069-5. [DOI] [PubMed] [Google Scholar]
- 4.Ravaioli E, Oie KS, Kiemel T, et al. Nonlinear postural control in response to visual translation. Exp Brain Res. 2005;160:450–459. doi: 10.1007/s00221-004-2030-y. [DOI] [PubMed] [Google Scholar]
- 5.Cenciarini M, Peterka RJ. Stimulus-dependent changes in the vestibular contribution to human postural control. J Neurophysiol. 2006;95:2733–2750. doi: 10.1152/jn.00856.2004. [DOI] [PubMed] [Google Scholar]
- 6.Mergner T, Maurer C, Peterka RJ. Sensory contributions to the control of stance: a posture control model. Adv Exp Med Biol. 2002;508:147–152. doi: 10.1007/978-1-4615-0713-0_18. [DOI] [PubMed] [Google Scholar]
- 7.Speers RA, Kuo AD, Horak FB. Contribution of altered sensation and feedback responses to changes in coordination of postural control due to aging. Gait Posture. 2002;16:20–30. doi: 10.1016/s0966-6362(02)00003-6. [DOI] [PubMed] [Google Scholar]
- 8.Nashner L, Berthoz A. Visual contribution to rapid motor responses during postural control. Brain Res. 1978;150:403–407. doi: 10.1016/0006-8993(78)90291-3. [DOI] [PubMed] [Google Scholar]
- 9.Whitney SL, Sparto PJ, Hodges LF, et al. Responses to a virtual reality grocery store in persons with and without vestibular dysfunction. Cyberpsychol Behav. 2006;9:152–156. doi: 10.1089/cpb.2006.9.152. [DOI] [PubMed] [Google Scholar]
- 10.Malfait N, Gribble PL, Ostry DJ. Generalization of motor learning based on multiple field exposures and local adaptation. J Neurophysiol. 2005;93:3327–3338. doi: 10.1152/jn.00883.2004. [DOI] [PubMed] [Google Scholar]
- 11.Sills AW, Baloh RW, Honrubia V. Caloric testing 2: results in normal subjects. Ann Otol Rhinol Laryngol Suppl. 1977;86(5 pt Suppl 43):7–23. doi: 10.1177/00034894770865s302. [DOI] [PubMed] [Google Scholar]
- 12.Baloh RW, Honrubia V, Yee RD, et al. Changes in the human vestibuloocular reflex after loss of peripheral sensitivity. Ann Neurol. 1984;16:222–228. doi: 10.1002/ana.410160209. [DOI] [PubMed] [Google Scholar]
- 13.Keshner EA, Kenyon RV, Langston J. Postural responses exhibit multisensory dependencies with discordant visual and support surface motion. J Vestib Res. 2004;4:307–319. [PubMed] [Google Scholar]
- 14.Winter DA. Biomechanics and Motor Control of Human Movement. John Wiley & Sons, Inc; New York, NY: 1991. [Google Scholar]
- 15.Cavanaugh JT, Mercer VS, Stergiou N. Approximate entropy detects the effects of a secondary cognitive task on postural control in healthy young adults: a methodological report. J Neuroeng Rehabil. 2007;4 doi: 10.1186/1743-0003-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberger AL, Amaral LAN, Glass L, et al. PhysioBank, physiotoolkit, and physionet: components of a new research resource for complex physiological signals. Circulation. 2000;101:215–220. doi: 10.1161/01.cir.101.23.e215. [DOI] [PubMed] [Google Scholar]
- 17.Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci U S A. 1991;88:2297–2301. doi: 10.1073/pnas.88.6.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pincus SM, Goldberger AL. Physiological time-series analysis: what does regularity quantify? Am J Physiol. 1994;266:H1643–H1656. doi: 10.1152/ajpheart.1994.266.4.H1643. [DOI] [PubMed] [Google Scholar]
- 19.Buzzi UH, Ulrich BD. Dynamic stability of gait cycles as a function of speed and system constraints. Motor Control. 2004;8:241–254. doi: 10.1123/mcj.8.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mochizuki L, Duarte M, Amadio AC, et al. Changes in postural sway and its fractions in conditions of postural instability. J Appl Biomech. 2006;22:51–60. doi: 10.1123/jab.22.1.51. [DOI] [PubMed] [Google Scholar]
- 21.Fujiwara K, Kiyota T, Maeda K, et al. Postural adaptability to floor oscillations in the elderly. J Physiol Anthropol. 2007;26:485–493. doi: 10.2114/jpa2.26.485. [DOI] [PubMed] [Google Scholar]
- 22.Bugnariu N, Fung J. Aging and selective sensorimotor strategies in the regulation of upright balance. J Neuroeng Rehabil. 2007;4:19. doi: 10.1186/1743-0003-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krakauer JW, Ghilardi MF, Gehz C. Independent learning of internal models for kinematic and dynamic control of reaching. Nat Neurosci. 1999;2:578–585. doi: 10.1038/14826. [DOI] [PubMed] [Google Scholar]
- 24.Boyd LA, Winstein CJ. Impact of explicit information on implicit motor-sequence learning following middle cerebral artery stroke. Phys Ther. 2003;83:976–989. [PubMed] [Google Scholar]
- 25.Shumway-Cook A, Woollacot M. Attentional demands and postural control: the effect of sensory context. J Gerontol Series A Biol Sci Med Sci. 2000;55:M10–M16. doi: 10.1093/gerona/55.1.m10. [DOI] [PubMed] [Google Scholar]
- 26.Stergiou N, Harbourne RT, Cavanaugh JT. Optimal movement variability: a new theoretical perspective for neurological physical therapy. J Neurol Phys Ther. 2006;30:120–129. doi: 10.1097/01.npt.0000281949.48193.d9. [DOI] [PubMed] [Google Scholar]
- 27.Cavanaugh JT, Guskiewicz KM, Stergiou N. A nonlinear dynamic approach for evaluating postural control: new directions for the management of sports-related cerebral concussion. Sports Med. 2005;35:935–950. doi: 10.2165/00007256-200535110-00002. [DOI] [PubMed] [Google Scholar]
- 28.Riley MA, Turvey MT. Variability and determinism in motor behavior. J Mot Behav. 2002;34:99–125. doi: 10.1080/00222890209601934. [DOI] [PubMed] [Google Scholar]
- 29.Donker SF, Roerdink M, Greven AJ, Beek PJ. Regularity of the center-of-pressure trajectories depends on the amount of attention invested in postural control. Exp Brain Res. 2007;181:1–11. doi: 10.1007/s00221-007-0905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahboobin A, Loughlin PJ, Redfern MS, Sparto P. Sensory re-weighting in human postural control during moving-scene perturbations. Exp Brain Res. 2005;167:260–267. doi: 10.1007/s00221-005-0053-7. [DOI] [PubMed] [Google Scholar]
- 31.Keshner EA, Kenyon RV. The influence of an immersive virtual environment on the segmental organization of postural stabilizing responses. J Vestib Res. 2000;10:207–219. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


