Abstract
Polo-like kinase 1 (Plk1), the best characterized member of the mammalian polo-like kinase family, is well regulated throughout the cell cycle at the protein expression level. Moreover, it is known that Plk1 kinase activity is also regulated at the post-translational level through phosphorylation. However, the upstream kinases of Plk1 have not been identified. Although the involvement of the p38 MAP kinase pathway in cellular responses to stress has been well documented, the role of this pathway in normal cell cycle progression is unclear. Here, we show that phosphorylated p38 and MAP kinase-activated protein kinase 2 (MK2) are colocalized with Plk1 to the spindle poles during prophase and metaphase. Specific depletion of various members of the p38 MAP kinase pathway by the use of RNA interference revealed that the pathway is required for mitotic progression under normal growth conditions. Furthermore, MK2 directly phosphorylates Ser326 of Plk1. Ectopic expression of Plk1-S326A completely blocked cells at mitosis, likely due to the defect of bipolar spindle formation and subsequent activation of the spindle checkpoint. Only Plk1-S326E, but not the Plk1-S326A, efficiently rescued the p38 or MK2-depletion-induced mitotic defects, further solidifying the requirement of S326 phosphorylation during mitotic progression.
Keywords: Plk1, MK2, mitosis, phosphorylation
Introduction
Extensive studies have shown that polo-like kinase 1 (Plk1) expression is elevated in breast cancer, esophageal and gastric cancer, melanomas, ovarian cancer, non-small cell lung cancer, head and neck cancer, endometrial cancer, colorectal cancer and thyroid cancer (Eckerdt et al., 2005; Takai et al., 2005). A close correlation between lower survival rates and higher Plk1 expression levels was found in patients with several types of cancers (Knecht et al., 1999, 2000; Takai et al., 2005). Therefore, it was proposed that Plk1 could serve as a novel diagnostic marker for several types of cancers (Eckerdt et al., 2005; Takai et al., 2005).
At the cellular level, Plk1 has emerged as a vital regulator in many aspects of cell cycle progression, such as centrosome maturation, sister chromatid segregation and cytokinesis (Barr et al., 2004). In addition to the N-terminal kinase domain, all Plk family members, including mammalian Plk1, Xenopus Plx1, fission yeast Plo1 and budding yeast Cdc5, have a highly conserved region in the C-terminal domain, denoted by the polo box (Barr et al., 2004). The major function of the polo box domain has been shown to interact with Plk substrates. In the absence of a bound substrate, the polo box domain inhibits the basal activity of the kinase domain. Phosphorylation-dependent binding of the polo box domain to its ligands releases the kinase domain, while simultaneously localizing polo kinases to specific subcellular structures (Lowery et al., 2005).
Both the protein abundance and kinase activity of Plk1 are cell cycle dependent. Plk1 expression is low throughout G1/S phases, begins to rise at G2, reaches a peak during M phase (Golsteyn et al., 1994) and returns to the basal level upon exit from mitosis. Similar to several other mitotic regulators, Plk1 is degraded through the ubiquitin–proteasome pathway at the onset of the metaphase–anaphase transition (Shirayama et al., 1998). Common to many other protein kinases, the activity of Plk1 is also regulated by phosphorylation. However, the nature of these upstream kinases remains unknown (Barr et al., 2004).
Emerging evidence suggests a link between chronic inflammation and various types of cancers, and importantly, the sustained activation of the stress pathway in diseases involving chronic inflammation raises the possibility that Plk1 might be a key player acting downstream of the stress pathway in inflammation-induced cancer development. In addition to an established role in inflammation, the p38 MAP kinase pathway is also involved in chemotherapeutic agent-induced cancer cell apoptosis (Olson and Hallahan, 2004). However, the role of this pathway in normal cell cycle progression is unclear and increasing evidence suggests that the pathway might be involved in normal mitosis (Fan et al., 2005; Zarubin and Han, 2005; Cha et al., 2007).
In this study, we analysed the connection between the p38 MAP kinase pathway and Plk1 in mammalian cells. Using the RNA interference (RNAi) approach, we showed that various members of the p38 MAP kinase pathway are required for mitotic progression. Furthermore, we demonstrated that MAP kinase-activated protein kinase 2 (MK2) is a Plk1 kinase in vivo and the MK2-associated phosphorylation of Plk1 regulates its commitment during mitotic progression.
Results
The p38 MAP kinase pathway is required for mitotic progression
To examine the function of endogenous p38 MAP kinase, we used vector-based RNAi to specifically deplete p38α in HeLa cells. The targeting sequencing of p38α is the coding region 326–346 relative to first nucleotide of the start codon. In brief, pBabe-puro, which expresses a puromycin-resistance gene, was cotransfected with pBS/U6-p38α at a ratio of 1:9 to permit selection of transfection-positive cells (Supplementary information). Puromycin was added for additional 1 or 2 days at 1-day post-transfection. After floating cells were washed away with phosphate-buffered saline, attached cells were harvested for phenotypic analysis. As indicated in Figure 1a, p38α was reduced by ~70% or by at least 95% at 2 or 3 days post-transfection, respectively. The requirement of p38α for cell proliferation and viability was then determined. Cells transfected with control vector grew at a normal rate, whereas the proliferation and viability of cells depleted of p38α were strongly decreased (Figures 1b and c). At 2 or 3 days post-transfection, cells were also harvested for fluorescence-activated cell sorting (FACS) analysis. Consistent with inhibition of proliferation, p38-depleted cells showed obvious G2/M-phase arrest followed by apoptosis (Figure 1d).
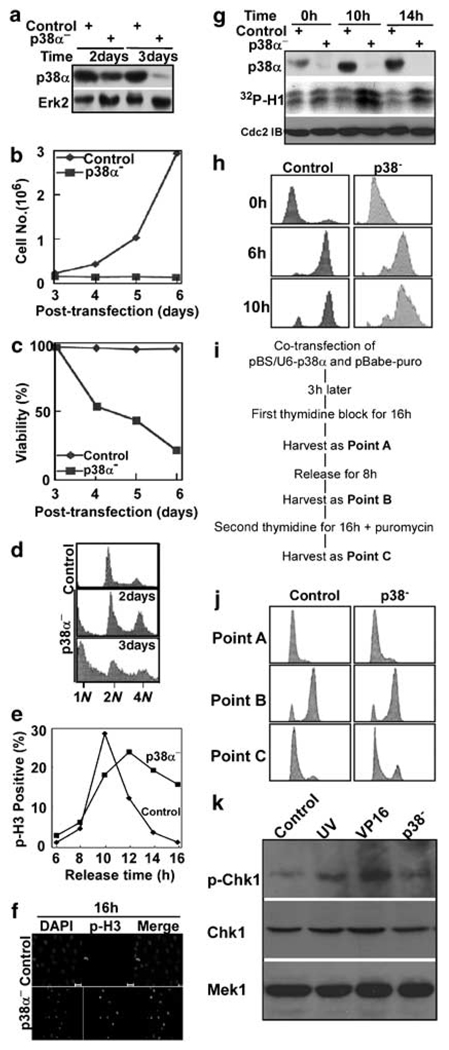
Figure 1.
p38 MAP kinase is required for cell proliferation and survival. (a) HeLa cells were co-transfected with pBS/U6-p38 and pBabe-puro at a ratio of 9:1. After 1 day of incubation, puromycin was added for additional 1 or 2 days to select transfection-positive cells. After floating cells were removed, the remaining attached cells were harvested, and lysates were subjected to western blot using antibodies indicated on the left. (b, c) Cells were transfected and selected with puromycin as in (a). Cells were harvested at times as indicated, and cell proliferation (b) and viability (c) were monitored. To determine cell viability, floating cells and attached cells were harvested and counted separately. Viability was calculated as a percentage of attached cells compared to total cells. (d) Fluorescence-activated cell sorting (FACS) profiles of p38-depleted cells harvested at 2 days or 3 days post-transfection. (e) p38 was depleted in a synchronized culture using a double thymidine block protocol (16 h of thymidine block, 8 h of release and followed by a second 16 h incubation with thymidine). After release from the block for different times, cells were analysed by a phospho-histone H3 (p-H3) antibody staining. (f) Representative images of cells at 16 h post-release. Scale bar, 20 µm. (g) p38α was depleted in synchronized cells as in (e). After release from thymidine block for different times as indicated, cells were harvested and lysates were subjected to an anti-p38α immunoblot (top panel) or an anti-Cdc2 (middle panel) immunoprecipitation (IP)/kinase assay, using histone H1 as a substrate. (h) p38α was depleted in synchronized cells as in (e). After release from thymidine block for different times as indicated, cells were harvested for FACS analysis. (i) The detailed protocol to deplete p38α in well synchronized cells. (j) HeLa cells were depleted of p38a as in (i), harvested at three different time points as indicated and analysed by FACS. Point A, after first thymidine block for 16 h. Point B, after release for 8 h from the first thymidine block. Point C, after second thymidine block for 16 h. (k) HeLa cells were depleted of p38a as in (i), harvested at point C, and analysed by anti-phospho-Chk1 western blot to examine DNA damage checkpoint activation. As two positive controls, HeLa cells were either UV irradiated with 100 J/m2 and harvested after 1.5 h incubation (lane 2) or treated with 25 µg/ml VP16 for 4 h (lane 3).
To distinguish whether p38α depletion led to G2- or M-phase arrest, cells were depleted of p38α, and synchronized with a double thymidine block protocol. Following release from the block for different times, p38-depleted cells were stained with a phospho-histone H3 antibody (Figure 1e) (Supplementary information). Control cells began to enter mitosis at 8 h, reached a peak of phospho-histone H3 staining at 10 h and had exited mitosis at 14 h after release. The p38α-depleted cells also entered mitosis at 8 h after release, but a significant difference was observed at later times. At 16 h post-release, less than 2% of control cells were phosphohistone H3 positive, whereas ~15% of p38-depleted cells remained in mitosis (Figures 1e and f). After release at different times from the thymidine block, p38α-depleted cells were harvested and lysates were subjected to an anti-p38α western blot or an anti-Cdc2 immunoprecipitation/kinase assay (Figure 1g). Depletion of p38α significantly increased the kinase activities of Cdc2, supporting a requirement for p38α for mitotic progression. Finally, as a control experiment, we used FACS to follow cell cycle progression after p38α depletion in cells synchronized by the double thymidine block protocol. We found that under this condition, the p38-depleted cells can continue to undergo cell cycle from G1 to M phase after release from the block (Figure 1h). To make sure that the population of p38α-depleted cells has gone through the same cell cycle progression as control cells during the double thymidine block, HeLa cells were depleted of p38α as in Figure 1i, and harvested at three different time points during the process; point A, after first thymidine block for 16 h; point B, after release for 8 h from the first thymidine block and point C, after second thymidine block for 16 h. As indicated in Figure 1j by FACS, the p38α-depleted cells have gone through the similar cell cycle progression as control cells during the synchronization process. Finally, we also tested whether there is DNA damage in p38α-depleted cells at the end of block sequence. Both UV irradiation and VP16 treatment led to activation of Chk1, whereas p38α depletion did not cause significant DNA damage checkpoint activation, indicating that there is no serious DNA damage in p38α-depleted cells at the end of the synchronization(Figure 1k).
To test whether other members of the p38 MAP kinase pathway are also required for mitosis, we used siRNA to target various members of the pathway. The targeting sequences of MK2, MKK3, MKK6 or p38α are the coding region 1100–1120, 260–280, 395–415 or 212–232 relative to first nucleotide of the start codon, respectively. HeLa cells were transfected with siRNA targeting p38α or MK2, the major downstream target of p38. Cells were harvested at 2 days post-transfection, and lysates were used for western blot analysis. As indicated in Figure 2a, both p38 and MK2 were efficiently depleted. The p38α- or MK2-depleted cells were synchronized with the double thymidine block and released for different times. Cells were fixed and stained with a phospho-histone H3 antibody. Control cells showed normal mitotic progression and only 2% of cells remained phospho-histone H3 positive at 16 h post-release. However, both p38α- and MK2-depleted cells showed a similar phenotype of mitotic block. Even at 16 h post-release, ~20% of cells remained in mitosis (Figure 2b). Further characterization indicated that most of the MK2-depleted cells at 14 h post-release were blocked at prometaphase (Figure 2c). As a control experiment, we used FACS to follow cell cycle progression after MK2 depletion in the synchronized cells. We found that under this condition, the MK2-depleted cells can continue to undergo cell cycle from G1 to M phase after release from the block (Figure 2d).
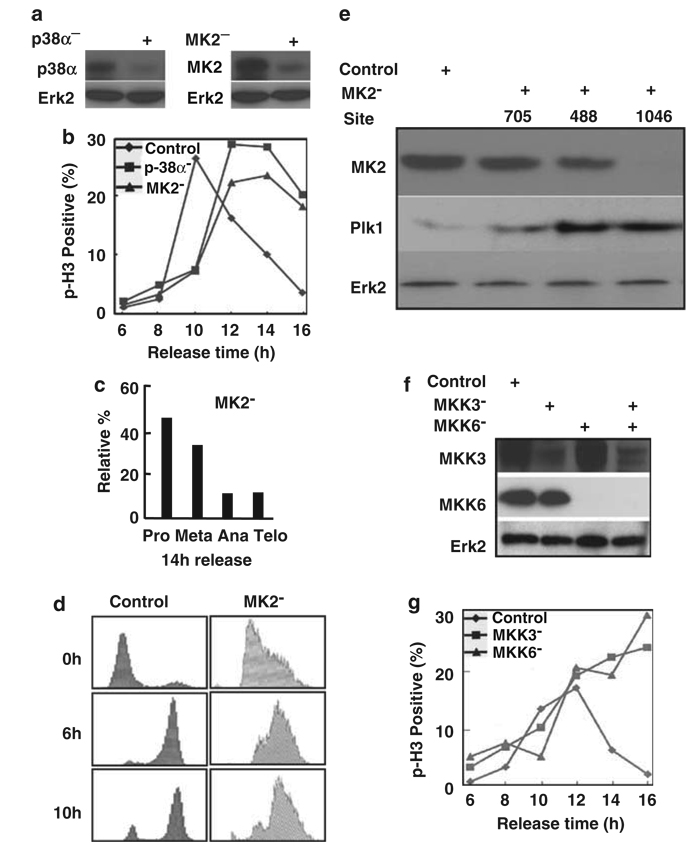
Figure 2.
The p38 MAP kinase pathway is required for mitotic progression. (a) HeLa cells were transfected with siRNA by targeting p38α (left panel), or targeting MK2 (right panel). Cells were harvested at 2 days post-transfection, and lysates were analysed by western blot. (b) Cells were depleted of p38α or MK2 as described in (a), subjected to the double thymidine block, released for different times and stained with a phospho-histone H3 (p-H3) antibody. (c) Relative percentages of different subphases of mitosis of MK2-depleted cells at 14 h post-release. (d) MK2 was depleted in synchronized cells as in (b). After release from thymidine block for different times as indicated, cells were harvested for fluorescence-activated cell sorting (FACS) analysis. (e) MK2 depletion leads to Plk1 stabilization. HeLa cells were transfected with pBS/U6-MK2 by targeting three different sites (nt 705, nt 488 and nt 1046). At 2 days post-transfection, cells were harvested and subjected to western blot using the antibodies indicated. (f) HeLa cells were transfected with siRNAs to deplete MKK3, MKK6 or MKK3/6. At 2 days post-transfection, cells were harvested and analysed by western blot. (g) HeLa cells were depleted of MKK3 or MKK6 as described in (f), subjected to the double thymidine block, released for different times and stained with a phospho-histone H3 (p-H3) antibody.
We also used vector-based RNAi to deplete MK2 by targeting three different sites (nt 705, nt 488 and nt 1046). At 2 days post-transfection, the cell lysates were prepared and analysed by an anti-Plk1 western blot (Figure 2e). Depletion of MK2 targeting three different sites all led to upregulation of Plk1, most likely due to mitotic arrest of these cells. Two upstream kinases of p38 MAP kinase, MKK3 and MKK6, were also depleted using siRNA. HeLa cells were transfected with siRNA to deplete MKK3, MKK6 or MKK3/6. At 2 days post-transfection, cells were harvested and analysed by western blot (Figure 2f); the levels of both MKK3 and MKK6 were significantly reduced. MKK3- or MKK6-depleted cells were then subjected to the double thymidine block, released for different times and stained with a phospho-histone H3 antibody. As expected, both MKK3 and MKK6 depletion led to mitotic block (Figure 2g). Although the RNAi-based knockdown of p38α and MK2 leads to a retarded mitotic progression, the downregulation of MKK3/6 seems to block cells completely in mitosis. This could be due to the existence of multiple downstream targets of MKK3/6 such as p38â, which may also have functions in mitosis. Finally, the requirement of the stress pathway for mitosis was confirmed by using SB203580, a p38 inhibitor. Chromosome lagging and mitotic block were observed in cells treated with SB203580 (Figure 3).
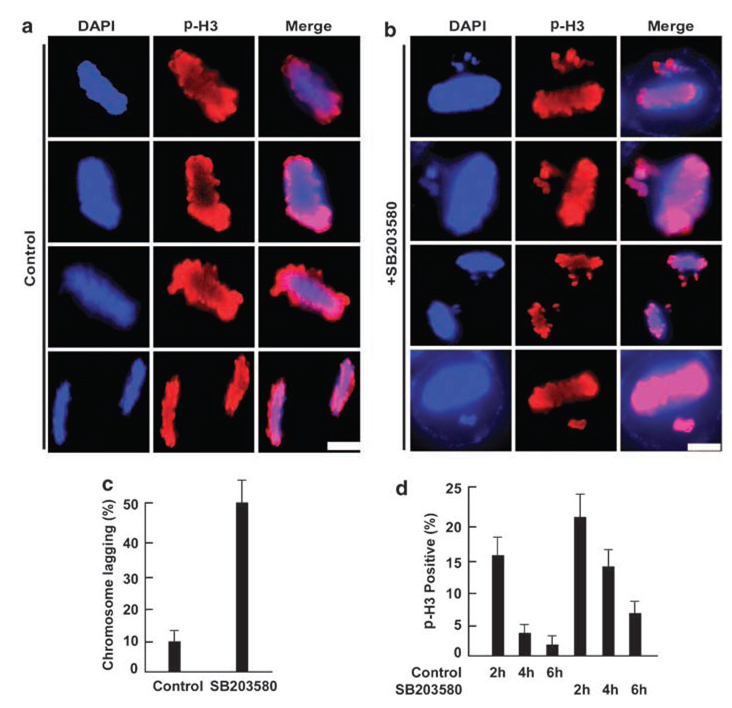
Figure 3.
Inhibition of the p38 MAP kinase pathway leads to mitotic defects. (a, b). HeLa cells were synchronized with the double thymidine block, released for 6 h, treated with SB203580 for additional 6 h and stained with a phospho-H3 antibody. Scale bar, 5 µm. (c) Histogram quantifying the chromosome lagging phenotype after SB203580 treatment. (d) Cells were synchronized with the double thymidine block, released for 8 h, treated with SB203580 for additional 2, 4 or 6 h, and stained with a phospho-H3 antibody.
Activation of the p38 MAP kinase pathway during mitosis
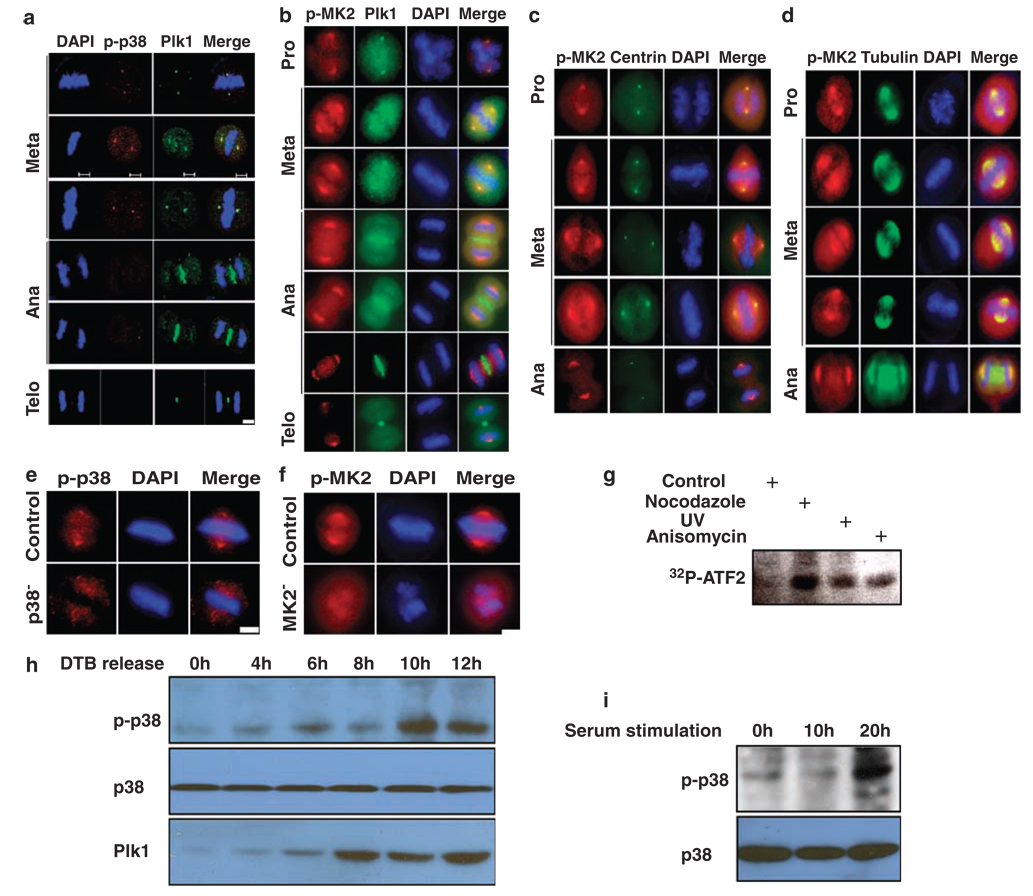
To further explore the involvement of the p38 MAP kinase pathway during mitosis, we analysed the subcellular localization of activated forms of p38α and MK2. HeLa cells were synchronized by the double thymidine block, and harvested at 10 h post-release. Harvested cells were co-stained with antibodies to Plk1 and phospho-p38α (Figure 4a). We found that phospho-p38α colocalized with Plk1 at spindle poles in metaphase. Furthermore, phospho-MK2 colocalized with Plk1 in centrosomes in prophase and spindle poles in metaphase. Plk1 re-distributed to mid-zones in anaphase and mid-bodies in telophase, whereas phospho-MK2 remained at spindle poles in late mitosis (Figure 4b). The spindle pole localization of phospho-MK2 during different stages of mitosis was further confirmed by using HeLa cells stably expressing green fluorescence protein (GFP)-centrin (Figure 4c) or GFP-tubulin (Figure 4d). The spindle pole localization of phospho-p38 and phospho-MK2 was lost upon p38 or MK2 depletion, indicating the specificity of staining (Figures 4e and f). During the preparation of this paper, p38 phosphorylation, activation and localization at the centrosomes during normal mitosis were also reported in HeLa cells, in agreement with what we described here (Cha et al., 2007). To directly address whether the p38 MAP kinase pathway is activated in mitosis, HeLa cells were treated with 200 ng/ml nocodazole for 12 h, 50 J/m2 UV irradiation or 50 ng/ml anisomycin for 4 h and harvested. Lysates were subjected to anti-p38α IP/kinase assays using purified GST-ATF2 as a substrate. As indicated, the p38 kinase activity of mitotic cells is significantly higher than that of interphase cells, supporting the notion that the p38 MAP kinase pathway is specifically activated during mitosis (Figure 4g). Moreover, HeLa cells were synchronized with the double thymidine block protocol, released for different times and harvested for western blotting. Both activation of p38 and increased levels of Plk1 were detected in G2/M phase, but not in G1/S phase (Figure 4h). Finally, NIH3T3 cells were serum starved for 2 days, released into cell cycle upon 15% serum stimulation for different times and harvested for phospho-p38 western blot. Again, activation of p38 was clearly detected at 20 h post-release, when majority of the cells reached mitosis (Figure 4i).
Figure 4.
Activation of the p38 MAP kinase pathway during mitosis. (a) Colocalization of Plk1 and phospho-p38α at the spindle poles during mitosis. HeLa cells were synchronized with the double thymidine block, released for 10 h and co-stained with Plk1 and phospho-p38α. (b) Cells were prepared as in (a), and co-stained with Plk1 and phospho-MK2. (c, d) HeLa cells stably expressing GFP-centrin (c) or GFP-tubulin (d) were prepared as in (a), and stained with phospho-MK2. Scale bar, 5 µm. (e, f) Spindle localization of phospho-p38α (p-p38)/MK2 is specific. HeLa cells were p38α- or MK2-depleted using RNA interference (RNAi) as in Figure 2a and stained with phospho-p38α (e) or phospho-MK2 (p-MK2) (f) antibody. Scale bar, 5 µm. (g) HeLa cells were treated with 200 ng/ml nocodazole for 12 h, 50 J/m2 UV irradiation or 50 ng/ml anisomycin for 4 h and harvested. Lysates were subjected to anti-p38α immunoprecipitation (IP)/kinase assays using purified GST-ATF2 as a substrate. (h) HeLa cells were synchronized with the double thymidine block protocol, released for different times and harvested for western blotting. (i) NIH3T3 cells were serum starved for 2 days, released into cell cycle upon 15% serum stimulation for different times and harvested for phospho-p38 western blot.
MK2 phosphorylates Plk1 in vitro and in vivo
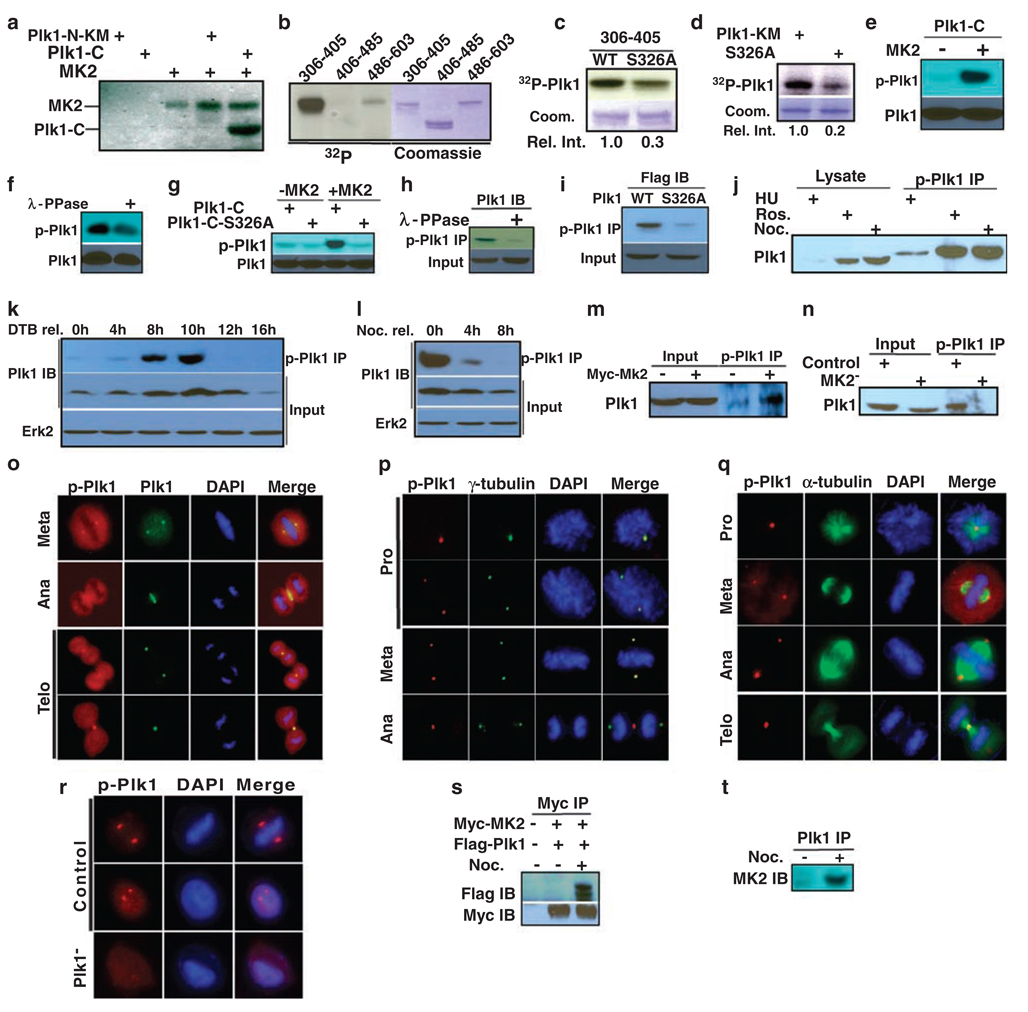
Considering the essential function of Plk1 during mitosis, we next investigated whether the p38 MAP kinase pathway and Plk1 are directly connected. Toward that end, purified p38 or MK2 was incubated with purified Plk1-KM (kinase defective mutant) in the presence of [γ-32P]ATP. The reaction mixtures were resolved by SDS-polyacrylamide gel electrophoresis, followed by autoradiography. In addition to the expected autophosphorylation of p38 and MK2, Plk1-KM was also clearly phosphorylated by MK2, but not by p38 (data not shown). To identify the phosphorylation site in Plk1, purified Plk1 fragments (either N-KM or C-terminal domain) were tested as substrates for MK2. Only the C-terminal domain of Plk1 yielded a strong phosphorylation signal (Figure 5a). To further narrow down the sites, three Plk1 fragments (aa306–405, aa406–485 and aa486–603) were subjected to the kinase assay, and only the domain containing aa306–405 was a robust substrate for MK2 (Figure 5b). Phosphoamino acid analysis indicated that serine is the major amino acid phosphorylated by MK2 (data not shown). Extensive site-directed mutagenesis was used to map the phosphorylation site to be Ser326. Compared to the phosphorylation level of the wild-type (WT) Plk1 fragment, phosphorylation of the S326A fragment by MK2 was significantly reduced, indicating that Ser326 is a major phosphorylation site (Figures 5c and d). In summary, we concluded that Ser326 of PlK1 is a major MK2 phosphorylation site in vitro. We might point out that S326 is not the only site phosphorylated by MK2. In fact, we also mapped S383 as another site phosphorylated by MK2 (data not shown) and are currently analysing the functional significance of Plk-S383 phosphorylation. The proposed MK2 consensus motif is L/F/I-X-R-X-X-S/T-Ø. Although Ø is a hydrophobic residue, Arg in the −3 position is criticalfor substrate recognition (Manke et al., 2005). With an Arg residue at −2 position, the sequence context of Plk1-S326, P-P-RF-S326-I partially matches the conserved MK2 consensus sequence.
Figure 5.
MK2 targets Ser326 of Plk1. (a) Purified MK2 was incubated with Plk1 fragments in the presence of [γ-32P]ATP. The reaction mixtures were analysed by polyacrylamide gel electrophoresis, then autoradiography. Plk1-N-KM (kinase defective mutant): aa1–305; Plk1-C: aa306–603. (b) MK2 was incubated with three Plk1 C-terminal fragments (aa306–405, aa406–485 and aa486–603). (c, d) MK2 was incubated with Plk1-KM or a Ser326A mutant of different lengths (c, aa306–405; d, aa1–603). (e) MK2 was incubated with Plk1-C in the presence of cold ATP. The reaction mixtures were analysed by anti-phospho-Plk1 (p-Plk1) western blot. (f) After Plk1-C was incubated with MK2, the 50 µl reaction mixture was treated with 400 U λ-phosphatase (400 U µl−1; New England Biolab, Ipswich, MA, USA, Catalog number P0753) for 3 h at room temperature and analysed as in (e). (g) Purified Plk1-C (WT or S326A mutant) was incubated with MK2 and analysed by western blot. (h) HeLa cells were treated with nocodazole and harvested. After incubation with λ-phosphatase, lysates were subjected to anti-phospho-Plk1 immunoprecipitation (IP), followed by anti-Plk1 western blot. (i) HEK293 cells were transfected with Flag-Plk1 (WT or S326A mutant), and treated with nocodazole. Lysates were subjected to anti-phospho-Plk1 IP, followed by anti-Flag western blot. (j) HeLa cells were treated with hydroxyurea (lanes 1 and 4) or nocodazole (lanes 3 and 6) to block at S or M phase, respectively. To block cells at G2 phase, cells were released from the double thymidine block for 6 h, then incubated for 2 h in the presence of roscovitine (lanes 2 and 5). (k, l) HeLa cells were synchronized with the double thymidine block (k) or nocodazole treatment (l), released for the different times, and harvested. (m, n) HeLa cells were either MK2-overexpressed (m) or -depleted (n). (o–q) Mitotic cells were co-stained with a phospho-Plk1 antibody and antibodies against Plk1 (o), γ-tubulin (p) or α-tubulin (q). DNA was stained with DAPI. (r) Phospho-Plk1 staining is specific. Cells were Plk1-depleted with dsRNA and stained with phospho-Plk1 antibody. Scale bar, 5 µm. (s) HEK293 cells were co-transfected with Myc-MK2 and Flag-Plk1. At 1 day post-transfection, cells were treated with nocodazole for 12 h, and then harvested. Lysates were subjected to anti-Myc IP, followed by anti-Flag western blot analysis. (t) HeLa cells were treated with nocodazole, and harvested. Lysates were subjected to Plk1 IP, followed by anti-MK2 western blot analysis.
Using a peptide containing phospho-S326 to immunize rabbits, a phospho-Plk1-specific antibody was generated, and affinity purified. A series of experiments utilizing the phospho-Plk1 antibody were also performed to test whether S326 is phosphorylated by MK2. First, GST-Plk1-C (aa306–603), purified from bacteria, was incubated with MK2 in the presence of cold ATP, and then analysed by western blot. Phospho-Plk1 signal was detected only in the sample that was pre-incubated with MK2 (Figure 5e) and disappeared after λ-phosphatase treatment (Figure 5f), suggesting that the antibody specifically recognized the phosphorylated form of Plk1. To confirm that the phospho-Plk1 antibody recognizes phospho-S326 specifically, either purified GST-Plk1-C-WT or S326A mutant was incubated with MK2. Positive phospho-Plk1 signal was detected in only WT-Plk1, but not S326A mutant, indicating the specificity of the antibody (Figure 5g).
Next, lysates from nocodazole-treated HeLa cells were incubated with or without λ-phosphatase, subjected to anti-phospho-Plk1 IP, followed by anti-Plk1 western blot. Disappearance of Plk1 western signal in the anti-phospho-Plk1 IP sample upon phosphatase treatment indicated that phosphorylation of S326 occurs in vivo (Figure 5h). Moreover, HEK293 cells were transfected with Flag-Plk1 (WT or S326A mutant) and treated with nocodazole. Lysates were subjected to anti-phospho-Plk1 IP, followed by Flag western blot (Figure 5i). The fact that only WT-Plk1, but not the S326A mutant, was detected indicated that S326 is phosphorylated in vivo. To test whether phosphorylation of S326 is cell cycle dependent, cells were synchronized at S, G2 or M phase using various drug treatments, and phosphorylation of S326 was analysed. Phosphorylation of S326 in Plk1 clearly occurs in G2 and M phases, but not in S phase (Figure 5j). Cells were also synchronized at late G1 or M phase then released for different times. Again, phosphorylation of S326 starts to be detected in G2 and peaks in mitosis and disappears upon mitotic exit (Figures 5k and l). Phosphorylation of Plk1 at S326 was increased upon MK2 overexpression (Figure 5m), and inhibited upon MK2 depletion (Figure 5n), indicating that MK2 is responsible for the phosphorylation. Finally, co-staining with various mitotic markers and a phospho-Plk1 antibody was performed on cells growing on coverslips. Data indicated that phosphorylation of S326 occurred throughout mitosis, as phospho-Plk1 signal was detected in typical mitotic structures, such as spindle poles, mid-zones and mid-bodies (Figures 5o–q). As a control, phospho-Plk1 signal was disappeared upon Plk1 depletion (Liu and Erikson, 2002) (Figure 5r).
Physical association of Plk1 with MK2
We wanted to test whether Plk1 associates with MK2 directly. Toward that end, HeLa cells were co-transfected with Myc-MK2 and Flag-Plk1. At 1 day post-transfection, cells were treated with nocodazole for 12 h and harvested. Lysates were prepared, subjected to anti-Myc IP and probed with a Flag antibody (Figure 5s). As indicated, Plk1 was co-immunoprecipitated with MK2 in nocodazole-treated cells, suggesting that the association might be M phase specific. The association of endogenous Plk1 and MK2 proteins in HeLa cells was also showed by co-IP of MK2 with a Plk1 antibody in mitotic cells (Figure 5t).
Phosphorylation of S326 regulates Plk1 functions during mitosis
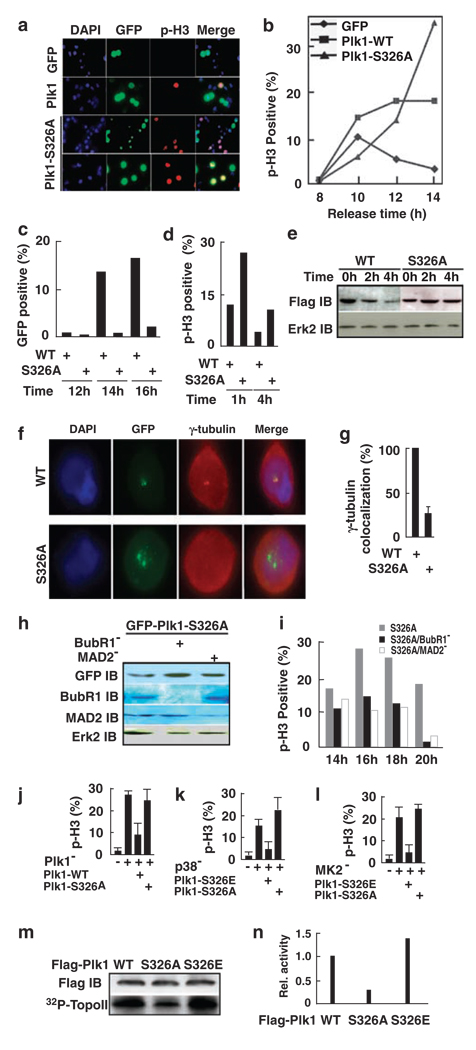
Considering the requirement of the p38 MAP kinase pathway during mitosis as described above, we were interested to determine whether the essential role of Plk1 is regulated by MK2-associated phosphorylation at Ser326. Thus, we assessed whether expression of the S326A mutant would cause mitotic block. For that purpose, HeLa cells were transfected with Plk1 constructs. At 1 day post-transfection, cells were stained with a phospho-histone H3 antibody (Figure 6a). Compared with GFP alone, expression of WT Plk1 led to a slight increase in phospho-histone H3 staining, whereas Plk1-S326A expression caused a much more severe mitotic block. Next, Plk1 was expressed in cells synchronized with the double thymidine block. Upon release for different times, mitotic progression was monitored by phospho-histone H3 staining (Figure 6b). Again, expression of WT Plk1 slowed down the mitotic exit, whereas cells with Plk1-S326A expression were completely blocked at M phase. We also analysed the ability of cells expressing Plk1 or Plk1-S326A to reach the stage of cytokinesis. For that purpose, cells were processed as in Figure 6b. Upon release from the block for different times, cells were stained with an α-tubulin antibody. Mid-body, a unique structure formed between two dividing cells during cytokinesis, can be monitored by α-tubulin staining. Analysis of cells with mid-body structure indicated that Plk1-expressing cells eventually exited mitosis, whereas cells expressing Plk1-S326A did not reach cytokinesis over the entire releasing period (Figure 6c).
Figure 6.
Phosphorylation of S326 regulates Plk1 function during mitosis. (a) HeLa cells were transfected with GFP-Plk1 (WT or S326A). At 1 day post-transfection, cells were stained with a phospho-histone H3 (p-H3) antibody. (b, c) Cells were synchronized with the double thymidine block and transfected with GFP-Plk1 or GFP-Plk1-S326A during the 8-h interval. Upon release for the times indicated, cells were stained with a phospho-histone H3 antibody (b) or an α-tubulin antibody (c). In (b), only GFP-positive cells were counted. In (c), only the cells with mid-body structure were counted. (d, e) Cells were transfected with GFP-Plk1 (d) or Flag-Plk1 (e) and treated with nocodazole. The M-phase cells were collected by mechanic shake off, released for different times and stained with a phospho-histone H3 antibody (d) or analysed by western blot (e). In (d), only GFP-positive cells were counted. (f, g) At 1 day post-transfection with GFP-Plk1, cells were stained with a γ-tubulin antibody. (h, i) Cells were transfected with siRNA to deplete BubR1 or MAD2 (Tang et al., 2006a), then synchronized and transfected with GFP-Plk1-S326A as in (b). Upon release for the times indicated, cells were analysed by western blot (h) or stained with a phospho-histone H3 antibody (i). (j–l) Cells were transfected with siRNA to deplete Plk1 (j), p38a (k) or MK2 (l), then subjected to the double thymidine block with transfection of GFP fusion Plk1 during the 8-h interval. Cells were released from the block for 16 h and stained with a phospho-histone H3 antibody. (m, n) COS-7 cells were transfected with Flag-Plk1 (WT, S326A or S326E), treated with 200 ng/ml nocodazole for 12 h and harvested. Lysates were subjected to anti-Flag immunoprecipitation (IP)/kinase assays using a purified GST-topoisomerase IIα fragment as a substrate. The quantification results were shown in (n).
To further address the significance of S326 phosphorylation in mitotic exit, cells were transfected with Plk1 constructs, treated with nocodazole and subjected to the mitotic shake-off protocol. Mitotic cells were collected, reseeded on polylysine-coated coverslips and stained with a phospho-histone H3 antibody after different releasing times (Figure 6d). Compared to cells expressing WT Plk1, the mitotic exit of cells expressing Plk1-S326A was significantly delayed. It was established that Plk1, a substrate for the anaphase-promoting complex, is degradated at the onset of anaphase (Lindon and Pines, 2004). As expression of the Plk1-S326A mutant led to mitotic arrest, we speculated that Plk1 might be stabilized under these conditions. To test this possibility, cells were transfected with Flag-Plk1 constructs and treated with nocodazole. Mitotic cells were collected by mechanic shake off, released into fresh medium for different times and analysed by western blot (Figure 6e). The level of WT Flag-Plk1 gradually decreased as cells exited from mitosis. Compared to the protein level of the starting point, the level of Plk1 was decreased to about 50 or 15% after 2 or 4 h release, respectively. In contrast, the protein level of Plk1-S326A remained fairly constant through the entire releasing period, most likely due to the inactivation of anaphase-promoting complex in these cells. We also tried to address the potential connection between S326 phosphorylation and Plk1 stability. Unfortunately, our data indicated that S326 phosphorylation does not directly regulate the function of the destruction box that controls the stability of Plk1 (data not shown).
Next, we tried to examine the potential spindle pole defects in cells expressing Plk1-S326A by staining with a γ-tubulin antibody, a centrosome marker (Figures 6f and g). In WT Plk1-expressing cells, Plk1 was colocalized with γ-tubulin to the centrosomes. In contrast, γ-tubulin staining was diffuse and delocalized from the centrosomes in cells expressing Plk1-S326A, suggesting the requirement of S326 phosphorylation for centrosomal recruitment of γ-tubulin. Finally, we tested whether silencing of the spindle checkpoint machinery rescued the mitotic arrest induced by Plk1-S326A expression. After depletion of BubR1 or MAD2 as described previously (Tang et al., 2006a), two classical spindle checkpoint proteins, cells expressing Plk1-S326A were able to exit mitosis, suggesting that the spindle checkpoint was activated in these cells (Figures 6h and i).
To further evaluate the significance of Ser326 phosphorylation during mitosis, we compared the ability of Plk1 constructs with different phosphorylation states at S326 to rescue the mitotic defects induced by depletion of Plk1, p38α or MK2. We previously reported that Plk1 depletion led to G2/M arrest in cancer cells. Because of the high specificity of the RNAi technique, the mouse Plk1-expressing constructs will not be affected by RNAi targeting the human sequence (Liu and Erikson, 2003). Expression of WT mouse Plk1, but not the Plk1-S326A mutant, was able to rescue the Plk1 depletion-induced mitotic arrest in HeLa cells (Figure 6j). Moreover, mitotic block induced by the depletion of p38 and MK2 was rescued only by Plk1-S326E (phosphorylation mimicking mutant), but not S326A, supporting the notion that Plk1 might act downstream of MK2 through phosphorylation at S326 during mitotic progression (Figures 6k and l). Finally, we tried to determine whether phosphorylation of Ser326 alters the kinase activity of Plk1. Accordingly, COS-7 cells were transfected with Flag-Plk1 (WT, S326A or S326E) for 1d, treated with nocodazole and harvested. Lysates were subjected to an anti-Flag IP, followed by a kinase assay using purified GST-topoisomerase II fragment (aa1259–1350) as a substrate (Li et al., 2008). As indicated, the kinase activity of Plk1-S326A mutant was about one-third of that of WT Plk1, whereas the kinase activity of Plk1-S326E mutant increased by 50% compared to that of WT Plk1, confirming that MK2 is an upstream kinase for Plk1 (Figures 6m and n).
Discussion
The role of the p38 MAP kinase pathway in the regulation of G2/M phases in the absence of stress has been controversial. In one study, it was shown that inhibiting p38 activity does not influence the rate at which normal cells enter and exit mitosis (Mikhailov et al., 2004). In contrast, another study provided evidence that p38 MAP kinase is activated during normal mitosis (Campos et al., 2002). The mitotic profiles in the developing retina were highly enriched for phosphorylated p38, and inhibition of p38 activity arrested cells at the metaphase–anaphase transition (Campos et al., 2002). Using the small inhibitor to treat HeLa cells, Cha et al. (2007) proposed a functional role for p38 MAP kinase in modulating mitotic transit in the absence of stress. Here, we provide additional evidence to support the involvement of the p38 MAP kinase pathway in normal mitosis. In agreement with what Cha et al. (2007) reported, phosphorylated p38α was found to colocalize with Plk1 at centrosomes/spindle poles during pro/metaphase. Moreover, we show that phospho-MK2 also localizes at spindle poles throughout the entire mitosis, including anaphase/telophase. Although the results based on experiments using small chemical inhibitor led to the conclusion emphasizing the role of the p38 pathway in the G2/M transition, our data on phospho-MK2 localization and loss-of-function phenotype analyses of p38α, MK2 and MKK3/6 support a role of the stress pathway in mitotic progression. Considering that small chemical inhibitor likely prevents the activation of entire pathway, whereas RNAi approach only specifically knocks down one of the pathway members, it is possible that the requirement of the pathway in later stage of mitosis might not be easily observed in the approach using inhibitors.
Extensive in vitro kinase assays and experiments utilizing a phospho-Plk1-S326 antibody support the notion that MK2 targets Plk1 at S326. The connection between the stress pathway and polo kinase was previously observed in fission yeast (Petersen and Hagan, 2005). The p38 stress pathway promotes phosphorylation of Ser402 in Plo1, the polo kinase in fission yeast, and phosphorylation of Ser402 promotes Plo1 recruitment to the spindle pole body (Petersen and Hagan, 2005). However, sequence alignment indicates that Ser402 is not conserved in Plk1, thus it cannot be the target site of the p38 MAP kinase pathway in mammalian cells. As Ser326 of mammalian Plk1 is also not conserved in fission yeast Plo1, and protein level of Plo1 is not regulated throughout the cell cycle (Mulvihill et al., 1999), we propose that the p38 MAP kinase pathway might regulate functions of polo kinases differently in different organisms.
It has been well established that Plk1 is required for centrosome maturation and subsequent bipolar spindle formation, and phosphorylation of Plk1 plays a pivotal role in controlling its activity (Barr et al., 2004). Very little is known about its upstream kinases, however. Here, we have identified MK2 as a major Plk1 kinase toward Ser326, whose phosphorylation is critical to recruit γ-tubulin to centrosomes and subsequent establishment of functional bipolar spindles. To our knowledge, this is the first direct evidence to demonstrate that the essential function of Plk1 in centrosome maturation and bipolar spindle formation is controlled by its upstream kinase.
Materials and methods
Antibodies
To generate the phospho-Plk1-S326 antibody, a peptide-containing phospho-S326 (ITCLTIPPRFSpIAPSSLDPSS) was synthesized and used to immunize rabbits. The antibody was then affinity purified by Proteintech Group (Chicago, IL, USA). The phospho-p38α antibody (9211) and phospho-MK2 antibody (3041) used in these studies were obtained from Cell Signaling Technology (Danvers, MA, USA). The phosphor-histone H3 antibody (06-570) and Cdc2 antibody (06-923) were from Upstate Biotechnology (Charlottesville, VA, USA). The γ-tubulin antibody (T-6557) used for centrosome detection and Myc antibody (M-5546) were purchased from Sigma (St Louis, MO, USA). The antibodies against BubR1 (A300–386A) and Mad2 (ab11690) were from Bethyl Laboratories (Montgomery, TX, USA) and Abcam (Cambridge, MA, USA) respectively. The other antibodies, such as Plk1 (F-8), Erk2 (C-14), p38 MAP kinase (A-12), MK2 (H-66), MKK3 (N-20), MKK6 (N-19) and α-tubulin (H-300), were ordered from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
RNAi
Twenty-one-nucleotide RNAs were designed to specifically deplete endogenous p38α, MK2, MKK3 and MKK6. The targeting sequences are as follows: for human p38α (accession no. L35253), ACTGCGGTTACTTAAACATA, corresponding to the coding region 212–232 relative to the first nucleotide of the start codon; for human MK2 (accession no. NM_032960), ACGAGCAGATCAAGATAAAAA, corresponding to the coding region 1100–1120 relative to the first nucleotide of the start codon; for human MKK3 (accession no. L36719), GCACGGTCGACTGTTTCTACA, corresponding to the coding region 260–280 relative to the first nucleotide of the start codon; for human MKK6 (accession no. U39656), GGATACATCACTAGATAAAGG, corresponding to the coding region 395–415 relative to the first nucleotide of the start codon. Plk1, BubR1 and Mad2 were depleted as described previously (Liu and Erikson, 2002; Tang et al., 2006a, b).
Vector-based RNAi was also used to deplete p38α and MK2. Plasmids pBS/U6-p38 and pBS/U6-MK2 were constructed as described previously (Sui et al., 2002). The target sequence of human p38 was GGGGGCAGATCTGAACAACAT, corresponding to the coding region 326–346 relative to the start codon. To specifically deplete MK2, targeting sequences were GGGTGATGTGTGGATCTGCAT, TCCAGAGAAGTATGACAA and GGGAGGATGTCAAGGAGGAGA, corresponding to the coding region 488–508, 705–725 and 1046–1066 relative to the first nucleotide of the start codon, respectively.
Supplementary Material
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Acknowledgements
We are grateful to Dr Raymond Erikson, in whose laboratory the preliminary experiments were performed, for generously providing many cell lines and plasmids. We appreciate Eleanor Erikson, Xiaoyi Zhang and Hongchang Li for helpful discussions and critical reading of the paper. XL is a recipient of the Howard Temin Award from the National Cancer Institute (K01 CA114401).
Abbreviations
- MK2
MAP kinase-activated protein kinase 2
- Plk1
polo-like kinase 1
- RNAi
RNA interference
References
- Barr FA, Sillje HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–440. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- Campos CB, Bedard PA, Linden R. Activation of p38 mitogen-activated protein kinase during normal mitosis in the developing retina. Neuroscience. 2002;112:583–591. doi: 10.1016/s0306-4522(02)00096-9. [DOI] [PubMed] [Google Scholar]
- Cha H, Wang X, Li H, Fornace AJ. A functional role for p38 MAPK in modulating mitotic transit in the absence of stress. J Biol Chem. 2007;282:22984–22992. doi: 10.1074/jbc.M700735200. [DOI] [PubMed] [Google Scholar]
- Eckerdt F, Yuan J, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene. 2005;24:267–276. doi: 10.1038/sj.onc.1208273. [DOI] [PubMed] [Google Scholar]
- Fan L, Yang X, Du J, Marshall M, Blanchard K, Ye X. A novel role of p38 alpha MAPK in mitotic progression independent of its kinase activity. Cell Cycle. 2005;4:1616–1624. doi: 10.4161/cc.4.11.2125. [DOI] [PubMed] [Google Scholar]
- Golsteyn RM, Schultz SJ, Bartek J, Ziemiecki A, Ried T, Nigg EA. Cell cycle analysis and chromosomal localization of human Plk1, a putative homologue of the mitotic kinases Drosophila polo and Saccharomyces cerevisiae Cdc5. J Cell Sci. 1994;107(Part 6):1509–1517. doi: 10.1242/jcs.107.6.1509. [DOI] [PubMed] [Google Scholar]
- Knecht R, Elez R, Oechler M, Solbach C, von Ilberg C, Strebhardt K. Prognostic significance of polo-like kinase (PLK) expression in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:2794–2797. [PubMed] [Google Scholar]
- Knecht R, Oberhauser C, Strebhardt K. PLK (polo-like kinase), a new prognostic marker for oropharyngeal carcinomas. Int J Cancer. 2000;89:535–536. [PubMed] [Google Scholar]
- Li H, Wang Y, Liu X. Plk1-dependent phosphorylation regulates functions of DNA topoisomerase IIalpha in cell cycle progression. J Biol Chem. 2008;283:6209–6221. doi: 10.1074/jbc.M709007200. [DOI] [PubMed] [Google Scholar]
- Lindon C, Pines J. Ordered proteolysis in anaphase inactivates Plk1 to contribute to proper mitotic exit in human cells. J Cell Biol. 2004;164:233–241. doi: 10.1083/jcb.200309035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Erikson RL. Activation of Cdc2/cyclin B and inhibition of centrosome amplification in cells depleted of Plk1 by siRNA. Proc Natl Acad Sci USA. 2002;99:8672–8676. doi: 10.1073/pnas.132269599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Erikson RL. Polo-like kinase (Plk)1 depletion induces apoptosis in cancer cells. Proc Natl Acad Sci USA. 2003;100:5789–5794. doi: 10.1073/pnas.1031523100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery DM, Lim D, Yaffe MB. Structure and function of Polo-like kinases. Oncogene. 2005;24:248–259. doi: 10.1038/sj.onc.1208280. [DOI] [PubMed] [Google Scholar]
- Manke IA, Nguyen A, Lim D, Stewart MQ, Elia AE, Yaffe MB. MAPKAP kinase-2 is a cell cycle checkpoint kinase that regulates the G2/M transition and S phase progression in response to UV irradiation. Mol Cell. 2005;17:37–48. doi: 10.1016/j.molcel.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Mikhailov A, Shinohara M, Rieder CL. Topoisomerase II and histone deacetylase inhibitors delay the G2/M transition by triggering the p38 MAPK checkpoint pathway. J Cell Biol. 2004;166:517–526. doi: 10.1083/jcb.200405167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill DP, Petersen J, Ohkura H, Glover DM, Hagan IM. Plo1 kinase recruitment to the spindle pole body and its role in cell division in Schizosaccharomyces pombe. Mol Biol Cell. 1999;10:2771–2785. doi: 10.1091/mbc.10.8.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JM, Hallahan AR. p38 MAP kinase: a convergence point in cancer therapy. Trends Mol Med. 2004;10:125–129. doi: 10.1016/j.molmed.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Petersen J, Hagan IM. Polo kinase links the stress pathway to cell cycle control and tip growth in fission yeast. Nature. 2005;435:507–512. doi: 10.1038/nature03590. [DOI] [PubMed] [Google Scholar]
- Shirayama M, Zachariae W, Ciosk R, Nasmyth K. The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J. 1998;17:1336–1349. doi: 10.1093/emboj/17.5.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui G, Soohoo C, Affar EB, Gay F, Shi Y, Forrester WC, et al. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci USA. 2002;99:5515–5520. doi: 10.1073/pnas.082117599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai N, Hamanaka R, Yoshimatsu J, Miyakawa I. Polo-like kinases (Plks) and cancer. Oncogene. 2005;24:287–291. doi: 10.1038/sj.onc.1208272. [DOI] [PubMed] [Google Scholar]
- Tang J, Erikson RL, Liu X. Checkpoint kinase 1 (Chk1) is required for mitotic progression through negative regulation of polo-like kinase 1 (Plk1) Proc Natl Acad Sci USA. 2006a;103:11964–11969. doi: 10.1073/pnas.0604987103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Erikson RL, Liu X. Ectopic expression of Plk1 leads to activation of the spindle checkpoint. Cell Cycle. 2006b;5:2484–2488. doi: 10.4161/cc.5.21.3411. [DOI] [PubMed] [Google Scholar]
- Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)