Abstract
Conjugation of gonadotropin-releasing hormone (GnRH) analogues GnRH-III, MI-1544, and MI-1892 through lysyl side chains and a tetrapeptide spacer, Gly-Phe-Leu-Gly (X) to a copolymer, poly(N-vinylpyrrolidone-co-maleic acid) (P) caused increased antiproliferative activity toward MCF-7 and MDA-MB-231 breast, PC3 and LNCaP prostate, and Ishikawa endometrial cancer cell lines in culture and against tumor development by xenografts of the breast cancer cells in immunodeficient mice. MCF-7 cells treated with P-X-1544 and P-X-1892 displayed characteristic signs of apoptosis, including vacuoles in the cytoplasm, rounding up, apoptotic bodies, bleb formation, and DNA fragmentation. Conjugates, but not free peptides, inhibited cdc25 phosphatase and caused accumulation of Ishikawa and PC3 cells in the G2/M phase of the cell cycle after 24 h at lower doses and in the G1 and G2 phases after 48 h. Since P-X-peptides appear to be internalized, the increased cytotoxicity of the conjugates is attributed to protection of peptides from proteolysis, enhanced interaction of the peptides with the GnRH receptors, and/or internalization of P-X-peptide receptor complexes so that P can exert toxic effects inside, possibly by inhibiting enzymes involved in the cell cycle. The additional specificity of P-X-peptides compared with free peptides for direct antiproliferative effects on the cancer cells but not for interactions in the pituitary indicates the therapeutic potential of the conjugates.
Gonadotropin-releasing hormone (GnRH, Glp-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH2) was originally identified and has long been recognized as the central regulator of the hypothalamic–pituitary–gonadal axis. GnRH agonist analogues such as buserelin, goserelin, lupron, and decapeptyl inhibit the actions of sex hormones at their target organs and decrease sex hormone synthesis and secretion in long-term treatment. GnRH analogues are used to treat sex hormone-dependent cancers of the breast, prostate, and ovaries (1–3). Beside having their chemical castration effects, GnRH analogues may suppress tumor growth in 33% of patients with estrogen-receptor-poor cancers (4).
The finding that peripheral tissues locally produce GnRH and GnRH-like peptides and express GnRH-binding sites suggests that these peptides have an autocrine or paracrine role at sites distal to the pituitary. Evidence is mounting that GnRH in extrapituitary tissue may somehow be involved in apoptosis or programmed cell death. In the ovary, gonadotropins, estrogens, growth hormones, and growth factors IGF1, EGF/TGF-α, basic FGF, interleukin-1β, and NO act in concert to ensure the survival of preovulatory follicles. By contrast, androgens, interleukin-6, and gonadal GnRH-like peptide are apoptotic factors (5).
GnRH receptor mRNA is expressed in human pituitary, breast, breast tumor (6), ovary, ovarian tumor (7), prostate, prostate tumor, endometrium, and endometrial tumor (8). Human uterine leiomyomata and myometrium also possess specific binding sites for GnRH (9). GnRH receptor genes were found in MDA-MB-231 (10), MCF-7 breast (11), LNCaP (12) and PC3 prostate, Ishikawa endometrial (13, 14), and ovarian (15) cancer cell lines. These cell lines contain high- and low-affinity binding sites for GnRH (16, 17). The high-affinity GnRH-binding sites are commonly regarded as the same as the GnRH receptor of the pituitary gland. It was, thus, proposed that the effects of GnRH analogues in these cancers are mediated by interactions with specific GnRH receptors.
We had previously synthesized GnRH analogues MI-1544 (Ac-d-Trp-d-Cpa-d-Trp-Ser-Tyr-d-Lys-Leu-Arg-Pro-d-Ala-NH2) (18) and MI-1892 [Ac-d-Trp-d-Cpa-d-Trp-Ser-d-Lys-β-Asp(α-DEA)-Leu-Gln-Pro-d-Ala-NH2, in which Cpa indicates p-Cl-Phe and DEA, diethylamide] (19), which were found to have highly selective direct growth-inhibitory activity against breast and prostate tumors (20–23). We also studied the relationships between structure and luteinizing hormone (LH)-releasing activities as well as direct suppression of tumor growth (19). The results suggest that a minor structural change can differently affect LH release in the pituitary and direct suppression of the growth of cancer cells. MI-1544 has both high endocrine activity in the pituitary and direct antitumor activity, whereas MI-1892 has strong direct antitumor activity but low endocrine activity.
A variant, GnRH-III, Glp-His-Trp-Ser-His-Asp-Trp-Lys-Pro-Gly-NH2, from the sea lamprey, Petromyzon marinus, was isolated and synthesized (24, 25). It directly suppresses growth of breast, prostate, and endometrial cancer cells but does not have endocrine activity at the concentrations effective against growth of cancer cells (26–29). Radioreceptor assay with 3H-labeled GnRH-III showed the presence of high- and low-affinity binding sites in the membrane suspensions of these cells (29, 30).
Peptide hormones are rapidly degraded in vivo by proteinases. To protect against proteolysis and improve therapeutic potential, the lysyl side chains of MI-1544 and MI-1892 were coupled (31) through a Gly-Phe-Leu-Gly (X) spacer, to a copolymer, poly(N-vinylpyrrolidone-co-maleic acid) (P; ref. 32). Conjugation substantially increased the antitumor activities of both peptides in vitro and in vivo. The surviving fraction, 60–95%, of human tumor cell lines treated with free GnRH analogues in vitro fell to 0–5% when the cells were treated with conjugates. The antiproliferative effect of analogues in vitro was also enhanced by conjugation (21). The antitumor activity in vivo, however, increased markedly (20, 33). The peptide conjugates appear to bind specifically to GnRH receptors on cancer cells (30), as do the free peptides (29).
The present work with cancer cells that have GnRH receptors was undertaken to examine the effects of analogue conjugates on in vivo as well as in vitro suppression of growth of cancer cells, on the progress of the cell cycle, and on the enzyme, cdc25 phosphatase, that controls the G2/M transition (34).
MATERIALS AND METHODS
Peptides.
Previously described methods were used to synthesize GnRH-III, MI-1544, and MI-1892 (18, 19). Buserelin was obtained from Hoechst, Frankfurt/Main, Germany.
Conjugates.
Peptides MI-1544, MI-1892, and GnRH-III were conjugated through a spacer (X) to a copolymer (P), previously referred to as NVP-MA when details of syntheses were reported (31, 32), to form P-X-1544, P-X-1892, and P-X-GnRH-III, respectively, with ≈15% peptide and average molecular weight 12,000.
Tumor Cell Cultures.
Human MCF-7 and MDA-MB-231 breast, PC3 and LNCaP prostate, and Ishikawa endometrium tumor cell lines (American Type Culture Collection) with GnRH receptors (16, 17) were maintained in Dulbecco’s modified Eagle’s minimal essential medium (DMEM; GIBCO/BRL) supplemented with 10% fetal calf serum (GIBCO). PC3 cells were cultured in RPMI medium 1640 (GIBCO). Cells were kept at 37°C and subcultured twice a week.
Drug Sensitivity Assays.
The effect of compounds on growth of the above tumor cell cultures was examined in clonogenic, cell proliferation, and sulforhodamine B (SRB) assays (35) as described previously (21). In the clonogenic assay, colony formation reflects survival of cells. In the cell proliferation assay, cells were directly counted after release from Petri dishes by trypsinization, and in the SRB assay, dye bound to cells was quantified spectrophotometrically, with results expressed as percentage of control values.
Apoptosis Assays.
Cytomorphological changes in treated cells were observed with an Olympus inverted phase-contrast microscope. DNA strand breaks were quantified by using IdT-mediated X-dUTP nick end labeling with the ApopTag kit (Oncor) and with an in situ cell death detection kit (POD; Boehringer Mannheim). The kits produce a yellow-brown stain within apoptotic cells. These cells could be further examined under the light microscope after counterstaining with hematoxylin or methyl green. A cell death detection ELISA kit (Boehringer Mannheim) was also used for qualitative and quantitative detection of cytoplasmic histone-associated DNA fragments.
cdc25 Phosphatase Assay.
The cdc25 phosphatase assay is recommended by the New Drug Development Office (NDDO, Amsterdam) of the European Organization for Research and Treatment of Cancer (EORTC) as a mechanism-based screening for antimitotic compounds, which have potential as anticancer drugs, because the enzyme is responsible for entry of cells from G2 to M phase (35). The effects of compounds studied here on the phosphatase activity were measured by S. Leclerc and L. Meijer (Station Biologique, Roscoff, France) as described previously (34).
Flow Cytometry.
Cell cycle phase distributions of control and treated cultures were analyzed by measurements of relative DNA contents of individual cells with a fluorescence-activated cell sorter. Samples were prepared according to the method of Shapiro (36). PC3 or Ishikawa cells were treated with various amounts of P-X-1544, in 50-ml plastic flasks (Greiner; Frickenhausen, Germany) for 24- and 48-h periods. Cells were released by trypsinization, washed with 0.04 M NaH2PO4/Na2HPO4, pH 7.4, containing 0.14 M NaCl (PBS), fixed in 70% (vol/vol) ethanol, and stored at −20°C. Before flow cytometry, cells were collected by centrifugation and incubated for 30 min at room temperature in PBS (1 ml) containing propidium iodide (20 μg) and RNase (100 μg). Measurements were carried out with a FACStar Plus flow cytometer (Becton Dickinson Immunocytometry Systems) and with a FACStar Plus Research Program connected with a Doublets Discrimination signal processor. The quality of the setup was checked with lysed, propidium iodide-stained normal human lymphocytes. Cell cycle analysis was accomplished with Multi-Cycle software (Phoenix Flow Systems, San Diego).
In Vivo Experiments.
The experimental procedure for the in vivo antitumor experiments is described in ref. 17. Female CBA/Ca HRIJ-T6 and BDF1 mice weighing 19–22 g were immunosuppressed by thymectomy, whole-body irradiation, and syngeneic bone marrow transplantation. The animals were kept under specific pathogen-free (SPF) conditions. Human MDA-MB-231 breast tumor cells were xenografted into the mice. Pieces of adequate tumor tissue (1.5–2 mm3) were transplanted subcutaneously (s.c.) into the mice. Experiments with compounds were started 3–4 weeks after transplantation when the xenografts had grown to a volume of approximately 0.15–0.20 cm3. The mice were randomized and divided into experimental groups (10 mice per group). GnRH-III or P-X-GnRH-III in PBS was administered daily at doses of 4 mg/kg of body weight s.c., on the side opposite the tumor. Tumors were measured weekly and their volumes were calculated as described earlier (33).
RESULTS
Clonogenic Assay.
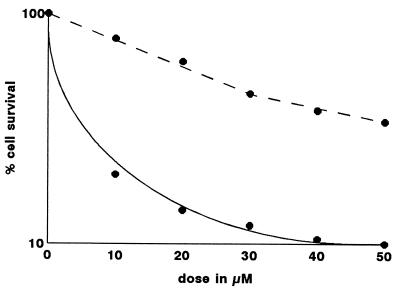
The effect of GnRH-III, P-X-GnRH-III, and buserelin on colony formation by five human tumor cell lines is summarized in Table 1. MCF-7 and MDA-MB-231 cells were the most sensitive to GnRH-III, while the Ishikawa cells were the least affected. Buserelin had a comparatively weak effect on the clonogenicity of both breast cancer cell lines. Fig. 1 illustrates survival curves obtained by exposure of MCF-7 cells to concentrations of GnRH-III and its conjugate. The free peptide was about one-fourth as efficient as the conjugate.
Table 1.
Effects of GnRH analogues and P-X-GnRH-III on colony formation by human tumor cell lines
| Cell line | Surviving colonies, % of control
|
||
|---|---|---|---|
| GnRH-III | Buserelin | P-X-GnRH-III | |
| MCF-7 | 42 ± 8 | 90 ± 2 | 18 ± 10 |
| MDA-MB-231 | 34 ± 7 | 92 ± 2 | 12 ± 7 |
| PC3 | 83 ± 5 | NT | 70 ± 4 |
| Ishikawa | 88 ± 3 | NT | 55 ± 6 |
| LNCaP | 75 ± 5 | NT | 22 ± 10 |
The treatment dose was 50 μM conjugated peptide or free peptide. The number of colonies is expressed as percentage of numbers of control colonies. NT, not tested.
Figure 1.
Effect of GnRH-III (broken line) and P-X-GnRH-III (solid line) on colony formation of MCF-7 cells.
Antiproliferation Effect.
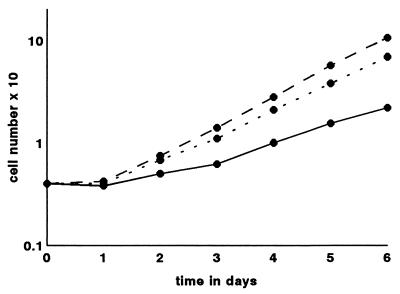
The effects of GnRH-III, P-X-GnRH-III, and buserelin on proliferation of four human cancer cell lines are shown in Table 2. The breast cancer cell lines were more sensitive to the peptides than were the prostate and endometrial cells. Buserelin was less effective in decreasing cell numbers than was GnRH-III. The antiproliferation effect of P-X-GnRH-III was about twice that of GnRH-III. Again, the proliferation of the breast cancer cells was inhibited more by the conjugate than was proliferation of the other two cell lines. Fig. 2 shows results of a typical experiment using MDA-MB-231 cells. The cultures were treated on the next day after seeding with 4 × 104 cells in 50-mm-diameter Petri dishes with 50 μM conjugated peptide at the beginning only and with the free peptide on alternate days for 5 days. The GnRH-III conjugate retarded cell proliferation much more strongly than did the free peptide.
Table 2.
Effect of GnRH analogues and P-X-GnRH-III on proliferation of human tumor cell lines
| Cell line | Cells, % of control
|
||
|---|---|---|---|
| GnRH-III | Buserelin | P-X-GnRH-III | |
| MCF-7 | 68 ± 5 | 87 ± 2 | 23 ± 7 |
| MDA-MB-231 | 64 ± 6 | 80 ± 4 | 21 ± 6 |
| PC3 | 82 ± 3 | NT | 66 ± 5 |
| Ishikawa | 79 ± 4 | NT | 52 ± 5 |
Cells were treated with 50 μM conjugated peptide or free peptide at the beginning only with P-X-GnRH-III, or at the beginning and on alternate days of incubation with the free peptides for 5 days. Cell numbers are expressed as percentage of cell numbers in control cultures. NT, not tested.
Figure 2.
Effect of GnRH-III (dotted line) and P-X-GnRH-III (solid line) on the proliferation of MDA-MB-231 cells. Control cells (broken line) were similarly incubated. Seeding concentration was 4 × 104 cells per 50-mm-diameter Petri dish. Details of the treatments are provided with Table 2.
The higher antiproliferation activity of the P-X-GnRH-III than of GnRH-III was also shown with the SRB assay (Table 3). GnRH-III reduced cell proliferation slightly, in the range 11–19%.
Table 3.
Effect of GnRH-III and P-X-GnRH-III on the proliferation of human tumor cell lines (SRB assay)
| Cell line | Cells, % of control
|
|
|---|---|---|
| GnRH-III | P-X-GnRH-III | |
| MCF-7 | 81 ± 5 | 43 ± 8 |
| MDA-MB-231 | 86 ± 4 | 55 ± 7 |
| PC3 | 89 ± 3 | 66 ± 6 |
| Ishikawa | 85 ± 4 | 55 ± 6 |
| LNCaP | 87 ± 3 | 51 ± 7 |
Cells were treated with 50 μM GnRH-III or conjugated GnRH-III at the start of incubation for 2 days, except LNCaP cells, which were cultured for 3 days. SRB incorporated in the cells is expressed as percentage of the dye incorporated in untreated control cells.
Apoptosis.
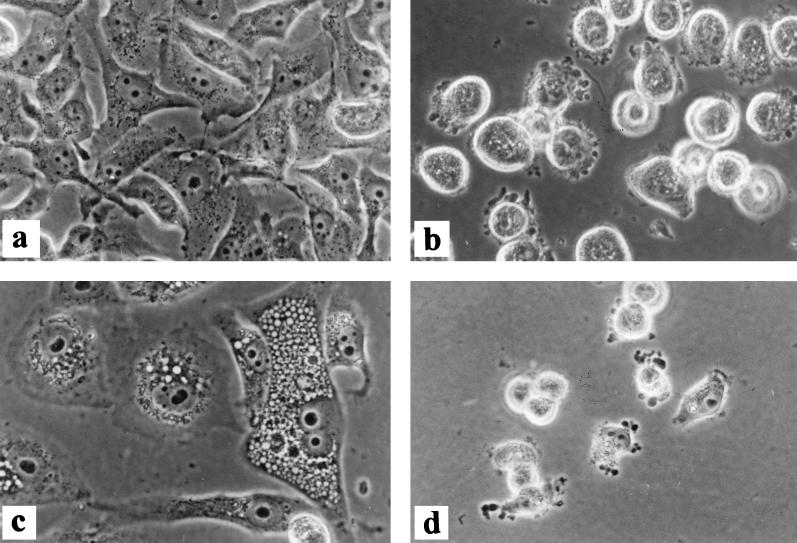
MCF-7 cells treated with either P-X-GnRH-III or P-X-1544 showed morphologic signs of programmed cell death. Representative results with the latter conjugate are shown in Fig. 3. Control cells had normal morphology (Fig. 3a). Vacuoles in the cytoplasm, rounding-up of cells, apoptotic bodies, and bleb formation were the most characteristic signs of the apoptotic process (Fig. 3b). Cells were enlarged, heavily vacuolated, and multinucleated after treatment with 200 μM conjugated peptides for 72 h (Fig. 3c). The few cells remaining after the prolonged (144-h) treatment with the largest dose show disintegration by bleb formation (Fig. 3d).
Figure 3.
Effects of P-X-1544 on cytomorphology of MCF-7 cell cultures observed by phase-contrast microscopy. (×200.) Cells were untreated (a) or were treated with conjugated peptide at 50 μM for 48 h (b), 200 μM for 72 h (c), or 200 μM for 144 h (d).
The ApopTag kit produced a strong brown color in cells treated with P-X-1544 and P-X-GnRH-III (25, 50, and 100 μM conjugated peptide) as well as with cells treated with adriamycin (0.05, 0.1, and 0.5 μg/ml). With untreated cultures, no brown coloration was seen. Similar results were obtained with the POD test.
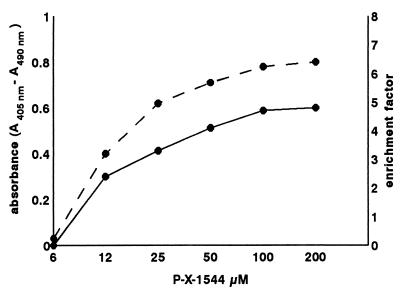
Apoptosis was also revealed, by the cell death detection ELISA method, in cells treated with conjugated peptide when oligonucleosome content of the cytoplasm increased. Absorbance values increased in direct proportion to the amount of conjugated peptide used (Fig. 4).
Figure 4.
Formation of oligonucleosomes as measured by the cell death detection ELISA kit after treatment of MCF-7 cells, for 24 h, with P-X-GnRH-III as shown by absorbance (solid line) and enrichment factor (broken line).
cdc25 Phosphatase Activity.
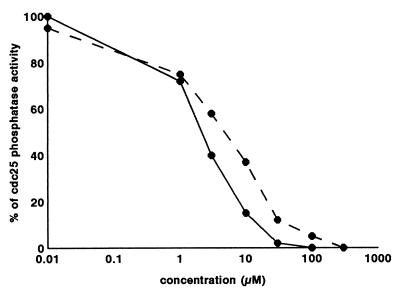
GnRH-III, MI-1544, and MI-1892 failed to cause an inhibition of phosphatase activity at 50 μM, which is the concentration above which compounds are not considered to be cytotoxic by the European Organization for Research and Treatment of Cancer. Inhibition in response to P-X-1544 and P-X-1892, however, is shown in Fig. 5, and IC50 values are shown in Table 4. The most efficient compound was the P-X-1544 conjugate, followed by P-X-1892.
Figure 5.
Effects of P-X-1544 (solid line) and P-X-1892 (broken line) on cdc25 phosphatase activity measured as described in the text.
Table 4.
Effect of GnRH analogues and their conjugates on cdc25 phosphatase activity
| Compound | IC50, μM
|
|
|---|---|---|
| Peptide | Conjugate | |
| MI-1544 | 1,000 | — |
| P-X-1544 | 1.33* | 0.86 |
| MI-1892 | 1,000 | — |
| P-X-1892 | 3.33* | 2.07 |
| P-X-OH | — | 37.5 |
Compounds were incubated with the enzyme as described in ref. 34.
The concentration of peptide conjugated.
Flow Cytometry.
The effects of P-X-1544 on cell cycle phase distribution of PC3 and Ishikawa cell lines are summarized in Table 5. The conjugate caused minor changes in the proportions of PC3 cells in the G2/M phase after 24 h. The proportion of cells in G2 phase tended to increase at the expense of those in G0/G1 phase. Differences in the proportion of cell cycle phases were more pronounced with the Ishikawa cell line. Accumulation of cells in the G1 and G2 phases and a decrease in the proportion of S phase cells was observed only after 48 h. The significant decrease in the proportion of S and increase in the proportion of G2/M phase cells became evident at the lower dose, 50 μM.
Table 5.
Effect of P-X-1544 on the distribution of cell cycle phases of PC3 and Ishikawa cell lines
| Cells | Conjugated peptide, μM | Phase distribution, %
|
|||||
|---|---|---|---|---|---|---|---|
| 24 hr
|
48 hr
|
||||||
| G1 | S | G2/M | G1 | S | G2/M | ||
| PC3 | 0 | 54 | 29 | 17 | 74 | 17 | 9 |
| 50 | 52 | 26 | 22 | 76 | 16 | 8 | |
| 100 | 48 | 29 | 23 | 83 | 11 | 6 | |
| Ishikawa | 0 | 44 | 33 | 23 | 49 | 33 | 18 |
| 50 | 47 | 31 | 22 | 55 | 14 | 31 | |
| 100 | 44 | 33 | 23 | 58 | 12 | 30 | |
Cells were grown for 24 and 48 hr in the presence or absence of conjugated peptide before flow cytometry as described in the text.
Effects on Xenografts.
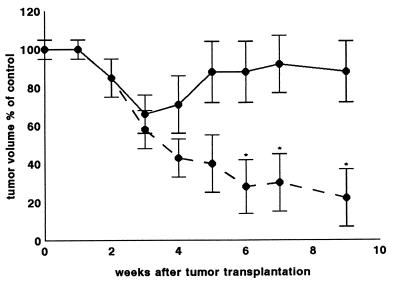
Daily doses of GnRH-III (4 mg/kg) had no influence on the growth of MDA-MB-231 xenografts in mice, whereas treatment with P-X-GnRH-III containing 4 mg of conjugated GnRH-III per kg of body weight decreased tumor volume to 0.30 ± 0.15 cm3, 57% of control volumes (0.70 ± 0.15 cm3) by the end of fourth week (Fig. 6). Treatment for 56 days caused a 78% decrease in tumor volume and eventually complete regression of tumors.
Figure 6.
Effects of GnRH-III (4 mg/kg; solid line) and P-X-GnRH-III (4 mg of conjugated peptide per kg; broken line) on the volumes of MDA-MB-231 xenografts in immunosuppressed mice expressed as a percentage of the volume of control tumors in age-matched animals. Results for 10 animals in each group are given as means ± SD with significance calculated by using the Student t test. ∗, P < 0.01.
DISCUSSION
The lower rate of inhibition of proliferation, observed with the SRB assay compared with that observed by the cell counting method is attributed to the shorter test procedure (3 days vs. 6 days).
The higher antitumor activity of peptide conjugates than of free peptides in vitro may be due to enhanced binding of peptide conjugates through supplementary nonbiospecific interactions of the carrier (P) with external domains of the receptor and adjacent membrane structures and/or even to internalization of receptor-conjugate complexes. The copolymer structure could then exert toxic effects inside the cell, as discussed below. The results support the suggestion that polypeptide hormones are protected from proteolytic degradation and can remain active after conjugation (21, 33). The loss of antitumor activity of GnRH-III in vivo is, thus, attributed to proteolysis.
The accumulation of PC3 and Ishikawa cells in the G2/M phase after treatment with P-X-1544 was more pronounced than with the GnRH analog SB-75 (37). The results are in contrast with the slowing by lupron of the transition of G0/G1 to S phase of the ovarian carcinoma cell line 2774 (38) and with the increase in G0/G1 and decrease in G2/M phase of MCF-7 cancer cells caused by buserelin (39). The pronounced effects of peptide conjugates on phase distributions in cell cycles probably reflect the greater suppression of in vitro growth of cancer cells by peptide conjugates than by free peptides. The accumulation of cells in the G2/M phase probably reflects the inhibition of cdc25 phosphatase by peptide conjugates. The slight accumulation of Ishikawa cells in the G1 phase, however, may indicate that the peptide conjugate inhibits another enzyme responsible for entry of cells to the DNA-synthesis phase. The observation that the peptide conjugates and not the free peptides inhibit activity of cdc25 phosphatase may have biological significance if conjugates but not free peptides are internalized after interaction with receptors on cell membranes. It is clear, however, from the IC50 values for the copolymer contents of the conjugates (Table 4) that the peptides contribute to the inhibitory properties of the conjugates. Preliminary evidence for such receptor-based internalization has been observed (B.V., unpublished results). Once inside the cell, the P-X component of P-X peptide could contribute toxicity. While P-X-OH significantly inhibits the cdc25 phosphatase, this molecular species would not enter cells through a GnRH receptor-based mechanism. P-X-OH is without effect on cell growth. Evidence that the presence of receptors on cell membranes correlates with hormone-induced apoptosis is accumulating (40–42).
Retention of biological activity of peptides, observed here and previously (21, 33) after conjugation with P-X, reflects similar retention of cytotoxicity in conjugated antitumor drugs (43–50), including neocarcinostatin, doxorubicin, daunomycin, methotrexate, and mephalan, after conjugation with various polymers. Thus, conjugation of GnRH analogues GnRH-III, MI-1544, and MI-1892 with P-X remains a promising ploy by protecting the bound peptide from proteolysis in the circulation and thereby increasing the availability for receptor interactions on the tumor cell membranes. The observation by J. Horváth (personal communication) that the low endocrine activities of GnRH-III (29) and MI-1892 (19) in the pituitary are further diminished by conjugation with P-X indicates increased selectivity for direct action of peptide conjugates on the tumor cells. This is further evidence of the strong therapeutic potential of specific and stable GnRH conjugates that act directly to suppress growth of breast, prostate, ovarian, and endometrial cancer cells without endocrine side effects involving the pituitary.
Acknowledgments
We acknowledge the cdc25 phosphatase assay performed by S. Leclerc and L. Meijer and the skillful assistance of Judit Szász, Vilma Pályi, Irén Nemes, Edit Szöke, Csilla Kazatsay, and Rozália Hunyadi. This work was supported by U.S.–Hungarian Joint Fund Grant 455, by Hungarian Technological Research Development Project (OMFB) Grant 94-97-48-0735, by Hungarian National Research Fund (OTKA) Grant T-016323, and by the Carpenter Chair, Creighton University.
ABBREVIATIONS
- GnRH
gonadotropin-releasing hormone
- P
poly(N-vinylpyrrolidone-co-maleic acid) copolymer
- X
Gly-Phe-Leu-Gly
- SRB
sulforhodamine B
References
- 1.Vickery B H. Endocr Res. 1986;7:115–124. doi: 10.1210/edrv-7-1-115. [DOI] [PubMed] [Google Scholar]
- 2.Höffken K. Recent Results Cancer Res. 1992;124:91–104. doi: 10.1007/978-88-470-2186-0_9. [DOI] [PubMed] [Google Scholar]
- 3.Weinbauer G F, Nieschlag E. Recent Results Cancer Res. 1992;124:113–136. doi: 10.1007/978-88-470-2186-0_11. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann M, Jonat W, Kleeberg U, Eiermann W, Jänicke F, Hilfrich J, Kreienberg R, Albrecht M, Weitzel H K, Schmid H, et al. J Clin Oncol. 1989;7:1113–1119. doi: 10.1200/JCO.1989.7.8.1113. [DOI] [PubMed] [Google Scholar]
- 5.Hsueh A J W, Eisenhauer K, Chun S-Y, Hsu S-Y, Billig H. Recent Prog Horm Res. 1996;51:433–456. [PubMed] [Google Scholar]
- 6.Baumann K H, Kiesel L, Kaufmann M, Baster G, Runnebaum B. Breast Cancer Res Treat. 1993;25:37–46. doi: 10.1007/BF00662399. [DOI] [PubMed] [Google Scholar]
- 7.Ohno T, Imai A, Furui T, Takahashi K, Tamaya T. Am J Obstet Gynecol. 1993;169:605–610. doi: 10.1016/0002-9378(93)90630-2. [DOI] [PubMed] [Google Scholar]
- 8.Imai A, Ohno T, Iida K, Fuseya T, Furui T, Tamaya T. Gynecol Oncol. 1994;55:144–148. doi: 10.1006/gyno.1994.1264. [DOI] [PubMed] [Google Scholar]
- 9.Marinaccio M N, Reshkin S, Pinto V, Paradiso A. Minerva Ginecol. 1994;46:519–526. [PubMed] [Google Scholar]
- 10.Harris N, Dutlow C, Eidne K, Dong K-W, Roberts J, Millar R. Cancer Res. 1991;51:2577–2581. [PubMed] [Google Scholar]
- 11.Kakar S S, Grizzle W E, Neill J D. Mol Cell Endocrinol. 1994;106:145–149. doi: 10.1016/0303-7207(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 12.Limonta P, Dondi D, Roberta M, Moretti R M, Fermo D, Garattini E, Motta M. J Clin Endocrinol Metab. 1993;76:797–800. doi: 10.1210/jcem.76.3.8445038. [DOI] [PubMed] [Google Scholar]
- 13.Srkalovich G, Wittliff J L, Schally A V. Cancer Res. 1990;50:1841–1846. [PubMed] [Google Scholar]
- 14.Emons G, Schröder B, Ortmann O, Westphalen S, Schulz K-D, Schally A V. J Clin Endocrinol Metab. 1993;77:1458–1464. doi: 10.1210/jcem.77.6.8263128. [DOI] [PubMed] [Google Scholar]
- 15.Irmer G, Bürger C, Müller R, Ortmann O, Peter U, Kakar S S, Neill J D, Schulz K D, Emons G, Müller G. Cancer Res. 1995;55:817–822. [PubMed] [Google Scholar]
- 16.Vincze B, Pályi I, Daubner D, Kremmer T, Számel I, Bodrogi I, Sugár J, Seprödi J, Mezö I, Teplán I, et al. J Steroid Biochem Mol Biol. 1991;38:119–126. doi: 10.1016/0960-0760(91)90116-m. [DOI] [PubMed] [Google Scholar]
- 17.Vincze B, Pályi I, Daubner D, Kálnay A, Mezö G, Hudecz F, Szekerke M, Teplán I, Mezö I. J Cancer Res Clin Oncol. 1994;120:578–584. doi: 10.1007/BF01212811. [DOI] [PubMed] [Google Scholar]
- 18.Kovács M, Mezö I, Flerkó B, Teplán I, Nikolics K. Biochem Biophys Res Commun. 1984;118:351–355. doi: 10.1016/0006-291x(84)91108-2. [DOI] [PubMed] [Google Scholar]
- 19.Mezö I, Seprödi J, Vincze B, Pályi I, Kéri G, Vadász Z, Tóth G, Kovács M, Koppán M, Horváth J, et al. Biomed Pept Proteins Nucleic Acids. 1996;2:33–40. [PubMed] [Google Scholar]
- 20.Pályi, I., Vincze, B., Kálnay, A., Gaál, D., Mezö, I., Seprödi, J. & Teplán, I. (1994) Ann. Oncol.5, Suppl. 5, 85.
- 21.Pályi I, Vincze B, Kálnay A, Turi G, Mezö I, Teplán I, Seprödi J, Pató J, Móra M. Cancer Detect Prev. 1996;20:146–152. [PubMed] [Google Scholar]
- 22.Vincze B, Pályi I, Prajda N, Daubner D, Kálnay A, Mezö I, Seprödi J, Teplán I. Cell Prolif. 1992;25:518. [Google Scholar]
- 23.Mezö I, Seprödi J, Vadász Z, Teplán I, Vincze B, Pályi I, Gaál D, Kálnay A, Pató J, Móra M. In: Peptides 1994. Maia H L S, editor. Leiden, The Netherlands: ESCOM; 1995. pp. 763–764. [Google Scholar]
- 24.Sower S A, Chiang Y-C, Lovas S, Conlon J M. Endocrinology. 1993;132:1125–1131. doi: 10.1210/endo.132.3.8440174. [DOI] [PubMed] [Google Scholar]
- 25.Lovas, S., Conlon, J. M., Vincze, B., Pályi, I., Gaál, D., Kálnay, A., Mezö, I., Teplán, I., Tóth, G. & Kovács, M. (1997) U.S. Patent 5,593,965.
- 26.Pályi I, Vincze B, Kálnay A, Turi G, Gaál D, Mezö I, Teplán I, Pató J, Móra M, Lovas S, et al. In: 5th International Congress on Hormones and Cancer. Labrie J, editor. Leiden, the Netherlands: ESCOM; 1995. p. 87. [Google Scholar]
- 27.Pályi I, Vincze B, Kálnay A, Turi G, Gaál D, Mezö I, Teplán I, Pató J, Móra M, Kovács M, et al. Ann Oncol. 1996;7:83. (abstr.). [Google Scholar]
- 28.Pályi I, Mezö I, Lovas S, Vincze B, Turi G, Kálnay A, Vadász Z, Teplán I, Murphy R F. Proc Am Assoc Cancer Res. 1997;38:612. (abstr.). [Google Scholar]
- 29.Lovas S, Pályi I, Vincze B, Horváth J, Kovács M, Mezö I, Tóth G, Teplán I, Murphy R F. J Pept Res. 1998;52:384–389. doi: 10.1111/j.1399-3011.1998.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 30.Vincze B, Pató J, Mezö I, Tóth G, Pályi I, Gaál D, Kálnay A, Stefik I, Lovas S, Teplán I, et al. Proc Am Assoc Cancer Res. 1997;38:433. (abstr.). [Google Scholar]
- 31.Móra M, Pató J, Tüdös F. Makromol Chem. 1989;190:1967–1974. [Google Scholar]
- 32.Mezö, I., Pató, J., Lovas, S., Pályi, I., Vincze, B., Murphy, R. F., Tóth, G., Gaál, D., Kálnay, A., Teplán, I., et al. (1995) Patent Cooperation Treaty (PCT), Pub. no. W096/04927.
- 33.Vincze B, Pályi I, Gaál D, Pató J, Móra M, Mezö I, Teplán I, Seprödi J, Kovács M. Cancer Detect Prevent. 1996;20:153–159. [PubMed] [Google Scholar]
- 34.Baratte B, Meijer L, Galaktionov K, Beach D. Anticancer Res. 1992;12:873–880. [PubMed] [Google Scholar]
- 35.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren J T, Bokesch H, Kenney S, Boyd M R. J Natl Cancer Inst. 1990;82:1107–1111. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro H M. Practical Flow Cytometry. New York: Liss; 1988. [Google Scholar]
- 37.Kleinman D, Douvdevani A, Schally A V, Levy J, Sharoni Y. Am J Obstet Gynecol. 1994;170:96–102. doi: 10.1016/s0002-9378(94)70391-4. [DOI] [PubMed] [Google Scholar]
- 38.Thompson M A, Adelson M D, Kaufman L M. J Clin Endocrinol Metab. 1991;72:1036–1041. doi: 10.1210/jcem-72-5-1036. [DOI] [PubMed] [Google Scholar]
- 39.Mullen P, Scott W N, Miller W R. Br J Cancer. 1991;63:930–932. doi: 10.1038/bjc.1991.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szende B, Zalatnai A, Schally A V. Proc Natl Acad Sci USA. 1989;86:1643–1647. doi: 10.1073/pnas.86.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takebayashi H, Oida H, Fujisawa K, Yamagachi M, Hikida T, Fukumoto M, Narumiya S, Kakizuka A. Cancer Res. 1996;56:4164–4170. [PubMed] [Google Scholar]
- 42.Imai A, Horibe S, Takagi A, Ohno T, Tamaya T. Eur J Obstet Gynecol. 1997;74:73–78. doi: 10.1016/s0301-2115(97)02750-4. [DOI] [PubMed] [Google Scholar]
- 43.Maeda H. Adv Drug Deliv Rev. 1991;6:181–202. [Google Scholar]
- 44.Maeda H, Seymour L W, Miyamato Y. Bioconjug Chem. 1992;3:351–362. doi: 10.1021/bc00017a001. [DOI] [PubMed] [Google Scholar]
- 45.Duncan R, Dimitrijevic S, Evagaron E G. S T P Pharma Sci. 1996;6:237–263. [Google Scholar]
- 46.Seymour L W. Crit Rev Ther Drug Carrier Syst. 1992;9:135–187. [PubMed] [Google Scholar]
- 47.Duncan R, Seymour L W, O’Hare K B, Flanagan P A, Wedge S R, Hume I C, Ulbrich K, Strohalm J, Subr V, Spreafico F, et al. J Controlled Release. 1992;19:331–346. [Google Scholar]
- 48.Gaál D, Hudecz F. Eur J Cancer. 1998;34:155–161. doi: 10.1016/s0959-8049(97)00338-9. [DOI] [PubMed] [Google Scholar]
- 49.Hudecz F, Clegg J A, Kajtár J, Embleton M J, Pimm M V, Szekerke M, Baldwin R W. Bioconjug Chem. 1993;4:25–33. doi: 10.1021/bc00019a004. [DOI] [PubMed] [Google Scholar]
- 50.Duncan R, Hume I C, Yardley H J, Flanagan P A, Ulbrich K, Subr V, Strohalm J. J Controlled Release. 1991;16:121–136. [Google Scholar]