Abstract
Background
Gastric Electrical Stimulation (GES) with short pulses improves nausea and vomiting in patients with gastroparesis, whereas GES with long pulses improves gastric motility.
Aims
To assess the effects of a novel method of GES using dual pulse (both short and long pulses) on gastric tone, compliance and sympathovagal activity in dogs.
Materials and Methods
The study was performed in 7 dogs implanted with a gastric cannula and a pair of gastric serosal electrodes for dual pulse GES. The study was composed of a number of sessions on different days with different stimulation parameters, including variations in the number of short pulses and stimulation amplitude.
Results
1) Dual pulse GES of one short pulse and one long pulse with various amplitudes inhibited gastric tone (p<0.05) but did not alter sympathetic or vagal activity; 2) Dual pulse GES with five short pulses and one long pulse not only inhibited gastric tone, but also reduced sympathetic activity and increased vagal activity (p<0.05). 3) Dual pulse GES with five short pulses and one long pulse significantly increased gastric compliance.
Conclusions
Dual pulse GES reduces gastric tone and increases gastric compliance. The variation in the number of short pulse affects the sympathetic and vagal activities, whereas, the increase in stimulation strength enhances its effects on gastric tone.
Keywords: gastric electrical stimulation, gastric tone, gastric compliance, sympathetic and vagal activity
1. Introduction
Gastric electrical stimulation (GES) has been introduced as an innovative therapy for patients with refractory gastroparesis. The results of various studies suggest that GES with appropriate parameters may affect gastric myoelectrical activity, gastric motility and gastrointestinal symptoms in animals and humans [1–18]. The effects of GES are known to be associated with stimulation configurations, sites and parameters [2, 3]. Available data from clinic and animal research have shown that GES with long pulses (in the order of millisecond, ms) improves gastric motility [1, 2, 4–11], whereas GES with short pulses (in the order of microsecond, μs) improves symptoms of nausea and vomiting in patients with gastroparesis [12–16] and in dogs [17, 18].
Recently a novel method, called dual pulse GES has been introduced [19]. In this new method, GES is performed using both short pulses and long pulses. It was hypothesized that dual pulse GES would improve symptoms by the action of short pulses and alter gastric motility via the action of long pulses. An initial study in our lab did show a reduction in both gastric dysrhythmia and emetic responses induced by vasopressin with dual pulse GES in dogs [19]. These preliminary but exciting findings suggest that the dual pulse GES may be a better therapy than the conventional GES for functional dyspepsia or gastroparesis in which both dyspeptic symptoms and dysmotility are present, and symptoms and dysmotility are often disassociated. In addition to dyspeptic symptoms and dysmotility, gastric accommodation or gastric compliance is also often impaired in functional dyspepsia and gastroparesis [20, 21]. Accordingly, it is also important to know the effects of any potential GES therapy on gastric tone and compliance before it can be applied in these groups of patients.
It is well known that the tone of the proximal stomach plays a key role in distribution, storage and emptying of food. Gastric tone is maintained by the balance between the excitatory cholinergic and the inhibitory nitrergic pathways [22–24]. Disruption of this balance alters gastric tone. Previous studies have indicated that GES with long pulses reduced gastric tone, and the inhibitory effect was mediated via the vagal and nitrergic pathways [2, 25]. However, it is unknown whether dual pulse GES is also capable of reducing gastric tone and whether the sympathovagal pathway is involved with the effect of dual pulse GES.
The aims of this study were therefore to assess the effects of dual pulse GES on gastric tone and compliance and to investigate the possible mechanism of dual pulse GES involving sympathovagal activities in a canine model.
2. Material and methods
2.1. Animal preparation
After an overnight fast, seven healthy female hound dogs weighing 18–22 kg were anesthetized with intravenous infusion of sodium thiopental (5mg/kg) and maintained with inhalation isoflurane (1–2%) carrier gases delivered from a ventilator after endotracheal intubation. A midline laparotomy was performed and a gastric cannula was implanted on the anterior side of the stomach 6 cm above the pylorus. The gastric cannula was exteriorized through the abdominal wall, providing direct access to the inside of the stomach once opened. One pair of 28-gauge stainless steel cardiac pacing wires was implanted into the seromuscular layer of the stomach 14 cm above the pylorus along the greater curvature. The two electrodes in the pair were separated by approximately 1 cm. These electrodes were tunneled to the back of the chest subcutaneously, exteriorized, secured and numbered for attachment to the stimulation equipment. Studies were performed after the animals completely recovered from surgery, usually two weeks after surgery. The experimental protocol was approved by the Institutional Animal Care and Use Committee of the Veterans Research Administration, Oklahoma City, OK.
2.2. Experimental protocols
The animals were overnight fasted on the day of experiments. Each dog was studied in six sessions under identical conditions on six separate days in a randomized order. Four sessions were for studying gastric tone. Each session was composed of a 30-min baseline recording, followed by a 30-min period with dual pulse GES with one of the four sets of stimulation parameters. The other two sessions were designed to study gastric compliance: one serving as a control session without dual pulse GES and the other with dual pulse GES performed using the set of parameters soliciting the most significant effects on gastric tone and vagal activity.
2.3. Dual pulse gastric electrical stimulation
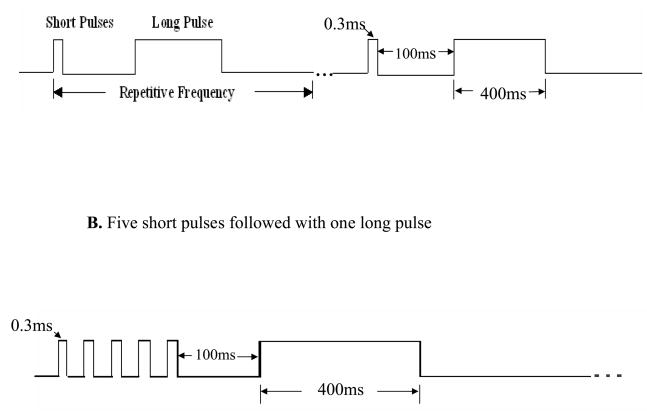
Dual pulse GES was performed via the pair of electrodes on the greater curvature using an adjustable electrical stimulator (Model A310, World Precision Instruments, Sarasota, FL, USA). The stimuli were composed of a number of short pulses followed with one long pulse. The four sets of stimulation parameters for the gastric tone study were as follows: Set 1: one short pulse (0.3ms) followed with one long pulse (400ms) with an interval of 100ms between the short pulse and the long pulse (Fig. 1A), amplitude of 2mA and repeated at the frequency of the intrinsic gastric slow waves; Sets 2 and 3: same as set 1 except a pulse amplitude of 4mA and 6mA, respectively; Set 4: same as Set 1 but the number of short pulse was increased from one to five (width of 0.3ms, frequency of 40 Hz) and the amplitude that led to the most alteration in gastric volume was selected. The interval between the last short pulse and the long pulse was 100ms (Figure 1B). All of these were repeated at the frequency of the intrinsic gastric slow waves. The stimulation parameters which resulted in the most significant effect on gastric volume and sympathovagal activity were chosen for the gastric compliance study.
Fig. 1.
A. One short pulse (0.3ms) followed with a long pulse (400ms) with an interval of 100ms between the short pulse and long pulse, amplitude of 2mA and repeated at the frequency of the intrinsic gastric slow waves. B. Increase the number of short pulse to five (width of 0.3ms, frequency of 40 Hz). The interval between the last short pulse and the long pulse was 100ms.
2.4. Placement of Barostat balloon
During the experiment, the animal was put on a test table, standing and slightly restrained from movement. The gastric cannula was opened, and the stomach was cleaned by rinsing with warm water and completely drained. A double-lumen catheter with a spherical polyethylene balloon (700 ml, 10 cm in length) was inserted into the stomach through the gastric cannula. The balloon was placed to the direction of the fundus, and the position of the balloon was considered appropriate once a slight resistance was met (reaching the fundic wall). The catheter was secured once the desired position of the balloon was verified. When inflated, the balloon covered the fundus and most area of gastric corpus.
2.5. Assessment of gastric tone
Gastric tone was assessed with an electronic Barostat system (Distender Series II, Toronto, Canada). The catheter was connected to the barostat and the folded balloon was fully opened by briefly inflating the balloon which was then deflated. The minimal distending pressure (MDP) was determined by inflating the balloon in 1 mmHg steps until a pressure at which evident respiratory excursions were recorded and a proper balloon volume was achieved (30 ml or more). The pressure that was 2 mmHg above the MDP was defined as the operating pressure and used for recording. The operating pressure was individualized among animals and maintained the same in the various sessions of a particular study in each dog.
2.6. Analysis of sympathetic and vagal activities
Sympathetic and vagal activities were assessed by overall power spectral analysis of the heart rate variability [26]. The power in the low frequency band (0.04–0.15 Hz), LF, stands mainly for sympathetic activity and part of parasympathetic activity. The power in the high frequency band (0.15–0.50 Hz), HF, represents pure parasympathetic activity. LF was defined as the area under the curve in the frequency range of 0.04–0.15 Hz and HF was defined as the area under the curve in the frequency range of 0.15–0.50 Hz. The LF/HF ratio reflects the balance between sympathetic activity and vagal activity.
2.7. Assessment of gastric compliance
Gastric compliance represents the pressure–volume relationship that reflects the elasticity of the stomach. It was tested by isobaric phasic distention using the intragastric balloon, with a stepwise increment of 2 mmHg in the balloon pressure, starting from 2 mmHg up to 16 mmHg. Each distention was maintained for 2 min with 1 min deflation in between. Gastric compliance was calculated by averaging the balloon volume over middle 1/3 of 2 min distention. A power exponential model was used to fit the nonlinear compliance curve, with parameter β representing the overall shape of the curve and κ essentially representing the change in volume as a function of 1/P (P stands for pressure) at any given point [27]. The estimated κ and β for each animal were used to calculate the pressure corresponding to half-maximal volume on the pressure-volume curve (P1/2). A shift of the compliance curve is appropriately reflected in a change in P1/2, and the values of P1/2 are considered to be the primary endpoint for compliance.
2.8. Data and Statistical Analysis
Gastric tone was expressed as the mean intragastric balloon volume averaged over each observation period. Paired Student’s t-test or One way analysis of variance (ANOVA) was used for comparisons where appropriate. All data are expressed as Mean±SE. A p value of less than 0.05 was considered as statistical significant.
3. Results
All dogs tolerated the procedures well. One dog was excluded from the gastric compliance study because gastric myoelectrical activity could not be recorded from gastric electrodes, suggesting a possible loss of contact between stimulation electrodes and gastric tissue. That is, the electrodes could not be used for delivering gastric electrical stimulation.
3.1. Effects of dual pulse GES with different amplitudes on gastric tone
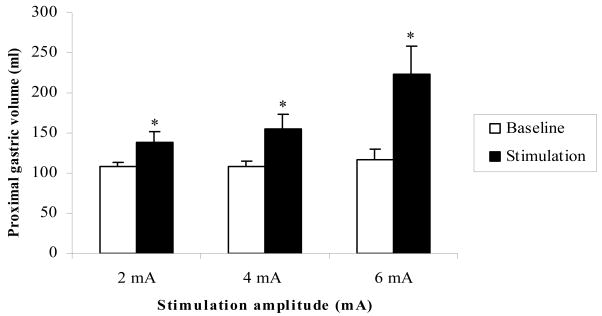
Gastric volume is inversely related to gastric tone. Dual pulse GES with one short pulse and amplitude of 2mA or 4mA or 6mA significantly increased gastric volume. That is, gastric tone was inhibited (Fig. 2). In addition, the highest increase in gastric volume was observed when dual pulse GES was applied with a pulse amplitude of 6mA. The percentage of the gastric volume increase during dual pulse GES was 27.6±8.3% with 2mA amplitude, 41.5±12.4% with 4mA, and 114.1±51.6% with 6mA.
Fig. 2.
Dual pulse GES with variation of amplitudes significantly induced the proximal gastric volume increase, which was gastric tone decrease. *p<0.05 vs Baseline.
3.2 Effects of dual pulse GES with different numbers of short pulses on gastric tone
In order to understand the effects of short pulses, we raised the number of short pulses from one short pulse to five short pulses at amplitude of 6mA. It was found that the effect of dual pulse GES with five short pulses on gastric tone was similar to that of one short pulse. Gastric volume significantly increased from 100.3 ± 2.4 ml at baseline to 168.8 ± 25.8ml during dual pulse GES with five short pulses (p=0.032). The changes in the percentage of gastric volume were comparable between one short pulse session and five short pulses session (114.1 ± 51.6% in one short pulse vs. 61.6 ± 23.2% in five short pulses, p=0.46) although dual pulse GES with five short pulses seemed less potent than the dual pulse GES with one short pulse.
3.3 Effects of dual pulse GES on sympathetic and parasympathetic (vagal) activity
As shown in Table 1, dual pulse GES with one short pulse did not alter sympathetic and parasympathetic (vagal) activities in compared with baseline (p>0.05). However, dual pulse GES with five short pulses at 6mA significantly reduced the sympathetic activity from 0.42 ± 0.02 at baseline to 0.32 ± 0.02 (p=0.006) and the sympathovagal balance from 0.72 ± 0.05 at baseline to 0.48 ± 0.04 (p=0.007), and increased the vagal activity from 0.58 ± 0.02 at baseline to 0.68 ± 0.02 (p=0.006). These data suggested that dual pulse GES with five short pulses significantly inhibited sympathetic activity and excited vagal activity.
Table 1.
Effects of dual pulse GES with one short pulse and different stimulation amplitudes on sympathetic and parasympathetic (vagal) activity
| Sympathetic and parasympathetic (vagal) activity | ||||||
|---|---|---|---|---|---|---|
| Baseline | Stimulation | |||||
| P1/(P1+P2) | P2/(P1+P2) | P1/P2 | P1/(P1+P2) | P2/(P1+P2) | P1/P2 | |
| 2mA | 0.43 ± 0.02 | 0.56 ± 0.02 | 0.78 ± 0.08 | 0.43 ± 0.02 | 0.57 ± 0.02 | 0.76 ± 0.06 |
| 4mA | 0.41 ± 0.02 | 0.589 ± 0.02 | 0.71 ± 0.06 | 0.406 ± 0.03 | 0.594 ± 0.03 | 0.70 ± 0.08 |
| 6mA | 0.42 ± 0.04 | 0.58 ± 0.04 | 0.81 ± 0.19 | 0.42 ± 0.03 | 0.58 ± 0.02 | 0.73 ± 0.08 |
3.4. Effects of dual pulse GES on gastric compliance
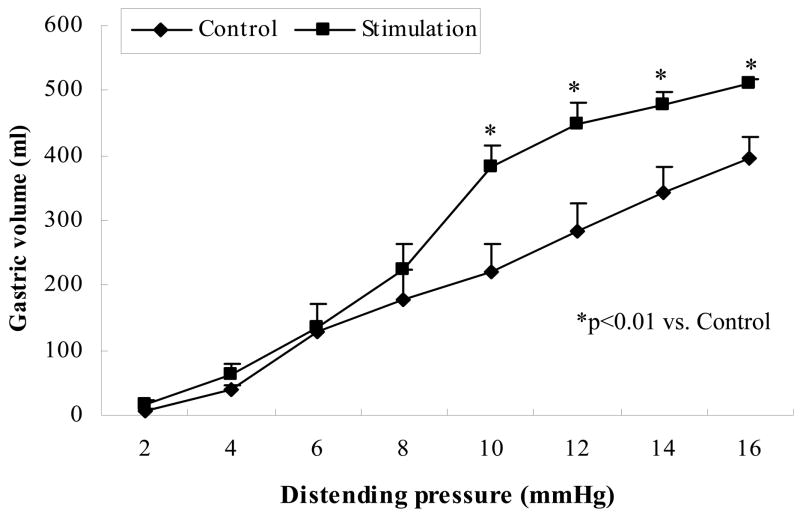
Based on the findings on gastric tone, and sympathetic and parasympathetic activities, dual pulse GES with five short pulses and amplitude of 6mA was used in the gastric compliance study. All three parameters related to the compliance were altered with dual pulse GES: was reduced from 28.5 ± 4.5 in the control session to 13.5 ± 1.8 (p<0.05); β was increased from 0.66 ± 0.13 to 1.1 ± 0.17 (p<0.05) and P1/2 was decreased from 11.8 ± 1.1 mmHg to 8.6 ± 0.6 mmHg (p<0.05). The pressure-volume curve is showed in Fig. 3. It is seen that the pressure corresponding to half-maximal volume on the pressure–volume curve (P1/2) was significantly reduced with dual pulse GES, suggesting an increase in gastric compliance.
Fig. 3.
The effect of dual pulse GES on gastric compliance (mean±SE). Dual pulse induced a significantly increase in gastric compliance compared with without dual pulse GES (*p<0.01).
4. Discussion
In this study, it was found that 1) dual pulse GES inhibited gastric tone, and the alteration of gastric tone was associated with stimulation energy but not the number of short pulses; 2) dual pulse GES with five short pulses at amplitude of 6mA increased gastric compliance; 3) dual pulse GES with five but not one short pulse significantly increased parasympathetic (vagal) activity and decreased sympathetic activity.
GES as a potential approach for treating refractory gastroparesis has received increasing attention and some promising results have been reported in both dogs and humans [6, 8, 12–16]. Long pulse GES has been shown to entrain gastric slow waves in humans and dogs [1, 4, 5, 8, 9], improve gastric emptying and gastrointestinal symptoms in patients with gastroparesis [6], and canine models of gastroparesis [10], induce gastric relaxation in dogs [2, 11]. Short pulse GES is not able to entrain gastric slow waves or alter gastric motility, but has recently been shown to prevent vomiting and reduce the symptom scores in a canine model [17, 18] and improved symptoms of nausea and vomiting in patients with gastroparesis [12–16]. In this study, we devised a novel stimulation method: dual pulse GES that consisted of short pulse followed by long pulse with different stimulation parameters including a variation in stimulation amplitude, and a variation in the number of short pulses. In theory, dual pulse GES, combined the characters of both short pulse and long pulse, should be capable of improving both gastric motility and gastrointestinal symptoms.
Gastric tone plays an important role in the regulation of accommodation, storage and emptying of food from the stomach. A number of studies showed that gastric motor disorders, such as gastroparesis [28] and functional dyspepsia [21, 29], were related to the alteration of gastric tone, and the treatment options of these functional disorders are usually very limited especially for refractory gastroparesis. Therefore, it is of great significance to study the therapeutic potential of GES for gastrointestinal motility diseases. It has recently been reported that GES with long pulses induced gastric relaxation [30, 31]. Similarly, the dual pulse GES also inhibited gastric tone, and the inhibitory effect was associated with the stimulation energy but not with the number of short pulses, suggesting that the effect was attributed to long but not short pulse. While the mechanisms involved in the inhibitory effect of dual pulse GES on gastric tone was not investigated in the present study, previous studies have indicated cholinergic vagal and nitrergic mechanisms [2.25].
Consistent with the finding on gastric tone, dual pulse GES was shown to increase gastric compliance. This is of great clinical significance as numerous studies have previously reported reduced gastric compliance and accommodation in patients with functional dyspepsia or gastroparesis [21, 28, 29].
GES with five but not one short pulse significantly excited vagal activity and inhibited sympathetic activity, suggesting that the effect of GES on sympthovagal activity was attributed to short pulses. In addition, gastric tone tended to increase, although not significant, during dual pulse GES with five short pulses, when compared with that of dual pulse GES with one short pulse; this might be attributed to the increase of vagal tone with GES of five short pulses.
The findings of the present study are suggestive of therapeutic potentials of dual pulse GES for functional dyspepsia and gastroparesis although clinical studies are needed to provide more direct evidence. Impaired gastric accommodation, antral hypomotility, gastric dysrhythmia and decreased vagal tone are considered as key players in the pathogenesis of gastroparesis [21, 32–35]. Our previous studies have shown that dual pulse GES normalized vasopressin-induced dysrhythmias, reduced the symptoms and prevented vomiting in the normal dogs [19]. The current study demonstrated that dual pulse GES seemed to be able to relax fundus, enhance gastric compliance and increase vagal activity. It is, therefore, believed that dual pulse GES may be able to improve both gastric motility and gastrointestinal symptoms. All these findings will benefit patients with gastroparesis.
It should also be noted that the current study had certain limitations. The sample size was relatively small and therefore the findings may not be well generalized. The other drawback of the study was that the used animal model did not represent functional dyspepsia or gastroparesis and therefore it is not clear whether these data could be translated into humans.
In summary, dual pulse GES reduces gastric tone and increases gastric compliance and the effects are attributed to the component of long pulses. Dual pulse GES also improves vagal activity and the effect is attributed to short pulses. These findings suggest that the combination of short and long pulses may be more applicable in treating dysmotility patients with different pathogenesis.
Practice Points
GES with long pulses improves gastric motility, whereas GES with short pulses improves symptoms of nausea and vomiting in patients with gastroparesis and in dogs.
Dual pulse GES with both short and long pulses reduces gastric tone, increases gastric compliance, and affects the sympathetic and vagal activities.
This study helps us better understand this innovative dual pulse GES approach for patients with gastroparesis, although the clinical significance of the findinging warrants further validation
Research Agenda
To assess the effect of dual pulse GES on gastric tone
To explore the effect of dual pulse GES on compliance
To investigate the possible mechanism of dual pulse GES involving sympathovagal activities in a canine model
Acknowledgments
This work is partially supported by a grant from National Institutes of Health (DK055437)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hocking MP, Vogel SB, Sninsky CA. Human gastric myoelectric activity and gastric emptying following gastric surgery and with pacing. Gastroenterology. 1992;103:1811–6. doi: 10.1016/0016-5085(92)91439-b. [DOI] [PubMed] [Google Scholar]
- 2.Xing JH, Chen JD. Effects and mechanisms of long-pulse gastric electrical stimulation on canine gastric tone and accommodation. Neurogastroenterol Motil. 2006;18:136–43. doi: 10.1111/j.1365-2982.2005.00748.x. [DOI] [PubMed] [Google Scholar]
- 3.Lei Y, Xing JH, Chen JD. The effect on gastric tone of gastric electrical stimulation with trains of short pulses varies with sites and stimulation conditions. Dig Dis Sci. 2008;53:2066–71. doi: 10.1007/s10620-008-0282-2. [DOI] [PubMed] [Google Scholar]
- 4.Eagon JC, Kelly KA. Effects of gastric pacing on canine gastric motility and emptying. Am J Physiol. 1993;265:G767–74. doi: 10.1152/ajpgi.1993.265.4.G767. [DOI] [PubMed] [Google Scholar]
- 5.Eagon JC, Kelly KA. Effect of electrical stimulation on gastric electrical activity, motility and emptying. Neurogastroenterol Motil. 1995;7:39–45. doi: 10.1111/j.1365-2982.1995.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 6.McCallum RW, Chen JD, Lin Z, Schirmer BD, Williams RD, Ross RA. Gastric pacing improves emptying and symptoms in patients with gastroparesis. Gastroenterology. 1998;114:456–61. doi: 10.1016/s0016-5085(98)70528-1. [DOI] [PubMed] [Google Scholar]
- 7.Xing JH, Brody F, Rosen M, Chen JD, Soffer E. The effect of gastric electrical stimulation on canine gastric slow waves. Am J Physiol Gastrointest Liver Physiol. 2003;284:G956–62. doi: 10.1152/ajpgi.00477.2002. [DOI] [PubMed] [Google Scholar]
- 8.Lin ZY, McCallum RW, Schirmer BD, Chen JD. Effects of pacing parameters on entrainment of gastric slow waves in patients with gastroparesis. Am J Physiol. 1998;274:G186–91. doi: 10.1152/ajpgi.1998.274.1.G186. [DOI] [PubMed] [Google Scholar]
- 9.Wang ZS, Qian LW, Ueno T, Chen JDZ. Gastric myoelectrical activity and autonomic nerve system responses to various gastric electrical stimulation. Dig Dis Sci. 2000;45:1252. [Google Scholar]
- 10.Bellahsene BE, Lind CD, Schirmer BD, Updike OL, McCallum RW. Acceleration of gastric emptying with electrical stimulation in a canine model of gastroparesis. Am J Physiol. 1992;262:G826–34. doi: 10.1152/ajpgi.1992.262.5.G826. [DOI] [PubMed] [Google Scholar]
- 11.Xu XH, Bringing DL, Chen JDZ. Effects of vasopressin and long pulse gastric electrical stimulation on gastric emptying, gastric and intestinal myoelectrical activity and symptoms in dogs. Neurogastroenterol Motil. 2005;17:236–44. doi: 10.1111/j.1365-2982.2004.00616.x. [DOI] [PubMed] [Google Scholar]
- 12.Familoni BO, Abell TL, Nemoto D, Voeller G, Johnson B. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig Dis Sci. 1997;42:892–7. doi: 10.1023/a:1018804128695. [DOI] [PubMed] [Google Scholar]
- 13.Abell T, McCallum R, Hocking M, Koch K, Abrahamsson H, Leblanc I, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–8. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 14.GEMS study group. Electrical stimulation for the treatment of gastroparesis: preliminary report of a multicenter international trial. Gastroenterology. 1996;110:A668. [Google Scholar]
- 15.Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of gastroparesis with electrical stimulation. Dig Dis Sci. 2003;48:837–48. doi: 10.1023/a:1023099206939. [DOI] [PubMed] [Google Scholar]
- 16.Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of diabetic gastroparesis by high-frequency gastric electrical stimulation. Diabetes Care. 2004;27:1071–6. doi: 10.2337/diacare.27.5.1071. [DOI] [PubMed] [Google Scholar]
- 17.Chen JD, Qian L, Ouyang H, Yin J. Gastric electrical stimulation with short pulses reduces vomiting but not dysrhythmias in dogs. Gastroenterology. 2003;124:401–9. doi: 10.1053/gast.2003.50048. [DOI] [PubMed] [Google Scholar]
- 18.Song GQ, Hou X, Yang B, Sun Y, Liu J, Qian W, et al. Efficacy and efficiency of gastric electrical stimulation with short pulses in the treatment of vasopressin-induced emetic responses in dogs. Neurogastroenterol Motil. 2006;18:385–91. doi: 10.1111/j.1365-2982.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu JS, Qiao X, Chen JD. Therapeutic potentials of a novel method of dual-pulse gastric electrical stimulation for gastric dysrhythmia and symptoms of nausea and vomiting. Am J Surg. 2006;191:255–61. doi: 10.1016/j.amjsurg.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Tack J, Coulie B, Van Custem E, Ryden J, Janssens J. The influence of gastric electrical stimulation on proximal gastric motor and sensory function in severe idiopathic gastroparesis. Gastroenterology. 1999;116:A-1090. [Google Scholar]
- 21.Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–52. doi: 10.1016/s0016-5085(98)70012-5. [DOI] [PubMed] [Google Scholar]
- 22.Azpiroz F, Malagelada JR. Importance of vagal input in maintaining gastric tone in the dog. J Physiol. 1987;384:511–24. doi: 10.1113/jphysiol.1987.sp016467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lidums I, Hebbard GS, Holloway RH. Effect of atropine on proximal gastric motor and sensory function in normal subjects. Gut. 2000;47:30–6. doi: 10.1136/gut.47.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paterson CA, Anvari M, Tougas G, Huizinga JD. Nitrergic and cholinergic vagal pathways involved in the regulation of canine proximal gastric tone: an in vivo study. Neurogastroenterol Motil. 2000;12:301–6. doi: 10.1046/j.1365-2982.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Chen JD. Gastric electrical stimulation inhibits postprandial antral tone partially via nitrergic pathway in conscious dogs. Am J Physiol Regul Integar Comp Physiol. 2006;290:R904–8. doi: 10.1152/ajpregu.00842.2004. [DOI] [PubMed] [Google Scholar]
- 26.Wang ZS, Chen JDZ. Robust ECG R-R wave detection using evolutionary-programming-based fuzzy inference system (EPFIS) and its application to assessing braingut interaction. IEEE Proc Sci, Meas Technol. 2000;147:351–6. [Google Scholar]
- 27.Bharucha AE, Camilleri M, Zinsmeister AR, Hanson RB. Adrenergic modulation of human colonic motor and sensory function. Am J Physiol. 1997;273:G997–1006. doi: 10.1152/ajpgi.1997.273.5.G997. [DOI] [PubMed] [Google Scholar]
- 28.Undeland KA, Hausken T, Aanderud S, Berstad A. Lower postprandial gastric volume response in diabetic patients with vagal neuropathy. Neurogastroenterol Motil. 1997;9:19–24. doi: 10.1046/j.1365-2982.1997.d01-3.x. [DOI] [PubMed] [Google Scholar]
- 29.Boeckxstaens GE, Hirsch DP, van den Elzen BD, Heisterkamp SH, Tytagat GN. Impaired drinking capacity in patients with functional dyspepsia: relationship with proximal stomach function. Gastroenterology. 2001;121:1054–63. doi: 10.1053/gast.2001.28656. [DOI] [PubMed] [Google Scholar]
- 30.Xing JH, Brody F, Brodsky J, Larive B, Ponsky J, Soffer E. Gastric electrical stimulation at proximal stomach induces gastric relaxation in dogs. Neurogastroenterol Motil. 2003;15:15–23. doi: 10.1046/j.1365-2982.2003.00385.x. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Chen JD. Gastric electrical stimulation reduces gastric tone energy dependently. Scand J Gastroenterol. 2005;40:154–9. doi: 10.1080/00365520410009582. [DOI] [PubMed] [Google Scholar]
- 32.Malagelada JR, Rees WD, Mazzotta LJ, Go VL. Gastric motor abnormalities in diabetic and postvagotomy gastroparesis: effect of meteoclopramide and bethanechol. Gastroenterology. 1980;78:286–93. [PubMed] [Google Scholar]
- 33.Koch KL, Stern RM, Stewart WR, Vasey MW. Gastric emptying and gastric myoelectrical activity in patients with diabetic gastroparesis: Effect of long-term domperidone treatment. Am J Gastroenterol. 1989;84:1069–75. [PubMed] [Google Scholar]
- 34.Undeland KA, Hausken T, Svebak S, Aanderud S, Berstad A. Wide gastric antrum and low vagal tone in patients with diabetes mellitus type I compared to patients with functional dyspepsia and healthy individuals. Dig Dis Sci. 1996;41:9–16. doi: 10.1007/BF02208577. [DOI] [PubMed] [Google Scholar]
- 35.Rothstein RD, Alavi A, Reynolds JC. Electrogastrography in patients with gastroparesis and effect of long-term cisapride. Dig Dis Sci. 1993;38:1518–24. doi: 10.1007/BF01308614. [DOI] [PubMed] [Google Scholar]