Abstract
IL-23 plays a critical role establishing inflammatory immunity and enhancing IL-17 production in vivo. However, an understanding of how it performs those functions has been elusive. In this report, using an IL-17-capture technique, we demonstrate that IL-23 maintains the IL-17-secreting phenotype of purified IL-17+ cells without affecting cell expansion or survival. IL-23 maintains the Th17 phenotype over multiple rounds of in vitro stimulation most efficiently in conjunction with IL-1β. However, in contrast to Th1 and Th2 cells, the Th17 phenotype is not stable and when long-term IL-23-stimulated Th17 cultures are exposed to Th1- or Th2-inducing cytokines, the Th17 genetic program is repressed and cells that previously secreted IL-17 assume the cytokine secreting profile of other Th subsets. Thus, while IL-23 can maintain the Th17 phenotype it does not promote commitment to an IL-17-secreting lineage.
Introduction
The cytokine environment generated in an immune response to self or foreign antigens directs the development of discrete T helper cell subsets that mediate specific types of inflammation as a mechanism for combating pathogens, with Th1 cells mediating immunity to intracellular bacteria and parasites, Th2 cells mediating immunity to extracellular parasites, and Th17 cells involved in resistance to extracellular bacteria and fungal infections (1, 2). Cytokines promote the development of effector subsets by activating STAT proteins that, in concert with lineage specific transcription factors, initiate a subset-specific effector program. Thus, IL-12-activated Stat4, and IFNγ-stimulated induction of T-bet, promote Th1 differentiation, and IL-4 activated Stat6, which induces GATA-3 expression, promotes Th2 development (3). While rapid progress has been made in understanding Th17 biology, and there are many parallels with Th1 and Th2 cells, many aspects of Th17 development and function are still unclear. TGFβ and IL-6, through the induction of IL-21, promote the development the IL-17-secreting phenotype (1, 2) by activating Stat3 and inducing Th17 lineage defining transcription factors including RORγt and RORα (4, 5). Other cytokines have been shown to contribute to the Th17 phenotype, though their precise function is not yet clear.
IL-23 is required for the in vivo function of Th17 cells. While some Th17 cells can develop in the absence of IL-23, mice deficient in IL-23p19 have a greatly diminished ability to mediate inflammation (1, 2). The initial descriptions of Th17 cells were based on cultures stimulated with IL-23, although only a small percentage of cells within these cultures were IL-17-positive (6, 7). This resulted from the inability of IL-23 to prime naïve cells to become Th17 cells, largely due to a lack of IL-23R on naïve cells (8). In short term cultures, IL-23 maintains a population of IL-17-secreting T cells, which has been interpreted as a function of promoting Th17 cell expansion or survival (9). However, this has not been extensively tested and in some early experiments IL-23 function was compared to control cultures incubated with IL-2, a cytokine now known to inhibit the Th17 phenotype (10). IL-23 induces the expression of IL-22 (11, 12), and most recently, IL-23 has also been shown to maintain a pathogenic Th17 phenotype compared to cells cultured in TGFβ and IL-6 due to an inability of IL-23 to induce IL-10 production (13). However, the long-term effects of IL-23 on Th17 cells, and whether IL-23 mediates commitment to the Th17 phenotype, as defined by the ability of these cells to maintain the Th17 phenotype in the presence of a Th1 or Th2 promoting environment, remain unclear. In this report we define the effects of IL-23 on IL-17-secreting T cells through multiple rounds of antigen receptor stimulation. Using cytokine-capture assays, we demonstrate that IL-23 maintains the Th17 phenotype without affecting proliferation or survival. Results suggest that while IL-23 is a maintenance factor for the Th17 phenotype, it does not mediate commitment to this phenotype.
Materials and Methods
Mice
Indiana University Institutional Animal Care and Use Committee approved all animal studies in this report. C57BL/6 or Balb/c mice were from Harlan Sprague Dawley (Indianapolis, IN).
Th cell differentiation
Naïve CD4+CD62L+ cells isolated from spleens using a MACS isolation system (Miltenyi Biotec) were activated with soluble anti-CD3 (4 μg/ml 145-2C11), soluble anti-CD28 (1 μg/ml; BD Pharmingen) in the presence of CD4+ depleted irradiated splenocytes (1:5). These conditions were found to be optimal over plate-bound anti-CD3 for Th17 generation, particularly in long-term cultures. Th1 and Th2 primed cultures were activated with plate bound anti-CD3 and CD4+ depleted irradiated splenocytes (1:5) and cultured with anti-IL-4 (10 μg/ml 11B11) and IL-12 (5 ng/ml; R&D Systems) or anti-IFN-γ (10 μg/ml XMG) and IL-4 (10 ng/ml; Peprotech), respectively. Th17 primed cultures were cultured with anti-IFN-γ, anti-IL-4, TGF-β1 (1 ng/ml; R&D Systems), IL-6 (100 ng/ml; Peprotech), and IL-1β (10 ng/ml; eBioscience). Cells were expanded on day three after stimulation by adding ½ the concentration of the original cytokines in fresh media. For Th1 and Th2 long-term cultures, cells on day 5 of each round of stimulation were re-plated (0.5×106 cells/ml) and stimulated with plate-bound anti-CD3 (0.5 μg/ml) in the presence of CD4+ depleted irradiated splenocytes (1:5) and cultured with original concentration of cytokines and antibodies. For long-term Th17 cultures, cells were re-plated (0.5×106 cells/ml) and stimulated with soluble anti-CD3 (1 μg/ml) in the presence of CD4+ depleted irradiated splenocytes (1:5) and cultured with IL-23 (10 ng/ml; R&D Systems) and IL-1β (10 ng/ml; eBioscience) as indicated and antibodies as described above.
Th cell analysis
On day 5 of each round of stimulation, 1×106 live cells/ml were restimulated with plate-bound anti-CD3 (4 μg/ml) or where indicated IL-18 (50 ng/ml; R&D Systems) + IL-23 (10 ng/ml) for 24 hours. Cell-free supernatants were collected and cytokine production was analyzed using ELISA (reagents from BD Pharmingen, Peprotech, or R&D Systems). Gene expression data was obtained by performing real-time PCR from RNA isolated from cells activated with plate-bound anti-CD3 for 4 hours (reagents from Applied Biosystems) and analyzed as previously described (14). For intracellular cytokine staining, T cells were stimulated for 4 hr with PMA (50ng/ml, Sigma) and Ionomycin (500 ng/ml, Sigma) in the presence of Golgistop (BD Pharmingen) at the recommended concentration. Cells were fixed with 2% paraformaldehyde for 15 min and permeabilized in PBS containing 0.1% Saponin. Cells were stained with PE anti-IL-17 and APC anti-IFN-γ (BD Pharmingen) before analysis. T cells were stained with 10 μM CFSE (Molecular Probes) for 10 min at 37°C before culture as indicated.
Th17 enrichment
Th17 cells were activated with plate-bound anti-CD3 (4 μg/ml). After 4 hr, Th17 cells were labeled with 75 μl of previously crosslinked (Controlled Protein-Protein Crosslinking Kit; Pierce) anti-CD45 (clone 30-F11; BD Pharmingen) and anti-IL17 (clone Tc11-18H10; BD Pharmingen) antibodies (0.2 mg/ml) for 5 min on ice. Labeled cells were then diluted in prewarmed complete RPMI 1640 to a concentration of 105 cells/ml and rotated for 1 hr at 37°C. After capture, the Th17 cells were stained with 100 μl of biotin-labeled anti-IL-17 (0.2 mg/ml; clone Tc11-8H4.1; BD Pharmingen) for 15 min before washing and incubating 10 min with Streptavidin-PE (BD Pharmingen). IL-17 captured cells were sorted using a FACS Aria cell sorter (Becton Dickinson).
Phospho-STAT protein analysis
T cells were stimulated for 30 minutes with IL-23 (10 ng/ml) or IL-4 (10 ng/ml). Following stimulation cells were fixed and then permeabilized by methanol. Cells were then stained for phospho-Stat3 or phospho-Stat6 (BD Pharmingen) before analysis. For immunoblot analysis, cells differentiated for three rounds of stimulation were restimulated with IL-12 (5 ng/ml) for 1 hour. Whole-cell protein lysates were immunoblotted with phospho-Stat4 (Zymed), total Stat4 (C-20; Santa Cruz) and actin (Calbiochem) as a control.
Results
IL-23 maintains IL-17 secretion without affecting Th17 cell proliferation or expansion
While the requirement for IL-23 in the function of Th17 cells in vivo is established, the precise role of this cytokine in affecting the Th17 phenotype is unclear. Among other functions, IL-23 was proposed to act as a Th17 cell proliferation or survival factor. To directly test these functions, we developed a cytokine capture assay for IL-17-secreting cells to compare IL-23 functions in enriched IL-17-high and –low secretor populations. Naïve CD4 T cells were cultured with TGFβ1+IL-6+IL-1β for 5 days before stimulation with anti-CD3 prior to selection of IL-17-high and –low cells by cell sorting (Fig. 1A). Following sorting there was a 10-12-fold enrichment for IL-17 secreting cells in the IL-17-high population (Fig.1A). The separation of cells into distinct populations was confirmed by demonstrating segregated expression of IL-17, IL-22, Il23r and Rorc, while IFNγ production was indistinguishable between the two populations (Fig. 1B and C). Intracellular staining for IFN-γ in these populations demonstrated less than 0.5% in any population (data not shown).
FIGURE 1.

Cytokine selection of IL-17+ Th17 cells. A, Naïve CD4+ T cells were activated, cultured in TGF-β+IL-6+IL-1β and blocking antibodies (anti-IFN-γ and anti-IL-4) for five days and activated before surface staining for IL-17 using cytokine capture. Cells were then sorted into IL-17-high and –low populations. B, Supernatants from IL-17-high and -low cells stimulated with anti-CD3 were tested for cytokines using ELISA. C, RNA was isolated from cells treated in (B) and gene expression was assessed using real-time PCR.
One of the proposed functions of IL-23 is promoting the proliferation or expansion of Th17 cells. To test this directly, naïve CD4 T cells were cultured with TGFβ1+IL-6+IL-1β for 5 days, separated based on IL-17 production and labeled with CFSE (Fig. 2A). CFSE-labeled IL-17-high and –low populations were cultured in blocking antibodies (anti-IFNγ and anti-IL-4) alone or blocking antibodies plus IL-23 for 24 or 48 hours to assess proliferation. While IL-17-high cells had an intrinsically higher rate of proliferation than IL-17-low cells, there was not a significant difference in proliferation between cultures incubated with or without IL-23 (Fig. 2B and C), suggesting that IL-23 does not promote a robust proliferative response.
FIGURE 2.
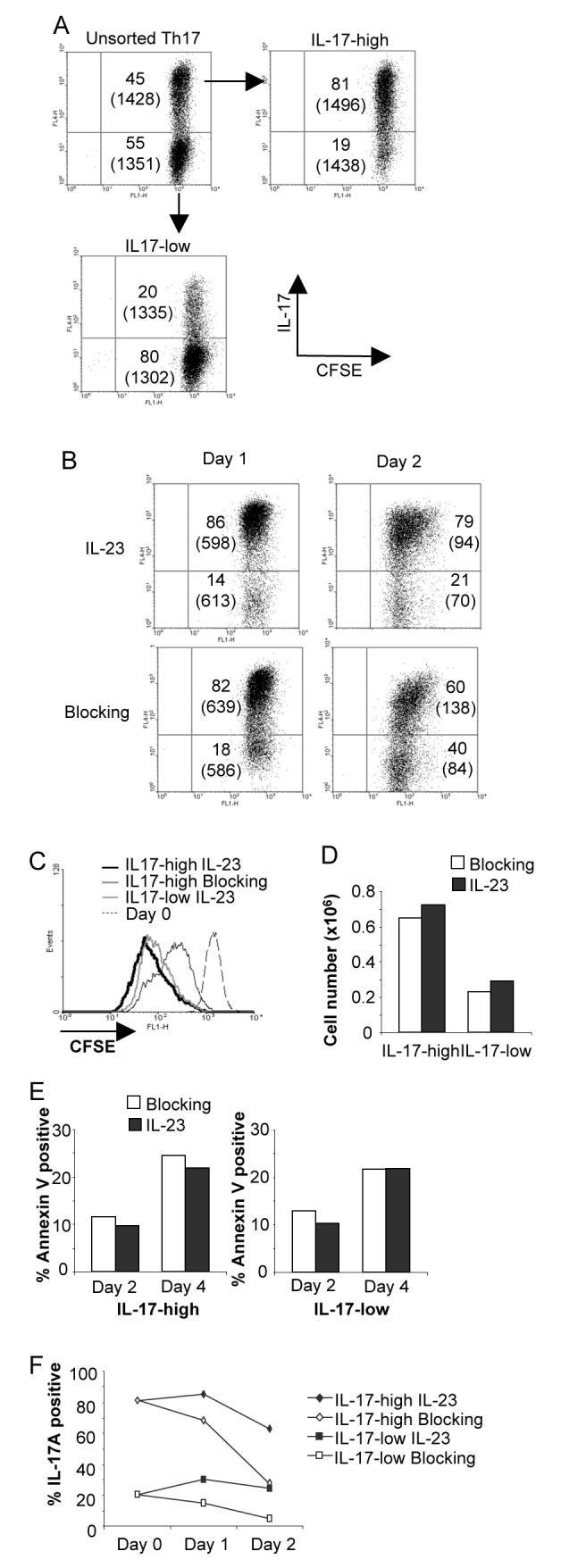
IL-23 maintains the IL-17 secreting phenotype without affecting cell expansion or survival. A, Naïve CD4+ T cells were activated, cultured in TGF-β+IL-6+IL-1β and blocking antibodies (anti-IFN-γ and anti-IL-4) for five days before sorting into IL-17-high and –low populations. Cells were then labeled with CFSE and for intracellular IL-17 following stimulation with PMA + ionomycin. Numbers indicate percent of cells in each quadrant and bracketed numbers indicate CFSE MFI. B, IL-17-high cells were cultured with IL-23 and blocking antibodies or blocking antibodies alone for the indicated times before cells were stimulated and stained for intracellular IL-17. Numbers indicate percent of cells in each quadrant and bracketed numbers indicate CFSE MFI. C, IL-17-high or -low CFSE-stained cells prepared as in B were cultured for two days with blocking antibodies in the presence or absence of IL-23 as indicated. CFSE staining is shown from freshly stained cells (Day 0) for comparison. D, IL-17-high and -low cells cultured as in B were counted after 48 hours. E, IL-17-high and -low cells were cultured as in B and were analyzed for Annexin V staining after two or four days of culture in the presence or absence of IL-23. F, IL-17-high and-low cells were stimulated with anti-CD3 and cultured with blocking antibodies with or without IL-23. Cells were stimulated with PMA+Ionomyocin for 4 hours and stained for intracellular IL-17.
IL-23 was also proposed to affect Th17 survival. However, in examining the overall cell growth in IL-17-high and –low cells, we observed the increased proliferative capacity of IL-17-high cells that resulted in a 2-3 fold increase in cell number compared to IL-17-low cells, but only minor effects of IL-23 (Fig. 2D). Similarly, IL-17-high and -low cells cultured in the presence or absence of IL-23 had similar percentages of Annexin V+ cells after two or four days of culture with little effect of IL-23 culture (Fig. 2E).
In contrast, we did note that in IL-17-high populations cultured with IL-23, a higher percentage of cells, with a higher intensity of IL-17 staining, was maintained compared to cells cultured in the absence of IL-23 (Fig. 2B). The effects of IL-23 on maintaining the IL-17-secreting phenotype were even more dramatic when cells were activated with anti-CD3. After two days of activation, IL-17-high cells cultured in IL-23 still had over 60% IL-17+ cells, while cultures incubated in the absence of IL-23 has less than 30% IL-17+ cells (Fig. 2F). This effect was also observed in the IL-17-low cultures where IL-17+ cells comprised less than 10% of the population when cultured in the absence of IL-23 (Fig. 2F). These data suggest that IL-23 maintains the IL-17-secreting phenotype without detectable effects on Th17 cell proliferation or expansion.
IL-23 maintains the Th17 phenotype in long-term cultures
To test the ability of IL-23 to maintain the Th17 phenotype over long term culture, we cultured naïve CD4 T cells for one week with TGFβ + IL-6 + IL-1β, the latter which we found amplifies IL-23 responsiveness in vitro ((9) and data not shown), and then for subsequent rounds of stimulation with either blocking antibodies alone or IL-23 with blocking antibodies. Despite a high level of IL-17 in the initial cultures, IL-23 was only partially effective in attenuating the decrease of IL-17 production from T cells following subsequent rounds of stimulation, compared to blocking antibodies alone (Fig. 3A). IL-23 was effective in limiting IFNγ production from these cultures suggesting that the decrease in IL-17 production observed over multiple rounds of stimulation is not due to coincident increases in IFNγ production or increases in the percentage of cells that are IFNγ+.
FIGURE 3.

IL-1β increases IL-23 stimulated maintenance of the Th17 phenotype. A, Naïve T cells were activated, primed with TGF-β+IL-6+IL-1β for the first round and cultured for 2 additional rounds of stimulation in the presence of blocking antibodies with or without IL-23. After each round of culture cells were stimulated and cell-free supernatants were tested for cytokine production using ELISA. B, Naïve T cells were activated and primed as in (A) and cultured for 2 additional rounds of stimulation with IL-23, IL-1β, or IL-23+IL-1β as indicated. Cytokine production was measured using ELISA.
Given the similarity in autoimmune disease phenotype between IL-1R1- and IL-23p19-deficient mice, and that IL-1 enhances IL-23 responsiveness (15-17), we next tested the ability of IL-1β to cooperate with IL-23 in long term cultures. Naïve CD4 T cells were cultured as in Fig. 3A for the first week and then cultured for subsequent rounds of stimulation with IL-23, IL-1β, or a combination of IL-23 and IL-1β. While IL-1β was no more effective than IL-23 in attenuating the loss of IL-17 secretion over multiple rounds of stimulation, the combination of IL-23 and IL-1β was able to maintain a high level of IL-17 secretion over three rounds of stimulation (Fig. 3B). There were not dramatic differences in the growth or survival among these cultures over several rounds of culture (data not shown), suggesting that the effects of these cytokines are not on survival or expansion, but rather on maintaining the phenotype of the cells. The combination of IL-23 and IL-1β was similarly capable of maintaining higher levels of IL-21 and IL-22 secretion than IL-23 alone, although there were decreases in these cytokines over rounds of stimulation (Fig. 3B).
To define the mechanism for the ability of IL-1β to augment IL-23 function we first analyzed IL-23R expression in cells cultured with IL-23, IL1β or both cytokines for the second and third rounds of stimulation. While culture with IL-23 maintained or enhanced Il23r mRNA expression, there was no increased expression in cells cultured with IL-1β alone or with both cytokines (Fig. 4A). We then examined IL-23 signaling using flow cytometry to assess levels of phospho-Stat3 following acute stimulation of cultures incubated for three rounds in IL-23, IL-1β or both cytokines. IL-23 stimulated Stat3 phosphorylation in cells from each of the conditions with insignificant differences in the phospho-Stat3 levels among the conditions, suggesting that the effect of IL-1β was not altering IL-23 signaling (Fig. 4B). To test if IL-1β had altered the Il17 gene to make it more responsive to IL-23, we took advantage of an assay we previously described for the acute stimulation of IL-17 production by a combination of IL-23 and IL-18 (18). Naïve CD4 T cells primed with TGFβ + IL-6 + IL-1β for the first round of stimulation and cultured in IL-23 or IL-23 + IL-1β for two rounds of stimulation were re-stimulated with IL-23 + IL-18. Cells that were cultured with IL-23 + IL-1β generated higher amounts of IL-17 than cells cultured in IL-23 alone in response to IL-23 + IL-18 (Fig. 4C). Since analysis of Il23r expression in these cultures (Fig. 4A) showed only minor differences in expression, with slightly lower levels in the IL-23 + IL-1β cultured cells, it suggests that culture in IL-23 + IL-1β is directly affecting the responsiveness of the Il17 locus, although we did not find differences in the level of total histone acetylation between cells that were cultured with or without IL-1β (data not shown). IL-1β may enhance IL-23 function by activating cooperative transcription factors, or through indirect mechanisms including the ability to limit the inhibitory effects of IL-2 on Th17 development (19).
FIGURE 4.

IL-1b increases responsiveness of the Il17 locus. A, Naïve T cells were activated, primed with TGF-β+IL-6+IL-1β for the first round and cultured for 2 additional rounds of stimulation with IL-23, IL-1β, or IL-23+IL-1β as indicated. At the end of the first, second and third round of stimulation RNA was isolated from cells stimulated with anti-CD3 for 4 hours. Quantitative PCR of Il23r expression is shown as relative to Th1 cultures after the third round of stimulation. B, Cells cultured and stimulated as in (A) for three rounds were stimulated with IL-23 for 30 minutes before intracellular staining for phospho-Stat3. C, Naïve cells primed and cultured as in (A) were stimulated with IL-18 and IL-23 for 24 hours. Cell free supernatants were measured for IL-17 using ELISA.
IL-23 does not mediate commitment to the Th17 lineage
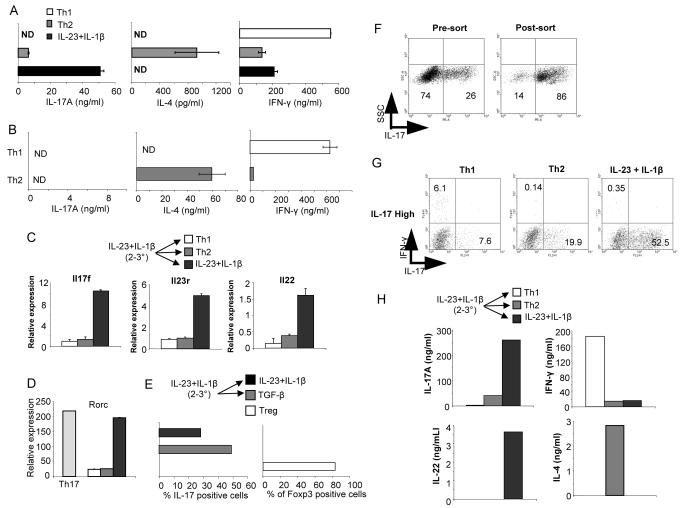
Since IL-23 was capable of maintaining the IL-17-secreting phenotype, it allowed us to determine if IL-23 mediated commitment to the Th17 lineage, commitment being defined as the ability of cells to maintain the IL-17-secreting phenotype in the presence of cytokines promoting the development of other subsets. Th1 and Th2 cells are committed to their respective lineages after three rounds of stimulation (20). Naïve CD4 T cells were primed with TGFβ + IL-6 + IL-1β for the first round, cultured for two rounds in IL-23 + IL-1β and then either maintained in IL-23 + IL-1β or switched to IL-12 or IL-4 containing media for the fourth round of stimulation before stimulation with anti-CD3 to assess cytokine production. While cells cultured for the fourth round in IL-23 + IL-1β maintained the ability to produce IL-17, cells switched to Th1 or Th2 promoting conditions showed diminished IL-17 production and the induction of IFNγ and IL-4, respectively (Fig. 5A and B). Similar results were generated using cultures derived from C57BL/6 or Balb/c mice (data not shown). The levels of IL-4 induced following switching into Th2 conditions were lower than seen from cells cultured for four weeks under Th2 conditions, though the levels of IFNγ secreted by Th17 cultures switched to Th1 conditions were comparable to long term Th1 cultures (Fig. 5A and B). Expression of other Th17 genes including Il17f, Il23r, Il22 and Rorc were also diminished in cultures switched to Th1 or Th2 conditions (Fig. 5C and D). In contrast, naïve CD4 T cells primed with TGFβ + IL-6 + IL-1β for the first round, cultured for two rounds in IL-23 + IL-1β, and subsequently switched to culture conditions that promote Treg development were unable to develop into Foxp3 expressing cells (Fig. 5E). Culture of cells with TGFβ + IL-2 increased the percentage of IL-17+ cells. Thus, Th17 cells cultured with IL-23 are not able to adopt all other CD4 T cell lineages.
FIGURE 5.
IL-23 does not program commitment to the Th17 lineage. A, Naïve T cells were activated, primed with TGF-β+IL-6+IL-1β for the first round followed by 2 rounds of stimulation in IL-23 + IL-1β, were cultured for an additional round of stimulation in Th1 or Th2 conditions, or IL-23+IL-1β. Supernatants from anti-CD3 stimulated cells were tested for cytokine production using ELISA. B, Naïve CD4+ T cells were activated and cultured under Th1 or Th2 priming conditions for four rounds of stimulation. At the end of the fourth round of stimulation, supernatants from anti-CD3 stimulated cells were tested for cytokine production using ELISA. C, Cells stimulated and cultured as in (A) were stimulated with anti-CD3 for 4 hours and RNA was isolated for qPCR. D, Cells were stimulated and cultured for three rounds as in (A). After three days of culture in Th1, Th2 or IL-23+IL-1β conditions, RNA was isolated from control or switched cultures for qPCR. E, Naïve T cells were activated, primed with TGF-β+IL-6+IL-1β for the first round followed by 2 rounds of stimulation in IL-23 + IL-1β, were cultured for an additional round of stimulation in IL-23 + IL-1β or TGF-β + IL-2. The percentages of cells positive for Foxp3 or IL-17 intracellular staining are indicated with cells cultured for one week in TGF-β + IL-2 shown as a control for Foxp3 expression. F, Naïve CD4+ T cells were primed and cultured as in (A) and after the third round of culture, cells were enriched for IL-17-secreting cells by cytokine selection. Surface staining for IL-17 is shown pre- and post-sort. G, IL-17-high cells from (F) were cultured in Th1, Th2 or IL-23+IL-1β for an additional round of stimulation. Cells were stimulated for 4 hours and stained for intracellular IL-17 and IFN-γ. H, Supernatants from anti-CD3 stimulated cells cultured as in (G) were tested for cytokine production using ELISA.
While T cells cultured for three rounds under these Th17 conditions produce large amounts of IL-17, there is still some production of IFNγ and it remained possible that a contaminating population of cells was expanding and overtaking the Th17 cells upon switching cultures to Th1 or Th2 conditions. To eliminate this possibility, we used the cytokine capture protocol to isolate IL-17-high cells from Th17 cultures after three rounds of stimulation (Fig. 5F) and then maintained the cultures with IL-23+IL-1β or switched to conditions promoting Th1 or Th2 development. As observed with unseparated Th17 cultures, IL-17-high cells maintained their phenotype with continued culture in IL-23 + IL-1β, but showed decreased IL-17 and IL-22 production, and increased IFNγ and IL-4 production in Th1 and Th2 conditions, respectively (Fig. 5G and H). Thus, IL-17-high cells are not stable secretors of IL-17 and upon exposure to conditions promoting the development of other Th subsets, they acquire new cytokine secreting characteristics.
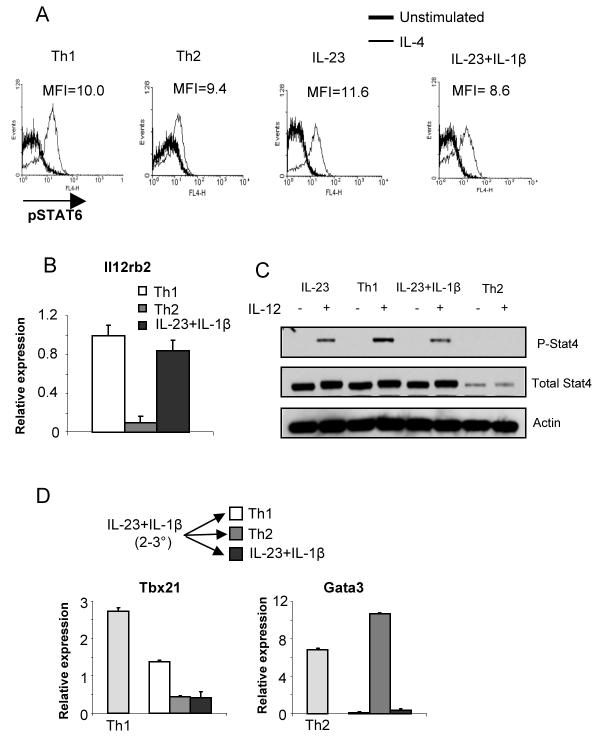
We then defined if the switch from Th17 to Th1 or Th2 was characterized by induction of the standard pathways and lineage determining factors. IL-4 signaling is qualitatively altered in Th1 cells through mechanisms that are still not clear but do not involve altered Stat6 activation (21) and IL-4 was able to activate Stat6 in cells cultured under Th1, Th2 or Th17 conditions (Fig. 6A). During Th2 development, IL-12 signaling is extinguished, contributing to commitment in the Th2 lineage (22). However, while Il12rb2 expression was greatly decreased in cells cultured for three rounds in Th2 conditions, cells cultured in IL-23 + IL-1β demonstrated expression of Il12rb2 similar to Th1 cells (Fig. 6B). To determine if IL-12 signaling was functional, we examined Stat4 expression and IL-12-induced phosphorylation of Stat4 in Th1, Th2 and Th17 cultures after three rounds of stimulation. While Stat4 expression is reduced and IL-12-induced Stat4 phosphorylation is eliminated in Th2 cultures, normal expression of Stat4 was retained in Th17 cultures. IL-12-induced Stat4 activation was only modestly diminished in IL-23+IL-1β and IL-23 cultured cells compared to Th1 cultures (Fig. 6C). Despite the reduction in IL-12-induced Stat4, Th1 conditions were still able to promote an increase in Tbx21 and Gata3 expression in Th17 cultures switched to Th1 or Th2 conditions, respectively (Fig. 6D). Thus, Th17 cells, even after long-term culture, are competent to assume a Th1 or Th2 phenotype.
FIGURE 6.
Signals promoting Th1 or Th2 development are intact in Th17 cultures. A, Cells stimulated and cultured as in Figure 5A and 5B were incubated with IL-4 for 30 minutes and stained for phospho-Stat6. B, RNA from cells stimulated as in (A) for three rounds of stimulation was examined for relative levels of Il12rb2 expression. Levels are relative to three-round Th1 cultures. C, Cells stimulated and cultured as in (A) for 3 rounds of stimulation were then stimulated with IL-12 for 1 hour. Phospho-Stat4, total Stat4 and actin were detected by immunoblot. D, Cells were stimulated and cultured for three rounds as in (A). After three days of culture in Th1, Th2 or IL-23+IL-1β, RNA was isolated from control or switched cultures for qPCR to test for expression of the indicated genes. Expression is relative to the level of expression of each gene in IL-23 + IL-1β cultured cells before the fourth round of culture.
Discussion
IL-23 is a critical cytokine in the development of inflammation. However, the cellular activities of IL-23 have not been well defined. In this report we demonstrate that IL-23 maintains a Th17 phenotype in the absence of promoting proliferation or survival, but that IL-23 cultured cells are not committed and can assume other cytokine secreting phenotypes. There are several reasons that it may be advantageous to have a transient Th17 phenotype. In the absence of multiple levels of control, the potent pro-inflammatory activity of these cells, if left unrestrained, could result in excessive tissue damage in vivo. In that respect, requiring several cytokines to establish and maintain the phenotype, while the presence of IL-4 or IL-12 effectively diminishes the phenotype, potentially limits the effect of Th17 cells and allows Th1 or Th2 cells to mediate progressive inflammation. The sensitivity of Th17 cells to the cytokine environment is consistent with the earliest reports of Th17 cells that demonstrated the importance of neutralizing interferons and IL-4 in the culture of IL-17-secreting T cells (6, 7). Our work takes this further by showing Th1- and Th2-promoting cytokines repress an established Th17 gene program. This sensitivity to other cytokines may also be reflected in vivo. In EAE models, where Th17 cells are clearly important for the initiation of disease, analysis of lymphocytes from the target organ show a heterogeneous population of IL-17- and IFNγ-secreting cells (23). Similarly, while IL-17 is required at early time points in the establishment of allergic inflammation, Th2 cells establish the cytokine environment characteristic of the allergic response. IL-17 administered to mice with established allergic disease inhibits inflammation (24). Together, these data suggest that, as we have shown for in vitro cultured cells, established Th17 cells in vivo may be inhibited by the appearance of other Th subsets where a changing cytokine environment results in repression of the Th17 phenotype.
IL-23 production may promote IL-17 production in at least two ways, by maintaining the IL-17-secreting potential of Th17 cells following antigen receptor stimulation and by directly activating Il17 in conjunction with IL-18 (18). The mechanism of IL-23 function at the level of the gene remains unclear. IL-23 requires Stat3 for activity and several reports have noted that IL-23-induced Stat3 can bind to the Il17 promoter and this might play a role in chromatin remodeling and histone acetylation at the locus (18, 25). Conversely, enhanced Stat3 activity potentiates IL-23 activity (18, 25). However, as other Stat3 activating cytokines have distinct effects on Th cell cytokine production, IL-23 must provide a qualitatively unique signal. As the synergy between IL-12 and IL-18 depends on the engagement of MAPK and NF-κB signals to cooperate with Stat4 signaling (3), it is likely that IL-23-activated Stat3 collaborates with other pathways activated by IL-18 or IL-1β. It is also possible that IL-23 inhibits the expression or function of other factors that inhibit the Th17 phenotype. For example, T-bet is a potent inhibitor of IL-17 production and anti-CD3 induced T-bet expression is responsible for changing cells from a Th17 to a Th1 cytokine secreting pattern in IL-23-stimulated cultures (14). In that culture system antigen receptor stimulation was responsible for decreasing IL-17 production and cells cultured in IL-23 without antigen receptor stimulation maintained IL-17-secreting potential. Thus, in the absence of commitment, the balance of activating and inhibitory signals a Th17 cell receives may determine the level of cytokine it will subsequently produce. It is still possible that IL-23 has effects on Th17 cell expansion or survival in vivo, though our results suggest that those effects must be indirect.
The plasticity of Th subsets is of considerable interest and is important in understanding how T cells regulate inflammation over time. In the long-term cultures we have described, the percentages of IL-17+ cells, and total IL-17 secretion, decrease over rounds of stimulation. Importantly, in the absence of cytokines that promote skewing to other phenotypes, cells that were secreting IL-17 do not appear to take on other phenotypes. We did not observe increases in IFNγ-secreting or Foxp3+ cells (data not shown) and the population seems to acquire cells that do not secrete any of the cytokines examined. Whether this is conversion of Th17 cells to non-secretor phenotypes or expansion of uncommitted cells is unclear. Following exposure to skewing conditions, Th17 cells appear to have differing abilities to acquire other phenotypes. For example, while IL-23-cultured IL-17+ Th17 cells may become Th1 or Th2 phenotype cells, we observe that culture of long-term Th17 cells with TGFβ + IL-2 did not convert them into Foxp3 expressing cells, but rather increased IL-17 production (Fig. 5), suggesting that IL-23 cultured cells are not capable of subsequently becoming adaptive Tregs. Conversely, natural Tregs have been shown to adapt a Th17 phenotype when cultured with IL-6 (26, 27), though there is still not a consensus on the ability of adaptive Tregs to undergo this change of phenotype (27, 28). Because of the phenotypic overlap, IL-23 may also be important for polarizing Th cells to distinguish the Th17 and Tfh phenotypes (29-31). Even with our culture conditions optimized for maintaining IL-17 secretion, IL-21 levels still decrease over time, and IL-22 levels are lower than in some previous reports (Fig. 3)(11,12). While some of these effects might arise from the specific culture conditions, including the concentrations of cytokines added to the culture, it is possible that IL-23, as it maintains IL-17 production, limits IL-21 production, as it does for IL-10 production (13). Thus, even though IL-23 does not limit conversion to Th1 or Th2 lineages, it might restrict cells from adopting Treg or Tfh phenotypes.
The ability of IL-23 to promote inflammation in vivo likely involves several of the functions we have described including the acute induction of IL-17, functioning in concert with IL-18 or IL-1, and the ability of IL-23 to preserve the IL-17-secreting potential of T cells. However, in the absence of IL-23 and the presence of other cytokine environments, IL-17 production can be diminished in favor of establishing new patterns of cytokine secretion. The transient Th17 phenotype may be a key to understanding how Th cell control may evolve during inflammation.
Acknowledgements
The authors thank J Blum, C-H Chang, A Mathur and A Dent for their suggestions on this manuscript and M Mwanthi for technical assistance.
Footnotes
This work was supported by U.S. Public Health Service Award AI45515 (to M.H.K.) from the National Institutes of Health. GLS and NY were supported by a Training Grant in Immunology and Infectious Disease (T32AI060519) and NY was supported by the DeVault Fellowship from the IU Cancer Biology Training Program.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 2.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 4.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 8.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 9.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O'Shea J J. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 13.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 14.Mathur AN, Chang HC, Zisoulis DG, Kapur R, Belladonna ML, Kansas GS, Kaplan MH. T-bet is a critical determinant in the instability of the IL-17-secreting T-helper phenotype. Blood. 2006;108:1595–1601. doi: 10.1182/blood-2006-04-015016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 17.Cho ML, Kang JW, Moon YM, Nam HJ, Jhun JY, Heo SB, Jin HT, Min SY, Ju JH, Park KS, Cho YG, Yoon CH, Park SH, Sung YC, Kim HY. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol. 2006;176:5652–5661. doi: 10.4049/jimmunol.176.9.5652. [DOI] [PubMed] [Google Scholar]
- 18.Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O'Malley JT, Kapur R, Levy DE, Kansas GS, Kaplan MH. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 19.Kryczek I, Wei S, Vatan L, Escara-Wilke J, Szeliga W, Keller ET, Zou W. Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J Immunol. 2007;179:1423–1426. doi: 10.4049/jimmunol.179.3.1423. [DOI] [PubMed] [Google Scholar]
- 20.Murphy E, Shibuya K, Hosken N, Openshaw P, Maino V, Davis K, Murphy K, O'Garra A. Reversibility of T helper 1 and 2 populations is lost after long-term stimulation. J Exp Med. 1996;183:901–913. doi: 10.1084/jem.183.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang H, Paul WE. Impaired interleukin 4 signaling in T helper type 1 cells. J Exp Med. 1998;187:1305–1313. doi: 10.1084/jem.187.8.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O'Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 27.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 29.Suto A, Kashiwakuma D, Kagami S, Hirose K, Watanabe N, Yokote K, Saito Y, Nakayama T, Grusby MJ, Iwamoto I, Nakajima H. Development and characterization of IL-21-producing CD4+ T cells. J Exp Med. 2008;205:1369–1379. doi: 10.1084/jem.20072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]