Abstract
Objective
Glomerular mesangial cells are active participants in pathogenesis of lupus glomerulonephritis (GN). Thus, targeted delivery of therapeutics to mesangial cells would be an attractive approach to treatment. However, lack of unique mesangial cell surface markers has hampered this process. The objective of this study was to identify mesangial marker(s) and develop a system for targeted drug delivery to the glomerulus.
Methods
Based on literature, alpha 8 (α8) integrin, expressed on surface of glomerular mesangial cells, was selected as a target molecule for delivery. Two mouse strains susceptible to lupus GN, NZM2328 and NZM2328×NOD F1 were studied. Glomerular expression of α8 integrin in normal and nephritic mice was confirmed by immunofluorescence and QPCR. Liposomes were formulated and conjugated with an anti-α8 integrin antibody. These immuno-liposomes (ILs) were loaded with DiI, a red fluorescent dye, to allow tracking in vivo and injected into the tail vein of female mice at different ages. Specificity of targeting was studied by fluorescence microscopy and flow cytometry.
Results
α8 integrin is expressed in glomeruli of normal and nephritic mice. Anti-α8 integrin ILs injected into the tail vein, traffic to the glomerulus and glomerular mesangial cells in normal and nephritic mice. The DiI delivery by anti-α8 integrin ILs was tissue specific, predominantly to glomeruli with some non-specific uptake by CD11b cells.
Conclusions
This is the first demonstration of specific delivery to mesangium following tail vein injection in mice. The anti-α8 integrin ILs offer a novel approach for targeted drug therapy in lupus and other glomerular diseases.
Keywords: Kidney, Lupus, Drug delivery, immunoliposomes, alpha 8 integrin
Renal failure contributes significantly to the morbidity associated with Systemic Lupus Erythematosus (SLE). However, the molecular mechanisms of renal injury and progressive renal failure are complex and not completely understood. Recently, there has been increasing evidence that end organ susceptibility to disease, local milieu in the kidney and active participation by renal cells play important roles in pathogenesis of lupus glomerulonephritis (GN) (1-6). This, in turn, identifies a clear role for end organ targeted therapies in treatment of lupus GN and a new area for investigation.
In SLE, systemic autoimmune responses lead to glomerular immune complexes and GN. In MRL lpr/lpr mice, glomerular immune complex deposition is associated with a rapid increase in MCP-1 and RANTES production by glomerular mesangial cells (7). This is followed by inflammatory cell infiltration into the glomeruli and progressive renal disease characterized by glomerulosclerosis, interstitial inflammation, fibrosis, and tubular atrophy. Thus, mesangial cell responses in the form of inflammatory cytokine secretion, proliferation, and extracellular matrix production have been implicated as critical elements for progressive GN (8). Our studies in NZM2328, a murine model of spontaneous SLE, also implicate an important role for a local immune response in disease progression (2). Clearly, drug delivery specifically to the mesangium and modulation of mesangial cell responses are potential avenues for therapy. However, targeting of mesangial cells using antibodies or receptor ligands has been hampered because there are no currently identified cell surface markers unique to the murine or human mesangial cells.
Liposomes are a vehicle of choice for targeted drug delivery (9). Liposomes allow incorporation of hydrophobic drugs within the lipid bilayer and hydrophilic drugs in the central aqueous void volume. Significantly, liposomes can be conjugated to antibodies on their surface to form immuno-liposomes (ILs). ILs have been used for site-specific drug delivery in cancer treatments (10, 11). In this study, we have explored the use of ILs as vehicles for targeted delivery to the glomerulus, specifically to the glomerular mesangial cells. Since human and murine mesangial cells lack unique cell surface markers, our first task was to identify suitable target molecules on the mesangial cells. The integrin family of receptors is expressed on surface of mesangial cells (12). On the mesangial cells, the β1 integrin combines with α1, α3, αv, or α8 integrin chains to form the functional heterodimeric proteins. These integrins have critical functions in glomerular development and interactions with extracellular matrix proteins. Several of the integrins are present on many different cell types including the vascular endothelium (13). In comparison, α8 integrin expression is relatively restricted on glomerular mesangial cells in mice (and humans), interstitial smooth muscle cells, and alveolar myofibroblasts in lung (14, 15). α8 integrin is also expressed on hippocampal dentate hilar neurons in the brain (16). Therefore, we selected α8 integrin as a molecule on the mesangial cells for immuno-liposomal targeting. Our study is the first demonstration of targeted mesangial delivery in mice following systemic injection into the tail vein and offers a new avenue for therapeutic strategies in renal disease.
Materials and Methods
Preparation of liposomes
Liposomes were prepared following standard procedures (17) Briefly, Lipids: 1,2-Distearoyl-sn-Glycero-3-Phosphocholine (DSPC), Cholesterol, 1,2-Distearoyl-sn-Glycero-3-Phosphoethanolamine-N[Amino(PolyethyleneGlycol)2000] (PEG), 1,2-Distearoyl-sn-Glycero-3-Phosphoethanolamine-N-[Maleimide (Polyethylene Glycol)2000] (PEG-MAL) (Avanti Polar) were dissolved in chloroform and mixed in a glass tube in the following percentages: 63.0% (DSPC), 27.0% (Cholesterol), 7.3% (PEG), 2.7% (PEG-MAL). A lipophilic red fluorescent dye DiI (1,1V-dioctadecyl-d,d,d′,d′-tetramethylindocarbocyanine) (Invitrogen Corporation) was added to the lipid mixture at a concentration of 4μg/mg of lipid. After mixing, the solvents were dried under nitrogen and the lipids were hydrated in 300mM citrate buffer, pH 4 at 65°C for 30 min. The liposomes were then sized by extrusion sequentially through 400nM and 80nM polycarbonate filters. Size and homogeneity of the liposomal preparation was confirmed by electron microscopy. The anti-α8 integrin antibody (Santa Cruz Biotechnologies) was thiolated using 2-Iminothiolane HCl and mixed with the sized liposomes overnight at room temperature (18). The liposome-antibody conjugate was dialyzed against PBS and sized ILs stored at 4°C till use. Purified rabbit IgG (Pierce Corporation) was used to prepare control rabbit IgG ILs.
Mice
Female NZM2328 and NZM2328×NOD (NZM/NOD) F1 mice used for these studies were bred and maintained in a specific pathogen free mouse facility at the University of Virginia. All experimental procedures were approved by the Institutional Animal Care and Use Committee. The NZM2328 mouse strain is a well-established model of spontaneous SLE (19, 20). Female NZM2328 mice develop autoantibodies and lupus-like glomerulonephritis leading to renal failure. (NZM/NOD) F1 female mice have been recently described by our group and develop SLE with nephritis similar to the NZM2328 strain (21). To study α8 integrin expression, mice were sacrificed at different times and tissues harvested. For IL targeting studies, female mice were injected twice, eight hours apart, with anti-α8 integrin ILs (0.63mg lipid/mouse) I.V. in the tail vein and studied 18hrs after the last injection. Mice were perfused with saline and tissues harvested. As previously described, one kidney was cut into pieces and collected in 10% buffered formalin for histopathology, 4% paraformaldehyde for immunostaining or snap frozen in liquid nitrogen for direct immunofluorescence and RNA isolation (2, 22). The other kidney was processed for glomerular isolation. Glomerular trafficking of anti-α8 integrin ILs was tested in pre-nephritic (n=4) and nephritic (n=9) female (NZM/NOD) F1 mice. Pre-nephritic (n=2) and nephritic (n=7) age matched mice injected with rabbit IgG ILs were used as controls. Similar experiments were also carried out in NZM2328 females (anti-α8 integrin ILs n=6; rabbit IgG ILs n=5).
Isolation of glomeruli
Mice were perfused with saline and kidneys harvested. The renal cortex was cut into pieces and digested with 1.2mg/ml collagenase (Worthington Biochemicals) for 20mins at 37°C. The suspension was washed in HBSS, passed twice through 100 μM filters, and purified glomeruli harvested off 50 μM filters. Glomeruli were either studied directly or after immunostaining by fluorescence microscopy. For some experiments, isolated glomeruli were also used to obtain primary podocyte cultures using standard methods (23). In some experiments, a rapid method of isolation for enriched glomerular preparations was used (24). Mice were perfused with 5μM magnetic beads (Invitrogen Corporation) that get trapped in the glomerular capillaries. Following collagenase digestion and two passes through 100 μM filters, the suspension was held against a strong magnet. Because of the trapped magnetic beads, glomeruli adhered to the walls of the tube. The enriched glomerular isolates were washed twice with HBSS and used for RNA isolation.
Immunostaining of isolated whole glomeruli was carried out to study the distribution of liposomal uptake. The glomeruli were fixed in 4% paraformaldehyde and treated with 0.3% triton×100. Non-specific staining was blocked with normal goat serum, and the glomeruli were incubated with rabbit anti-vimentin antibody (Santa Cruz Biotechnologies). Bound antibody was detected using biotinylated goat anti-rabbit IgG antibody (Southern Biotechnology Associates) followed by neutravidin –FITC (Molecular Probes). All incubations and washes were carried out in Perm wash buffer (BD Biosciences). Images were captured on a Zeiss Confocal Microscope, and analyzed with LSM5 software. Staining of podocytes in glomeruli and in culture was done using goat anti-synaptopodin antibody (Santa Cruz Biotechnology) followed by biotinylated rabbit anti-goat IgG (Southern Biotechnology Associates). Bound antibody was detected with neutravidin –FITC.
Immunostaining of kidney
IgG immune complex and C3 complement deposition was studied by direct immunofluorescence staining of frozen kidney sections as previously described (2,22). Distribution of α8 integrin in the kidney was studied on paraformaldehyde fixed kidney sections. The sections were incubated with rabbit anti-α8 integrin antibody (Santa Cruz Biotechnologies) followed by biotinylated goat anti-rabbit IgG. Staining was detected by Neutravidin FITC and images captured on a fluorescence microscope. Control sections were incubated with polyclonal rabbit IgG.
Flow cytometry
Single cell suspensions from blood, spleen, liver, lung, and brain were prepared. Cells were incubated with anti-CD16/32 (Fc block - Clone 93, eBiosciences) and stained with cychrome-conjugated anti-CD11b (clone M1/70). Data were collected on BD FACSCAN using Cell Quest software and 50-100,000 events/sample were acquired. The data were analyzed using FlowJo Software. Data are presented in Table 1 as percent DiI positive (FL2) of total cells and percent CD11b (FL3) of the DiI positive gate.
Table 1.
Uptake of ILs in non-renal tissues is predominantly by CD11b positive cells.
| Frequency | % DiI positive of total | %CD11b of DiI gate | ||
|---|---|---|---|---|
| Immunoliposomes | Rabbit IgG | Anti-α8 integrin | Rabbit IgG | Anti-α8 integrin |
| Spleen | 5.85±1.2* | 5.87±1.7 | 95.3 ±6.7 | 89.3 ±9.1 |
| Liver | 1.52±0.3 | 1.33±0.6 | 82.9 ±3.3 | 90.8 ±1.7 |
| Lung | 5.08±1.6 | 3.99±1.3 | 85.9± 8.4 | 91.4 ±4.4 |
| Blood | 2.15±0.5 | 2.67±0.7 | 79.9 ±9.5 | 90.1 ±5.0 |
| Brain | 0.16±0.04 | 0.09±0.02 | 91.7 ±4.6 | 80.5 ±17.0 |
Values are mean ± SD from 3 independent experiments.
Results
Alpha 8 integrin, a potential target molecule on mesangial cells, is expressed in glomeruli of lupus-susceptible mice
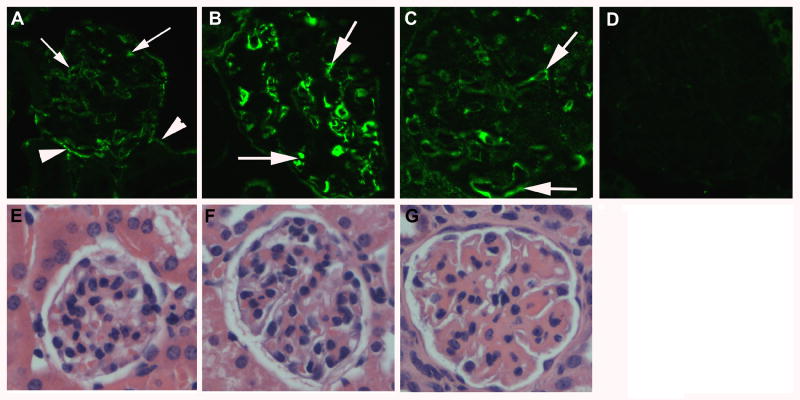
We first tested the expression of α8 integrin in the glomeruli of kidneys of two murine models of spontaneous SLE, NZM2328, and (NZM/NOD) F1. All experiments described in this paper were carried out in both mouse strains with similar results. The data from (NZM/NOD) F1 are presented. (NZM/NOD) F1 mice spontaneously develop SLE and lupus nephritis with kinetics similar to the NZM2328 mice (21). Glomerular immune complex deposition is seen starting at 5 months of age. Onset of acute proliferative GN occurs by 6-7 months that progresses to chronic GN by 7-10 months. To investigate the expression of α8 integrin protein at different stages of GN, kidney sections from mice at 3, 6, and 9 months of age were stained with an anti-α8 integrin antibody. Three female mice were studied at each time point. Alpha 8 integrin was detected in glomeruli at all ages. Cells positive for α8 integrin expression were also detected outside the glomeruli and in the interstitial regions. These likely represent the α8 integrin expressing interstitial smooth muscle cells. Representative pictures at each time point are shown (Figures 1A-C). Kidney sections incubated with control rabbit IgG did not stain (D). The mice were also evaluated for histopathological changes in the kidney to confirm the severity of renal disease. As shown in the bottom panel (figure 1), no pathological changes were evident at 3 months of age (E). At 6 months, early changes of mesangial expansion indicative of acute proliferative GN were seen (F). By 9 months, the glomeruli had undergone chronic changes (G). These data show that α8 integrin is expressed in normal as well as diseased glomeruli. Therefore, α8 integrin could be used as a potential target for drug delivery in nephritic mice.
Figure 1. α8 integrin is expressed in glomeruli of normal and nephritic mice.
Representative photomicrographs showing indirect immunofluorescence staining of sections from paraformaldehyde fixed kidneys of 3 (A), 6 (B) and 9 (C) month old (NZM/NOD) F1 female mice stained with a rabbit anti-α8 integrin antibody. Glomeruli show expression of α8 integrin (arrows) at all ages. Some interstitial cells (arrowheads) are also positive for α8 integrin expression. Section stained with rabbit IgG was used as negative control (D). Bottom panel (E-G) shows H&E stained sections of formalin fixed kidneys from the same mice showing normal glomerulus (E), early acute GN (F) and chronic GN (G).
Design of anti-α8 integrin immuno-liposomes
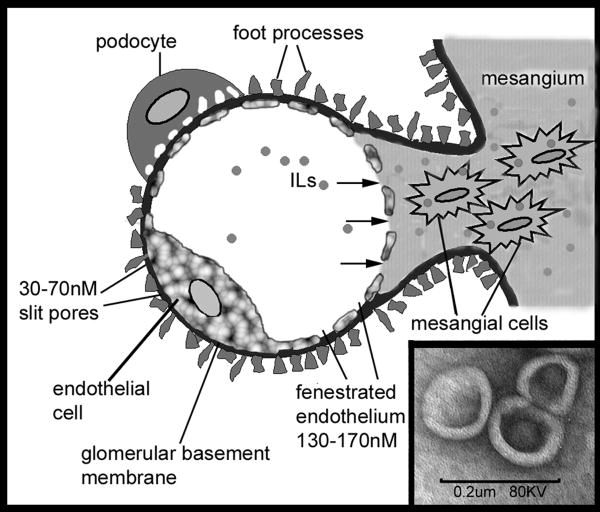
Anatomical structure of renal glomeruli and the unique interaction of mesangium with the vasculature form the basis of our immuno-liposomal targeting strategy (12, 25). A schematic diagram of a cross section through one glomerular capillary loop is shown in Figure 2. The blood flowing in the capillary lumen is filtered through the glomerular filtration assembly consisting of fenestrated endothelium, glomerular basement membrane, and podocyte epithelium. In the central regions of the glomerulus, the basement membrane between endothelial cells and the mesangial space is absent. This creates a unique communication between the blood and mesangial space through the endothelial fenestrations that are between the 130-170nM in size. Thus, liposomes <130nM in size can pass freely into the mesangium and can be taken up by the mesangial cells. In the filtration assembly, the slits between the podocyte foot processes range from 30-70nM. Thus, liposomes >70nM would not pass into the urinary space. Therefore, in our study, the liposomal preparation was extruded through serial filters to obtain liposomes between 70-130nM in diameter. An electron micrograph of one liposomal preparation with unilamellar liposomes about 100nM in diameter is shown in Figure 2 Inset.
Figure 2. Schematic representation of a glomerular capillary loop and the immuno-liposomal targeting strategy.
The capillary lumen is surrounded by a fenestrated endothelium, glomerular basement membrane and podocyte foot processes forming the filtration assembly. In the center, the glomerulus lacks the basement membrane allowing direct communication between the mesangial space with the circulation. Sized immunoliposomes (∼100nM diameter) from the circulation shown in the capillary lumen enter the mesangial space through the 130-170nM endothelial fenestrations (arrows) and are taken up by mesangial cells. The slit pores (30-70nM) between podocyte foot processes prevent loss of the liposomes into the urinary space. Inset: Electron micrograph of sized liposomal preparation showing unilamellar vesicles. Scale bar = 200nM.
Targeting of anti-α8 integrin ILs to mouse glomeruli
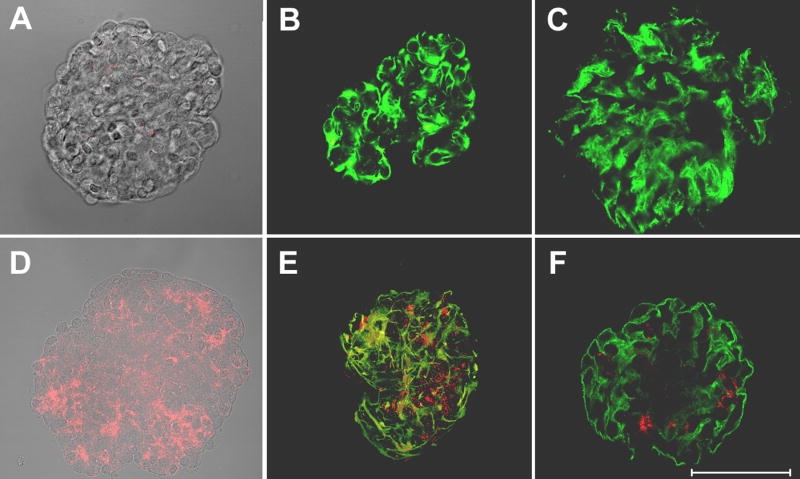
Anti-α8 integrin ILs and control rabbit IgG ILs were prepared as described in the methods section. All liposomal preparations included DiI, a red fluorescent dye, to allow tracking of liposomal uptake in vivo. Two-month-old (NZM/NOD) F1 mice were given two injections of either anti-α8 integrin IL or control rabbit IgG ILs in the tail vein, eight hours apart. The mice were sacrificed 18 hours later, glomeruli isolated using conventional filtration methods, and studied. Results from mice injected with control rabbit IgG ILs (Figure 3A-C) and anti-α8 integrin ILs (Figure 3D-F) are shown. All glomeruli isolated from anti-α8 integrin IL treated mice showed a speckled red fluorescence indicating liposomal delivery of DiI. (Figure 3D-F). Optical sectioning (Z-stack) through the glomeruli showed that the delivery of DiI was present throughout the glomeruli (data not shown). DiI was not detected in glomeruli from rabbit IgG IL treated mice (Figure 3A-C). Occasional specks of red fluorescence were seen in some glomeruli from these mice and an example is shown in Figure 3A. Fluorescence with phase overlays of representative isolated glomeruli are shown in 3A and 3D. Unconjugated, sized, DiI loaded liposomal preparations did not traffic to the kidney (data not shown).
Figure 3. Delivery of DiI, a fluorescent dye to glomerular mesangium and mesangial cells by anti-α8 integrin ILs in young, non-nephritic mice.
Glomeruli were isolated from mice injected with control rabbit IgG ILs (A-C) or anti-α8 integrin ILs (D-F). Red fluorescence distributed through out the glomeruli is due to liposomal delivery of DiI. The glomeruli were studied unstained (A, D), stained with anti-vimentin antibody (B,E) or anti-synaptopodin antibody. Photographs captured by optical sectioning on Zeiss confocal microscope using LSM software show 3D images of one representative glomerulus in each experiment. Panels A & D show a superimposition of phase and fluorescence images. Scale bar = 50μM.
To determine distribution of liposomal DiI delivery, isolated glomeruli were stained with antibodies to vimentin (Figure 3 B, E). Vimentin is an intermediate filament protein present in mesangial cells and podocytes in the glomeruli (26). The 3D images of stained glomeruli captured by confocal microscopy and optical sectioning are shown. Figure 3E shows large areas of yellow showing co-localization of the DiI (red) with vimentin staining (green). This clearly demonstrates successful intracellular delivery by the anti-α8 integrin ILs. In addition, there is significant accumulation of the DiI in the mesangial space.
To further discriminate between delivery into mesangial cells or podocytes, glomeruli were stained with antibody to synaptopodin, a podocyte cell-specific marker (23). There was either minimal or no colocalization of DiI with synaptopodin (Fig 3C, F). Thus, the anti-α8 integrin ILs are capable of specific delivery to the mesangium and the mesangial cells.
For additional evidence supporting mesangial targeting, mice were injected with anti-α8 integrin ILs, glomeruli isolated, and cultured on collagen-coated coverslips (23). By day 2, podocytes migrated out of the glomeruli. On day 4, these primary podocyte cultures on coverslips were stained with synaptopodin antibody to identify the podocytes, and studied by fluorescence confocal microscopy. None of the podocytes were positive for DiI (data not shown).
Anti-α8 integrin ILs can traffic into a nephritic kidney
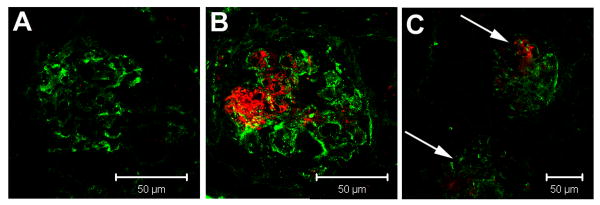
Ability to reverse or prevent progression of ongoing disease is the major requirement of any therapy in SLE. Thus, it was important to determine whether the specificity of targeting could be seen in nephritic mice. Therefore, anti-α8 integrin ILs were injected into 6-month-old (NZM/NOD) F1 female mice. At 6 months, female mice spontaneously have glomerular IgG immune complex deposits (Figure 4). Rabbit IgG ILs injected into age matched nephritic mice did not traffic into the glomeruli (Figure 4A). However, the presence of deposits did not interfere with trafficking of anti-α8 integrin ILs into the glomeruli (Figure 4B). Despite the pathologic changes in the kidney, liposomal delivery of DiI was restricted predominantly to the glomerulus. DiI was rarely seen outside the glomeruli or in the renal interstitium (Figure 4C). It should be noted that DiI uptake by the endothelial cells lining blood vessels was not seen.
Figure 4. Glomerular immune complex deposition does not prevent anti-α8 integrin immuno-liposomal targeting.
Six month old (NZM/NOD) F1 female mice were injected with DiI loaded control rabbit IgG ILs (A) or anti-α8 integrin ILs (B,C) and snap frozen kidney sections were stained for IgG immune complex deposits by direct immunofluorescence. Fluorescence microscopy showed glomerular IgG immune complex deposits in all the mice. Glomerular delivery of DiI was seen in anti-α8 integrin IL treated mice (B). Significantly, DiI in the nephritic mice was restricted to glomeruli (indicated by arrows) and not in the surrounding renal tubules or interstitium as shown at a lower magnification (C). Scale bar = 50μM.
Ability of anti-α8 integrin ILs to deliver DiI specifically into the glomeruli of 12-month-old surviving (NZM/NOD) F1 females was also studied. These females had persistent proteinuria >1000mg/dl indicating that they were severely nephritic. Injection of anti-α8 integrin ILs followed by staining of isolated glomeruli with anti-vimentin antibody showed large areas of co-localization with DiI. Few DiI positive cells also showed co-localization with synaptopodin staining. Thus, unlike normal mice, some leaking of the anti-α8 integrin ILs occurs into the podocytes of severely nephritic mice. However, these data clearly show the feasibility of using anti-α8 integrin ILs for targeted delivery to glomeruli even at advanced stages of disease.
Specificity of targeting
It was expected that the large size of the liposomes would block their passage through intact endothelium into other organs. This would prevent interaction between the anti-α8 integrin ILs with interstitial smooth muscle cells that express α8 integrin. To determine this, 6-month old female mice were injected with anti-α8 integrin or control rabbit IgG ILs. Single cell suspensions were obtained from spleen, liver, lung, brain, and peripheral blood and analyzed for DiI uptake by flow cytometry. Frequencies of cells positive for DiI uptake were low and ranged from 6% in spleen to 0.09% in brain of the total cells and were not significantly different between the two groups (Table 1). These data indicate that the uptake was not mediated through α8 integrin expression. Since macrophages are known to rapidly clear liposomes, the cells were stained with anti-CD11b antibody. As shown in figure 5, the cells taking up DiI were also CD11b positive in mice injected with either rabbit IgG ILs (Figure 5A) or anti-α8 integrin ILs (Figure 5B). This was also seen in blood, liver, lung, and brain where 80 to 95% of the cells in the DiI positive gate were CD11b positive (Table 1). Therefore, anti-α8 integrin liposomal delivery is infrequent in non-glomerular tissues. Moreover, such uptake is non-specific and by phagocytic cells.
Figure 5. Trafficking of DiI loaded anti-α8 integrin ILs into different tissues by flow cytometry and fluorescence microscopy.
Top Panel: Spleen cells from (NZM/NOD) F1 mice injected with rabbit IgG ILs (A) or anti-α8 integrin ILs (B) were stained with anti-CD11b antibodies and analyzed by flow cytometry. Representative dot plots of spleen cells from one mouse in each group showing DiI (FL2) and CD11b (FL3) positive cells. Note that DiI positive cells are also CD11b positive in both groups. Bottom Panel: Tissue distribution of DiI in interstitial regions was studied by fluorescence microscopy of DAPI stained frozen sections of livers (C-E) and spleens (F-H) from untreated (C&F), control rabbit IgG ILs treated (D&G), and anti-α8 integrin ILs treated mice (E&H).
In the glomeruli, liposomal delivery of DiI into the mesangial cells is clearly seen in our studies (Figure 3). However, Figure 3E also shows that significant amounts of liposomal DiI is extracellular and delivered into the mesangial space. Thus, organs like spleen and liver with open circulations might allow passive liposomal entry into the interstitium. To address this, the frozen sections from different organs were treated with DAPI for nuclear staining and studied by fluorescence microscopy. As shown in Figure 5, there was some red fluorescence in livers (top panels) and spleens (bottom panel) of mice that were injected with anti-rabbit IgG ILs or (5D, 5G) anti-α8 integrin IL (5E, 5H). However, this was not different from autofluorescence in livers and spleens from untreated mice (5C, 5F). Thus, liposomal delivery into the interstitial spaces other than the glomerulus was not detected with anti-α8 integrin ILs.
Discussion
Glomerular mesangial cells are critical players in renal function and disease. Mesangial cells are either myelomonocyte derived or of smooth muscle lineage (8). There are currently no known unique surface markers on murine and human mesangial cells adding to the difficulty in development of targeted drug delivery systems to these cells. In this paper, we have demonstrated that α8 integrin on the surface of mesangial cells can be used as a potential target for delivery of therapeutics selectively to the kidney. Although α8 integrin is expressed on other cell types in the body, it is not present on endothelial cells. Therefore, the use of sized immunoliposomes as vehicles for delivery circumvents the requirement for a unique cell surface marker. Since human mesangial cells are known to express α8 integrin, this approach can be extended for human therapy (12).
Three other liposomal systems have been used for delivery to glomeruli. However, these have significant disadvantages for human therapy. Delivery of oligonucleotides to murine mesangial cells has been achieved by injecting inactivated Sendai virus containing liposomes into the renal artery (27, 28). Due to the potential viral toxicity, immunogenicity, as well as the complex route of administration, this method cannot be used repeatedly and is not viable for clinical application.
In rats, using the strategy of sized ILs with a surface antibody to Thy1.1 (OX7), a cytotoxic drug doxorubicin was delivered to the mesangium (25). Thy1.1 antigen is expressed on rat mesangial cells. However, it is also expressed on many different tissues including T cells and will be easily accessible to the ILs in circulation. In addition, another limitation of OX7 ILs is that Thy1.1 is not expressed on human (or murine) mesangial cells. Thus, the OX7 ILs have limited application in treatment of human disease or study of murine models of nephritis.
Recently, dexamethasone-carrying immuno-liposomes with an antibody to E-selectin were used for treatment in an induced model of immune complex glomerulonephritis (29,30). Since E-selectin is upregulated on all inflamed vascular endothelium, this method will have limited capabilities in clinical setting that is accompanied by systemic inflammatory involvement. Thus, the currently available modalities cannot be extrapolated to therapeutic use in humans.
Our studies demonstrate that α8 integrin is a feasible candidate for targeted delivery. It is expressed in normal as well as nephritic kidneys in lupus. Analysis of gene expression by quantitative PCR in glomerular RNA also gave similar results (data not shown). Importantly, delivery by anti-α8 integrin ILs was specific to the kidney even in severely nephritic mice. Thus, targeted delivery by anti-α8 integrin ILs opens up a novel method for treatment of glomerular diseases in humans and will expand the repertoire of possible agents for therapy. For example, imantinib, a tyrosine kinase inhibitor of the Platelet Derived Growth Factor (PDGF) receptor increases survival in the NZB/W F1 mouse model of spontaneous SLE and would be potentially important in treatment of renal disease (31). However, systemic administration of PDGF tyrosine kinase inhibitor has shown to induce cardiac toxicity (32). Thus, targeted therapy will clearly be beneficial. TGFβ is another molecule that plays an important role in SLE. However, neutralizing systemic TGFβ in SLE is complicated because of its dual role in autoimmunity and end organ damage (33, 34). Systemically, TGFβ can activate regulatory T cells and act as an immunosuppressant; in the kidney, TGFβ is an important mediator of glomerular fibrosis. Thus, the anti-α8 integrin ILs could be used for modulating of TGFβ gene expression in the mesangial cells. In addition to lupus GN, this method can be used for treatment of a diverse range of chronic glomerular diseases and will facilitate the concept of end organ targeted therapies in renal disease.
Acknowledgments
The authors would like to thank Jan Redick, Advanced Microscopy Services, University of Virginia for help with confocal and electron microscopy. The authors acknowledge the helpful technical suggestions by Amy Vergis and Konstantine Khutsishvili, and critical comments by Dr. Mark Okusa, Division of Nephrology, University of Virginia. A US Provisional Patent Application has been filed by the University of Virginia, Patent Foundation on June 26, 2008, titled” Immunoliposomes: a Novel System or Delivery of Therapeutic Agents to the Renal Glomerulus.
Grant Support: National Institutes of Health NIDDK R01 DK069769
Footnotes
Author contributions: Dr. Bagavant had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Study Design: Scindia, Deshmukh, Bagavant
Acquisition of data: Scindia, Thimmalapura, Bagavant
Analysis and interpretation of data: Scindia, Thimmalapura, Deshmukh, Bagavant
Manuscript Preparation: Scindia, Deshmukh, Bagavant
Statistical Analysis: Scindia, Bagavant
References
- 1.Xie C, Zhou XJ, Liu X, Mohan C. Enhanced susceptibility to end-organ disease in the lupus-facilitating NZW mouse strain. Arthritis Rheum. 2003;48:1080–92. doi: 10.1002/art.10887. [DOI] [PubMed] [Google Scholar]
- 2.Bagavant H, Deshmukh US, Wang H, Ly T, Fu SM. Role for nephritogenic T cells in lupus glomerulonephritis: progression to renal failure is accompanied by T cell activation and expansion in regional lymph nodes. J Immunol. 2006;177:8258. doi: 10.4049/jimmunol.177.11.8258. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Bagavant H, Deshmukh US, Fu SM. Glomerular gene expression profiles in different stages of renal disease in a mouse model of systemic lupus erythematosus. Arthritis Rheum. 2006;9S:S788. [Google Scholar]
- 4.Qing X, Zavadil J, Crosby MB, Hogarth MP, Hahn BH, Mohan C, et al. Nephritogenic anti-DNA antibodies regulate gene expression in MRL/lpr mouse glomerular mesangial cells. Arthritis Rheum. 2006;54:2198–210. doi: 10.1002/art.21934. [DOI] [PubMed] [Google Scholar]
- 5.Tesch GH, Maifert S, Schwarting A, Rollins BJ, Kelley VR. Monocyte chemoattractant protein 1-dependent leukocytic infiltrates are responsible for autoimmune disease in MRL-Fas(lpr) mice. J Exp Med. 1999;190:1813–24. doi: 10.1084/jem.190.12.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shultz PJ, DiCorleto PE, Silver BJ, Abboud HE. Mesangial cells express PDGF mRNAs and proliferate in response to PDGF. Am J Physiol Renal Physiol. 1998;255:F674–8. doi: 10.1152/ajprenal.1988.255.4.F674. [DOI] [PubMed] [Google Scholar]
- 7.Lema P, Maier H, Nieto E, Vielhaur V, Luckow B, Mampaso F. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J Am Soc Nephrol. 2001;12:1369–82. doi: 10.1681/ASN.V1271369. [DOI] [PubMed] [Google Scholar]
- 8.Herrera GA. Plasticity of mesangial cells: a basis for understanding pathological alterations. Ultrastruct Pathol. 2006;30:471–9. doi: 10.1080/01913120600932594. [DOI] [PubMed] [Google Scholar]
- 9.Samad A, Sultana Y, Aqil M. Liposomal Drug Delivery Systems: An Update Review. Current Drug Delivery. 2007;4:297–305. doi: 10.2174/156720107782151269. [DOI] [PubMed] [Google Scholar]
- 10.Nellis DF, Giardina SL, Janini GM, Shenoy SR, Marks JD, Tsai R, et al. Preclinical manufacture of anti-HER2 liposome-inserting, scFv-PEG-lipid conjugate. 2. Conjugate micelle identity, purity, stability, and potency analysis. Biotechnol Prog. 2005;21:221–32. doi: 10.1021/bp049839z. [DOI] [PubMed] [Google Scholar]
- 11.Kontermann RE. Immunoliposomes for cancer therapy. Curr Opin Mol Ther. 2006;8:39–45. [PubMed] [Google Scholar]
- 12.Madsen K, Tisher CC. Anatomy of the kidney. Elements of Normal Renal Structure and Function. Brenner & Rector's The Kidney. (7th) 2004:3–72. [Google Scholar]
- 13.Kramer RH, Cheng YF, Clyman R. Human Microvascular Endothelial Cells Use β1 And β3 Integrin Receptor Complexes To Attach To Laminin. J Cell Bio. 1990;111:1233–43. doi: 10.1083/jcb.111.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnapp LM, Breuss JM, Ramos DM, Sheppard D, Pytela R. Sequence and tissue distribution of the human integrin alpha 8 subunit: a beta1-associated alpha subunit expressed in smooth muscle cells. J Cell Sci. 1995;108:537–544. doi: 10.1242/jcs.108.2.537. [DOI] [PubMed] [Google Scholar]
- 15.Wagner TE, Frevert CW, Herzog EL, Schnapp LM. Expression of the integrin subunit alpha8 in murine lung development. J Histochem Cytochem. 2003;51:1307–1315. doi: 10.1177/002215540305101008. [DOI] [PubMed] [Google Scholar]
- 16.Einheber S, Pierce JP, Chow D, Znamensky V, Schnapp LM, Milner TA. Dentate hilar mossy cells and somatostatin-containing neurons are immunoreactive for the alpha8 integrin subunit: characterization in normal and kainic acid-treated rats. Neuroscience. 2001;105:619–38. doi: 10.1016/s0306-4522(01)00205-6. [DOI] [PubMed] [Google Scholar]
- 17.Huwyler J, Wu D, Pardridge WM. Brain Drug Delivery Of Small Molecules Using Immunoliposomes. Proc Natl Acad Sci USA. 1996;93:14164–9. doi: 10.1073/pnas.93.24.14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pardridge WM. Gene Targeting In Vivo with Pegylated Immunoliposomes. Meth Enzymol. 2003;373:507–28. doi: 10.1016/S0076-6879(03)73032-8. [DOI] [PubMed] [Google Scholar]
- 19.Waters ST, Fu SM, Gaskin F, Deshmukh US, Sung SSJ, Kannapell CC, et al. NZM2328: A new mouse model of systemic lupus erythematosus with unique genetic susceptibility loci. Clin Immunol. 2001;100:372–83. doi: 10.1006/clim.2001.5079. [DOI] [PubMed] [Google Scholar]
- 20.Waters ST, McDuffie M, Bagavant H, Deshmukh US, Gaskin F, Jiang C, et al. Breaking tolerance to double stranded DNA, nucleosome and other nuclear antigens is not required for the pathogenesis of lupus glomerulonephritis. J Exp Med. 2004;199:255–64. doi: 10.1084/jem.20031519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sim D, Bagavant H, Scindia YM, Ge Y, Gaskin F, Fu SM, et al. Crossing of lupus-susceptible NZM2328 mice with diabetes prone NOD mice results in a hybrid strain with diverse autoantibody population and severe renal disease. 2008 Manuscript submitted. [Google Scholar]
- 22.Bagavant H, Tung KSK. Failure of CD25+ T cells from lupus-prone mice to suppress lupus glomerulonephritis and sialoadenitis. J Immunol. 2005;175:944–50. doi: 10.4049/jimmunol.175.2.944. [DOI] [PubMed] [Google Scholar]
- 23.Katsuya K, Yaoita E, Yoshida Y, Yamamoto Y, Yamamoto T. An improved method for primary culture of rat podocytes. Kidney Int. 2006;69:2101–6. doi: 10.1038/sj.ki.5000398. [DOI] [PubMed] [Google Scholar]
- 24.Takemoto M, Asker N, Gerhardt N, Lundkvist A, Johansson BR, Saito Y, et al. A New Method For Large Scale Isolation Of Kidney Glomeruli From Mice. American Journal Of Pathology. 2002;161:799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuffin G, Waelti E, Huwyler J, Hammer C, Marti HP. Immunoliposome targeting to mesangial cells: a promising strategy for specific drug delivery to the kidney. J Am Soc Nephrol. 2005;16:3295–305. doi: 10.1681/ASN.2005050485. [DOI] [PubMed] [Google Scholar]
- 26.Cortes P, Mendez M, Riser BL, Guerin CJ, Guez-Barbero AR, Hassett C, et al. F-Actin Fiber Distribution In Glomerular Cells: Structural And Functional Implications. Kidney Int. 2000;58:2452–61. doi: 10.1046/j.1523-1755.2000.00428.x. [DOI] [PubMed] [Google Scholar]
- 27.Isaka Y. Gene therapy targeting kidney diseases: routes and vehicles. Clin Exp Nephrol. 2006;10:229–235. doi: 10.1007/s10157-006-0442-7. [DOI] [PubMed] [Google Scholar]
- 28.Ito K, Chen J, Asano T, Vaughan ED, Jr, Poppas DP, Hayakawa M, et al. Liposome-mediated gene therapy in the kidney. Hum Cell. 2004;17:17–28. doi: 10.1111/j.1749-0774.2004.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 29.Asgeirsdottir SA, Zwiers PJ, Morselt HW, Moorlag HE, Bakker HI, Heeringa P, et al. Inhibition of proinflammatory genes in anti-GBM glomerulonephritis by targeted dexamethasone-loaded AbEsel liposomes. Am J Physiol Renal Physiol. 2008;294:554–61. doi: 10.1152/ajprenal.00391.2007. [DOI] [PubMed] [Google Scholar]
- 30.Asgeirsdóttir SA, Kamps JA, Bakker HI, Zwiers PJ, Heeringa P, van der Weide K, et al. Site-specific inhibition of glomerulonephritis progression by targeted delivery of dexamethasone to glomerular endothelium. Mol Pharmacol. 2007;72:121–31. doi: 10.1124/mol.107.034140. [DOI] [PubMed] [Google Scholar]
- 31.Zoja C, Corna D, Rottoli D, Zanchi C, Abbate M, Remuzzi G. Imatinib ameliorates renal disease and survival in murine lupus autoimmune disease. Kidney Int. 2006;70:97–103. doi: 10.1038/sj.ki.5001528. [DOI] [PubMed] [Google Scholar]
- 32.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011–9. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saxena V, Lienesch DW, Zhou M, Bommireddy R, Azhar M, Doetschman T, et al. Dual 2008. Roles of Immunoregulatory Cytokine TGF-β in the Pathogenesis of Autoimmunity-Mediated Organ Damage. J Immunol. 2008;180:1903–12. doi: 10.4049/jimmunol.180.3.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Albuquerque DA, Saxena V, Adams DE, Boivin GP, et al. An ACE inhibitor reduces Th2 cytokines and TGF-beta1 and TGF-beta2 isoforms in murine lupus nephritis. Kidney Int. 2004;65:846–9. doi: 10.1111/j.1523-1755.2004.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]